Is Hydrogen Peroxide Generated in Infusions of Medicinal Herbs?
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Plant Material
2.3. Preparation of Infusions
2.4. Determination of Hydrogen Peroxide
- AS,B—absorbance of a sample added with the Blank Reagent;
- AW,XO—absorbance of water added with the Xylenol Orange Reagent;
- AS,B—absorbance of water added with the Blank Reagent.
2.5. Determination of Hydrogen Peroxide Scavenging by Herbal Extracts
2.6. Determination of Polyphenols by UPLC-PDA-MS/MS
2.7. Detection of Semiquinone Radicals
2.8. Detection of Superoxide Generation
2.9. Cell Culture
2.10. Estimation of Cytotoxicity of Herbal Infusions
2.11. Effect of Catalase on the Cytotoxicity of Herbal Infusions
2.12. Statistical Analysis
3. Results
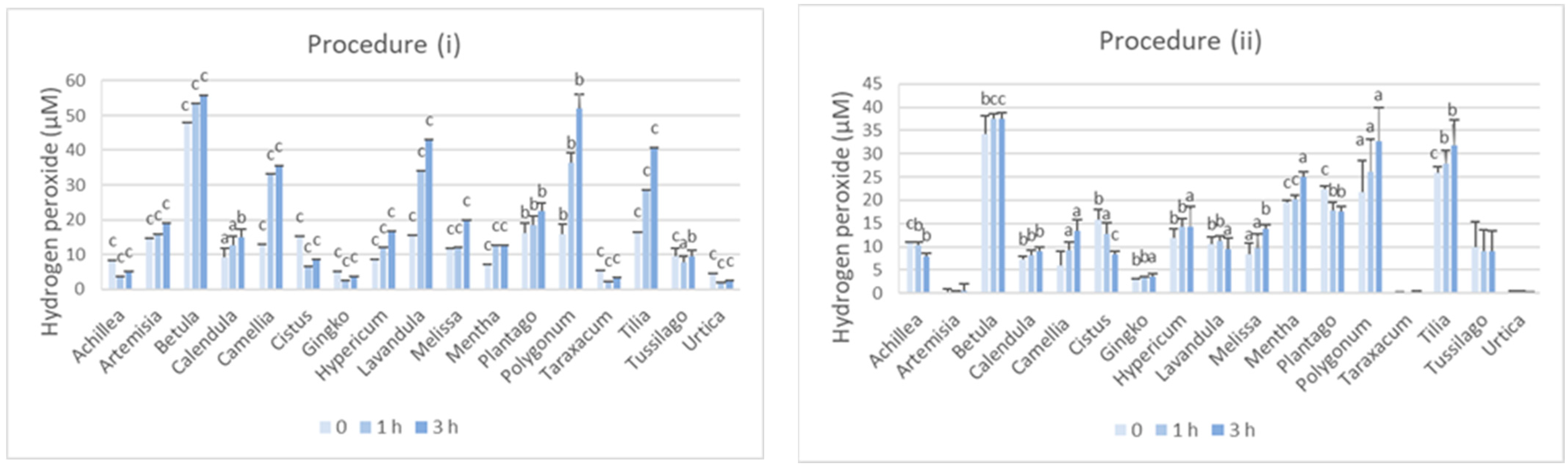
3.1. Hydrogen Peroxide Is Generated in Herbal Infusions
3.2. Herbal Infusions Contain Phenolic Compounds
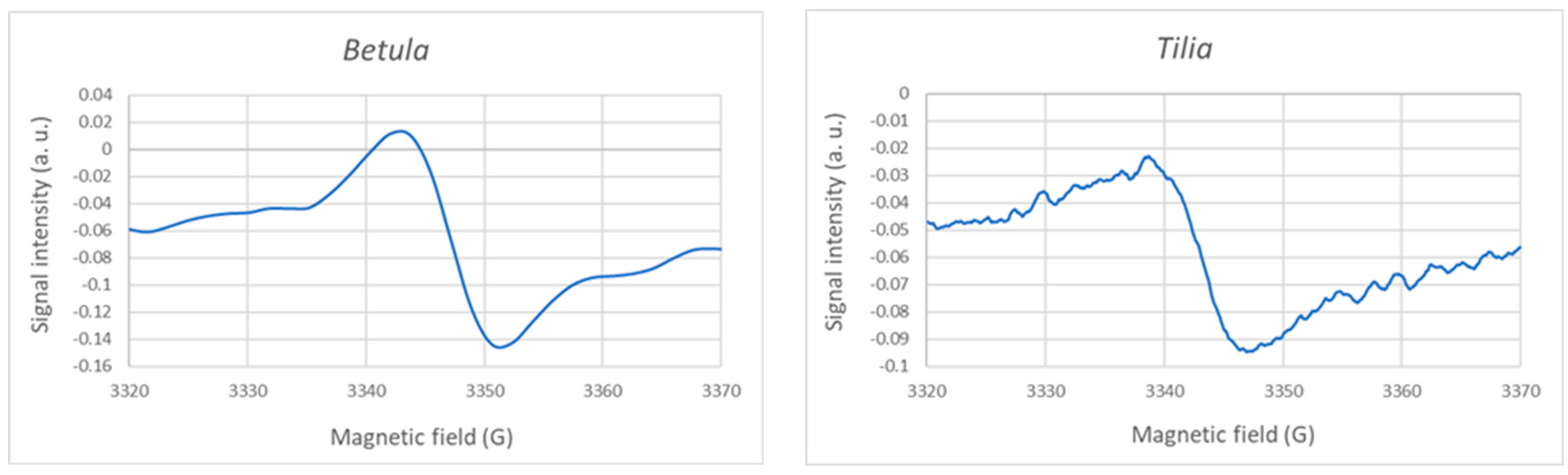
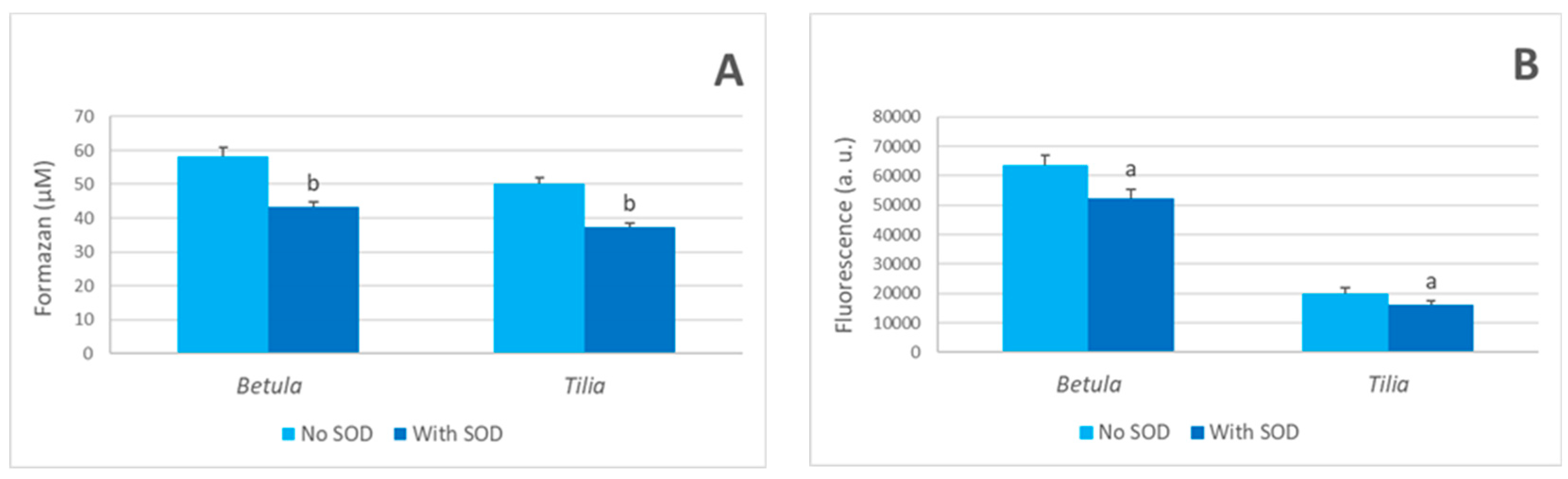
3.3. Mechanism of Hydrogen Peroxide Generation
3.4. Herbal Infusions Scavenge Hydrogen Peroxide
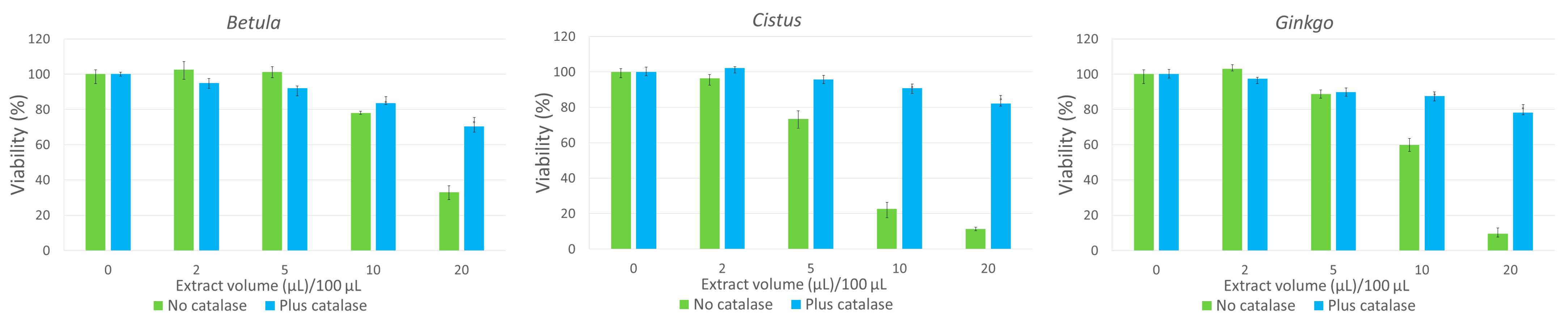
3.5. Hydrogen Peroxide Contributes to the Cytotoxic Action of Herbal Infusions
4. Discussion
4.1. Formation and Scavenging of Hydrogen Peroxide by Herbal Infusions
4.2. Biomedical Relevance of the Presence of Hydrogen Peroxide in Infusions of Medicinal Herbs
4.3. Generation of Hydrogen Peroxide May Introduce Artifacts in Cellular In Vitro Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciriminna, R.; Albanese, L.; Meneguzzo, F.; Pagliaro, M. Hydrogen peroxide: A key chemical for today’s sustainable development. ChemSusChem 2016, 9, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- Baldry, M.G.C. The bactericidal, fungicidal and sporicidal properties of hydrogen peroxide and peracetic acid. J. Appl. Bacteriol. 1983, 54, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Zonta, W.; Mauroy, A.; Farnir, F.; Thiry, E. Virucidal efficacy of a hydrogen peroxide nebulization against murine norovirus and feline calicivirus, two surrogates of human norovirus. Food Environ. Virol. 2016, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Mileto, D.; Mancon, A.; Staurenghi, F.; Rizzo, A.; Econdi, S.; Gismondo, M.R.; Guidotti, M. Inactivation of SARS-CoV-2 in the liquid phase: Are aqueous hydrogen peroxide and sodium percarbonate efficient decontamination agents? ACS Chem. Health Saf. 2021, 28, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.V.; Rath, T.; Radtke, C. Hydrogen peroxide (H2O2): A review of its use in surgery. Wien. Med. Wochenschr. 2017, 169, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.C.; Friedman, A.J. Hydrogen peroxide and cutaneous biology: Translational applications, benefits, and risks. J. Am. Acad. Dermatol. 2019, 81, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Alkahtani, R.; Stone, S.; German, M.; Waterhouse, P. A review on dental whitening. J. Dent. 2020, 100, 103423. [Google Scholar] [CrossRef] [PubMed]
- Alshammery, S. Evaluation of light activation on in-office dental bleaching: A systematic review. J. Contemp. Dent. Pract. 2019, 20, 1355–1360. [Google Scholar] [CrossRef]
