Abstract
Metronidazole, ornidazole, tinidazole, and secnidazole are 5-nitroimidazoles. The purpose of this work was to propose a new economical TLC–densitometric method to evaluate the chemical stability of metronidazole, secnidazole, ornidazole, and tinidazole under stress conditions. A forced degradation study was performed on silica gel and aqueous solutions at various pH values; the metronidazole, secnidazole, ornidazole, and tinidazole solutions were prepared in saline and in hydrogen peroxide, respectively. The samples of the 5-nitroimidazoles were heated. TLC analyses were performed on silica gel 60F254 using chloroform–methanol (9:1, v/v) as the mobile phase. As the TLC–densitometric method can effectively separate the metronidazole, secnidazole, ornidazole, and tinidazole from their degradation products which formed as a result of the stress studies, it is considered to can be a good alternative and important tool in the routine quality control and stability testing of metronidazole, secnidazole, ornidazole, and tinidazole in pharmaceutical formulations. The results indicate that the proposed TLC–densitometric method is cost-effective, rapid, specific, accurate, and precise; the TLC–densitometric method also realizes the criterion of the linearity. A major advantage of the proposed method is its low cost and ability to analyze the 5-nitroimidazole which was investigated and all its degradation products simultaneously.
1. Introduction
The interest in nitroimidazoles began with the isolation of a natural antibiotic, azomycin, from Nocardia mesenterica in the 1950s. Chemically, this compound is in fact a 2-nitroimidazole. Since its discovery, synthetic analogues have been developed, being derivatives of 4-nitro and 5-nitroimidazole, which are now widely used in medicine [1]. The nitro group at position 5 on the imidazole ring is the most common positional isomer. Some examples of this type of drug are metronidazole, ornidazole, tinidazole, secnidazole (Figure 1). These drugs have a bactericidal effect, mainly against anaerobic and microaerophilic bacteria and protozoa. Table 1 summarizes the selected physicochemical properties of metronidazole, secnidazole, ornidazole, and tinidazole [2,3,4,5,6,7].
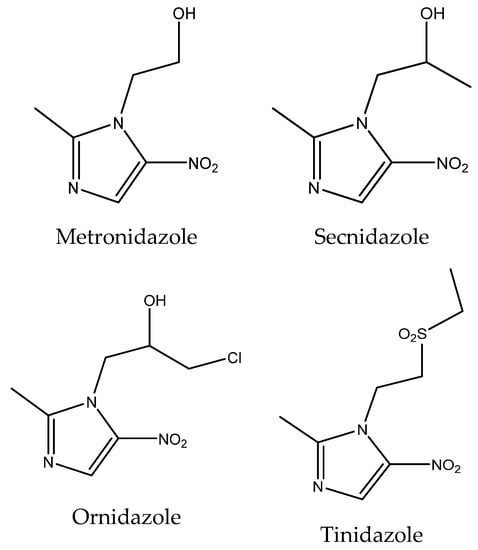
Figure 1.
Structural formulas of metronidazole, secnidazole, ornidazole, and tinidazole.

Table 1.
Selected physicochemical properties of metronidazole, secnidazole, ornidazole, and tinidazole [2,3,4,5,6,7].
Durability, i.e., the drug’s stability, refers to the drug substance and the finished drug form and determines the resistance to unfavorable processes occurring during the production, storage under certain conditions and time, as well as during the drug’s transport and distribution. The development of a medicinal substance, taking into account its physicochemical form, is closely related to its physical and chemical stability. These features are the basis for determining the conditions of the technological procedure, the selection of auxiliary agents in the development of drug formulations, and specifying the conditions for their storage. The reasons for the instability of drugs are divided into biological reasons, which include the loss of asepticity, as well as the related chemical changes; physical phenomena, such as the evaporation, sublimation, water sorption, change in consistency, formation of sediments, sedimentation of suspensions, separation of suspensions, separation of emulsions, and mechanical damage; and finally chemical phenomena, such as hydrolysis, oxidation and reduction, and spatial rearrangements. A large number of drugs decompose during storage, causing them to lose their biological activity and medicinal value. They undergo hydrolysis, oxidation, reduction, and racemization. The rate of these processes depends to a large extent on the temperature, pH, and the presence of light. Therefore, it is important to determine the optimal storage conditions for drugs and pharmaceutical active ingredients [8,9,10,11,12,13].
The study of the stability of metronidazole, secnidazole, ornidazole, and tinidazole has been the subject of numerous scientific papers [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. Various analytical techniques were used to analyze the stability of 5-nitroimidazoles: high-performance liquid chromatography (HPLC) [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32], ultra-performance liquid chromatography (UPLC) [33,34], liquid chromatography with mass spectrometry (LC-MS) [25], liquid chromatography with tandem photo diode array detection and mass spectrometry (LC-PDA-MS) [35], UV-spectrophotometry [23,32,36,37,38,39,40], FT-IR-spectrophotometry [41], thin layer chromatography (TLC) [32,42], TLC–densitometry [23,43,44,45], and calorimeters [23,46]. Metronidazole (two degradation products were found) [43], secnidazole (two degradation products were found) [23], and tinidazole (one degradation product was found) [44] were tested using the TLC technique in conjunction with densitometry. In individual publications, only one of the listed 5-nitroimidazoles was the subject of research. These publications mainly give the amounts of 5-nitroimidazole that remained (not that which was degraded or what percentage was decomposed) under stress conditions. Only in a few publications do the authors indicate to what number of degradation products the tested 5-nitroimidazole is converted [16,21,22,23,24,25,27,29,35,43,44]. In addition, there are no scientific reports in which the stability of metronidazole, secnidazole, ornidazole, and tinidazole was tested under exactly the same stress conditions and using exactly the same research method.
Therefore, the aim of this study was to evaluate the chemical stability of metronidazole, secnidazole, ornidazole, and tinidazole exposed to identical stress conditions which exist during the storage of these drugs and their analysis by using a new TLC method coupled with densitometry. The degradation studies of metronidazole, secnidazole, ornidazole, and tinidazole were assessed by means both TLC–densitometric and spectrodensitometric procedures, which are of rapid and inexpensive. Tee estimation of the effectiveness of the proposed TLC method for the separation of metronidazole, secnidazole, ornidazole, and tinidazole as pharmaceutical active ingredients from their degradation products was performed in this study. The presented results my help to us understand the impact of temperature exposure on the stability of these commonly used active pharmaceutical ingredients through a simple TLC method, without needing complex equipment such as HPLC or something similar.
2. Materials and Methods
2.1. Chemicals and Reagents
Pharmaceutical reference standard of metronidazole, secnidazole, ornidazole, and tinidazole, as well as 2-methyl-5-nitroimidazole, were supplied by Sigma-Aldrich (Saint Louis, MO, USA). Metronidazole and tinidazole were pharmaceutical primary standards with a purity according with the United States Pharmacopeia and European Pharmacopoeia, respectively. Secnidazole and ornidazole were analytical standards with a quality level equal to 100. The hydrogen peroxide (3%) was from Hasco-Lek (Wrocław, Poland). The physiological salt solution (0.9% NaCl) was from Gilbert (Hérouville-Saint-Clair, France). Chloroform, methanol, acetone, ethyl acetate, glacial acetic acid, 0.1 M of solution of HCl, and 0.1 M of solution of NaOH were purchased from POCh (Gliwice, Poland). All of the chemicals and reagents used in this study were of HPLC grade.
