Effects of Soybean and Tempeh Water Extracts on Regulation of Intestinal Flora and Prevention of Colon Precancerous Lesions in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Media
2.2. Preparation of Tempeh and Its Water Extract
2.3. Inhibitory Effect on the Proliferation of Colon Cancer Cell Line
2.4. Effect of Tempeh and Soybeans on Intestinal Functions in SD Rats
2.4.1. Animals
2.4.2. Hematology Examination
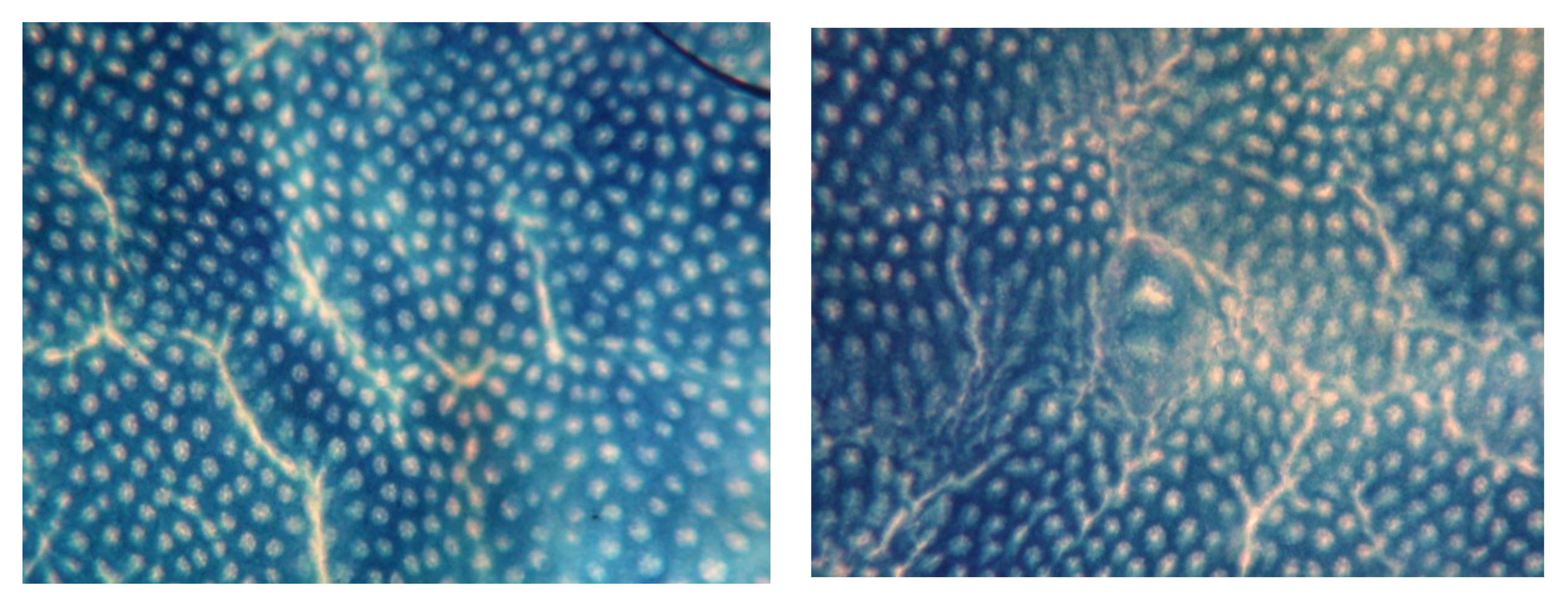
2.4.3. Determination of Precancerous Colon Lesions
2.4.4. Microbiota Composition in Cecal Content
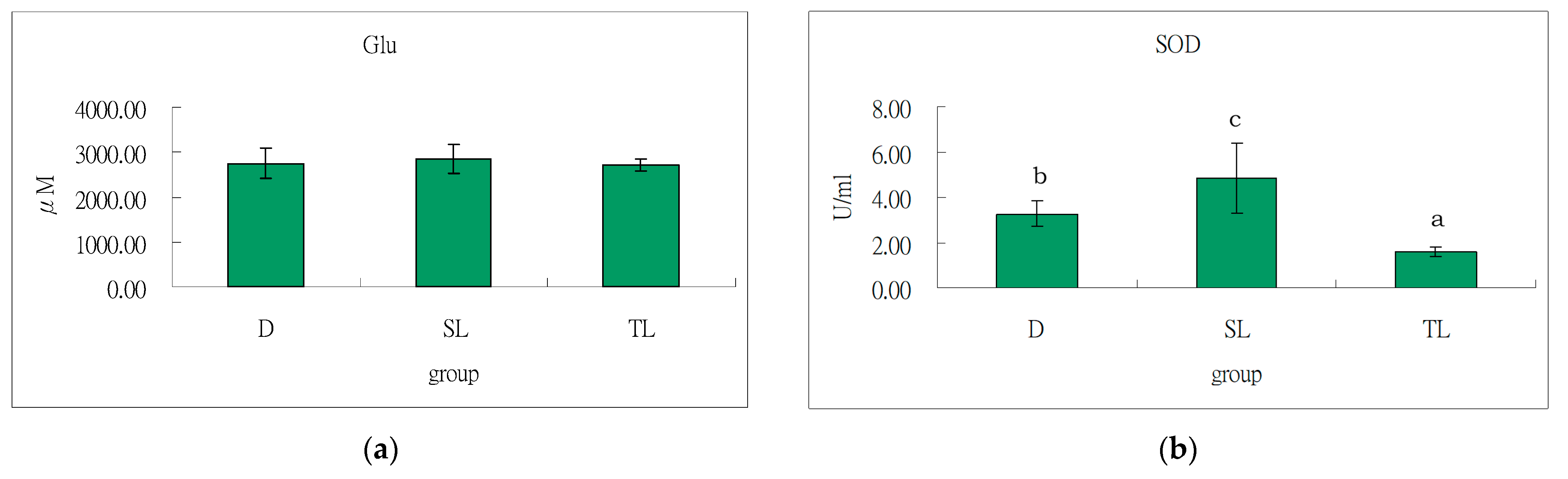
2.4.5. Evaluation of the Antioxidant Status
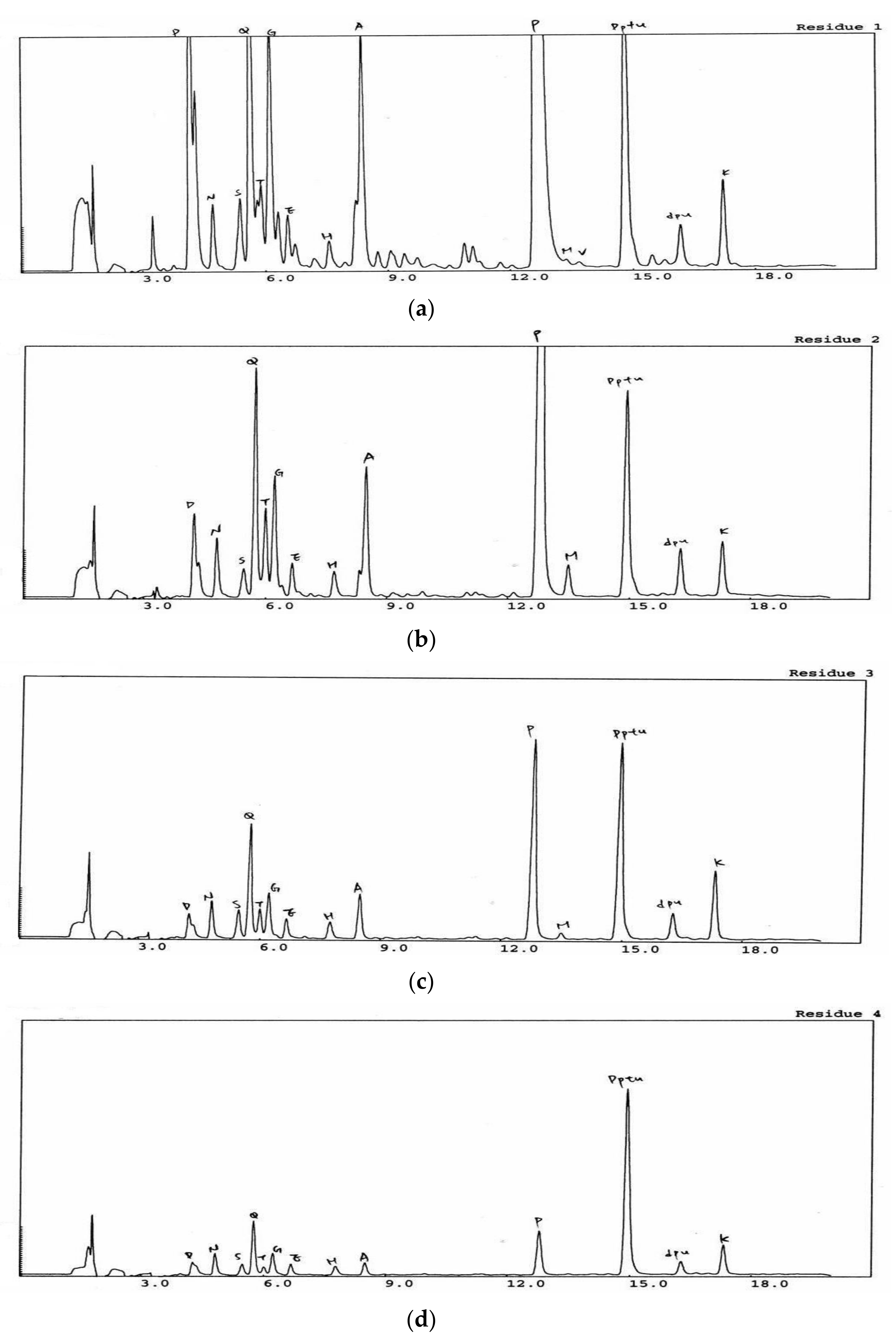
2.5. Separation, Purification, and Identification of Antioxidant Peptides from Tempeh
2.6. Statistical Analysis
3. Results
3.1. Antioxidative Oligopeptides in WET
3.2. In Vitro Experiments
Inhibitory Effect of WET on the Proliferation of Colon Cancer Cell Line
3.3. In Vivo Experiments
3.3.1. General Physiological and Biochemical Status of Experimental Rats
3.3.2. Aberrant Crypt Foci Formation
3.4. Microbiota Populations in the Cecum Contents
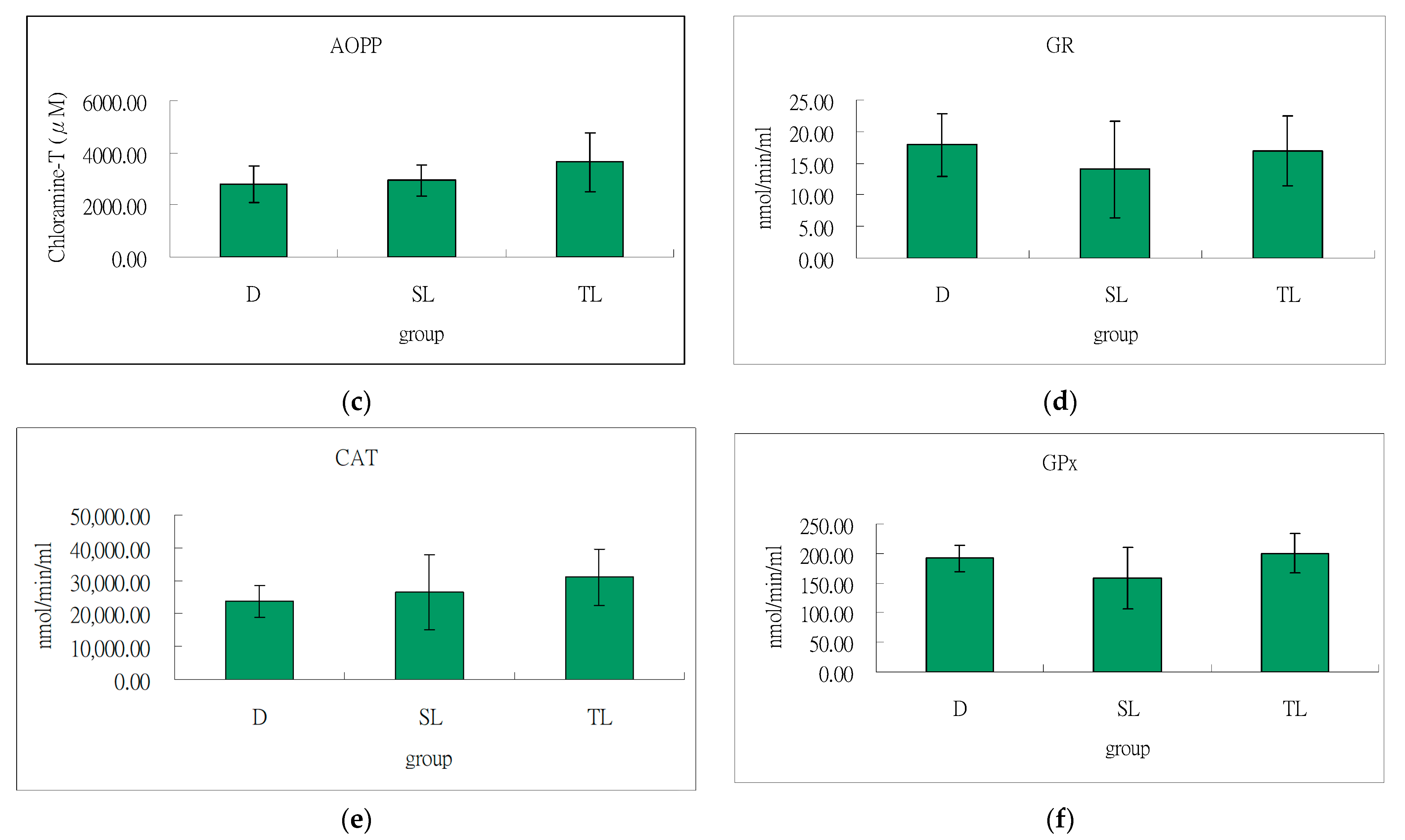
3.5. Antioxidant Effect of Soybeans and Tempeh on Liver of SD Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wong, M.C.; Ding, H.; Wang, J.; Chan, P.S.; Huang, J. Prevalence and risk factors of colorectal cancer in Asia. Intest. Res. 2019, 17, 317–329. [Google Scholar] [CrossRef]
- Ko, K.P.; Shin, A.; Cho, S.; Park, S.K.; Yoo, K.Y. Environmental contributions to gastrointestinal and liver cancer in the Asia–Pacific region. J. Gastroenterol. Hepatol. 2018, 33, 111–120. [Google Scholar] [CrossRef]