- Ortega, K.L.; Rech, B.D.O.; El Haje, G.L.C.; Gallo, C.D.B.; Pérez-Sayáns, M.; Braz-Silva, P.H. Do hydrogen peroxide mouthwashes have a virucidal effect? A systematic review. J. Hosp. Infect. 2020, 106, 657–662. [Google Scholar] [CrossRef]
- Henry, M.C.; Wheeler, J.; Mofenson, H.C.; Caraccio, T.R.; Marsh, M.; Comer, G.M.; Singer, U.J. Hydrogen peroxide 3% exposures. J. Toxicol. Clin. Toxicol. 1996, 34, 323–327. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, L.D.; Leff, J.A. Catalase and hydrogen peroxide cytotoxicity in cultured cardiac myocytes. J. Mol. Cell. Cardiol. 1995, 27, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.X.; Wen, J.L.; Wang, L.; Wang, X.P.; Chen, T.S. Intracellular catalase activity instead of glutathione level dominates the resistance of cells to reactive oxygen species. Cell Stres Chaperones 2019, 24, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Pieńkowska, N.; Bartosz, G.; Pichla, M.; Grzesik-Pietrasiewicz, M.; Gruchala, M.; Sadowska-Bartosz, I. Effect of antioxidants on the H2O2-induced premature senescence of human fibroblasts. Aging 2020, 12, 1910–1927. [Google Scholar] [CrossRef] [PubMed]
- Al-Shehri, S.S.; Knox, C.L.; Liley, H.G.; Cowley, D.M.; Wright, J.R.; Henman, M.G.; Hewavitharana, A.K.; Charles, B.G.; Shaw, P.N.; Sweeney, E.L.; et al. Breastmilk-saliva interactions boost innate immunity by regulating the oral microbiome in early infancy. PLoS ONE 2015, 10, e0135047. [Google Scholar] [CrossRef] [PubMed]
- Al-Kerwi, E.A.; Al-Hashimi, A.H.; Salman, A.M. Mother’s milk and hydrogen peroxide. Asia Pac. J. Clin. Nutr. 2005, 14, 428–431. [Google Scholar] [PubMed]
- Akagawa, M.; Shigemitsu, T.; Suyama, K. Production of hydrogen peroxide by polyphenols and polyphenol-rich beverages under quasi-physiological conditions. Biosci. Biotechnol. Biochem. 2003, 67, 2632–2640. [Google Scholar] [CrossRef]
- Arakawa, H.; Maeda, M.; Okubo, S.; Shimamura, T. Role of hydrogen peroxide in bactericidal action of catechin. Biol. Pharm. Bull. 2004, 27, 277–281. [Google Scholar] [CrossRef]
- Grzesik, M.; Bartosz, G.; Stefaniuk, I.; Pichla, M.; Namieśnik, J.; Sadowska-Bartosz, I. Dietary antioxidants as a source of hydrogen peroxide. Food Chem. 2019, 278, 692–699. [Google Scholar] [CrossRef]
- Wang, J.Q.; Gao, Y.; Long, D.; Yin, J.F.; Zeng, L.; Xu, Y.Q.; Xu, Y.Q. Effects of hydrogen peroxide produced by catechins on the aroma of tea beverages. Foods 2022, 11, 1273. [Google Scholar] [CrossRef]
- Hegele, J.; Münch, G.; Pischetsrieder, M. Identification of hydrogen peroxide as a major cytotoxic component in Maillard reaction mixtures and coffee. Mol. Nutr. Food Res. 2009, 53, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Wakabayashi, K.; Nagao, M.; Sugimura, T. Implication of hydrogen peroxide in the mutagenicity of coffee. Mut. Res. Lett. 1985, 144, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Fujita, Y.; Wakabayashi, K.; Nukaya, H.; Kosuge, T.; Sugimura, T. Mutagens in coffee and other beverages. Environ. Health Perspect. 1986, 67, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G.; Baran, S.; Grzesik-Pietrasiewicz, M.; Sadowska-Bartosz, I. The antioxidant capacity and hydrogen peroxide formation by black and orange carrots. Agric. Food Sci. 2022, 31, 71–77. [Google Scholar] [CrossRef]