2.2. Preparation of Stock and Working Standard Solution
Standard solutions (S1) containing 1 mg·mL−1 of metronidazole, secnidazole, ornidazole, and tinidazole, respectively, were prepared by dissolving 50 mg of metronidazole, secnidazole, ornidazole, and tinidazole, respectively, in 50 mL of methanol. In order to assess the effect of heating (temperature) on the chemical stability of the examined metronidazole, secnidazole, ornidazole, and tinidazole, respectively, the six solutions of metronidazole, secnidazole, ornidazole, and tinidazole (I–VI) prepared in various solvents were used: I—in water at pH = 2.62; II—in water at pH = 5.56; III—in water at pH = 8.21; IV—in hydrogen peroxide (3%); V—in physiological salt (0.9% NaCl); and VI—in methanol. All of the solutions were prepared in the appropriate measuring flasks in an amount of 10 mL by mixing of 5 mL of the standard stock solutions S1 (equivalent to 5 mg of metronidazole, secnidazole, ornidazole, and tinidazole) and 5 mL of the proper solvent. Methanolic solutions of metronidazole, secnidazole, ornidazole, and tinidazole, respectively, (VII) were used in the case of a stability study on silica gel which were prepared by mixing 5 mL of the stock standard solutions (equivalent to 5 mg of metronidazole, secnidazole, ornidazole, and tinidazole) and 5 mL of methanol. Methanolic solutions (VII) of metronidazole, secnidazole, ornidazole, and tinidazole were also used as the reference standard solutions.
2.3. Chromatographic Conditions
Chromatography was performed on aluminum foil plates coated with 0.20 mm layers of silica gel 60F254 (#1.05570, Merck, Germany). Before use, the plates were activated at 120 °C for 20 min. Five microliters of solutions were spotted by the use of a micropipette (Camag, Switzerland) onto chromatographic plates in each case. Three mobile phases: acetone + chloroform + ethyl acetate (4:4:1, v/v/v); chloroform + acetone + glacial acetic acid (7.5:2.5:0.1, v/v/v); and chloroform + methanol (9:1, v/v) were tested. The mobile phase of chloroform: methanol (9:1, v/v) was chosen as the best, and it was finally used in the entire experiment. The chamber was pre-saturated with mobile phase vapors for 30 min at room temperature (20 ± 1 °C). The development distance was approximately 75 mm.
2.4. Stability of Metronidazole, Secnidazole, Ornidazole, and Tinidazole
2.4.1. Effect of Temperature on the Chemical Stability of Metronidazole, Secnidazole, Ornidazole, and Tinidazole on Silica Gel
The standard solutions of metronidazole, secnidazole, ornidazole, and tinidazole were spotted in the amount of 5 μL on chromatographic plates after their activation at 120 °C for 20 min. Next, these plates with spotted compounds were heated at 40 °C, 80 °C, and 120 °C for 24 h. After this time, the standard solutions of metronidazole, secnidazole, ornidazole, and tinidazole were spotted on chromatographic plates near the compounds which were heated. The plates were then developed with mobile phase chloroform: methanol (9:1, v/v). The experiments were performed in six different analyses.
2.4.2. Effect of Temperature on the Chemical Stability of Metronidazole, Secnidazole, Ornidazole, and Tinidazole in Form of Various Solutions
The solutions of metronidazole, secnidazole, ornidazole, and tinidazole prepared in solvents I–VI in volume flasks (10 mL) were heated at 40 °C for 100, 250, 500, and 1000 h. After these times, the solutions were spotted on chromatographic plates and developed in chloroform: methanol (9:1, v/v) was the mobile phase. The experiments were performed in six different analyses.
2.5. Validation of Method
2.5.1. Linearity of Detector Response and Range
The linearity of the TLC method was evaluated by an analysis series of the standard solutions of metronidazole, secnidazole, ornidazole, and tinidazole at the following concentrations: 0.005, 0.010, 0.015, 0.020, 0.025, 0.030, 0.035; 0.040, 0.045, 0.050, 0.06, 0.080, 0.100, 0.120, 0.140, 0.160, 0.180, 0.200, 0.220, 0.240, 0.260, 0.280, and 0.300 mg·mL−1. The solutions (5 μL) were applied to the same plate. The plates were developed using mobile phase chloroform–methanol in a volume composition of 9:1. The calibration plots were developed by plotting the peak area versus the concentration of metronidazole, secnidazole, ornidazole, and tinidazole, respectively.
The experiments were performed in six different analyses.
2.5.2. Accuracy
The accuracy of the TLC method was determined by investigating the recovery percentage of the studied metronidazole, secnidazole, ornidazole, and tinidazole at three concentration levels covering the low, medium, and high regions of the calibration plot by spotting on a chromatographic plate (n = 5 for each concentration level), where n represents the number of analyses. The resulting spots were analyzed by the use of the TLC procedure described above.
2.5.3. Precision
The precision was evaluated in terms of the repeatability (intra-day) and inter-day precision. The repeatability of the proposed method was determined by the analysis of three replicates of the sample solutions at three concentration levels under the same operating conditions over a short interval of time (the same day). Intermediate (inter-day) precision was determined for three sample solutions at three concentration levels by an analyst who performed the analysis over a period of one week. To determine the precision of the procedure, the concentrations were prepared independently and experiments were performed in three different analyses. The precision was evaluated as the coefficient of variation, CV [%]. The peak areas obtained were used to calculate the mean and CV [%].
2.5.4. Limit of Detection (LOD) and Limit of Quantification (LOQ) Based on the Special Calibration Curve
A specific calibration curves were prepared using samples containing metronidazole, secnidazole, ornidazole, and tinidazole, respectively, in the low range of their concentrations. The limit of detection and quantification were calculated from the equation of the graph of the area obtained spots of 5-nitroimidazole versus the concentration. The formulas used were:
where σ is the standard deviation of the response and S is the slope of the calibration curve.
The experiments were performed in six different analyses.
2.6. Densitometric and Spectrodensitometric Analysis
Densitometric and spectrodensitometric investigations were done using a TLC Scanner 3 (Camag, Muttenz, Switzerland) operated in the absorbance mode and controlled by winCATS 1.4.2 software. Densitometric scanning was performed in a multi-wavelength mode in the range of 200 to 380 nm (precisely at seven wavelengths, i.e., 200 nm, 230 nm, 260 nm, 290 nm, 320 nm, 350 nm, and 380 nm), at wavelength intervals of 30 nm at each step. In the densitograms, each line corresponds to a different wavelength at which the densitometry analysis was performed.
2.7. Statistical Analysis
The statistical evaluation of the obtained results was performed with the use of the computer software Statistica 13.0 (StatSoft, Kraków, Poland). The results are the averages of the six analyzes performed.
3. Results and Discussion
Controlling the chemical stability of a drug substance is an extremely important aspect that allows the drug to work properly. The studies conducted to check the durability of the drugs when exposed to various chemical and physical factors provide valuable information to manufacturers and pharmacists who can warn patients about the dangers of the improper storage or transport of drugs to countries where the average daily temperatures are higher than in the country where the drug is distributed.
3.1. Effect of Temperature on the Chemical Stability of Metronidazole, Secnidazole, Ornidazole, and Tinidazole on Silica Gel
The amounts of metronidazole, secnidazole, ornidazole, and tinidazole that remained after heating on silica gel for 24 h at 40 °C, 80 °C, and 120 °C are compared in Figure 2. On silica gel at 40 °C, all of the tested 5-nitroimidazoles are stable. Metronidazole and secnidazole show a similar stability at 80 °C and 120 °C. The full description of the chromatographic peaks of the examined metronidazole, secnidazole, ornidazole, and tinidazole and their degradation products formed during pharmaceutical active ingredient heating on silica gel at 120 °C for 24 h is presented in Table S1. In the case of metronidazole and secnidazole, it was found that at 120 °C they degrade into two products (Table S1, Figures S1 and S2). The amount of metronidazole remains at 87.54% and secnidazole at 89.02%. Earlier studies also indicated the formation of two degradation products, metronidazole [43] and secnidazole [23].
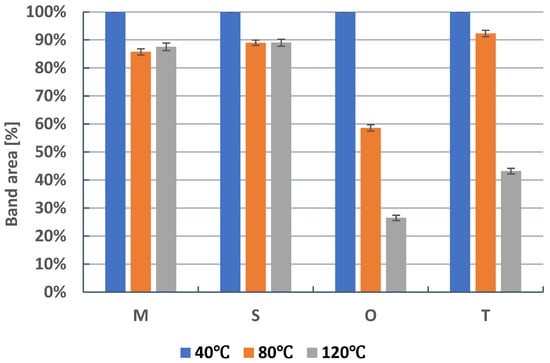
Figure 2.
