Non-Conventional Cuts in Batch Distillation to Brazilian Spirits (cachaça) Production: A Computational Simulation Approach
Abstract
1. Introduction
2. Methods
2.1. Simulation of Pot Still Distillation
2.2. Vapor–Liquid Equilibrium
2.3. Validation
2.4. Fermented Must Composition
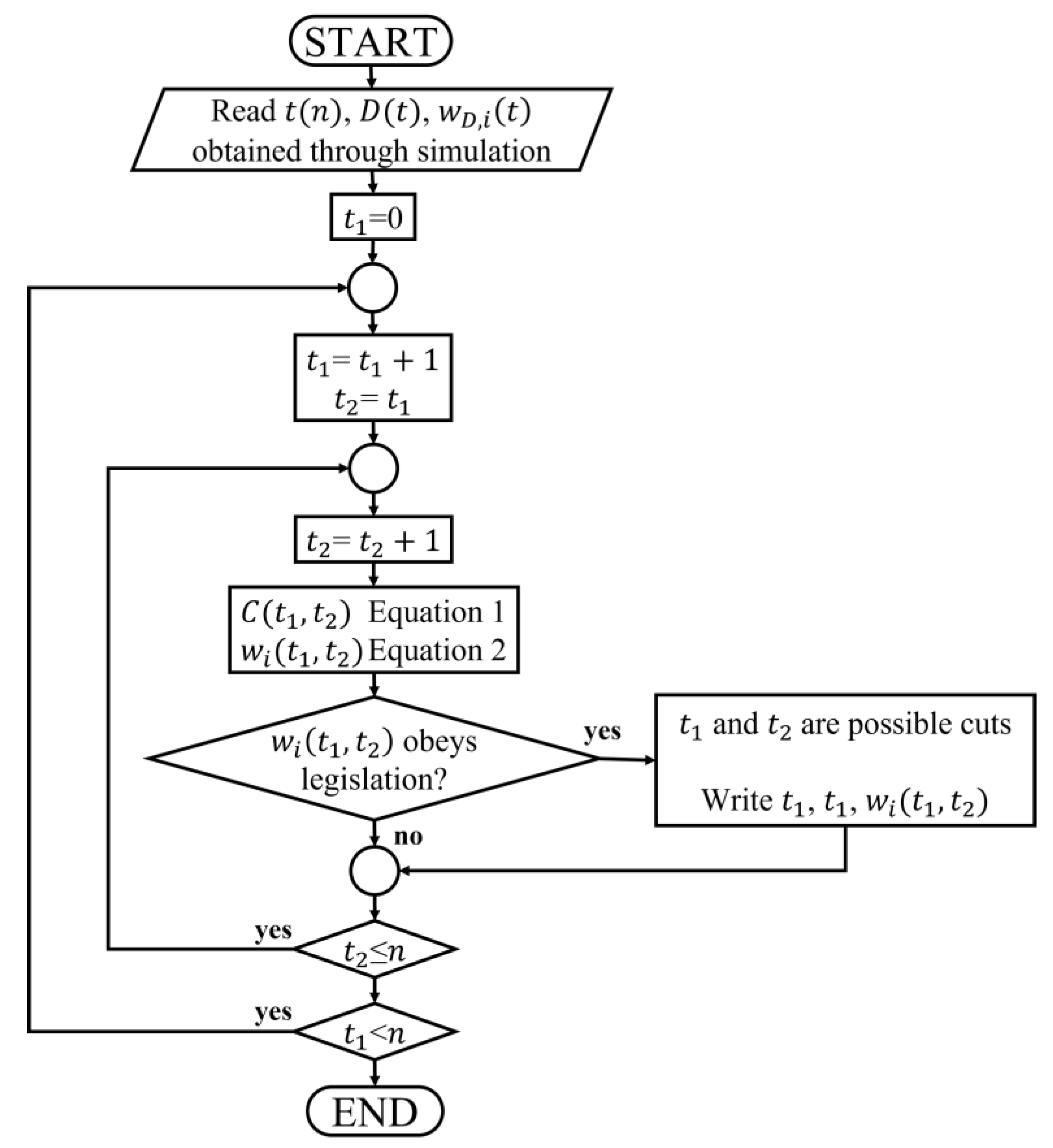
2.5. Algorithm to Determine Distilling Cuts
3. Results
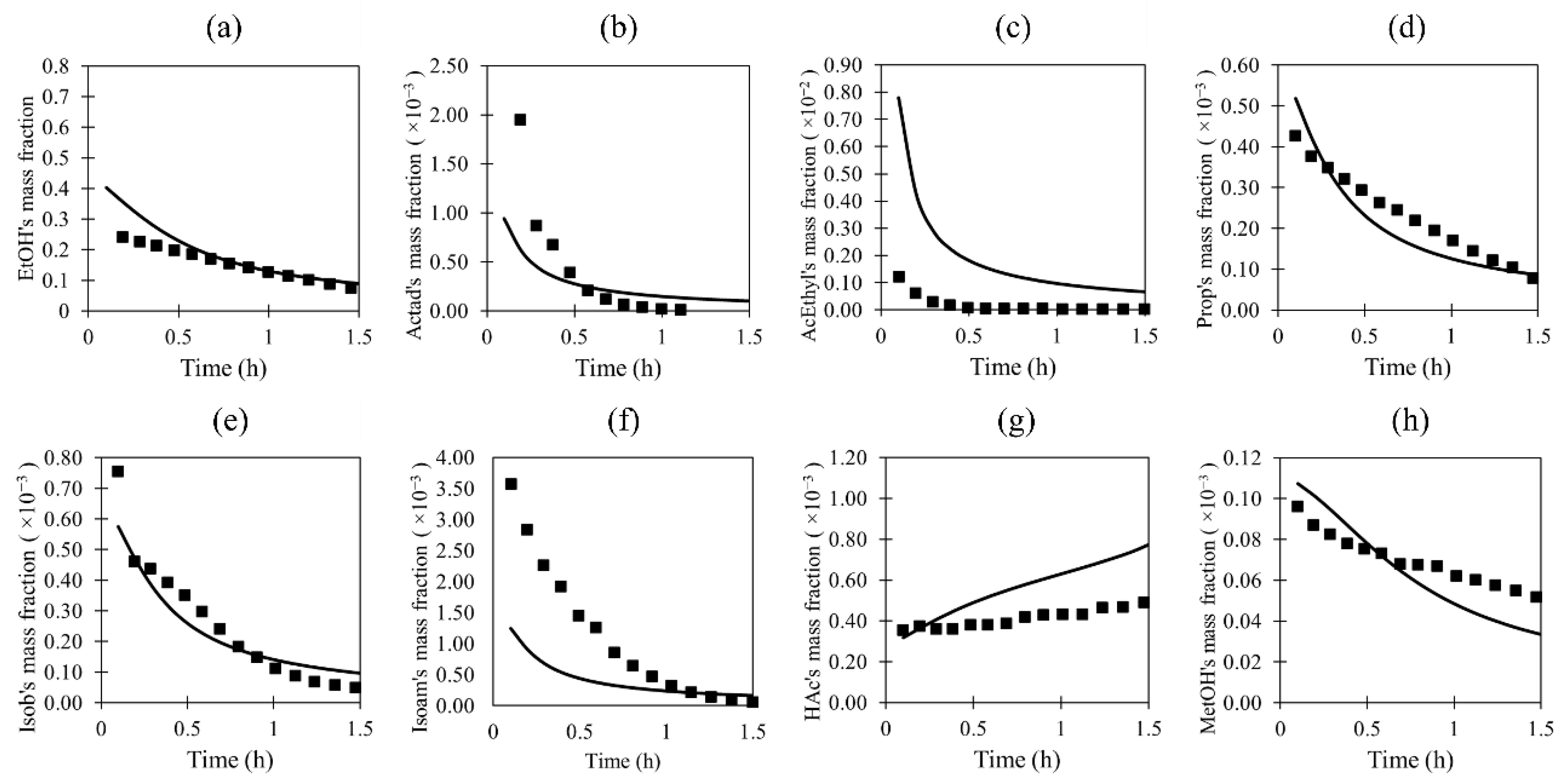
3.1. Validation
3.2. Simulation of Traditional Distilling Cuts
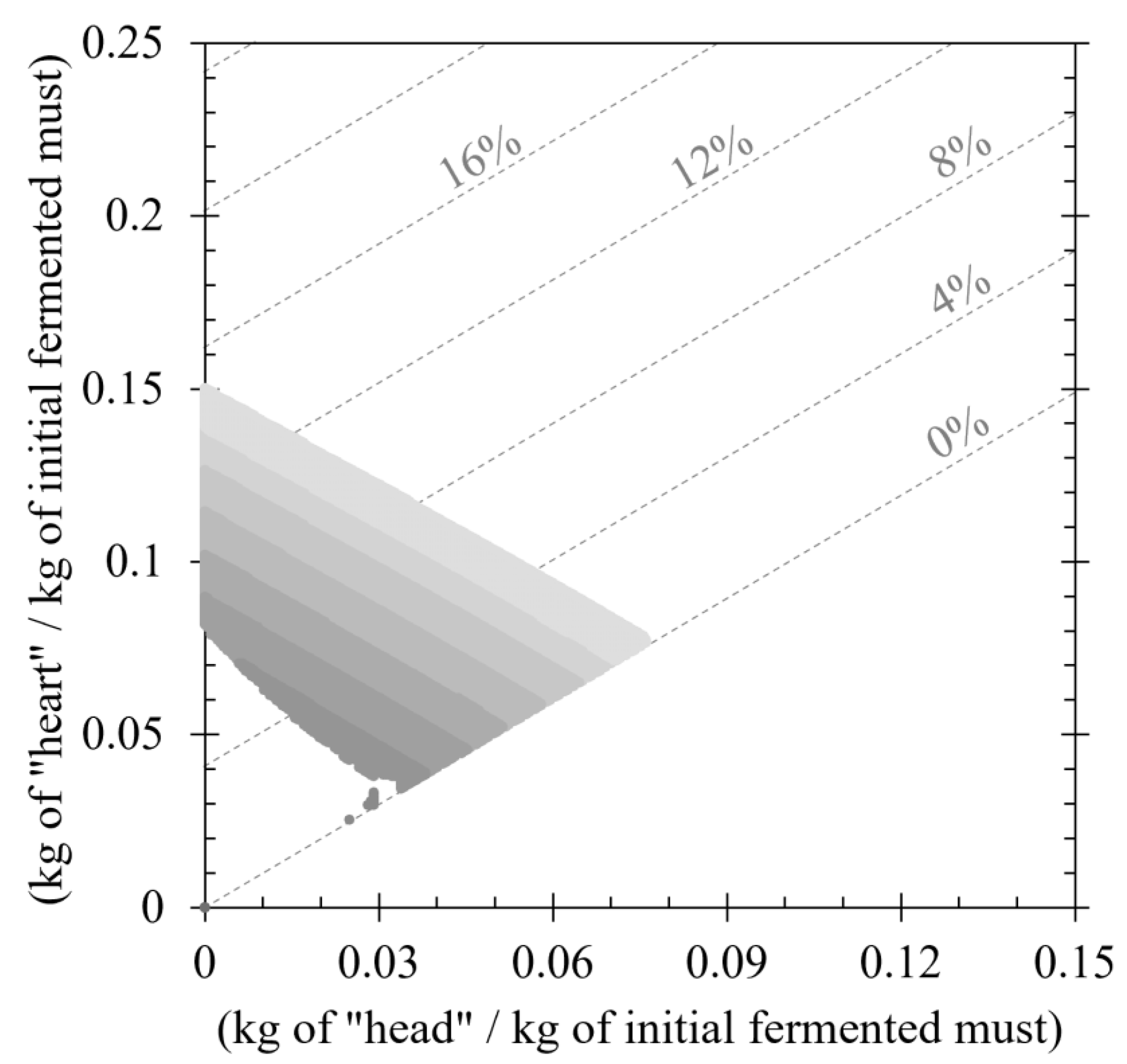
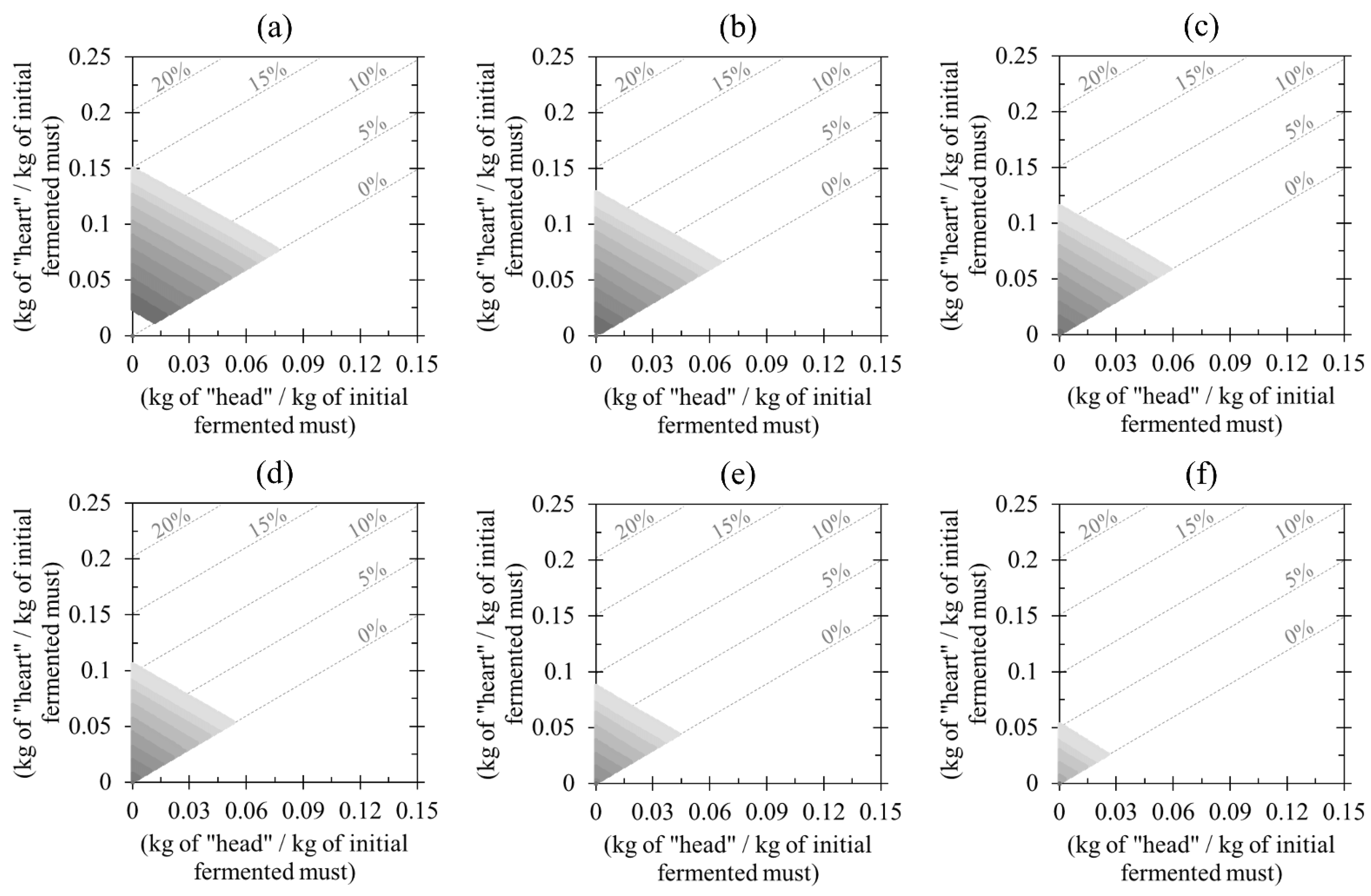
3.3. Evaluation of Nonconventional Distilling Cuts
3.4. Influence of Minor Compounds on Spirit Quality
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| NRTL | Non-Random Two Liquids model |
| HOC | Hayden O’Connell model |
| Mass fraction of distillate for compound i | |
| Mass fraction of cachaça for compound i | |
| C | Total amount of cachaça |
| D | Total amount of distillate |
| Time of first cut | |
| Time of Second cut | |