- Yang, G.; Shu, X.-O.; Li, H.; Chow, W.-H.; Cai, H.; Zhang, X.; Gao, Y.-T.; Zheng, W. Prospective cohort study of soy food intake and colorectal cancer risk in women. Am. J. Clin. Nutr. 2009, 89, 577–583. [Google Scholar] [CrossRef]
- Cao, Z.-H.; Green-Johnson, J.M.; Buckley, N.D.; Lin, Q.-Y. Bioactivity of soy-based fermented foods: A review. Biotechnol. Adv. 2019, 37, 223–238. [Google Scholar] [CrossRef]
- Roelofsen, P.; Talens, A. Changes in some B vitamins during molding of soybeans by Rhizopus oryzae in the production of tempeh kedelee. J. Food Sci. 1964, 29, 224–226. [Google Scholar] [CrossRef]
- Ahnan-Winarno, A.D.; Cordeiro, L.; Winarno, F.G.; Gibbons, J.; Xiao, H. Tempeh: A semicentennial review on its health benefits, fermentation, safety, processing, sustainability, and affordability. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1717–1767. [Google Scholar] [CrossRef]
- Yang, Y.; Kameda, T.; Aoki, H.; Nirmagustina, D.E.; Iwamoto, A.; Kato, N.; Yanaka, N.; Okazaki, Y.; Kumrungsee, T. The effects of tempe fermented with Rhizopus microsporus, Rhizopus oryzae, or Rhizopus stolonifer on the colonic luminal environment in rats. J. Funct. Foods 2018, 49, 162–167. [Google Scholar] [CrossRef]
- Esaki, H.; Onozaki, H.; Kawakishi, S.; Osawa, T. New antioxidant isolated from tempeh. J. Agric. Food Chem. 1996, 44, 696–700. [Google Scholar] [CrossRef]
- Kuligowski, M.; Pawłowska, K.; Jasińska-Kuligowska, I.; Nowak, J. Isoflavone composition, polyphenols content and antioxidative activity of soybean seeds during tempeh fermentation. CYTA-J. Food 2017, 15, 27–33. [Google Scholar] [CrossRef]