The comparison of means area (±SD) (%) of chromatographic bands obtained of metronidazole (M), secnidazole (S), ornidazole (O), and tinidazole (T) which were heated on silica gel at 40 °C, 80 °C, and 120 °C for 24 h and developed in the chloroform–methanol (9:1, v/v) as the mobile phase, where SD is the standard deviation.
Ornidazole, which was heated on silica gel at 80 °C, remains less than 60%. It degrades into four products (Figure S3). At 120 °C, the degradation of ornidazole occurs to an even greater extent (Figure 3, Table S1). Ornidazole remains at 26.49% and it degrades into four products. At the same time, the most substance with the value of RF = 0.19, i.e., 40.25% (Figure 3), is formed. Bakshi at al. [29], by HPLC, as well as Santurio et al. [32], by HPLC and TLC, found the presence of two or three degradation products of ornidazole, which was subjected to different stress conditions. It follows that the TLC–densitometric method proposed in this paper enables the separation of a larger amount, i.e., four ornidazole degradation products and three traces.
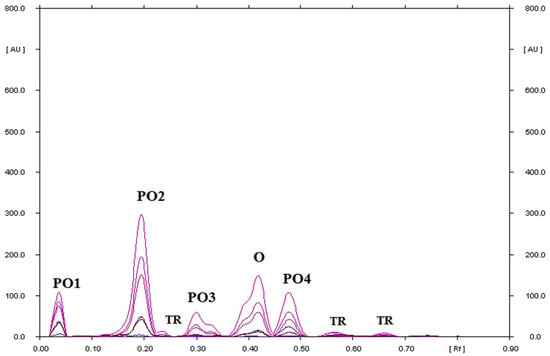
Figure 3.
TLC-UV densitograms recorded in the multi wavelength from 200 to 380 nm (at wavelength intervals of 30 nm at each step) of ornidazole heated on silica gel at 120 °C for 24 which was next separating using chloroform–methanol (9:1, v/v) as mobile phase, where O is ornidazole, PO1, PO2, PO3, and PO4 are the degradation products of ornidazole, and TR is the traces.
At 80 °C, tinidazole is relatively stable, and 92.33% of it remains (Figure 2). However, at 120 °C, it further degrades to a single product (Figure 4). Under these conditions, only 43.19% of tinidazole remains (Figure 2, Table S1). The degradation product of tinidazole was identified as 2-methyl-5-nitroimidazoles (Figure 5). These results are consistent with the studies of Vaghela and Rao [24]. Namely, the dry heating of tinidazole at 80 °C for 5 h resulted in a loss of 1.2% of tinidazole. At the same time, 2-methyl-5-nitroimidazole was found as a degradation product of tinidazole [24]. Sneha et al. [34] heated a solid sample at 60 °C for 24 h, which resulted in the thermal decomposition of tinidazole at the level of 0.98%. In contrast, heating a solid sample of tinidazole at 50 °C for 3 months was not significant for the degradation of tinidazole [25].
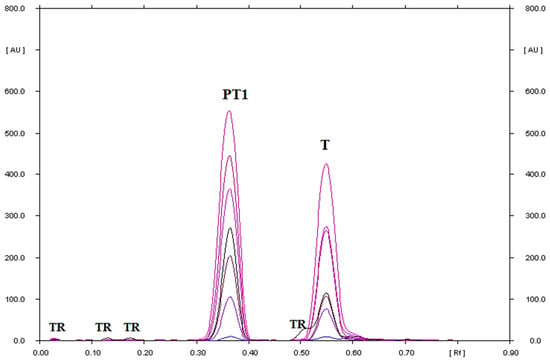
Figure 4.
TLC-UV densitograms recorded in the multi wavelength from 200 to 380 nm (at wavelength intervals of 30 nm at each step) of tinidazole heated on silica gel at 120 °C for 24 which was next separating using chloroform–methanol (9:1, v/v) as mobile phase, where: T is tinidazole, PT1 is the degradation product of tinidazole identified as 2-methyl-5-nitroimidazole, and TR is the traces.
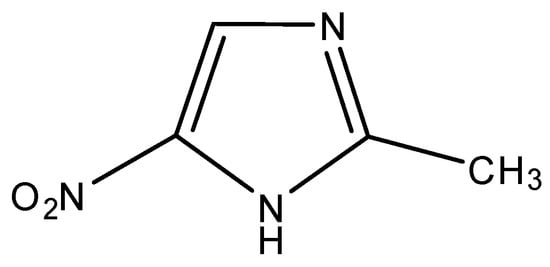
Figure 5.
Structural formula of 2-methyl-5-nitroimidazole.
The new chromatographic conditions were used: the plates precoated with silica gel 60F254 and the mobile phase chloroform–methanol (9:1, v/v) are effective for separating the individual tested 5-nitroimidazole and their degradation products. The densitograms (Figures S1–S3, Figure 3 and Figure 4) show traces (TR) of the potential next degradation products of the tested 5-nitroimidazoles.
3.2. Effect of Temperature on the Chemical Stability of Metronidazole, Secnidazole, Ornidazole, and Tinidazole in Form of Various Solutions
3.2.1. Stability of Metronidazole in Solutions
The stability study of metronidazole in solutions heated at 40 °C showed the stability of the test compound in solution I at pH = 2.62, solution II at pH = 5.56, and solution III at pH = 8.21 for 1000 h (Figure 6, Table S2). There is information in the scientific literature that supports these results. According to Wang and Yeh [15], metronidazole is stable in the pH range of 3.1–9.9, and they indicated pH = 5.6 as the environment with a maximum stability. Similarly, the stability of metronidazole benzoate after 30 min in 1M HCl, 1M NaOH, and heating at 50 °C for 30 min was also demonstrated [36]. Chandra et al. [46] concluded that the metronidazole was found to be most stable between pH 4 and 6. In the case of solution V containing saline (0.9% NaCl) and solution VI containing methanol as a solvent, no degradation of metronidazole was found under the conditions used for 1000 h. In a solution containing 3% H2O2 (hydrogen peroxide, solution IV), the decomposition products of metronidazole after heating at 40 °C for 100 h, 250 h, 500 h, and 1000 h were noted. The percentage of metronidazole that degraded was 5.66%, 7.34%, 13.56%, and 26.10%, respectively. After heating solution IV for 1000 h, the presence of five degradation products of metronidazole and two traces was noted (Figure 7).
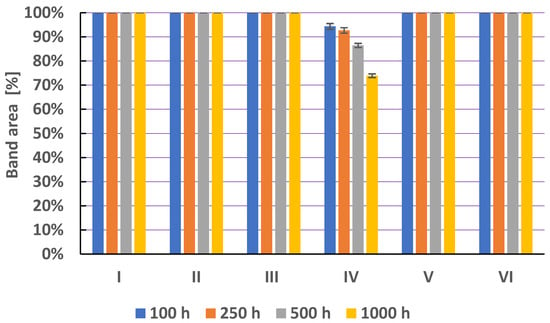
Figure 6.
The comparison of means area (±SD) (in %) of chromatographic bands obtained from metronidazole (M) in different solvents (I—in water at pH = 2.62; II—in water at pH = 5.56; III—in water at pH = 8.21; IV—in hydrogen peroxide (3%); V—in physiological salt (0.9% NaCl); and VI—in methanol) which were heated at 40 °C during 100, 250, 500, and 1000 h, where SD is the standard deviation.
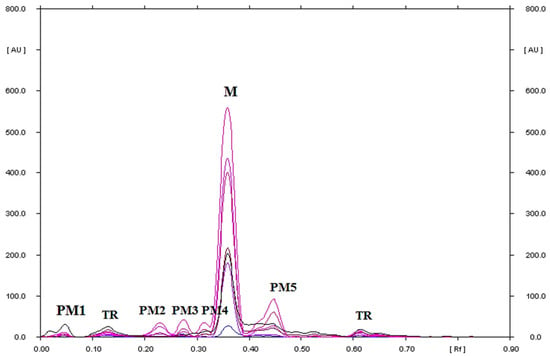
Figure 7.