- Bartosz, G.; Rajzer, K.; Grzesik-Pietrasiewicz, M.; Sadowska-Bartosz, I. Hydrogen peroxide is formed upon cooking of vegetables. Acta Biochim. Pol. 2022, 69, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Chai, P.C.; Long, L.H.; Halliwell, B. Contribution of hydrogen peroxide to the cytotoxicity of green tea and red wines. Biochem. Biophys. Res. Commun. 2003, 304, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Tama, A.; Bartosz, G.; Sadowska-Bartosz, I. Is hydrogen peroxide generated in wine? Food Biosci. 2022, 45, 101487. [Google Scholar] [CrossRef]
- Babich, H.; Ackerman, N.J.; Burekhovich, F.; Zuckerbraun, H.L.; Schuck, A.G. Gingko biloba leaf extract induces oxidative stress in carcinoma HSC-2 cells. Toxicol. In Vitro 2009, 23, 992–999. [Google Scholar] [CrossRef]
- Mohamed, I.K.; Osama, M.A.; Samiha, M.; Zahrat, E.M. Biochemical studies on Plantago major L. and Cyamopsis tetragonoloba L. Int. J. Biodivers. Conserv. 2011, 3, 83–91. [Google Scholar]
- Rezadoost, M.H.; Kumleh, H.H.; Ghasempour, A. Cytotoxicity and apoptosis induction in breast cancer, skin cancer and glioblastoma cells by plant extracts. Mol. Biol. Rep. 2019, 46, 5131–5142. [Google Scholar] [CrossRef] [PubMed]
- Zarringhalami, R.; Hanachi, P.; Ramezani Tamijani, R. Cytotoxic effect of Tilia dasystyla and Polygonatum orientale Desf extracts on AGS and SKOV-3 cancer cell lines. Iran. J. Pharm. Sci. 2020, 16, 9–16. [Google Scholar]
- Ivanov, M.; Gašić, U.; Stojković, D.; Kostić, M.; Mišić, D.; Soković, M. New evidence for Artemisia absinthium L. application in gastrointestinal ailments: Ethnopharmacology, antimicrobial capacity, cytotoxicity, and phenolic profile. Evid. Based Compl. Altern. Med. 2021, 2021, 9961089. [Google Scholar] [CrossRef] [PubMed]
- Gay, C.A.; Gebicki, J.M. Measurement of protein and lipid hydroperoxides in biological systems by the ferric-xylenol orange method. Anal. Biochem. 2003, 315, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Wang, H.C.; Deguire, S.N.; Jenkins, M.; Lawson, M.; Brumaghim, J.L. Kinetics of iron oxidation upon polyphenol binding. Dalton Trans. 2010, 39, 9982–9987. [Google Scholar] [CrossRef] [PubMed]
- Krych, J.; Gebicka, L. Catalase is inhibited by flavonoids. Int. J. Biol. Macromol. 2013, 58, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Żurek, N.; Karatsai, O.; Rędowicz, M.J.; Kapusta, I. Polyphenolic compounds of Crataegus berry, leaf, and flower extracts affect viability and invasive potential of human glioblastoma cells. Molecules 2021, 26, 2656. [Google Scholar] [CrossRef] [PubMed]
- Oritani, T.; Fukuhara, N.; Okajima, T.; Kitamura, F.; Ohsaka, T. Electrochemical and spectroscopic studies on electron-transfer reaction between novel water-soluble tetrazolium salts and a superoxide ion. Inorgan. Chim. Acta 2004, 357, 436–442. [Google Scholar] [CrossRef]
- Nazarewicz, R.R.; Bikineyeva, A.; Dikalov, S.I. Rapid and specific measurements of superoxide using fluorescence spectroscopy. J. Biomol. Screen. 2013, 18, 498–503. [Google Scholar] [CrossRef]
