An Overview on Production of Lignocellulose-Derived Platform Chemicals Such as 5-Hydroxymethyl Furfural, Furfural, Protocatechuic Acid
Abstract
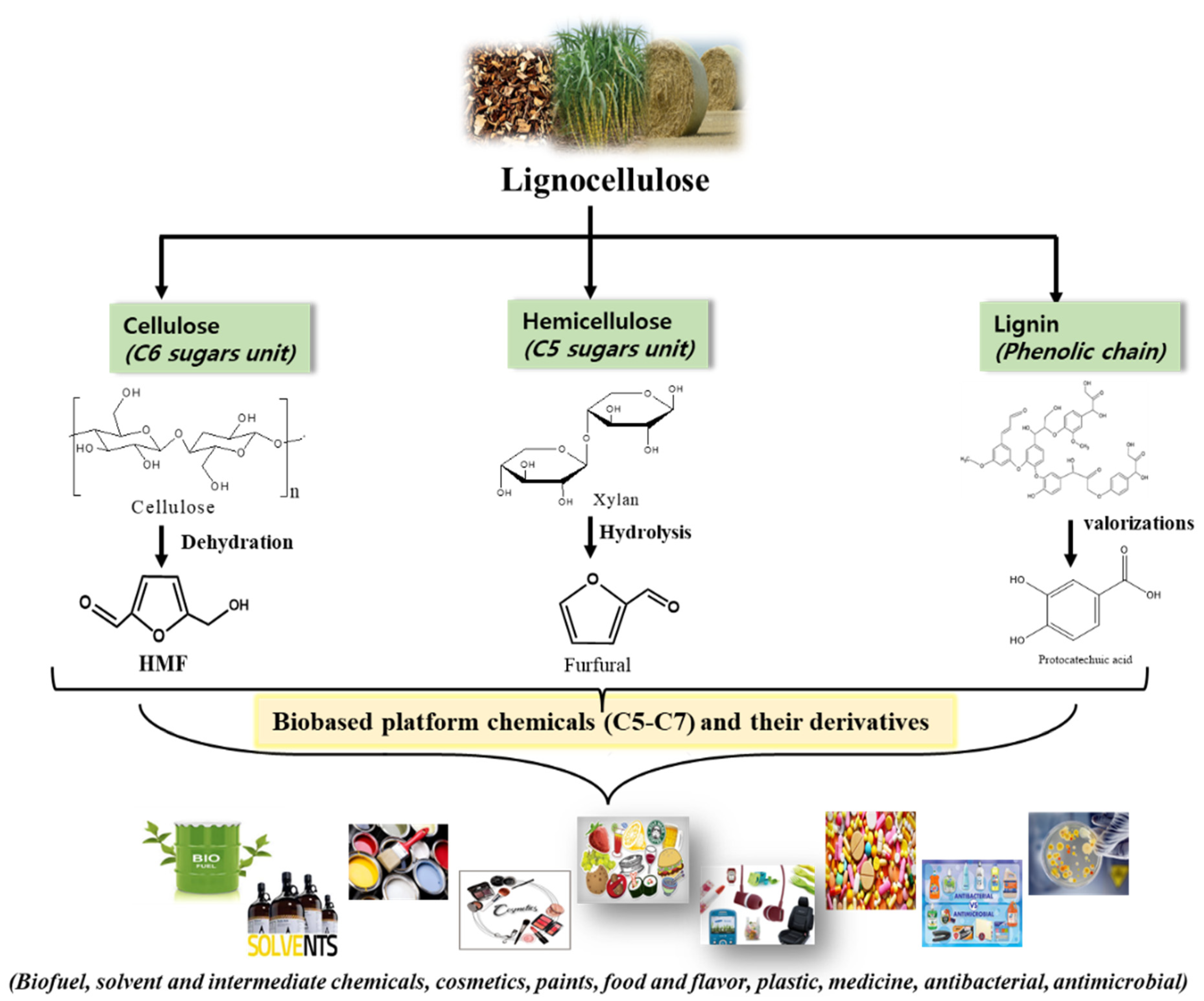
1. Introduction
2. Biomass-Derived Platform Chemicals
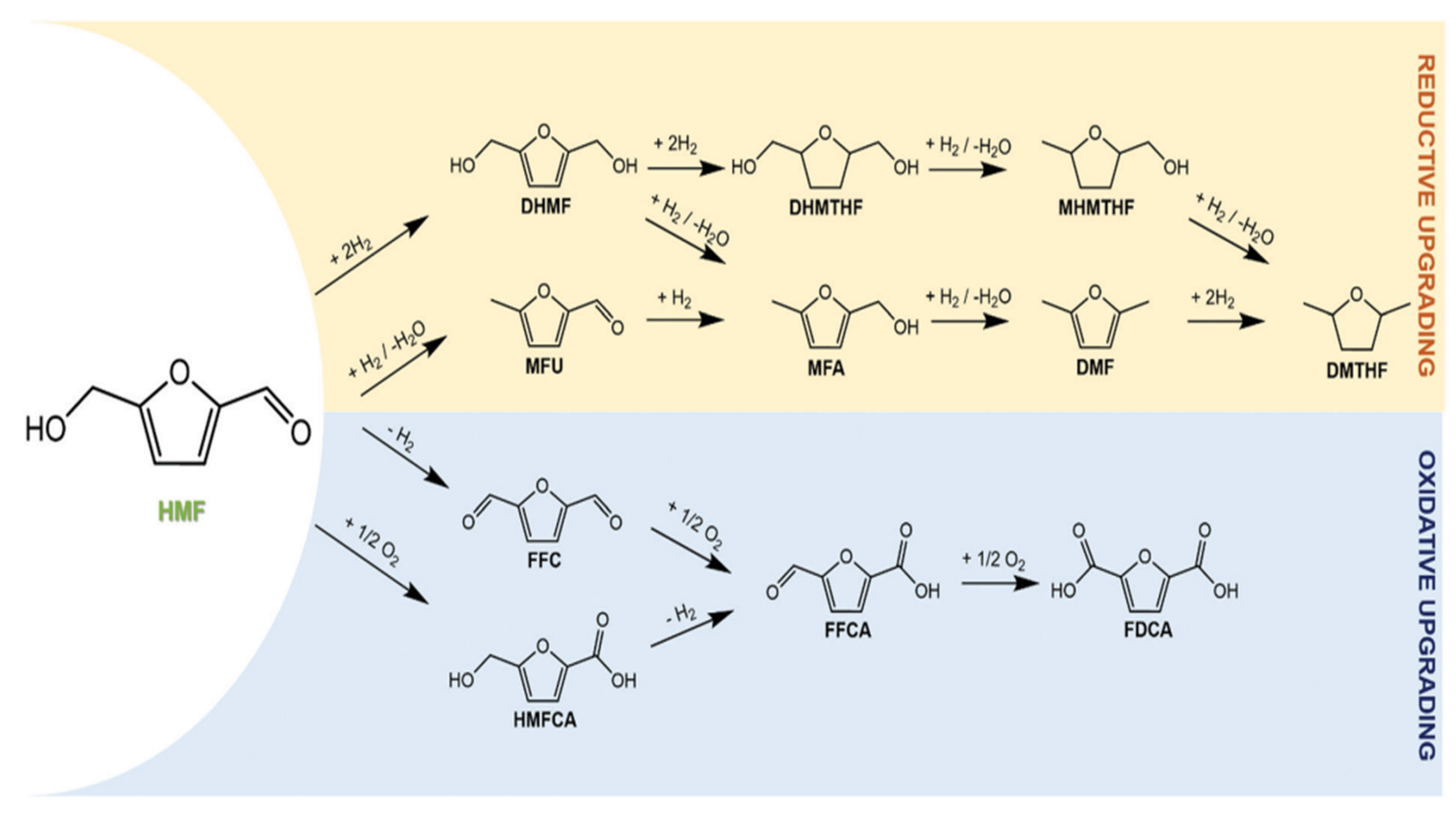
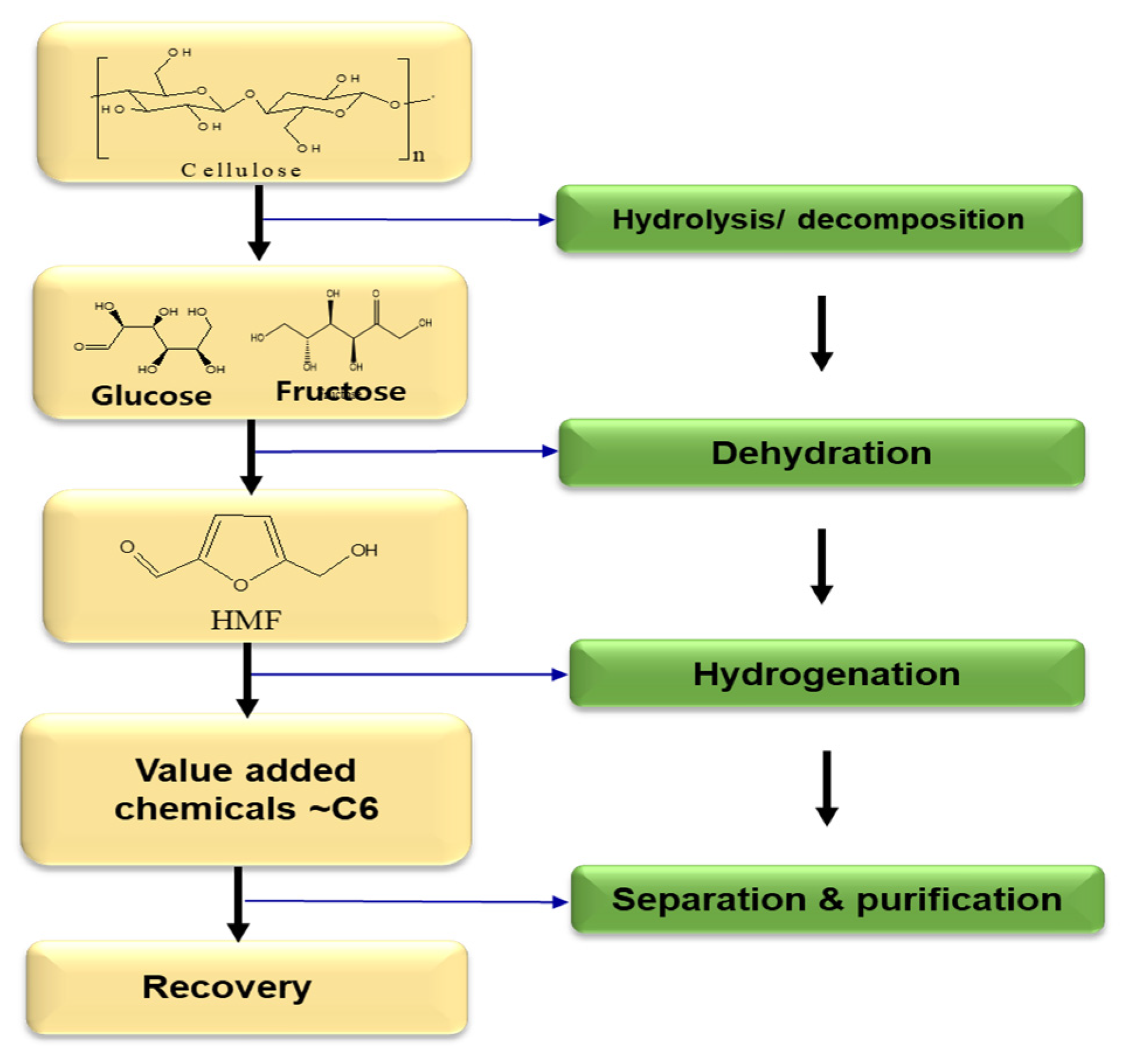
2.1. 5-Hydroxymethylfurfural (HMF)
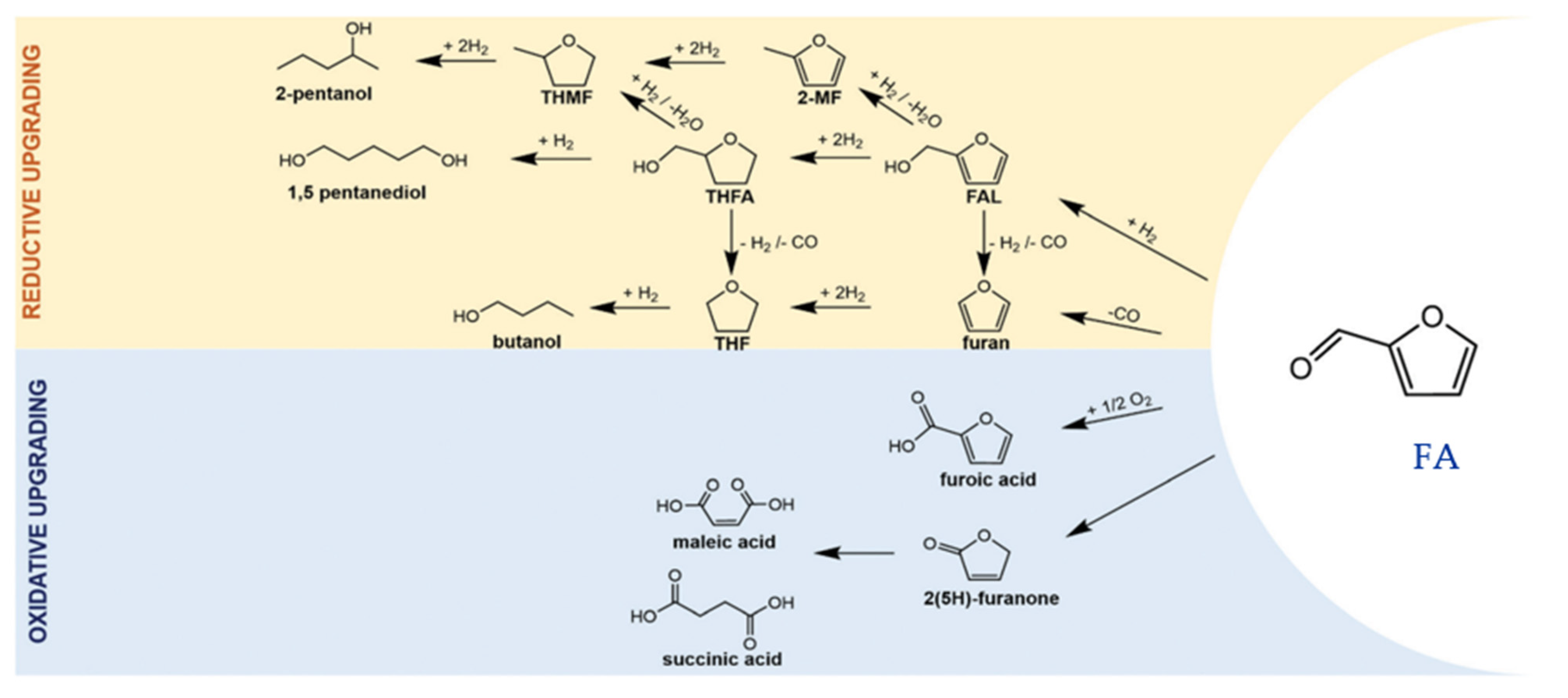
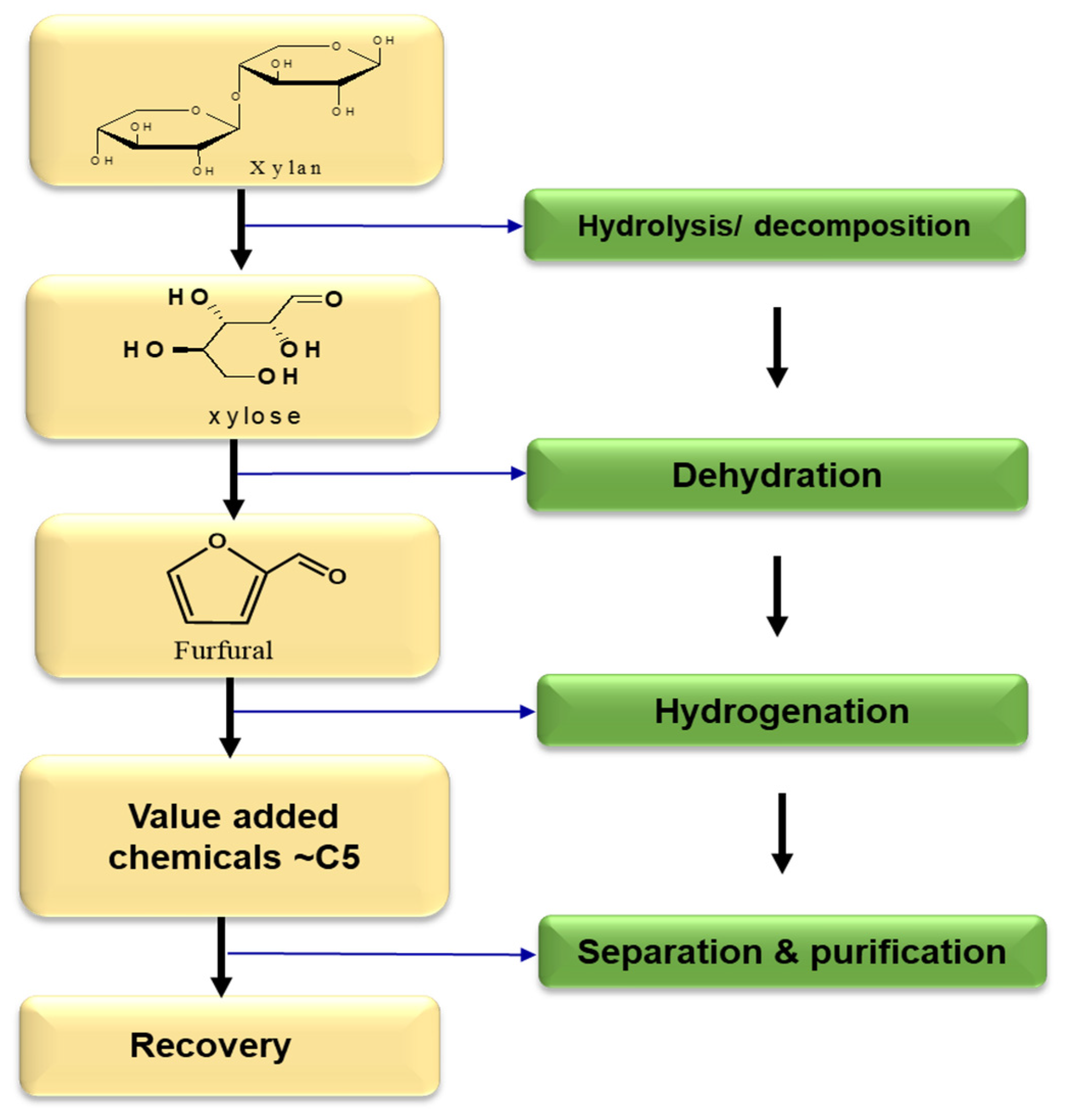
2.2. Furfural (FA)
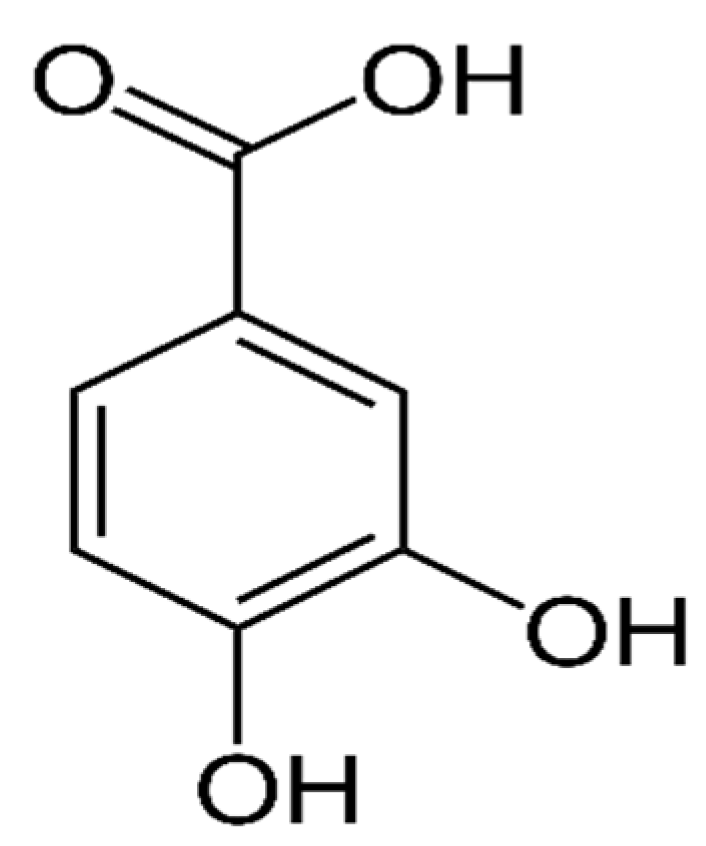
2.3. Protocatechuic Acid (PCA)
2.3.1. Synthesis of PCA from Lignin and Plants
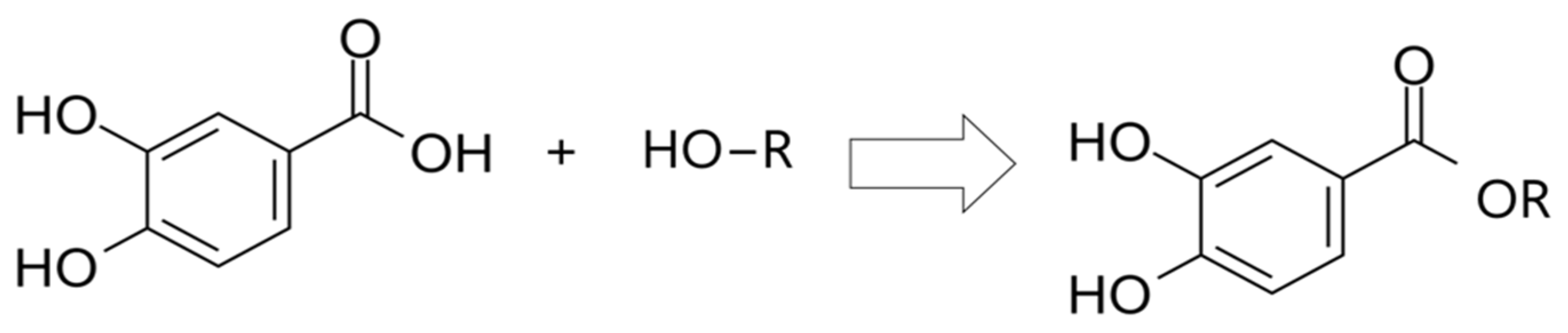
2.3.2. Protocatechuic Acid Derivatives
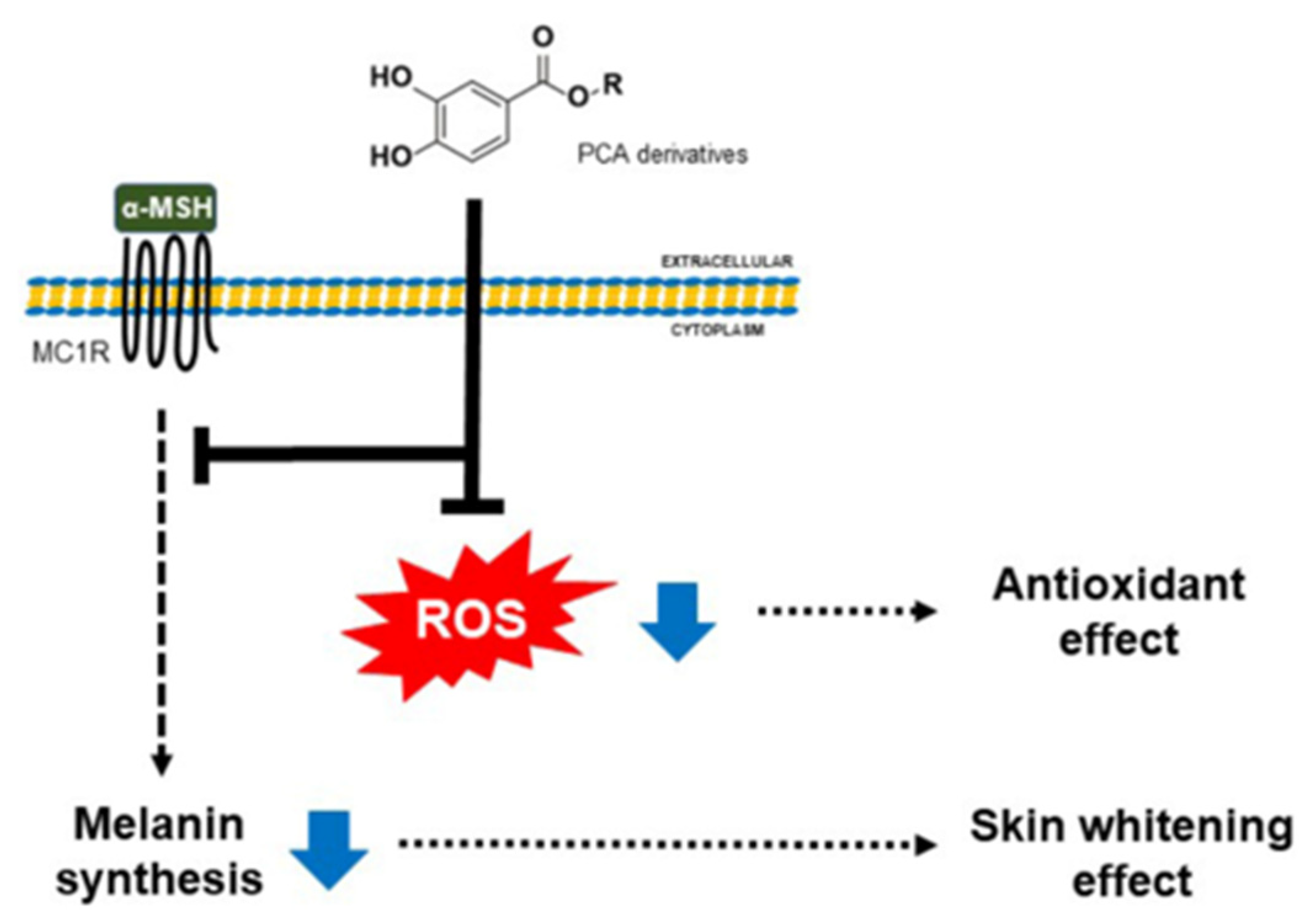
2.3.3. Effects of PCA Derivatives
3. Summary and Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem. 2013, 15, 584–595. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Dumesic, J.A. Catalytic routes for the conversion of biomass into liquid hydrocarbon transportation fuels. Energy Environ. Sci. 2011, 4, 83–99. [Google Scholar] [CrossRef]