TLC-UV densitograms recorded in the multi wavelength from 200 to 380 nm (at wavelength intervals of 30 nm at each step) of metronidazole in hydrogen peroxide (3%) solution, which after heating at 40 °C for 1000 h was separated on silica gel using a mobile phase chloroform–methanol (9:1, v/v), where M is metronidazole, PM1, PM2, PM3, PM4, and PM5 are the degradation products of metronidazole, and TR is the traces.
The RF value of metronidazole identified by the standard was in the range of 0.36–0.39 for all of the heated solutions. Starek et al. [43] using TLC found the presence of two unidentified degradation products in the heated alkaline solution of metronidazole. Verma et al. [16] using HPLC found five degradation products in a sample of metronidazole in hydrogen peroxide after 48 h, which is consistent with the results obtained in this work. Chen et al. [35] in a sample of metronidazole after photodegradation, using the LC-PDA-MS technique, also found the presence of five of its degradation products. Therefore, the proposed TLC–densitometry method is selective in relation to the analysis of metronidazole.
3.2.2. Stability of Secnidazole in Solutions
Figure 8 shows a comparison of the area of secnidazole chromatographic bands, obtained as a result of heating solutions I–VI at 40 °C for 100, 250, 500, and 1000 h. Secnidazole in water at pH = 5.56, in water at pH = 8.21, in saline, and in methanol is not degraded (Figure 8, Table S3). Secnidazole in water at pH = 2.62 is stable up to 250 h of heating. After 500 and 1000 h of heating this solution, a loss of secnidazole is observed. After 1000 h of heating secnidazole at 40 °C, it was found that 96.26% of secnidazole remained and one degradation product was formed (Table S3). These results are consistent with research by Jain et al. [20]. Secnidazole in 5 N HCl after 24 h at 60 °C causes its degradation, as a result of which, 98.25% of secnidazole remains [20]. Goncalves et al. [22] also showed the presence of one secnidazole degradation product using the HPLC method.
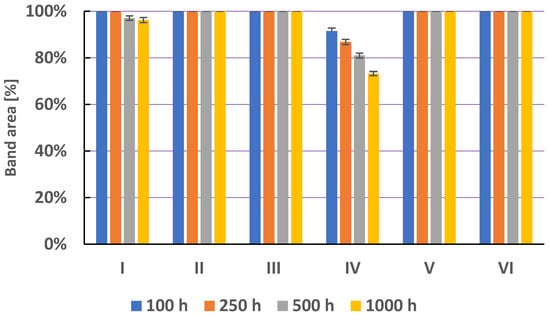
Figure 8.
The comparison of means area (±SD) (in %) of chromatographic bands obtained from secnidazole (S) in different solvents (I—in water at pH = 2.62; II—in water at pH = 5.56; III—in water at pH = 8.21; IV—in hydrogen peroxide (3%); V—in physiological salt (0.9% NaCl); and VI—in methanol) which were heated at 40 °C for 100, 250, 500, and 1000 h, where SD is the standard deviation.
The addition of hydrogen peroxide to the methanolic solution of secnidazole (solution IV) accelerates the degradation of secnidazole. The amount of secnidazole decreases with an increasing heating time (Figure 8), and the share of its degradation products increases. After 1000 h of heating secnidazole in the presence of hydrogen peroxide, 73.25% of secnidazole and four degradation products and three traces remained (Figure 9). The degradation product with an RF value of 0.37 was identified as 2-methyl-5-nitroimidazoles (Figure 5). Jain et al. [20] also found a loss of about 3% of secnidazole in hydrogen peroxide. Bakshi and Singh [21] concluded that secnidazole is stable in 3% H2O2 for 6 h at room temperature. However, almost 70–75% of the drug degradation was observed on an exposure to 30% H2O2 for 48 h. Two very small degradation products were observed. This showed that the drug was degraded in oxidative conditions to non-chromophoric compounds [21].
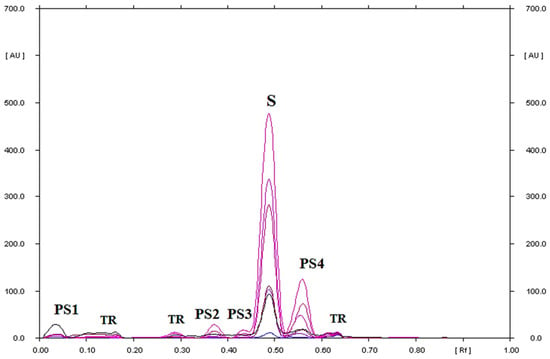
Figure 9.
TLC-UV densitograms recorded in the multi wavelength from 200 to 380 nm (at wavelength intervals of 30 nm at each step) of secnidazole in hydrogen peroxide (3%) solution, which after heating at 40 °C for 1000 h was separated on silica gel using a mobile phase chloroform–methanol (9:1, v/v), where S is secnidazole, PS1, PS2, PS3, and PS4 are the degradation products of secnidazole, and TR is the traces; PS2 was identified as 2-methyl-5-nitroimidazole.
3.2.3. Stability of Ornidazole in Solutions
The stability results, the amount of ornidazole remaining in the tested solutions after heating, are presented in Figure 10. After 100 and 250 h of heating, ornidazole is stable in solutions I (pH = 2.62) and IV (3% H2O2). In the remaining solutions, ornidazole is degraded. The smallest amount of ornidazole after 100 h of heating remains in solution III at pH = 8.21. This result is confirmed in the literature. Santurio et al. [32] confirmed that the alkaline environment has a very large impact on the degradation of ornidazole. This drug degrades at pH >6 to form two major degradation products, namely epoxide and diol. The latter is formed from the epoxide by opening its ring and attaching any nucleophile [32,47]. Heating solution III for 250 and 500 h does not result in the further loss of ornidazole. It is only after 1000 h of heating that the ornidazole content decreases to about 80% and two degradation products appear on the densitogram (Table S4, Figure S4).
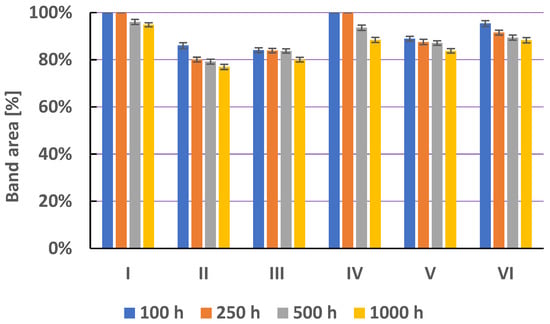
Figure 10.
The comparison of means area (±SD) (in %) of chromatographic bands obtained from ornidazole (O) in different solvents (I—in water at pH = 2.62; II—in water at pH = 5.56; III—in water at pH = 8.21; IV—in hydrogen peroxide (3%); V—in physiological salt (0.9% NaCl); and VI—in methanol) which were heated at 40 °C during 100, 250, 500, and 1000 h, where SD is the standard deviation.
Ornidazole degrades in all solutions after 500 h of heating. However, after 1000 h of heating, most ornidazole (94.87%) remains in solution I (pH = 2.56). One product of its degradation and two traces are visible on the densitogram (Table S4, Figure S5). Hizarcioglu et al. [47] showed that ornidazole under extremely acidic conditions or during the long-term exposure to a moderately acidic environment loses its structure and thus the properties characteristic of nitroimidazoles.
After 1000 h of heating, the least amount of ornidazole (77%) remains in solution II at pH = 5.56 and then three degradation products and three traces are visible on the densitogram (Figure 11). In the remaining solutions IV (3% H2O2), V (saline), and VI (methanol solution) after 1000 h of heating, about 83–89% of ornidazole remains. In solutions IV and V, ornidazole degrades to three products (Figures S6 and S7), and in the methanol solution to two degradation products (Figure S8). Bakshi et al. [29] using the HPLC method also reported two or three degradation products of ornidazole. The stability of ornidazole against oxidizing agents has already been studied in the scientific literature. During a 24 h exposure to H2O2 at room temperature, the ornidazole content in the solution decreased by 8% [29]. The degradation of ornidazole increased to 53% in 30% hydrogen peroxide [29]. At room temperature and in the environment of 0.9% NaCl, ornidazole is stable for over 300 h for 2 weeks [47]. On the other hand, ornidazole shows the greatest stability in an acidic environment (solution I). This result is consistent with the results reported by numerous authors in the literature and scientific publications [32,47].