| AA | Anhydrous Alcohol |
| EtOH | Ethanol |
| Actad | Acetaldehyde |
| Isob | Isobutanol |
| Isoam | Isoamyl alcohol |
| AcEthyl | Ethyl acetate |
| Prop | 1-propanol |
| HAc | Acetic acid |
| MetOH | Methanol |
| AAD | Average Absolute Deviation |
References
- Batista, F.R.M.; Meirelles, A.J.A. Computer Simulation Applied to Studying Continuous Spirit Distillation and Product Quality Control. Food Control. 2011, 22, 1592–1603. [Google Scholar] [CrossRef]
- Różański, M.; Pielech-Przybylska, K.; Balcerek, M. Influence of Alcohol Content and Storage Conditions on the Physicochemical Stability of Spirit Drinks. Foods 2020, 9, 1264. [Google Scholar] [CrossRef]
- Hodel, J.; O’Donovan, T.; Hill, A.E. Influence of Still Design and Modelling of the Behaviour of Volatile Terpenes in an Artificial Model Gin. Food Bioprod. Process. 2021, 129, 46–64. [Google Scholar] [CrossRef]
- Douady, A.; Puentes, C.; Awad, P.; Esteban-Decloux, M. Batch Distillation of Spirits: Experimental Study and Simulation of the Behaviour of Volatile Aroma Compounds. J. Inst. Brew. 2019, 125, 268–283. [Google Scholar] [CrossRef]