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S.; Liu, S. Chemocatalytic hydrolysis of cellulose into glucose over solid acid catalysts. Appl. Catal. B Environ. 2015, 174–175, 225–243. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, G.; Hao, W.; Tang, X.; Sun, Y.; Lin, L.; Liu, S. Catalytic conversion of biomass-derived carbohydrates into fuels and chemicals via furanic aldehydes. RSC Adv. 2012, 2, 11184–11206. [Google Scholar] [CrossRef]
- De, S.; Saha, B.; Luque, R. Hydrodeoxygenation processes: Advances on catalytic transformations of biomass-derived platform chemicals into hydrocarbon fuels. Bioresour. Technol. 2015, 178, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Teong, S.P.; Yi, G.; Zhang, Y. Hydroxymethylfurfural production from bioresources: Past, present and future. Green Chem. 2014, 16, 2015–2026. [Google Scholar] [CrossRef]
- Rosini, E.; Molinari, F.; Miani, D.; Pollegioni, L. Lignin Valorization: Production of High Value-Added Compounds by Engineered Microorganisms. Catalysts 2023, 13, 555. [Google Scholar] [CrossRef]
- Zhang, R.-K.; Tan, Y.-S.; Cui, Y.-Z.; Xin, X.; Liu, Z.-H.; Li, B.-Z.; Yuan, Y.-J. Lignin valorization for protocatechuic acid production in engineered Saccharomyces cerevisiae. Green Chem. 2021, 23, 6515–6526. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Menegazzo, F.; Ghedini, E.; Signoretto, M. 5-Hydroxymethylfurfural (HMF) Production from Real Biomasses. Molecules 2018, 23, 2201. [Google Scholar] [CrossRef] [PubMed]
- Trapasso, G.; Mazzi, G.; Chícharo, B.; Annatelli, M.; Torre, D.D.; Aricò, F. Multigram synthesis of pure HMF and BHMF. Org. Process Res. Dev. 2022, 26, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, S.; Li, Q.; Zhou, G.; Xia, H. Recent advances in the conversion of furfural into bio-chemicals through chemo-and bio-catalysis. RSC Adv. 2021, 11, 27042–27058. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A.H.; Kim, H.; Hwang, I.T. Efficient selective dehydration of fructose and sucrose into 5-hydroxymethylfurfural (HMF) using dicationic room temperature ionic liquids as a catalyst. Catal. Commun. 2012, 21, 96–103. [Google Scholar] [CrossRef]