- Nakayama, T.; Ichiba, M.; Kuwabara, M.; Kajiya, K.; Kumazawa, S. Mechanisms and structural specificity of hydrogen peroxide formation during oxidation of catechins. Food Sci. Technol. Res. 2002, 8, 261–267. [Google Scholar] [CrossRef][Green Version]
- Morsy, M.A.; Khaled, M.M. Novel EPR characterization of the antioxidant activity of tea leaves. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2002, 58, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Polovka, M.; Brezová, V.; Stasko, A. Antioxidant properties of tea investigated by EPR spectroscopy. Biophys. Chem. 2003, 106, 39–56. [Google Scholar] [CrossRef]
- Polat, M.; Korkmaz, M. Detection of irradiated black tea (Camellia sinensis) and rooibos tea (Aspalathus linearis) by ESR spectroscopy. Food Chem. 2008, 107, 956–961. [Google Scholar] [CrossRef]
- Çam, S.T.; Engin, B. Identification of irradiated sage tea (Salvia officinalis L.) by ESR spectroscopy. Radiat. Phys. Chem. 2010, 79, 540–544. [Google Scholar]
- Bournonville, C.F.; Díaz-Ricci, J.C. Quantitative determination of superoxide in plant leaves using a modified NBT staining method. Phytochem. Anal. 2011, 22, 268–271. [Google Scholar] [CrossRef]
- Wohlgemuth, H.; Mittelstrass, K.; Kschieschan, S.; Bender, J.; Weigel, H.J.; Overmyer, K.; Kangasjärvi, J.; Sandermann, H.; Langebartels, C. Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant Cell Environ. 2002, 25, 717–726. [Google Scholar] [CrossRef]
- Fink, B.; Laude, K.; McCann, L.; Doughan, A.; Harrison, D.G.; Dikalov, S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am. J. Physiol. Cell Physiol. 2004, 287, C895–C902. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Kalyanaraman, B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: Another inconvenient truth. Free Radic. Biol. Med. 2010, 48, 983–1001. [Google Scholar] [CrossRef] [PubMed]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Özyürek, M.; Bektaşoğlu, B.; Güçlü, K.; Güngör, N.; Apak, R. A novel hydrogen peroxide scavenging assay of phenolics and flavonoids using cupric reducing antioxidant capacity (CUPRAC) methodology. J. Food Comp. Anal. 2010, 23, 689–698. [Google Scholar] [CrossRef]
- Mansouri, A.; Makris, D.P.; Kefalas, P. Determination of hydrogen peroxide scavenging activity of cinnamic and benzoic acids employing a highly sensitive peroxyoxalate chemiluminescence-based assay: Structure–activity relationships. J. Pharm. Biomed. Anal. 2005, 39, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, H.; Dong, J.; Qian, W. Determination of hydrogen peroxide scavenging activity of phenolic acids by employing gold nanoshells precursor composites as nanoprobes. Food Chem. 2011, 126, 698–704. [Google Scholar] [CrossRef]
- Yvonne, O.; Driss, F.; Dang, P.M.-C.; Elbim, C.; Gougerot-Pocidalo, M.-A.; Pasquier, C.; El-Benna, J. Antioxidant effect of hydroxytyrosol, a polyphenol from olive oil: Scavenging of hydrogen peroxide but not superoxide anion produced by human neutrophils. Biochem. Pharmacol. 2004, 68, 2003–2008. [Google Scholar]
- Sroka, Z.; Fecka, I.; Cisowski, W. Antiradical and anti-H2O2 properties of polyphenolic compounds from an aqueous peppermint extract. Z. Naturforsch. C 2005, 60, 826–832. [Google Scholar]