- Gaiser, M.; Bell, G.M.; Lim, A.W.; Roberts, N.A.; Faraday, D.B.F.; Schulz, R.A.; Grob, R. Computer Simulation of a Continuous Whisky Still. J. Food Eng. 2002, 51, 27–31. [Google Scholar] [CrossRef]
- Nose, A.; Murata, T.; Hamakawa, Y.; Shoji, H.; Kozaki, D.; Hojo, M. Effects of Solutes on the Alcohol-Stimulative Taste of Vodkas. Food Chem. 2020, 340, 128160. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.G. Chapter 25—A Short History of Rum. In Whisky and Other Spirits Technology, Production and Marketing, 3rd ed.; Russell, I., Stewart, G.G., Kellershohn, J.B.T.-W., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 457–462. ISBN 978-0-12-822076-4. [Google Scholar]
- Warren-Vega, W.M.; Fonseca-Aguiñaga, R.; González-Gutiérrez, L.V.; Carrasco-Marín, F.; Zárate-Guzmán, A.I.; Romero-Cano, L.A. Chemical Characterization of Tequila Maturation Process and Their Connection with the Physicochemical Properties of the Cask. J. Food Compos. Anal. 2021, 98, 103804. [Google Scholar] [CrossRef]
- Anjos, O.; Caldeira, I.; Roque, R.; Pedro, S.I.; Lourenço, S.; Canas, S. Screening of Different Ageing Technologies of Wine Spirit by Application of Near-Infrared (NIR) Spectroscopy and Volatile Quantification. Processes 2020, 8, 736. [Google Scholar] [CrossRef]
- Ferrari, G.; Lablanquie, O.; Cantagrel, R.; Ledauphin, J.; Payot, T.; Fournier, N.; Guichard, E. Determination of Key Odorant Compounds in Freshly Distilled Cognac Using GC-O, GC-MS, and Sensory Evaluation. J. Agric. Food Chem. 2004, 52, 5670–5676. [Google Scholar] [CrossRef]
- Brasil Technical Regulation for Setting Identity and Quality Standards for Cane Spirit and Cachaça (in Portuguese); Brasil. 2005. Available online: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-vegetal/legislacao-1/biblioteca-de-normas-vinhos-e-bebidas/instrucao-normativa-no-13-de-29-de-junho-de-2005.pdf/view (accessed on 25 October 2022).