- Kudou, S.; Shimoyamada, M.; Imura, T.; Uchida, T.; Okubo, K. A new isoflavone glycoside in soybean seeds (Glycine max MERRILL), glycitein 7-O-β-D-(6”-O-acetyl) glucopyranoside. Agric. Biol. Chem. 1991, 55, 859–860. [Google Scholar] [CrossRef]
- Chang, C.T.; Hsu, C.K.; Chou, S.T.; Chen, Y.C.; Huang, F.S.; Chung, Y.C. Effect of fermentation time on the antioxidant activities of tempeh prepared from fermented soybean using Rhizopus oligosporus. Int. J. Food Sci. Technol. 2009, 44, 799–806. [Google Scholar] [CrossRef]
- Vattem, D.; Lin, Y.-T.; Labbe, R.; Shetty, K. Antimicrobial activity against select food-borne pathogens by phenolic antioxidants enriched in cranberry pomace by solid-state bioprocessing using the food grade fungus Rhizopus oligosporus. Process Biochem. 2004, 39, 1939–1946. [Google Scholar] [CrossRef]
- Wang, H.L.; Ruttle, D.I.; Hesseltine, C. Antibacterial compound from a soybean product fermented by Rhizopus oligosporus. Proc. Soc. Exp. Biol. Med. 1969, 131, 579–583. [Google Scholar] [CrossRef]
- Alorda-Clara, M.; Torrens-Mas, M.; Morla-Barcelo, P.M.; Roca, P.; Sastre-Serra, J.; Pons, D.G.; Oliver, J. High Concentrations of Genistein Decrease Cell Viability Depending on Oxidative Stress and Inflammation in Colon Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 7526. [Google Scholar] [CrossRef]
- Sheih, I.; Wu, H.; Lai, Y.; Lin, C. Preparation of high free radical scavenging tempeh by a newly isolated Rhizopus sp. R-69 from Indonesia. Food Sci. Agric. Chem. 2000, 2, 35–40. [Google Scholar]
- Fernández-Tomé, S.; Xu, F.; Han, Y.; Hernández-Ledesma, B.; Xiao, H. Inhibitory effects of peptide lunasin in colorectal cancer HCT-116 cells and their tumorsphere-derived subpopulation. Int. J. Mol. Sci. 2020, 21, 537. [Google Scholar] [CrossRef]