- Nguyen, P.M.; Niemeyer, E.D. Effects of nitrogen fertilization on the phenolic composition and antioxidant properties of basil (Ocimum basilicum L.). J. Agric. Food Chem. 2008, 56, 8685–8691. [Google Scholar] [CrossRef] [PubMed]
- Sati, P.; Pandey, A.; Rawat, S.; Rani, A. Phytochemicals and antioxidants in leaf extracts of Ginkgo biloba with reference to location, seasonal variation and solvent system. J. Pharm. Res. 2013, 7, 804–809. [Google Scholar] [CrossRef]
- Toczewska, J.; Konopka, T. Activity of enzymatic antioxidants in periodontitis: A systematic overview of the literature. Dent. Med. Probl. 2019, 56, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Durak, I.; Ormeci, N.; Akyol, O.; Canbolat, O.; Kavutçu, M.; Bülbül, M. Adenosine deaminase 5’-nucleotidase xanthine oxidase superoxide dismutase and catalase activities in gastric juices from patients with gastric cancer ulcer and atrophic gastritis. Dig. Dis. Sci. 1994, 39, 721–728. [Google Scholar] [CrossRef]
- Damirchi, A.; Kiani, M.; Jafarian, V.; Sariri, R. Response of salivary peroxidase to exercise intensity. Eur. J. Appl. Physiol. 2010, 108, 1233–1237. [Google Scholar] [CrossRef]
- Caruso, A.A.; Del Prete, A.; Lazzarino, A.I. Hydrogen peroxide and viral infections: A literature review with research hypothesis definition in relation to the current covid-19 pandemic. Med. Hypoth. 2020, 144, 109910. [Google Scholar] [CrossRef]
- Hernandez, P.; Sager, B.; Fa, A.; Liang, T.; Lozano, C.; Khazzam, M. Bactericidal efficacy of hydrogen peroxide on Cutibacterium acnes. Bone Jt. Res. 2019, 8, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Zhang, J.; Cao, L.; Huang, T.T.; Zhang, J.X.; Mi, Y.N.; Xiao, X.; Yan, P.P.; Wu, M.L.; Yao, T.; et al. Hydrogen Peroxide-Mediated Oxygen Enrichment Eradicates Helicobacter pylori In Vitro and In Vivo. Antimicrob. Agents Chemother. 2020, 64, e02192–e02219. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, A.F.; Sobchak, C.; Shifrin, Y.; Pan, J.; Gonska, T.; Belik, J. Hydrogen peroxide promotes gastric motility in the newborn rat. Ped. Res. 2018, 84, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Craven, P.A.; Pfanstiel, J.; DeRubertis, F.R. Role of reactive oxygen in bile salt stimulation of colonic epithelial proliferation. J. Clin. Investig. 1986, 77, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.P.; Dean, R.T. Fragmentation of proteins by free radicals and its effect on their susceptibility to enzymic hydrolysis. Biochem. J. 1986, 234, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Benedek, B.; Kopp, B. Achillea millefolium L. s.l. revisited: Recent findings confirm the traditional use. Wien Med Wochensch. 2007, 157, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.; Olatunde, A.; El-Mleeh, A.; Hetta, H.F.; Al-Rejaie, S.; Alghamdi, S.; Zahoor, M.; Magdy Beshbishy, A.; Murata, T.; Zaragoza-Bastida, A.; et al. Bioactive compounds pharmacological actions and pharmacokinetics of wormwood (Artemisia absinthium). Antibiotics 2020, 9, 353. [Google Scholar] [CrossRef]