- Brexó, R.P.; Brandão, L.R.; Chaves, R.D.; Castro, R.J.S.; Câmara, A.A.; Rosa, C.A.; Sant’Ana, A.S. Yeasts from Indigenous Culture for Cachaça Production and Brewer’s Spent Grain: Biodiversity and Phenotypic Characterization for Biotechnological Purposes. Food Bioprod. Process. 2020, 124, 107–120. [Google Scholar] [CrossRef]
- IBGE Agricultural Census -Rural Agroindustry. Production, Sale and Value of Production and Value of Sale in Rural Agro-Industry in Agricultural Establishments, by Type, Products of Rural Agro-Industry and Groups of Total Area. (in Portuguese); Brasília. 2021. Available online: https://sidra.ibge.gov.br/pesquisa/censo-agropecuario/censo-agropecuario-2017 (accessed on 25 October 2022).
- Brasil COMEX STAT General Imports and Exports. NCM 2208.40.00; Brasília. 2022. Available online: http://comexstat.mdic.gov.br/pt/geral/57379 (accessed on 4 October 2021).
- Portugal, C.B.; de Silva, A.P.; Bortoletto, A.M.; Alcarde, A.R. How Native Yeasts May Influence the Chemical Profile of the Brazilian Spirit, Cachaça? Food Res. Int. 2017, 91, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Portugal, C.B.; Alcarde, A.R.; Bortoletto, A.M.; de Silva, A.P. The Role of Spontaneous Fermentation for the Production of Cachaça: A Study of Case. Eur. Food Res. Technol. 2016, 242, 1587–1597. [Google Scholar] [CrossRef]
- Viana, E.J.; de Carvalho Tavares, I.M.; Rodrigues, L.M.A.; das Graças Cardoso, M.; Júnior, J.C.B.; Gualberto, S.A.; de Oliveira, C.P. Evaluation of Toxic Compounds and Quality Parameters on the Aged Brazilian Sugarcane Spirit. Res. Soc. Dev. 2020, 9, e395985544. [Google Scholar] [CrossRef]
- Bogusz, S.; Ketzer, D.C.M.; Gubert, R.; Andrades, L.; Gobo, A.B. Chemical Composition of the Sugar Cane Spirit “Cachaça” Produced in the Northwest Area of Rio Grande Do Sul, Brazil. Ciencia e Tecnologia de Alimentos 2006, 26, 793–798. [Google Scholar] [CrossRef][Green Version]
- Scanavini, H.F.A.; Ceriani, R.; Meirelles, A.J.A. Cachaça Distillation Investigated on the Basis of Model Systems. Braz. J. Chem. Eng. 2012, 29, 429–440. [Google Scholar] [CrossRef]
- Oliveira, E.S.; Bolini Cardello, H.M.A.; Jeronimo, E.M.; Rocha Souza, E.L.; Serra, G.E. The Influence of Different Yeasts on the Fermentation, Composition and Sensory Quality of Cachaça. World J Microbiol. Biotechnol 2005, 21, 707–715. [Google Scholar] [CrossRef]
- Barbosa, E.A.; Souza, M.T.; Diniz, R.H.S.; Godoy-Santos, F.; Faria-Oliveira, F.; Correa, L.F.M.; Alvarez, F.; Coutrim, M.X.; Afonso, R.J.C.F.; Castro, I.M.; et al. Quality Improvement and Geographical Indication of Cachaça (Brazilian Spirit) by Using Locally Selected Yeast Strains. J. Appl. Microbiol. 2016, 121, 1038–1051. [Google Scholar] [CrossRef]
- Oliveira, E.S. Evaluation of Isolated Yeasts in Artisanal Distilleries Sugar Cane Spirit Depending on the Formation of Volatile Compounds. Ph.D. Thesis, University of Campinas, Campinas, Brazil, 2001. (In Portuguese). [Google Scholar]
- Pineau, N.J.; Magro, L.; van den Broek, J.; Anderhub, P.; Güntner, A.T.; Pratsinis, S.E. Spirit Distillation: Monitoring Methanol Formation with a Hand-Held Device. ACS Food Sci. Technol. 2021, 1, 839–844. [Google Scholar] [CrossRef]
- Matias-Guiu, P.; Rodríguez-Bencomo, J.J.; Pérez-Correa, J.R.; López, F. Aroma Profile Design of Wine Spirits: Multi-Objective Optimization Using Response Surface Methodology. Food Chem. 2018, 245, 1087–1097. [Google Scholar] [CrossRef]