- Bird, R.P. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer. Cancer Lett. 1995, 93, 55–71. [Google Scholar] [CrossRef]
- Perše, M.; Cerar, A. Morphological and molecular alterations in 1,2-dimethylhydrazine and azoxymethane induced colon carcinogenesis in rats. J. Biomed. Biotechnol. 2010, 2011, 473964. [Google Scholar] [CrossRef]
- Pozharisski, K.; Shaposhnikov, J.; Petrov, A.; Likhachev, A. Distribution and carcinogenic action of 1, 2-dimethylhydrazine (SDMH) in rats. J. Cancer Res. Clin. Oncol. 1976, 87, 67–80. [Google Scholar] [CrossRef]
- Penit, C.; Papiernik, M. Regulation of thymocyte proliferation and survival by deoxynucleosides. Deoxycytidine produced by thymic accessory cells protects thymocytes from deoxyguanosine toxicity and stimulates their spontaneous proliferation. Eur. J. Immunol. 1986, 16, 257–263. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Hsu, C.-K.; Ko, C.-Y.; Chan, Y.-C. Dietary intake of xylooligosaccharides improves the intestinal microbiota, fecal moisture, and pH value in the elderly. Nutr. Res. 2007, 27, 756–761. [Google Scholar] [CrossRef]
- McLellan, E.A.; Bird, R.P. Aberrant crypts: Potential preneoplastic lesions in the murine colon. Cancer Res. 1988, 48, 6187–6192. [Google Scholar] [PubMed]
- Pahle, J.; Menzel, L.; Niesler, N.; Kobelt, D.; Aumann, J.; Rivera, M.; Walther, W. Rapid eradication of colon carcinoma by Clostridium perfringens Enterotoxin suicidal gene therapy. BMC Cancer 2017, 17, 129. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Aikawa, K.; Takahashi, T.; Yamai, S. Influence of sodium chloride on the β-glucuronidase activity of Clostridium perfringens and Escherichia coli. Lett. Appl. Microbiol. 2000, 31, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Humblot, C.; Murkovic, M.; Rigottier-Gois, L.; Bensaada, M.; Bouclet, A.; Andrieux, C.; Anba, J.; Rabot, S. β-glucuronidase in human intestinal microbiota is necessary for the colonic genotoxicity of the food-borne carcinogen 2-amino-3-methylimidazo [4, 5-f] quinoline in rats. Carcinogenesis 2007, 28, 2419–2425. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Jin, Y.-H. Intestinal bacterial β-glucuronidase activity of patients with colon cancer. Arch. Pharm. Res. 2001, 24, 564–567. [Google Scholar] [CrossRef]
- Cheng, K.-W.; Tseng, C.-H.; Chen, I.-J.; Huang, B.-C.; Liu, H.-J.; Ho, K.-W.; Lin, W.-W.; Chuang, C.-H.; Huang, M.-Y.; Leu, Y.-L. Inhibition of gut microbial β-glucuronidase effectively prevents carcinogen-induced microbial dysbiosis and intestinal tumorigenesis. Pharmacol. Res. 2022, 177, 106115. [Google Scholar] [CrossRef]
- Arimochi, H.; Kataoka, K.; Kuwahara, T.; Nakayama, H.; Misawa, N.; Ohnishi, Y. Effects of β-glucuronidase-deficient and lycopene-producing Escherichia coli strains on formation of azoxymethane-induced aberrant crypt foci in the rat colon. Biochem. Biophys. Res. Commun. 1999, 262, 322–327. [Google Scholar] [CrossRef]
- Ahmad, A.; Ramasamy, K.; Majeed, A.B.A.; Mani, V. Enhancement of β-secretase inhibition and antioxidant activities of tempeh, a fermented soybean cake through enrichment of bioactive aglycones. Pharm. Biol. 2015, 53, 758–766. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, S.; Zheng, Y. Chinese bayberry fruit extract alleviates oxidative stress and prevents 1, 2-dimethylhydrazine-induced aberrant crypt foci development in rat colon carcinogenesis. Food Chem. 2011, 125, 701–705. [Google Scholar] [CrossRef]