- Rastogi, S.; Pandey, M.M.; Kumar Singh Rawat, A. Medicinal plants of the genus Betula - traditional uses and a phytochemical-pharmacological review. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef]
- Muley, B.P.; Khadabadi, S.S.; Banarase, N.B. Phytochemical constituents and pharmacological activities of Calendula officinalis Linn (Asteraceae) A Review. Trop. J. Pharm. Res. 2009, 8, 455–465. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Barrajón-Catalán, E.; Fernández-Arroyo, S.; Saura, D.; Guillén, E.; Fernández-Gutiérrez, A.; Segura-Carretero, A.; Micol, V. Cistaceae aqueous extracts containing ellagitannins show antioxidant and antimicrobial capacity and cytotoxic activity against human cancer cells. Food Chem. Toxicol. 2010, 48, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kaur, P.; Singh, G.R.D.; Ahuja, P.S. Biology and chemistry of Ginkgo biloba. Fitoterapia 2008, 79, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s wort (Hypericum perforatum L.): A review of its chemistry pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemi, V.; Ghannadi, A.; Sharif, B. Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J. Ethnopharmacol. 2003, 89, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.—A review of its traditional uses phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [PubMed]
- Herro, E.; Jacob, S.E. Mentha piperita (peppermint). Dermatitis 2010, 21, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, M.; Paper, D.H.; Hose, S.; Franz, G. Investigation of the antiinflammatory activity of liquid extracts of Plantago lanceolata L. Phytother. Res. 1998, 12, S33–S34. [Google Scholar] [CrossRef]
- Wegener, T.; Kraft, K. Der Spitzwegerich (Plantago lanceolata L.): Reizlinderung bei Infektionen der oberen Atemwege [Plantain (Plantago lanceolata L.): Anti-inflammatory action in upper respiratory tract infections]. Wiener Med Wochenschr. 1999, 149, 211–216. [Google Scholar]
- Sung, Y.Y.; Yoon, T.; Yang, W.K.; Kim, S.J.; Kim, D.S.; Kim, H.K. The antiobesity effect of Polygonum aviculare L. ethanol extract in high-fat diet-induced obese mice. Evid. Based Compl Altern Med. 2013, 2013, 626397. [Google Scholar]
- Mir, M.A.; Sawhney, S.S.; Jassal, M.M.S. Qualitative and quantitative analysis of phytochemicals of Taraxacum officinale. Wudpecker J. Pharm. Pharmacol. 2013, 2, 1–5. [Google Scholar]
- Siger, A.; Antkowiak, W.; Dwiecki, K.; Rokosik, E.; Rudzińska, M. Nutlets of Tilia cordata Mill. and Tilia platyphyllos Scop.—Source of bioactive compounds. Food Chem. 2021, 346, 128888. [Google Scholar] [CrossRef]
- Chen, S.; Dong, L.; Quan, H.; Zhou, X.; Ma, J.; Xia, W.; Zhou, H.; Fu, X. A review of the ethnobotanical value phytochemistry pharmacology toxicity and quality control of Tussilago farfara L. (coltsfoot). J. Ethnopharmacol. 2021, 267, 113478. [Google Scholar] [CrossRef]
- Joshi, B.C.; Mukhija, M.; Kalia, A.N. Pharmacognostical review of Urtica dioica L. Int. J. Green Pharm. 2014, 8, 202–209. [Google Scholar]


| Compound | [M-H] m/z | |||
|---|---|---|---|---|
| Rt (min) | λmax (nm) | MS | MS/MS | |
| Neo-chlorogenic acid | 2.27 | 322 | 353 | 191 |
| Betuloside | 2.68 | 276 | 327 | 165 |
| Coumaryl-quinic acid I | 2.79 | 310 | 337 | 163 |