- Pérez-Maqueda, J.; Arenas-Ligioiz, I.; López, Ó.; Fernández-Bolaños, J.G. Eco-friendly preparation of 5-hydroxymethylfurfural from sucrose using ion-exchange resins. Chem. Eng. Sci. 2014, 109, 244–250. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- 5 hydroxymethylfurfural5 HMF Market Report 2023 (Global Edition), in, 2023.
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S.; Liu, S. Recent advances in catalytic transformation of biomass-derived 5-hydroxymethylfurfural into the innovative fuels and chemicals. Renew. Sustain. Energy Rev. 2017, 74, 230–257. [Google Scholar] [CrossRef]
- Kläusli, T. AVA Biochem: Commercialising renewable platform chemical 5-HMF. Green Process. Synth. 2014, 3, 235–236. [Google Scholar] [CrossRef]
- Dashtban, M.; Gilbert, A.; Fatehi, P. Recent advancements in the production of hydroxymethylfurfural. RSC Adv. 2014, 4, 2037–2050. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Sharma, B.K. Bio-Based Chemicals from Renewable Biomass for Integrated Biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef]
- Iriondo, A.; Agirre, I.; Viar, N.; Requies, J. Value-Added Bio-Chemicals Commodities from Catalytic Conversion of Biomass Derived Furan-Compounds. Catalysts 2020, 10, 895. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Upare, P.P.; Yoon, J.-W.; Kim, M.Y.; Kang, H.-Y.; Hwang, D.W.; Hwang, Y.K.; Kung, H.H.; Chang, J.-S. Chemical conversion of biomass-derived hexose sugars to levulinic acid over sulfonic acid-functionalized graphene oxide catalysts. Green Chem. 2013, 15, 2935–2943. [Google Scholar] [CrossRef]
- van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Román-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Morales, I.; Moreno-Recio, M.; Santamaría-González, J.; Maireles-Torres, P.; Jiménez-López, A. Mesoporous tantalum oxide as catalyst for dehydration of glucose to 5-hydroxymethylfurfural. Appl. Catal. B Environ. 2014, 154–155, 190–196. [Google Scholar] [CrossRef]
- Nikolla, E.; Román-Leshkov, Y.; Moliner, M.; Davis, M.E. “One-Pot” Synthesis of 5-(Hydroxymethyl)furfural from Carbohydrates using Tin-Beta Zeolite. ACS Catal. 2011, 1, 408–410. [Google Scholar] [CrossRef]