- Bortoletto, A.M.; Alcarde, A.R. Assessment of Chemical Quality of Brazilian Sugar Cane Spirits and Cachaças. Food Control. 2015, 54, 1–6. [Google Scholar] [CrossRef]
- Puentes, C.; Joulia, X.; Vidal, J.P.; Esteban-Decloux, M. Simulation of Spirits Distillation for a Better Understanding of Volatile Aroma Compounds Behavior: Application to Armagnac Production. Food Bioprod. Process. 2018, 112, 31–62. [Google Scholar] [CrossRef]
- Rodríguez-Bencomo, J.J.; Pérez-Correa, J.R.; Orriols, I.; López, F. Spirit Distillation Strategies for Aroma Improvement Using Variable Internal Column Reflux. Food Bioproc. Tech. 2016, 9, 1885–1892. [Google Scholar] [CrossRef]
- Esteban-Decloux, M.; Dechatre, J.C.; Legendre, P.; Guichard, H. Double Batch Cider Distillation: Influence of the Recycling of the Separated Fractions. LWT 2021, 146, 111420. [Google Scholar] [CrossRef]
- Luna, R.; López, F.; Pérez-Correa, J.R. Design of Optimal Wine Distillation Recipes Using Multi-Criteria Decision-Making Techniques. Comput. Chem. Eng. 2021, 145, 107194. [Google Scholar] [CrossRef]
- Luna, R.; Matias-Guiu, P.; López, F.; Pérez-Correa, J.R. Quality Aroma Improvement of Muscat Wine Spirits: A New Approach Using First-Principles Model-Based Design and Multi-Objective Dynamic Optimisation through Multi-Variable Analysis Techniques. Food Bioprod. Process. 2019, 115, 208–222. [Google Scholar] [CrossRef]
- Balcerek, M.; Pielech-Przybylska, K.; Patelski, P.; Dziekońska-Kubczak, U.; Strąk, E. The Effect of Distillation Conditions and Alcohol Content in ‘Heart’ Fractions on the Concentration of Aroma Volatiles and Undesirable Compounds in Plum Brandies. J. Inst. Brew. 2017, 123, 452–463. [Google Scholar] [CrossRef]
- Scanavini, H.F.A.; Ceriani, R.; Cassini, C.E.B.; Souza, E.L.R.; Maugeri Filho, F.; Meirelles, A.J.A. Cachaça Production in a Lab-Scale Alembic: Modeling and Computational Simulation. J. Food Process. Eng. 2010, 33, 226–252. [Google Scholar] [CrossRef]
- Aspen Technology Incorporation Aspen Plus V12.1 2022. Available online: https://www.aspentech.com (accessed on 20 October 2022).
- Cobre Brasil Cobre Brasil Artesanatos. Available online: https://www.cobrebrasil.com.br/ (accessed on 1 December 2022).
- Valderrama, J.O.; Faúndez, C.A.; Toselli, L.A. Advances on Modeling and Simulation of Alcoholic Distillation. Part 1: Thermodynamic Modeling. Food Bioprod. Process. 2012, 90, 819–831. [Google Scholar] [CrossRef]
- Decloux, M.; Coustel, J. Simulation of a Neutral Spirit Production Plant Using Beer Distillation. Int. Sugar J. 2005, 107, 628–643. [Google Scholar]
- Batista, F.R.M.; Follegatti-Romero, L.A.; Meirelles, A.J.A. A New Distillation Plant for Neutral Alcohol Production. Sep. Purif. Technol. 2013, 118, 784–793. [Google Scholar] [CrossRef]
- Scanavini, H.F.A. Study of Cachaça Distillation: Evaluation of Processing Conditions in the Quality of the Final Product. Ph.D. Thesis, University of Campinas, Campinas, Brazil, 2010. (In Portuguese). [Google Scholar]
- Sacher, J.; García-Llobodanin, L.; López, F.; Segura, H.; Pérez-Correa, J.R. Dynamic Modeling and Simulation of an Alembic Pear Wine Distillation. Food Bioprod. Process. 2013, 91, 447–456. [Google Scholar] [CrossRef]