| Chlorogenic acid | 2.88 | 324 | 353 | 191 |
| 3-(4-Hydroxyphenyl)-1-methylpropyl | 2.99 | 276 | 165 | - |
| Feruloyl-quinic acid | 3.00 | 322 | 357 | 193 |
| Caffeic acid glucoside | 3.21 | 324 | 341 | 179 |
| Coumaryl-quinic acid II | 3.54 | 310 | 337 | 163 |
| Myricetin 3-O-glucoside | 3.89 | 253, 354 | 479 | 317 |
| Kaempferol 3-O-rhamnoside | 3.97 | 264, 342 | 431 | 285 |
| Myricetin 3-O-pentoside | 4.37 | 253, 354 | 449 | 317 |
| Quercetin 3-O-glucoside | 4.54 | 255, 355 | 463 | 301 |
| Quercetin 3-O-glucuronide | 4.57 | 255, 355 | 477 | 301 |
| Quercetin 3-O-pentoside I | 4.96 | 255, 355 | 433 | 301 |
| Quercetin 3-O-pentoside II | 5.06 | 255, 355 | 433 | 301 |
| Quercetin 3-O-pentoside III | 5.16 | 255, 355 | 433 | 301 |
| Quercetin 3-O-rhamnoside | 5.26 | 255, 355 | 447 | 301 |
| Compound | [M-H] m/z | |||
|---|---|---|---|---|
| Rt (min) | λmax (nm) | MS | MS/MS | |
| Neo-chlorogenic acid | 2.28 | 322 | 353 | 191 |
| Ferulic acid glucoside | 2.62 | 324 | 355 | 193 |
| Coumarylo-quinic acid I | 2.79 | 310 | 337 | 163 |
| Chlorogenic acid | 2.88 | 324 | 353 | 191 |
| Coumarylo-quinic acid II | 2.95 | 310 | 337 | 163 |
| Coumarylic acid glucoside | 310 | 310 | 325 | 163 |
| Procyanidin dimer | 3.23 | 274 | 577 | 289 |
| Procyanidin trimer I | 3.30 | 274 | 865 | 577, 289 |
| (+)Catechin | 3.39 | 274 | 289 | 141 |
| Coumaryl-quinic acid III | 3.53 | 310 | 337 | 163 |
| Undefined derivative | 3.67 | 284 | 401 | 189 |
| Procyanidin trimer II | 3.83 | 278 | 865 | 577, 289 |
| Procyanidin tetramer | 3.97 | 277 | 1152 | 865, 577 |
| Kaempferol 3-O-sophoroside | 4.29 | 264, 352 | 609 | 285 |
| Quercetin 3-O-rutinoside | 4.51 | 255, 355 | 609 | 301 |
| Quercetin 3-O-glucoside | 4.66 | 255, 355 | 463 | 301 |
| Quercetin 3-O-(6″-malonyl)-glucoside | 4.94 | 255, 352 | 549 | 505, 463, 301 |
| Kaempferol 3-O-rutinoside | 5.00 | 264, 347 | 593 | 285 |
| Kaempferol 3-O-glucoside | 5.21 | 264, 347 | 447 | 285 |
| Quercetin 3-O-glucuronide | 5.44 | 355, 350 | 477 | 301 |
| Chrysoeriol 7-O-rutinoside | 5.60 | 345 | 607 | 299 |
| Quercetin 4′-O-glucoside | 5.76 | 255, 355 | 463 | 301 |
| Kaempferol 3-O-glucuronide | 6.07 | 264, 342 | 461 | 285 |
| Kaempferol 3-O-(6″-acetyl)-rutinoside | 7.01 | 264, 333 | 637 | 593, 301 |
| Compound | [M-H] m/z | |||
|---|---|---|---|---|
| Rt (min) | λmax (nm) | MS | MS/MS | |
| Caftaric acid | 2.25 | 327 | 311 | 179 |
| Chlorogenic acid | 2.88 | 327 | 353 | 191 |
| tri-Caffeoyl-tartaric acid | 3.35 | 324 | 635 | 473, 311 |
| Coutaric acid | 3.66 | 309 | 295 | 163 |
| Chicoric acid | 4.32 | 324 | 473 | 311, 179 |
| Compound | [M-H] m/z | |||
|---|---|---|---|---|
| Rt (min) | λmax (nm) | MS | MS/MS | |
| Caffeic acid cinnamyl ester | 3.68 | 327 | 295 | 179 |
| p-Coumaric acid | 4.09 | 309 | 163 | 116 |
| Coumaroyl-malic acid I | 4.41 | 312 | 279 | 163 |
| Coumaroyl-malic acid II | 4.62 | 324 | 279 | 163 |
| Feruloyl-sinapoyl-tartaric acid | 4.85 | 324 | 531 | 309, 193 |
| Compound | Concentration (μg/mL) |
|---|---|
| Neo-chlorogenic acid | 12.08 ± 0.05 |
| Betuloside | 394.77 ± 18.87 |
| Chlorogenic acid | 46.73 ± 2.02 |
| 3-(4-Hydroxyphenyl)-1-methylpropyl | 78.38 ± 1.79 |