- Saenluang, K.; Thivasasith, A.; Dugkhuntod, P.; Pornsetmetakul, P.; Salakhum, S.; Namuangruk, S.; Wattanakit, C. In Situ Synthesis of Sn-Beta Zeolite Nanocrystals for Glucose to Hydroxymethylfurfural (HMF). Catalysts 2020, 10, 1249. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, L.; Zhao, S.; Wang, X.; Wang, S. High selective production of 5-hydroymethylfurfural from fructose by a solid heteropolyacid catalyst. Fuel 2011, 90, 2289–2293. [Google Scholar] [CrossRef]
- Upare, P.P.; Hwang, D.W.; Hwang, Y.K.; Lee, U.H.; Hong, D.-Y.; Chang, J.-S. An integrated process for the production of 2,5-dimethylfuran from fructose. Green Chem. 2015, 17, 3310–3313. [Google Scholar] [CrossRef]
- Upare, P.P.; Hwang, Y.K.; Hwang, D.W. An integrated process for the production of 2,5-dihydroxymethylfuran and its polymer from fructose. Green Chem. 2018, 20, 879–885. [Google Scholar] [CrossRef]
- Furfural Market Size, Share & Trends Analysis Report by Process (Quaker Batch Process), By Raw Material (Corn Cob), By Application (Furfuryl Alcohol), By End-use, By Region, And Segment Forecasts, 2023–2030, in, 2023.