- Hutchins, A.M.; Slavin, J.L.; Lampe, J.W. Urinary isoflavonoid phytoestrogen and lignan excretion after consumption of fermented and unfermented soy products. J. Am. Diet. Assoc. 1995, 95, 545–551. [Google Scholar] [CrossRef]
- Zhu, Q.; Meisinger, J.; Thiel, D.H.V.; Zhang, Y.; Mobarhan, S. Effects of soybean extract on morphology and survival of Caco-2, SW620, and HT-29 cells. Nutr. Cancer 2002, 42, 131–140. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.; Zuo, X.; Wang, M. Chemopreventive effects of some popular phytochemicals on human colon cancer: A review. Food Funct. 2018, 9, 4548–4568. [Google Scholar] [CrossRef]


| C | D | SL | SH | TL | TH | |
|---|---|---|---|---|---|---|
| Albumin (g/dL) | 4.48 ± 0.28 b | 4.35 ± 0.30 ab | 4.21 ± 0.36 a | 4.36 ± 0.22 ab | 4.28 ± 0.13 ab | 4.33 ± 0.19 ab |
| BUN (mg/dL) | 13.20 ± 1.55 | 11.70 ± 2.00 | 13.33 ± 1.94 | 12.50 ± 1.08 | 11.78 ± 2.28 | 12.56 ± 1.42 |
| Creatinine (mg/mL) | 0.44 ± 0.06 | 0.44 ± 0.04 | 0.44 ± 0.08 | 0.42 ± 0.05 | 0.40 ± 0.05 | 0.41 ± 0.08 |
| Glucose (mg/dL) | 290.80 ± 65.76 b | 206.40 ± 50.94 a | 232.44 ± 75.08 a | 224.40 ± 44.81 a | 188.78 ± 35.95 a | 196.00 ± 41.17 a |
| Protein, total (g/dL) | 6.02 ± 0.40 | 5.90 ± 0.34 | 5.70 ± 0.49 | 5.89 ± 0.40 | 5.81 ± 0.29 | 5.86 ± 0.35 |
| AST (U/L) | 95.91 ± 59.21 | 121.10 ± 45.25 | 143.78 ± 89.16 | 149.10 ± 105.27 | 140.44 ± 80.40 | 134.88 ± 59.13 |
| ALT (U/L) | 50.3 ± 32.64 | 47.90 ± 18.17 | 57.67 ± 21.51 | 73.60 ± 52.22 | 67.22 ± 38.82 | 76.11 ± 55.25 |