| Feruloyl-quinic acid | 2.85 ± 0.07 |
| Caffeic acid glucoside | 2.50 ± 0.01 |
| Coumarylo-quinic acid II | 5.58 ± 0.19 |
| Myricetin 3-O-glucoside | 66.20 ± 2.19 |
| Kaempferol 3-O-rhamnoside | 9.00 ± 0.01 |
| Myricetin 3-O-pentoside | 4.64 ± 0.29 |
| Quercetin 3-O-glucoside | 78.46 ± 0.96 |
| Quercetin 3-O-glucuronide | 62.25 ± 0.55 |
| Quercetin 3-O-pentoside I | 15.74 ± 0.40 |
| Quercetin 3-O-pentoside II | 8.46 ± 0.08 |
| Quercetin 3-O-pentoside III | 5.61 ± 0.13 |
| Quercetin 3-O-rhamnoside | 9.81 ± 0.05 |
| Total | 808.99 ± 12.51 |
| Compound | Concentration (μg/mL) |
|---|---|
| Neo-chlorogenic acid | 2.95 ± 0.03 |
| Ferulic acid glucoside | |
| Coumaryl-quinic acid I | 1.35 ± 0.07 |
| Chlorogenic acid | 3.06 ± 0.01 |
| Coumaryl-quinic acid II | 2.22 ± 0.10 |
| Coumarylic acid glucoside | 1.30 ± 0.03 |
| Procyanidin dimer | 1.68 ± 0.04 |
| Procyanidin trimer I | 2.97 ± 0.01 |
| (+)Catechin | 0.85 ± 0.03 |
| Coumaryl-quinic acid III | 5.07 ± 0.17 |
| Undefined derivative | 3.03 ± 0.00 |
| Procyanidin trimer II | 2.75 ± 0.18 |
| Procyanidin tetramer | 3.70 ± 0.05 |
| Kaempferol 3-O-sophoroside | 11.15 ± 0.10 |
| Quercetin 3-O-rutinoside | 21.92 ± 0.56 |
| Quercetin 3-O-glucoside | 48.33 ± 0.48 |
| Quercetin 3-O-(6″-malonyl)-glucoside | 8.88 ± 0.21 |
| Kaempferol 3-O-rutinoside | 6.71 ± 0.11 |
| Kaempferol 3-O-glucoside | 9.61 ± 0.25 |
| Quercetin 3-O-glucuronide | 10.54 ± 0.42 |
| Chrysoeriol 7-O-rutinoside | 6.76 ± 0.16 |
| Quercetin 4′-O-glucoside | 4.20 ± 0.05 |
| Kaempferol 3-O-glucuronide | 3.46 ± 0.06 |
| Kaempferol 3-O-(6″-acetyl)-rutinoside | 10.11 ± 0.17 |
| Total | 174.56 ± 1.02 |
| Compound | Concentration (μg/mL) |
|---|---|
| Caftaric acid | 88.19 ± 2.28 |
| Chlorogenic acid | 12.57 ± 0.50 |
| tri-Caffeoyl-tartaric acid | 5.28 ± 0.12 |
| Coutaric acid | 6.20 ± 0.07 |
| Chicoric acid | 54.14 ± 0.92 |
| Total | 163.24 ± 2.77 |
| Compound | Concentration (μg/mL) |
|---|---|
| Caffeic acid cinnamyl ester | 24.57 ± 0.30 |
| p-Coumaric acid | 2.32 ± 0.02 |
| Coumaroyl-malic acid I | 6.21 ± 0.16 |
| Coumaroyl-malic acid II | 9.77 ± 0.10 |
| Feruloyl-sinapoyl-tartaric acid | 43.35 ± 1.03 |
| Total | 86.22 ± 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tama, A.; Pieńkowska, N.; Stefaniuk, I.; Bartosz, G.; Kapusta, I.; Sadowska-Bartosz, I. Is Hydrogen Peroxide Generated in Infusions of Medicinal Herbs? Processes 2023, 11, 2855. https://doi.org/10.3390/pr11102855
Tama A, Pieńkowska N, Stefaniuk I, Bartosz G, Kapusta I, Sadowska-Bartosz I. Is Hydrogen Peroxide Generated in Infusions of Medicinal Herbs? Processes. 2023; 11(10):2855. https://doi.org/10.3390/pr11102855
Chicago/Turabian StyleTama, Anna, Natalia Pieńkowska, Ireneusz Stefaniuk, Grzegorz Bartosz, Ireneusz Kapusta, and Izabela Sadowska-Bartosz. 2023. "Is Hydrogen Peroxide Generated in Infusions of Medicinal Herbs?" Processes 11, no. 10: 2855. https://doi.org/10.3390/pr11102855
APA StyleTama, A., Pieńkowska, N., Stefaniuk, I., Bartosz, G., Kapusta, I., & Sadowska-Bartosz, I. (2023). Is Hydrogen Peroxide Generated in Infusions of Medicinal Herbs? Processes, 11(10), 2855. https://doi.org/10.3390/pr11102855