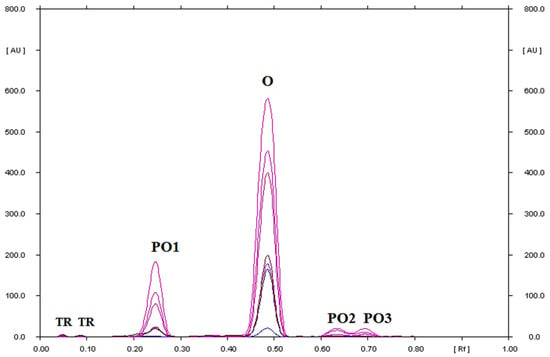
Figure 11.
TLC-UV densitograms recorded in the multi wavelength from 200 to 380 nm (at wavelength intervals of 30 nm at each step) of ornidazole in water at pH = 5.56 solution, which after heating at 40 °C for 1000 h was separated on silica gel using a mobile phase chloroform–methanol (9:1, v/v), where O is ornidazole, PO1, PO2, and PO3 are the degradation products of ornidazole, and TR is the traces.
3.2.4. Stability of Tinidazole in Solutions
Figure 12 shows a comparison of the areas of the chromatographic bands of tinidazole, obtained as a result of heating solutions I–VI at 40 °C for 100, 250, 500, and 1000 h. Solution I of tinidazole (in water at pH = 2.62) is stable throughout the heating period (Table S5). After 100 h of heating tinidazole in solutions II–VI, a comparable degradation of tinidazole occurs at the level of 5–9%, of which it is the highest in a solution with 3% hydrogen peroxide. However, after 1000 h of heating (Table S5), among the solutions in which tinidazole is degraded, it is in solution IV that the most tinidazole remains (Figure 12, Table S5). The gradual degradation of tinidazole proceeds evenly in solutions IV and VI. On the other hand, in solutions II, III, and V, after 500 h of heating, a more rapid than previously observed decrease in the area of the bands for tinidazole is observed. The results presented in the form of a graph (Figure 12) showing the areas of tinidazole bands in various solutions after heating them for 1000 h at 40 °C (Table S5) indicate that the addition of water at pH = 5.56, water at pH = 8.21, and saline lead to degradation of tinidazole at a comparable level. The areas of the tinidazole bands obtained are in the range of 57–65%. After 1000 h of heating, the lowest content of tinidazole (about 57%) (Table S5) was found in the solution with the addition of water at pH = 5.56 (II) (Figure 13). Under these conditions, four degradation products of tinidazole and three traces are observed in the densitogram. One of them with RF ≈ 0.34 was identified as 2-methyl-5-nitroimidazole. Vaghela and Rao [24] showed by HPLC that tinidazole can be degraded to two or three products depending on the stress conditions. Figures S9–S12 show the densitograms of tinidazole, which in solutions III (water at pH = 8.21), IV (3% H2O2), V (in physiological salt, 0.9% NaCl), and VI (in methanol) was heated at 40 °C for a period of 1000 h, respectively. In the densitograms of tinidazole from solutions III, V, and VI, one degradation product is observed in addition to tinidazole (Figures S9, S11 and S12). On the other hand, on the densitogram (Figure S10) of tinidazole in hydrogen peroxide, three degradation products are observed. In all these solutions, with the exception of hydrogen peroxide, the degradation product of tinidazole with an RF value of 0.34–0.38 was identified as 2-methyl-5-nitroimidazole. Bakshi and Singh stated, using HPLC and LC-MS methods [25], that tinidazole degrades to four products [48].
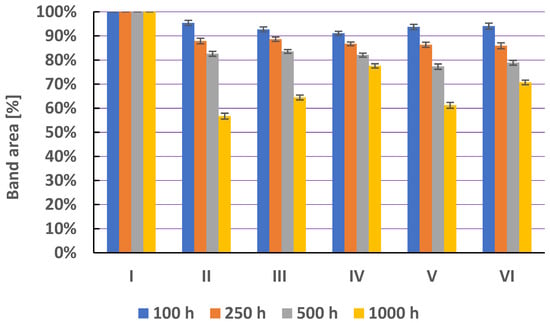
Figure 12.
The comparison of means area (±SD) (in %) of chromatographic bands obtained from tinidazole (T) in different solvents (I—in water at pH = 2.62; II—in water at pH = 5.56; III—in water at pH = 8.21; IV—in hydrogen peroxide (3%); V—in physiological salt (0.9% NaCl); and VI—in methanol) which were heated at 40 °C for 100, 250, 500, and 1000 h, where SD is the standard deviation.
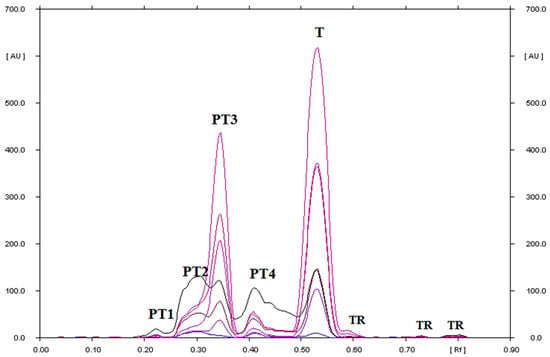
Figure 13.
TLC-UV densitograms recorded in the multi wavelength from 200 to 380 nm (at wavelength intervals of 30 nm at each step) of tinidazole in water at pH = 5.56 solution (II), which after heating at 40 °C for 1000 h was separated on silica gel using a mobile phase chloroform–methanol (9:1, v/v), where T is tinidazole, PT1, PT2, PT3, and PT4 are the degradation products of tinidazole, and TR is the traces; PT3 was identified as 2-methyl-5-nitroimidazole.
3.3. Comparison of the Obtained Degradation Products of Metronidazole, Secnidazole, Ornidazole and Tinidazole with Literature Data
The performed investigations showed that all tested 5-nitroimidazoles are subject to degradation. The number of degradation products depends on the stress conditions, i.e., the temperature (40 °C, 80 °C, and 120 °C) of heating 5-nitroimodazoles on silica gel and the solvent used and the time of the heating solutions of 5-nitroimidazoles (solutions from I to VI) at 40 °C. Table 2 contains information on the maximum number of degradation products of the tested 5-nitroimidazoles, which were detected in this work using the TLC–densitometric method, and the maximum number of degradation products of 5-nitroimidazoles found under stress conditions by other researchers who used various analytical techniques in their studies. Table 2 also contains information on the identification of the resulting 5-nitroimidazole degradation products. The chromatographic conditions used in this work allowed for the separation of more degradation products of metronidazole, secnidazole, ornidazole, and tinidazole than the stability studies of these compounds using the TLC technique described so far in the scientific literature (Table 2). In addition, the TLC conditions elaborated in this work allow for the detection of the same number of degradation products of metronidazole and a greater number of degradation products of secnidazole, ornidazole, and tinidazole than in the case of the analyzes carried out using the technique, e.g., HPLC. Traces are visible in the densitograms (Figures S1–S3, Figure 3, Figure 4, Figure 7, Figure 9 and Figure 11, Figures S4–S8, Figure 13, Figures S10 and S11). It is possible that even after heating for a longer period of time, there would be enough of them to be measurable with a densitometer which could contribute to the detection of even more of the degradation products of 5-nitroimidazoles.

Table 2.
Maximum number of degradation products of metronidazole, secnidazole, ornidazole, and tinidazole detected by various analytical techniques.