| Bilirubin-Total (mg/dL) | 0.42 ± 0.15 a | 0.63 ± 0.28 b | 0.55 ± 0.17 ab | 0.52 ± 0.18 ab | 0.54 ± 0.13 ab | 0.52 ± 0.12 ab |
| Cholesterol (mg/dL) | 43.9 ± 12.91 b | 33.30 ± 11.18 ab | 35.22 ± 10.52 ab | 34.00 ± 5.62 ab | 28.33 ± 5.41 a | 34.56 ± 13.79 ab |
| Triglyceride (mg/dL) | 160.00 ± 93.66 b | 99.20 ± 34.38 a | 115.11 ± 47.39 ab | 113.00 ± 33.79 ab | 102.67 ± 24.52 a | 101.33 ± 38.34 a |
| Group | C | D | SL | SH | TL | TH |
|---|---|---|---|---|---|---|
| colon weight (g) | 0.09 ± 0.03 a | 0.11 ± 0.02 ab | 0.11 ± 0.03 ab | 0.11 ± 0.02 ab | 0.11 ± 0.02 ab | 0.12 ± 0.03 b |
| cecum weight (g) | 3.83 ± 1.00 | 4.04 ± 1.55 | 4.26 ± 1.14 | 3.86 ± 0.72 | 4.03 ± 0.51 | 4.00 ± 0.87 |
| cecum content (g) | 2.77 ± 0.86 | 2.75 ± 1.51 | 2.54 ± 1.38 | 2.76 ± 0.66 | 2.41 ± 0.98 | 2.36 ± 1.15 |
| pH | 6.83 ± 0.08 | 6.74 ± 0.15 | 6.88 ± 0.10 | 6.83 ± 0.09 | 6.89 ± 0.12 | 6.78 ± 0.19 |
| fecal moisture (%) | 82.53 ± 4.98 b | 80.40 ± 1.38 ab | 78.04 ± 3.73 a | 79.42 ± 1.26 ab | 78.38 ± 3.67 a | 80.72 ± 2.96 ab |
| Number of Crypt/ACF | 1 Crypt | 2 Crypt | 3 Crypt | ≧4 Crypt | Total ACF |
|---|---|---|---|---|---|
| C | 0.1 ± 0.3 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.1 ± 0.3 a |
| D | 29.8 ± 13.4 c | 43.5 ± 19.4 c | 28.1 ± 15.4 b | 23.2 ± 17.2 c | 124.6 ± 54.9 c |
| SL | 11.2 ± 6.9 ab | 20.8 ± 13.9 b | 14.6 ± 11.9 b | 12.3 ± 12.9 bc | 58.9 ± 41.2 b |
| SH | 20.2 ± 19.4 bc | 33.0 ± 31.5 bc | 25.3 ± 28.9 b | 11.4 ± 12.8 abc | 89.9 ± 91.1 bc |
| TL | 15.9 ± 8.1 b | 28.6 ± 10.8 bc | 15.6 ± 6.1 b | 15.3 ± 8.9 bc | 75.3 ± 25.2 bc |
| TH | 17.6 ± 15.1 b | 27.2 ± 28.9 bc | 15.0 ± 11.7 b | 8.6 ± 13.0 ab | 68.3 ± 56.8 b |