- Campos, C.R.; Silva, C.F.; Dias, D.R.; Basso, L.C.; Amorim, H.V.; Schwan, R.F. Features of Saccharomyces Cerevisiae as a Culture Starter for the Production of the Distilled Sugar Cane Beverage, Cachaça in Brazil. J. Appl. Microbiol. 2010, 108, 1871–1879. [Google Scholar] [CrossRef]



| Ethanol | Acetic Acid | Acetaldehyde | Ethyl Acetate | n-Propanol | Isobutanol | Isoamyl Alcohol | Water | |
|---|---|---|---|---|---|---|---|---|
| c1 | 7.25 | 0.0273 | 1.35 × 10−3 | 6.40 × 10−4 | 2.13 × 10−3 | 1.35 × 10−3 | 9.74 × 10−3 | 92.71 |
| c2 | 7.07 | 0.0296 | 1.80 × 10−3 | 9.37 × 10−4 | 3.41 × 10−3 | 1.80 × 10−3 | 12.88 × 10−3 | 92.87 |
| c3 | 6.99 | 0.0436 | 1.58 × 10−3 | 7.87 × 10−4 | 3.37 × 10−3 | 2.78 × 10−3 | 14.29 × 10−3 | 92.95 |
| c4 | 6.99 | 0.0492 | 1.63 × 10−3 | 9.09 × 10−4 | 2.56 × 10−3 | 1.82 × 10−3 | 10.96 × 10−3 | 92.95 |
| c5 | 6.90 | 0.0460 | 0.95 × 10−3 | 6.73 × 10−4 | 2.93 × 10−3 | 1.77 × 10−3 | 11.38 × 10−3 | 93.04 |
| c6 | 6.90 | 0.0534 | 1.19 × 10−3 | 7.45 × 10−4 | 2.94 × 10−3 | 1.82 × 10−3 | 16.00 × 10−3 | 93.03 |
| c7 | 6.81 | 0.0409 | 1.26 × 10−3 | 8.46 × 10−4 | 2.53 × 10−3 | 2.54 × 10−3 | 14.53 × 10−3 | 93.13 |
| c8 | 6.81 | 0.0848 | 1.64 × 10−3 | 9.64 × 10−4 | 3.41 × 10−3 | 1.69 × 10−3 | 12.85 × 10−3 | 93.09 |
| c9 | 6.81 | 0.0401 | 1.32 × 10−3 | 8.15 × 10−4 | 2.97 × 10−3 | 1.81 × 10−3 | 16.42 × 10−3 | 93.13 |
| c10 | 6.72 | 0.0636 | 1.23 × 10−3 | 1.08 × 10−3 | 3.24 × 10−3 | 2.29 × 10−3 | 14.43 × 10−3 | 93.19 |
| c11 | 6.63 | 0.0887 | 1.39 × 10−3 | 5.85 × 10−4 | 4.25 × 10−3 | 1.46 × 10−3 | 10.79 × 10−3 | 93.26 |
| c12 | 6.54 | 0.0590 | 1.03 × 10−3 | 7.90 × 10−4 | 3.40 × 10−3 | 2.10 × 10−3 | 13.26 × 10−3 | 93.38 |
| c13 | 6.44 | 0.0537 | 1.22 × 10−3 | 7.12 × 10−4 | 2.71 × 10−3 | 1.75 × 10−3 | 10.69 × 10−3 | 93.49 |
| c14 | 6.44 | 0.0207 | 1.38 × 10−3 | 5.17 × 10−4 | 2.92 × 10−3 | 1.37 × 10−3 | 9.818 × 10−3 | 93.52 |
| c15 | 6.44 | 0.0308 | 1.66 × 10−3 | 6.15 × 10−4 | 2.30 × 10−3 | 1.78 × 10−3 | 9.877 × 10−3 | 93.51 |
| c16 | 6.36 | 0.0216 | 1.94 × 10−3 | 6.87 × 10−4 | 2.93 × 10−3 | 1.93 × 10−3 | 7.954 × 10−3 | 93.61 |
| c17 | 6.35 | 0.0502 | 2.16 × 10−3 | 6.06 × 10−4 | 3.95 × 10−3 | 1.50 × 10−3 | 12.18 × 10−3 | 93.58 |
| c18 | 6.27 | 0.0390 | 2.03 × 10−3 | 7.87 × 10−4 | 2.58 × 10−3 | 1.66 × 10−3 | 8.35 × 10−3 | 93.68 |
| c19 | 6.09 | 0.0320 | 1.28 × 10−3 | 6.52 × 10−4 | 2.46 × 10−3 | 1.40 × 10−3 | 9.49 × 10−3 | 93.86 |
| c20 | 6.00 | 0.0248 | 2.77 × 10−3 | 5.31 × 10−4 | 2.68 × 10−3 | 1.31 × 10−3 | 9.03 × 10−3 | 93.96 |
| c21 | 5.74 | 0.0480 | 2.53 × 10−3 | 5.81 × 10−4 | 3.10 × 10−3 | 1.94 × 10−3 | 9.49 × 10−3 | 94.20 |
| c22 | 5.48 | 0.0281 | 2.94 × 10−3 | 6.82 × 10−4 | 2.31 × 10−3 | 1.20 × 10−3 | 9.08 × 10−3 | 94.48 |
| c23 | 5.39 | 0.0237 | 1.63 × 10−3 | 5.01 × 10−4 | 4.91 × 10−3 | 2.07 × 10−3 | 13.63 × 10−3 | 94.56 |
| c24 | 5.13 | 0.0037 | 2.05 × 10−3 | 7.28 × 10−4 | 1.57 × 10−3 | 3.39 × 10−3 | 9.75 × 10−3 | 94.85 |
| Component | Unit | Limits | |
|---|---|---|---|
| Lower | Upper | ||
| Ethanol | % (v/v) at 20 °C | 38 | 48 |
| Volatile acidity (expressed in acetic acid) | mg/100 mL AA a | - | 150 |
| Total esters (expressed in ethyl acetate) | mg/100 mL AA a | - | 200 |
| Total aldehydes (in acetaldehyde) | mg/100 mL AA a | - | 30 |
| Higher alcohols b | mg/100 mL AA a | - | 360 |
| Methanol | mg/100 mL AA a | 20 | |
| Input Parameters | Value |
|---|---|
| Process configuration | Pot + Overhead condenser |