The data presented in Table 2 indicate that one or two degradation products were identified in samples of 5-nitroimidazoles subjected to stress conditions. However, using the TLC technique, only secnidazole degradation products have been identified so far [23]. In this manuscript, 2-methyl-5-nitroimidazole was identified as the degradation product of secnidazole and tinidazole using the TLC–densitometric method. Bakshi and Singh [48] reported that metronidazole can be degraded to simple compounds like ammonia and acetic acid; secnidazole and tinidazole can be degraded to 2-methyl-5-nitroimidazole; and ornidazole can be degraded to ornidazole epoxide and ornidazole diol. Tinidazole can also be converted to the 4-nitro isomer of tinidazole and to non-chromophoric compounds, invisible in the UV chromatogram [25].
The presented results may help us to understand the impact of a temperature exposure on the stability of 5-nitroimidazoles (metronidazole, secnidazole, ornidazole, and tinidazole) through a simple TLC method, without needing complex equipment such as HPLC or similar.
3.4. Validation of Method
3.4.1. Specificity
The obtained densitograms indicate that the chromatographic conditions used allow for the separation of potential impurities from metronidazole, secnidazole, ornidazole, and tinidazole, respectively. The chromatographic conditions used allowed for the separation of more of the degradation products of metronidazole, secnidazole, ornidazole, and tinidazole than the stability studies of these compounds described using the TLC technique so far in the scientific literature (Table 2) [23,43,44,45]. Namely, the chromatographic analysis of the degraded metronidazole samples enables the identification of five of the unknown degradation products of metronidazole; the secnidazole, ornidazole, and tinidazole samples enable the identification of the four degradation products of secnidazole, ornidazole, and tinidazole, respectively. 2-Methyl-5-nitroimidazole was identified in secnidazole and tinidazole samples. The specificity of the method is confirmed by the compatibility of the spectra of the 5-nitroimidazole standards with the spectra of the 5-nitroimidazoles after the stress test in various solutions and was heated at 40 °C for 1000 h (Figures S13–S16).
3.4.2. Accuracy
The accuracy of the method was evaluated by the measurement of the recovery. Metronidazole, secnidazole, ornidazole, and tinidazole content quantitative recoveries of 98.2% ÷ 99.4%, 98.8% ÷ 101.8%, 98.9% ÷ 101.3%, 97.6% ÷ 102.3% were obtained (Table 3). The low coefficient of variation values (CV < 3%) are indicative of the accuracy of the method.

Table 3.
Method validation results.
3.4.3. Range
The method was found to be linear for metronidazole in the concentration range of 0.20–0.90 μg·spot−1 (n = 10) and for secnidazole, ornidazole, and tinidazole in the concentration range of 0.20–1.00 μg·spot−1 (n = 12). The regression data shown in Table 3 reveal a good linear relationship over the concentration range studied.
3.4.4. Precision
The precision of the method was studied as the repeatability and intermediate of the system at three different concentrations of metronidazole, secnidazole, ornidazole, and tinidazole, respectively. The results from these experiments, expressed as the coefficients of variation (CV, %) of the, respectively, response factors (a relationship between the peak area and concentration of 5-nitroimidazole investigated), are presented in Table 3. Because the CV for the repeatability and intermediate were <3% the method was precise.
3.4.5. Limit of Detection (LOD) and Limit of Quantification (LOQ) Based on the Calibration Curve
Under the experimental conditions used, the lowest amounts of metronidazole, secnidazole, ornidazole, and tinidazole that could be detected as the LOD were 0.052, 0.063, 0.055, and 0.058 μg·spot−1, respectively. The limits of quantification (LOQ) of metronidazole, secnidazole, ornidazole, and tinidazole were 0.159, 0.190, 0.165, and 0.174 μg·spot−1, respectively. This indicates that the developed method is sensitive.
4. Conclusions
The TLC method combined with densitometry and spectrodensitometry developed in this work can be used as a very good tool for the estimation of the chemical stability of metronidazole, secnidazole, ornidazole, and tinidazole under different stress conditions. The results obtained in this study may be helpful in quality control as well as stability testing of the new pharmaceutical formulations containing metronidazole, secnidazole, ornidazole, and tinidazole as well as also in the degradation study of the metronidazole, secnidazole, ornidazole, and tinidazole residuals present in waste water. All tested 5-nitroimidazoles are stable when heated on chromatographic plates covered with silica gel at 40 °C for 24 h. However, at 80 °C and 120 °C, they degrade. Ornidazole degrades the most at 120 °C; four products of its degradation are formed. Metronidazole and secnidazole were stable for 1000 h of heating at 40 °C in aqueous solutions with pH = 2.62, pH = 5.56, pH = 8.21, saline, and methanol. In hydrogen peroxide, metronidazole and secnidazole degrade to five products and four products, respectively. Ornidazole is degraded in all solutions. The largest loss of ornidazole (23%) was found in water with pH = 5.56; three degradation products of ornidazole are formed. Meanwhile, only two ornidazole degradation products were detected in the neutral solution of ornidazole analyzed by HPLC [29]. Tinidazole is stable in an aqueous solution at pH = 2.62. In other solutions, tinidazole is degraded. Tinidazole is the most degraded in aqueous solution at pH = 5.56. Four degradation products are then formed. 2-Methyl-5-nitroimidazole as a degradation product with an RF value of 0.34–0.38 was identified in a solution of secnidazole dissolved in hydrogen peroxide and in solutions of tinidazole: aqueous with pH = 5.56, pH = 8.21, in a saline solution, and in a methanol solution of tinidazole. The chromatographic conditions used allowed for the separation of more of the degradation products of metronidazole, secnidazole, ornidazole, and tinidazole than the TLC stability studies of these compounds described so far [23,43,44,45]. Therefore, the results indicate that proposed TLC–densitometric method is cost-effective, rapid, specific, accurate, and precise. The TLC–densitometric method also realizes the criterion of the linearity in the required range of the metronidazole, secnidazole, ornidazole, and tinidazole concentrations. The presented results may help us to understand the impact of a temperature exposure on the stability of these commonly used active pharmaceutical ingredients through a simple method, without needing complex equipment such as HPLC or similar.