| C | D | SL | SH | TL | TH | |
|---|---|---|---|---|---|---|
| E. coli | 9.74 ± 0.94 | 9.95 ± 1.20 | 9.40 ± 1.13 | 9.70 ± 0.97 | 9.82 ± 0.86 | 10.1 ± 1.18 |
| Lactobacillus spp. | 11.78 ± 1.00 | 11.81 ± 0.74 | 11.82 ± 0.85 | 11.88 ± 0.78 | 11.70 ± 0.72 | 11.73 ± 0.87 |
| Bifidobacteria spp. | 8.43 ± 0.22 | 8.41 ± 0.33 | 8.19 ± 0.70 | 8.20 ± 0.50 | 7.91 ± 0.29 | 8.06 ± 0.25 |
| C. perfringens | ND # | 2.25 ± 0.52 | ND | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Divate, N.R.; Ardanareswari, K.; Yu, Y.-P.; Chen, Y.-C.; Liao, J.-W.; Chung, Y.-C. Effects of Soybean and Tempeh Water Extracts on Regulation of Intestinal Flora and Prevention of Colon Precancerous Lesions in Rats. Processes 2023, 11, 257. https://doi.org/10.3390/pr11010257
Divate NR, Ardanareswari K, Yu Y-P, Chen Y-C, Liao J-W, Chung Y-C. Effects of Soybean and Tempeh Water Extracts on Regulation of Intestinal Flora and Prevention of Colon Precancerous Lesions in Rats. Processes. 2023; 11(1):257. https://doi.org/10.3390/pr11010257
Chicago/Turabian StyleDivate, Nileema R., Katharina Ardanareswari, Yu-Ping Yu, Ya-Chen Chen, Jiunn-Wang Liao, and Yun-Chin Chung. 2023. "Effects of Soybean and Tempeh Water Extracts on Regulation of Intestinal Flora and Prevention of Colon Precancerous Lesions in Rats" Processes 11, no. 1: 257. https://doi.org/10.3390/pr11010257
APA StyleDivate, N. R., Ardanareswari, K., Yu, Y.-P., Chen, Y.-C., Liao, J.-W., & Chung, Y.-C. (2023). Effects of Soybean and Tempeh Water Extracts on Regulation of Intestinal Flora and Prevention of Colon Precancerous Lesions in Rats. Processes, 11(1), 257. https://doi.org/10.3390/pr11010257