- Li, X.; Jia, P.; Wang, T. Furfural: A Promising Platform Compound for Sustainable Production of C4 and C5 Chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- An, Z.; Li, J. Recent advances in the catalytic transfer hydrogenation of furfural to furfuryl alcohol over heterogeneous catalysts. Green Chem. 2022, 24, 1780–1808. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Rodríguez-Padrón, D.; Len, C. Recent Advances in Catalytic Hydrogenation of Furfural. Catalysts 2019, 9, 796. [Google Scholar] [CrossRef]
- Jaswal, A.; Singh, P.P.; Mondal, T. Furfural—A versatile, biomass-derived platform chemical for the production of renewable chemicals. Green Chem. 2022, 24, 510–551. [Google Scholar] [CrossRef]
- Jiang, Z.; Hu, D.; Zhao, Z.; Yi, Z.; Chen, Z.; Yan, K. Mini-Review on the Synthesis of Furfural and Levulinic Acid from Lignocellulosic Biomass. Processes 2021, 9, 1234. [Google Scholar] [CrossRef]
- Upare, P.P.; Hong, D.-Y.; Kwak, J.; Lee, M.; Chitale, S.K.; Chang, J.-S.; Hwang, D.W.; Hwang, Y.K. Direct chemical conversion of xylan into furfural over sulfonated graphene oxide. Catal. Today 2019, 324, 66–72. [Google Scholar] [CrossRef]
- Adom, F.; Dunn, J.B.; Han, J.; Sather, N. Life-cycle fossil energy consumption and greenhouse gas emissions of bioderived chemicals and their conventional counterparts. Environ. Sci. Technol. 2014, 48, 14624–14631. [Google Scholar] [CrossRef]
- Abdelmageed, M.E.; Shehatou, G.S.G.; Suddek, G.M.; Salem, H.A. Protocatechuic acid improves hepatic insulin resistance and restores vascular oxidative status in type-2 diabetic rats. Environ. Toxicol. Pharmacol. 2021, 83, 103577. [Google Scholar] [CrossRef]
- Habib, S.A.; Suddek, G.M.; Rahim, M.A.; Abdelrahman, R.S. The protective effect of protocatechuic acid on hepatotoxicity induced by cisplatin in mice. Life Sci. 2021, 277, 119485. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Palencia, L.A.; Talcott, S.T.; Safe, S.; Mertens-Talcott, S. Absorption and biological activity of phytochemical-rich extracts from açai (Euterpe oleracea Mart.) pulp and oil in vitro. J. Agric. Food Chem. 2008, 56, 3593–3600. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.-F.; An, L.-J.; Jiang, B.; Guan, S.; Bao, Y.-M. Alpinia protocatechuic acid protects against oxidative damage in vitro and reduces oxidative stress in vivo. Neurosci. Lett. 2006, 403, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Stagos, D.; Kazantzoglou, G.; Theofanidou, D.; Kakalopoulou, G.; Magiatis, P.; Mitaku, S.; Kouretas, D. Activity of grape extracts from Greek varieties of Vitis vinifera against mutagenicity induced by bleomycin and hydrogen peroxide in Salmonella typhimurium strain TA102. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2006, 609, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Antony, F.M.; Wasewar, K.L. The Sustainable Approach of Process Intensification in Biorefinery Through Reactive Extraction Coupled with Regeneration for Recovery of Protocatechuic Acid. Appl. Biochem. Biotechnol. 2023, 1–22. [Google Scholar] [CrossRef]
- Antony, F.M.; Wasewar, K.L. Ionic liquids as green solvents in process industry for reaction and separation: Emphasizing on protocatechuic acid recovery. Chem. Eng. Commun. 2023, 210, 2138–2145. [Google Scholar] [CrossRef]
- Upadhyay, P.; Lali, A. Protocatechuic acid production from lignin-associated phenolics. Prep. Biochem. Biotechnol. 2021, 51, 979–984. [Google Scholar] [CrossRef]
- Scazzocchio, E.; Figueras, F. Contemporary prediction of preeclampsia. Curr. Opin. Obstet. Gynecol. 2011, 23, 65–71. [Google Scholar] [CrossRef]
- Liu, Z.; Fang, S.; Moura, F.; Ding, J.; Jiang, N.; Di, J.; Zhang, M.; Lepró, X.; Galvao, D.; Haines, C. Hierarchically buckled sheath-core fibers for superelastic electronics, sensors, and muscles. Science 2015, 349, 400–404. [Google Scholar] [CrossRef]
- Okai, N.; Masuda, T.; Takeshima, Y.; Tanaka, K.; Yoshida, K.-I.; Miyamoto, M.; Ogino, C.; Kondo, A. Biotransformation of ferulic acid to protocatechuic acid by Corynebacteriumglutamicum ATCC 21420 engineered to express vanillate O-demethylase. Amb. Express 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Li, T.; Fan, J.; Wang, B.; Traugh, N.; Chen, Q.; Liu, J.S.; Li, B.; Liu, X.S. TIMER: A web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017, 77, e108–e110. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Protocatechuic Acid (CAS 99–50-3) Market Research Report 2023–2031, in 2022, p. 172.