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr11010170/s1, Table S1: Full description the chromatographic peaks of examined metronidazole, secnidazole, ornidazole, and tinidazole and their degradation products formed during pharmaceutical active ingredient heating on silica gel at 120 °C for 24 h; Figure S1: TLC-UV densitograms recorded in the multi wavelength from 200 to 380 nm (at wavelength intervals of 30 nm at each step) of metronidazole heated on silica gel at 120 °C for 24 h which was next separating using chloroform–methanol (9:1, v/v) as mobile phase, where M—metronidazole, PM1 and PM2—degradation products of metronidazole, and TR—traces; Figure S2: TLC-UV densitograms recorded in the multi wavelength from 200 to 380 nm (at wavelength intervals of 30 nm at each step) of secnidazole heated on silica gel at 120 °C for 24 h which was next separating using chloroform–methanol (9:1, v/v) as mobile phase, where S—secnidazole, PS1 and PS2—degradation products of secnidazole, TR—trace; Figure S3: TLC-UV densitograms recorded in the multi wavelength from 200 to 380 nm (at wavelength intervals of 30 nm at each step) of ornidazole heated on silica gel at 80 °C for 24 h which was next separating using chloroform–methanol (9:1, v/v) as mobile phase, where O—ornidazole, PO1, PO2, PO3, and PO4—degradation products of ornidazole, TR—traces; Table S2: Full description the chromatographic peaks of examined metronidazole and its degradation products formed during metronidazole heating in solutions at 40 °C for 1000 h; Table S3: Full description the chromatographic peaks of examined secnidazole and its degradation products formed during secnidazole heating in solutions at 40 °C for 1000 h; Table S4: Full description the chromatographic peaks of examined ornidazole and its degradation products formed during ornidazole heating in solutions at 40 °C for 1000 h; Figure S4: TLC-UV densitograms recorded in the multi wavelength from 200 to 380 nm (at wavelength intervals of 30 nm at each step) of ornidazole in water at pH = 8.21 solution, which after heating at 40 °C for 1000 h was separated on silica gel using a mobile phase chloroform–methanol (9:1, v/v), where O—ornidazole, PO1 and PO2—degradation products of ornidazole; Figure S5: TLC-UV densitograms recorded in the multi wavelength from 200 to 380 nm (at wavelength intervals of 30 nm at each step) of ornidazole in water at pH = 2.62 solution, which after heating at 40 °C for 1000 h was separated on silica gel using a mobile phase chloroform–methanol (9:1, v/v), where O—ornidazole, PO1—degradation product of ornidazole, TR—traces; Figure S6: TLC-UV densitograms recorded in the multi wavelength from 200 to 380 nm (at wavelength intervals of 30 nm at each step) of ornidazole in hydrogen peroxide (3%) solution, which after heating at 40 °C for 1000 h was separated on silica gel using a mobile phase chloroform–methanol (9:1, v/v), where O—ornidazole, PO1, PO2, and PO3—degradation products of ornidazole, TR—traces; Figure S7: TLC-UV densitograms recorded in the multi wavelength from 200 to 380 nm (at wavelength intervals of 30 nm at each step) of ornidazole in physiological salt (0.9% NaCl) solution, which after heating at 40 °C for 1000 h was separated on silica gel using a mobile phase chloroform–methanol (9:1, v/v), where O—ornidazole, PO1, PO2, PO3—degradation products of ornidazole; Figure S8: TLC-UV densitograms recorded in the multi wavelength from 200 to 380 nm (at wavelength intervals of 30 nm at each step) of ornidazole in methanolic solution, which after heating at 40 °C for 1000 h was separated on silica gel using a mobile phase chloroform–methanol (9:1, v/v), where O—ornidazole, PO1 and PO2—degradation products of ornidazole, TR—trace; Table S5: Full description the chromatographic peaks of examined tinidazole and its degradation products formed during tinidazole heating in solutions at 40 °C for 1000 h; Figure S9: TLC-UV densitograms recorded in the multi wavelength from 200 to 380 nm (at wavelength intervals of 30 nm at each step) of tinidazole in water at pH = 8.21 solution, which after heating at 40 °C for 1000 h was separated on silica gel using a mobile phase chloroform–methanol (9:1, v/v), where T—tinidazole, PT1—degradation product of tinidazole identified as 2-methyl-5-nitroimidazole; Figure S10: TLC-UV densitograms recorded in the multi wavelength from 200 to 380 nm (at wavelength intervals of 30 nm at each step) of tinidazole in hydrogen peroxide (3%) solution, which after heating at 40 °C for 1000 h was separated on silica gel using a mobile phase chloroform–methanol (9:1, v/v), where T—tinidazole, PT1, PT2, and PT3—degradation products of tinidazole, TR—traces; Figure S11: TLC-UV densitograms recorded in the multi wavelength from 200 to 380 nm (at wavelength intervals of 30 nm at each step) of tinidazole in physiological salt (0.9% NaCl) solution, which after heating at 40 °C for 1000 h was separated on silica gel using a mobile phase chloroform–methanol (9:1, v/v), where T—tinidazole, PT1—degradation product of tinidazole identified as 2-methyl-5-nitroimidazole, TR—traces; Figure S12: TLC-UV densitograms recorded in the multi wavelength from 200 to 380 nm (at wavelength intervals of 30 nm at each step) of tinidazole in methanolic solution, which after heating at 40 °C for 1000 h was separated on silica gel using a mobile phase chloroform–methanol (9:1, v/v), where T—tinidazole, PT1—degradation product of tinidazole identified as 2-methyl-5-nitroimidazole; Figure S13: Spectra comparison of standard metronidazole and metronidazole after heating in different solutions. Figure S14: Spectra comparison of standard secnidazole and secnidazole after heating in different solutions; Figure S15: Spectra comparison of standard ornidazole and ornidazole after heating in different solutions; Figure S16: Spectra comparison of standard tinidazole and tinidazole after heating in different solutions.
Funding
This research was funded by Medical University of Silesia grant number PCN-1-099/K/0/F and PCN-1-040/K/2/F.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dingsdag, S.A.; Hunter, N. Metronidazole: An update on metabolism, structure-cytotoxicity and resistance mechanisms. J. Antimicrob. Chemother. 2018, 73, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Drugbank. Available online: https://go.drugbank.com/drugs (accessed on 24 October 2022).
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 24 October 2022).
- Virtual Computational Chemistry Laboratory. Available online: http://www.vcclab.org/lab/alogps (accessed on 24 October 2022).
- Pyka, A.; Babuśka, M.; Zachariasz, M. A comparison of theoretical methods of calculation of partition coefficients for selected drugs. Acta Pol. Pharm. Drug Res. 2006, 63, 159–167. [Google Scholar]
- Schwartz, D.E.; Jeunet, F. Pharmacokinetic and metabolic studies with ornidazole in man. Comparison with metronidazole. In Chemotherapy. Parasites, Fungi, and Viruses; Williams, J.D., Geddes, A.M., Eds.; Springer: New York, NY, USA, 1976; Volume 6, pp. 49–59. [Google Scholar]
- Hansch, C.; Leo, A.; Hoekman, D. Exploring QSAR: Hydrophobic, Electronic and Steric Constants, 1st ed.; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Wu, Z. Drug stability testing and formulation strategies. Pharm. Dev. Technol. 2018, 23, 941. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, C.J.; Hage, D.S. Factors affecting the stability of drugs and drug metabolites in biological matrices. Bioanalysis 2009, 1, 205–220. [Google Scholar] [CrossRef]
- Yoshioka, S.; Stella, V.J. Stability of Drugs and Dosage Forms; Kluwer Academic Publishers: New York, NY, USA, 2002; pp. 4–138. [Google Scholar]
- Singh, S.; Junwal, M.; Modhe, G.; Tiwari, H.; Kurmi, M.; Parashar, N.S.P. Forced degradation studies to assess the stability of drugs and products. TrAC Trends Anal. Chem. 2013, 49, 71–88. [Google Scholar] [CrossRef]
- Ambhore, J.P.; Adhao, V.S.; Cheke, R.S.; Popat, R.R.; Gandhi, S.J. Futuristic review on progress in force degradation studies and stability indicating assay method for some antiviral drugs. GSC Biol. Pharm. Sci. 2021, 16, 133–149. [Google Scholar] [CrossRef]
- Chew, Y.L.; Hon-Kent, K.; Mei-Ann, L.; Kai-Bin, B.; Lokesh, A.G. Forced degradation of flibanserin bulk drug: Development and validation of stability indicating RP-HPLC method. Indian J. Pharm. Educ. Res. 2022, 56, 32–42. [Google Scholar] [CrossRef]