| Number of equilibrium stages | 1 |
| Vapor–liquid equilibrium model | NRTL-HOC |
| Condenser type | Total |
| Vaporization efficiency | Ideal |
| Pot Geometry | |
| Orientation | Vertical |
| Top geometry | Elliptical |
| Bottom geometry | Elliptical |
| Diameter | 0.33 m |
| Height | 0.35 m |
| Volume | 40 L |
| Initial Operation Conditions | |
| Total initial charge | 30 kg |
| Fermented must composition | Variable |
| Reflux ratio | 0.8 |
| Pot pressure | 1 atm |
| Pressure drop (between the pot still and the condenser) | 10% |
| Effective coil power | 2.5 kW |
| Stop condition | 31 wt% of EtOH in distillate |
| Acetaldehyde | Acetic Acid | Ethyl Acetate | Higher Alcohols | |
|---|---|---|---|---|
| c1 | 18.65 | 26.68 | 3.40 | 194.87 |
| c2 | 26.40 | 29.05 | 5.33 | 278.23 |
| c3 | 23.96 | 42.67 | 4.61 | 322.33 |
| c4 | 24.68 | 48.03 | 5.31 | 241.62 |
| c5 | 14.81 | 44.59 | 4.08 | 259.27 |
| c6 | 18.58 | 51.88 | 4.52 | 337.22 |
| c7 | 20.39 | 39.86 | 5.35 | 325.32 |
| c8 | 26.48 | 82.42 | 6.04 | 295.94 |
| c9 | 21.34 | 39.07 | 5.11 | 353.15 |
| c10 | 20.61 | 61.68 | 7.05 | 339.26 |
| c11 | 23.83 | 85.97 | 4.05 | 285.21 |
| c12 | 18.37 | 57.10 | 5.56 | 334.96 |
| c13 | 22.67 | 51.99 | 5.21 | 278.98 |
| c14 | 25.51 | 19.95 | 3.82 | 259.28 |
| c15 | 30.65 | 29.85 | 4.50 | 256.96 |
| c16 | 37.58 | 20.75 | 5.42 | 239.20 |
| c17 | 41.74 | 48.31 | 4.83 | 333.68 |
| c18 | 40.89 | 37.58 | 6.58 | 243.66 |
| c19 | 27.91 | 30.65 | 6.16 | 276.15 |
| c20 | 63.25 | 23.63 | 5.48 | 276.92 |
| c21 | 66.30 | 45.60 | 7.85 | 338.69 |
| c22 | 89.48 | 26.61 | 12.98 | 329.30 |
| c23 | 52.13 | 22.36 | 11.70 | 552.27 |
| c24 * | - | - | - | - |
| Yield a | Max. Yield b | Yield Gain c | Productivity Gain d | |
|---|---|---|---|---|
| c1 | 11.4 | 15.0 | 3.6 | 31.9 |
| c2 | 10.6 | 14.0 | 3.4 | 31.6 |
| c3 | 10.2 | 13.7 | 3.6 | 35.3 |
| c4 | 10.2 | 13.7 | 3.6 | 35.3 |
| c5 | 9.7 | 13.3 | 3.6 | 36.9 |
| c6 | 9.7 | 13.3 | 3.6 | 37.4 |
| c7 | 9.3 | 12.9 | 3.6 | 38.5 |
| c8 | 9.3 | 12.7 | 3.4 | 36.7 |
| c9 | 9.3 | 12.1 | 2.8 | 30.5 |
| c10 | 8.9 | 12.4 | 3.6 | 40.5 |
| c11 | 8.4 | 12.0 | 3.6 | 42.6 |
| c12 | 7.9 | 11.5 | 3.7 | 46.8 |
| c13 | 7.4 | 11.0 | 3.7 | 50.0 |
| c14 | 7.4 | 11.0 | 3.7 | 40.0 |
| c15 | NA | 8.6 | NA | NA |
| c19 | 5.5 | 8.6 | 3.1 | 56.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenorio, L.M.S.; Batista, F.R.M.; Monteiro, S. Non-Conventional Cuts in Batch Distillation to Brazilian Spirits (cachaça) Production: A Computational Simulation Approach. Processes 2023, 11, 74. https://doi.org/10.3390/pr11010074
Tenorio LMS, Batista FRM, Monteiro S. Non-Conventional Cuts in Batch Distillation to Brazilian Spirits (cachaça) Production: A Computational Simulation Approach. Processes. 2023; 11(1):74. https://doi.org/10.3390/pr11010074
Chicago/Turabian StyleTenorio, Lhucas M. S., Fabio R. M. Batista, and Simone Monteiro. 2023. "Non-Conventional Cuts in Batch Distillation to Brazilian Spirits (cachaça) Production: A Computational Simulation Approach" Processes 11, no. 1: 74. https://doi.org/10.3390/pr11010074
APA StyleTenorio, L. M. S., Batista, F. R. M., & Monteiro, S. (2023). Non-Conventional Cuts in Batch Distillation to Brazilian Spirits (cachaça) Production: A Computational Simulation Approach. Processes, 11(1), 74. https://doi.org/10.3390/pr11010074