- Tribot, A.; Amer, G.; Alio, M.A.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.-D.; Callois, J.-M.; Vial, C.; Michaud, P. Wood-lignin: Supply, extraction processes and use as bio-based material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Khan, A. Using films in the ESL classroom to improve communication skills of non-native learners. Elt. Voices 2015, 5, 46–52. [Google Scholar]
- Yang, X.-S.; He, X. Bat algorithm: Literature review and applications. Int. J. Bio-Inspired Comput. 2013, 5, 141–149. [Google Scholar] [CrossRef]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Durand, E.; Delavault, A.; Bourlieu, C.; Lecomte, J.; Baréa, B.; Espinoza, M.C.F.; Decker, E.A.; Salaun, F.M.; Kergourlay, G.; Villeneuve, P. Eleostearic phospholipids as probes to evaluate antioxidants efficiency against liposomes oxidation. Chem. Phys. Lipids 2017, 209, 19–28. [Google Scholar] [CrossRef][Green Version]
- Cuvelier, M.-E.; Richard, H.; Berset, C. Comparison of the antioxidative activity of some acid-phenols: Structure-activity relationship. Biosci. Biotechnol. Biochem. 1992, 56, 324–325. [Google Scholar] [CrossRef]
- Babcock, G.J.; Decker, L.L.; Volk, M.; Thorley-Lawson, D.A. EBV persistence in memory B cells in vivo. Immunity 1998, 9, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.J.; Nihei, K.-I.; Kubo, I. Lipoxygenase inhibitory activity of octyl gallate. J. Agric. Food Chem. 2004, 52, 3177–3181. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Fujita, K.-I.; Kubo, A.; Nihei, K.-I.; Ogura, T. Antibacterial activity of coriander volatile compounds against Salmonella choleraesuis. J. Agric. Food Chem. 2004, 52, 3329–3332. [Google Scholar] [CrossRef] [PubMed]
- Franks, N.; Lieb, W. Partitioning of long-chain alcohols into lipid bilayers: Implications for mechanisms of general anesthesia. Proc. Natl. Acad. Sci. USA 1986, 83, 5116–5120. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, K.H.; Song, Y.B.; Lee, J.-Y.; Lee, S.-J.; Lee, S.Y.; Kim, S.M.; Yun, K.H.; Cho, J.Y.; Kim, C.J. Intravascular imaging–guided or angiography-guided complex PCI. N. Engl. J. Med. 2023, 388, 1668–1679. [Google Scholar] [CrossRef]
- Cho, J.; Jung, H.; Kang, D.Y.; Sp, N.; Shin, W.; Lee, J.; Park, B.G.; Kang, Y.A.; Jang, K.-J.; Bae, S.W. The Skin-Whitening and Antioxidant Effects of Protocatechuic Acid (PCA) Derivatives in Melanoma and Fibroblast Cell Lines. Curr. Issues Mol. Biol. 2023, 45, 2157–2169. [Google Scholar] [CrossRef]
- de Jesus, A.A.; Hou, Y.; Brooks, S.; Malle, L.; Biancotto, A.; Huang, Y.; Calvo, K.R.; Marrero, B.; Moir, S.; Oler, A.J. Distinct interferon signatures and cytokine patterns define additional systemic autoinflammatory diseases. J. Clin. Investig. 2020, 130, 1669–1682. [Google Scholar] [CrossRef]
- Hills, G. Industrial use of lipases to produce fatty acid esters. Eur. J. Lipid Sci. Technol. 2003, 105, 601–607. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upare, P.P.; Clarence, R.E.; Shin, H.; Park, B.G. An Overview on Production of Lignocellulose-Derived Platform Chemicals Such as 5-Hydroxymethyl Furfural, Furfural, Protocatechuic Acid. Processes 2023, 11, 2912. https://doi.org/10.3390/pr11102912
Upare PP, Clarence RE, Shin H, Park BG. An Overview on Production of Lignocellulose-Derived Platform Chemicals Such as 5-Hydroxymethyl Furfural, Furfural, Protocatechuic Acid. Processes. 2023; 11(10):2912. https://doi.org/10.3390/pr11102912
Chicago/Turabian StyleUpare, Pravin P., Rachel E. Clarence, Hyungsub Shin, and Byung Gyu Park. 2023. "An Overview on Production of Lignocellulose-Derived Platform Chemicals Such as 5-Hydroxymethyl Furfural, Furfural, Protocatechuic Acid" Processes 11, no. 10: 2912. https://doi.org/10.3390/pr11102912
APA StyleUpare, P. P., Clarence, R. E., Shin, H., & Park, B. G. (2023). An Overview on Production of Lignocellulose-Derived Platform Chemicals Such as 5-Hydroxymethyl Furfural, Furfural, Protocatechuic Acid. Processes, 11(10), 2912. https://doi.org/10.3390/pr11102912