- Barnes, A.R.; Makohon, D.J. Correlation of degradation in metronidazole infusion with F0 appied during steam sterilization at 122 °C. Int. J. Pharm. 1993, 92, 233–236. [Google Scholar] [CrossRef]
- Wang, D.P.; Yeh, M.K. Degradation kinetics of metronidazole in solution. J. Pharm. Sci. 1993, 82, 95–98. [Google Scholar] [CrossRef]
- Verma, P.; Nambooding, V.; Mishra, S.; Bhagwat, A.; Bhoir, S. A stability indicating HPLC method for the determination of metronidazole using ecofriendly solvent as mobile phase component. Int. J. Pharm. Pharm. Sci. 2013, 5, 496–501. [Google Scholar]
- Nayak, S.; Goupale, D.C.; Dubey, A.; Shukla, V. Comparative stability study of metronidazole in aqueous and non aqueous vehicle. J. Appl. Pharm. 2011, 3, 295–300. [Google Scholar] [CrossRef]
- Wu, Y.; Fassihi, R. Stability of metronidazole, tetracycline HCl and famotidine alone and in combination. Int. J. Pharm. 2005, 290, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Attimarad, M.; Venugopala, K.N.; Chohan, M.S.; Shinu, P.; David, M.; Molina, E.I.P.; Nair, A.B.; Sreeharsha, N.; Altaysan, A.I.; Balgoname, A.A. Multivariate optimization of chromatographic conditions for rapid simultaneous quantification of antidiarrheal drugs in formulation using surface response methodology. Separations 2022, 9, 103. [Google Scholar] [CrossRef]
- Jain, P.S.; Lohar, T.R.; Kale, N.N.; Suran, S.J. Development and validation of stability-indicating HPTLC method for estimation of secnidazole in bulk drug and pharmaceutical doasage form. J. Adv. Drug Deliv. 2014, 1, 144–156. [Google Scholar]
- Bakshi, M.; Singh, S. ICH guidance in practice: Establishment of inherent stability of secnidazole and development of a validated stability-indicating high-performance liquid chromatographic assay method. J. Pharm. Biomed. Anal. 2004, 36, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Goncalves de Souza Lima, J.; Kogawa, A.C.; Salgado, H.R.N. Green analytical method for quantification of secnidazole in tablets by HPLC-UV. Drug Anal. Res. 2018, 2, 20–26. [Google Scholar] [CrossRef]
- Moustafa, A.A.; Bibawy, L.I. Stability-indicating assay of secnidazole in the presence of Its degradation products. Spectrosc. Lett. 1999, 32, 1073–1098. [Google Scholar] [CrossRef]
- Vaghela, B.K.; Rao, S.S. A novel validated stability indicating high performance liquid chromatographic method for estimation of degradation behavior of ciprofloxacin and tinidazole in solid oral dosage. J. Pharm. Bioallied Sci. 2013, 5, 298–308. [Google Scholar] [CrossRef]
- Bakshi, M.; Singh, S. HPLC and LC–MS studies on stress degradation behaviour of tinidazole and development of a validated specific stability-indicating HPLC assay method. J. Pharm. Biomed. Anal. 2004, 34, 11–18. [Google Scholar] [CrossRef]
- Ahmed, R.; Abdelaziz, M.; Saeed, A. Development and validation of stability indicating HPLC method for quantification of tinidazole. Eur. J. Chem. 2019, 10, 102–107. [Google Scholar] [CrossRef]
- Salomies, H.; Salo, J.P. An HPLC study of tinidazole hydrolysis. Chromatographia 1993, 36, 79–82. [Google Scholar] [CrossRef]
- Murali Krishna, M.; Venkateswarlu, R.; Harsha Vardhan, A.; Mahesh Reddy, A.; Geetha, C.; Suseela, P.; Rajeswari, Y.V.S. Validated RP-HPLC method for simultaneous estimation of tinidazole and diloxanide furoate in pharmaceutical formulations. World J. Pharm. Res. 2021, 7, 169–175. [Google Scholar]
- Bakshi, M.; Singh, B.; Singh, A.; Singh, S. The ICH guidance in practice: Stress degradation studies on ornidazole and development of a validated stability-indicating assay. J. Pharm. Biomed. Anal. 2001, 26, 891–897. [Google Scholar] [CrossRef]
- Prasad Babu, N.; Ramachandran, D. Development and validation of stability Indicating RP-HPLC method for quantitative estimation of ornidazole and its impurities in ornidazole injection. Res. J. Pharm. Technol. 2022, 15, 82–88. [Google Scholar] [CrossRef]
- D’Souza, K.; Syeda, A.; Khatal, P.; Sathyanarayana, M.B.; Vasantharayu, S.G. Stability indicating assay method development ana validation for simultaneous wstimation of ofloxacin and ornidazole by RP-HPLC in bulk: An application to tablet formulation and dissolution studies. Indian J. Pharm. Educ. Res. 2021, 55, 607–613. [Google Scholar] [CrossRef]
- Santurio, J.V.; Perez, J.; Pelaez, B.L.; Manchado, C. Influence of pH on the degradation of ornidazol isolation and identification of its degradation products. STP Pharma Sci. 1995, 5, 391–395. [Google Scholar]
- Pramar, Y.V.; Mandal, T.K.; Bostanian, L.A.; Le, G.; Morris, T.C.; Graves, R.A. Physicochemical and microbiological stability of compounded metronidazole suspensions in PCCA SuspendIt. Int. J. Pharm. Compd. 2021, 25, 169–175. [Google Scholar]
- Sneha, J.K.; Nirav, P.B.; Parag, P.R.; Nikita, P.N.; Hemant, D.T. Development and validation of stability indicating method for simultaneous estimation of ciprofloxacin hcl and tinidazole using rp-uplc method. IOSR J. Pharm. (IOSRPHR) 2012, 2, 12–19. [Google Scholar] [CrossRef]
- Chen, M.L.; Xu, H.X.; Yuan, W.F.; Zhao, S.H.; Li, X.; Zhu, L.X.; Shen, Z.Y.; Liu, Y.J.; Wang, M.J.; Ma, A.; et al. Identification of the major photodegradant in metronidazole by LC-PDA-MS and its reveal in compendial methods. Sci. Rep. 2022, 12, 11665. [Google Scholar] [CrossRef]
- Naveed, S.; Waheed, N.; Nazeer, S. Degradation study of metronidazole in active and different formulation by UV spectroscopy. J. Bioequiv. Bioavailab. 2014, 6, 124–127. [Google Scholar] [CrossRef]
- Aleanizy, F.S.; Al-Eid, H.A.; Tahir, E.E.; Alqahtani, F.Y.; Al-Gohary, O.M. Stability and in vitro dissolution studies of metronidazole tablets and infusions. Dissolution Technol. 2017, 24, 22–27. [Google Scholar] [CrossRef]
- Hassan, S.; Arsalan, A.; Tasawer Baig, M.; Syed, N.; Ibrahim, S.; Ali, S.I.; Huma, A.; Jabeen, A.; Naeem, M.; Arif, J. Factors affecting the formulation for the stabilization of secnidazole in gel preparations. Pharmacophore 2021, 11, 15–23. [Google Scholar]
- Khan, S.; Haseeb, M.; Baig, M.H.; Bagga, P.S.; Siddiqui, H.H.; Kamal, M.A.; Khan, M.S. Improved efficiency and stability of secnidazole—An ideal delivery system. Saudi J. Biol. Sci. 2015, 22, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Rencber, S.; Şenyigit, Z.; Özyazici, M. Stability studies of compression coated ornidazole tablets for colon specific drug delivery. J. Res. Pharm. 2019, 23, 34–43. [Google Scholar] [CrossRef]
- Trivedi, M.K. Spectroscopic characterization of biofield treated metronidazole and tinidazole. Med. Chem. 2015, 5, 340–344. [Google Scholar] [CrossRef]
- Sanyal, S.N.; Datta, A.K.; Chakrabarti, A. Stability indicating TLC method for the quantification of tinidazole in pharmaceutical dosage form—I.V. Fluid. Drug Dev. Ind. Pharm. 1992, 18, 2095–2100. [Google Scholar] [CrossRef]
- Starek, M.; Dąbrowska, M.; Chebda, J.; Żyro, D.; Ochocki, J. Stability of Metronidazole and Its Complexes with Silver(I) Salts under Various Stress Conditions. Molecules 2021, 26, 3582. [Google Scholar] [CrossRef]
- Meshram, D.; Patel, D.; Rohit, M.; Desai, S.; Tajne, M.R. Simultaneous determination of clotrimazole and tinidazole in tablet and cream by HPTLC. Int. J. Adv. Res. 2014, 2, 855–863. [Google Scholar]
- Mohammad, M.A.; Zawilla, N.H.; El-Anwar, F.M.; El-Moghazy Aly, S.M. Stability indicating methods for the determination of norfloxacin in mixture with tinidazole. Chem. Pharm. Bull. 2007, 55, 1–6. [Google Scholar] [CrossRef]
- Chandra, R.; Aggarwal, A.; Jain, D.V.S.; Kapoor, V.K.; Thakur, D.; Sharma, A. Degradation kinetics of metronidazole and its mutual prodrug with ciprofloxacin: A calorimetric analysis. Int. J. Biol. Chem. Sci. 2007, 1, 197–210. [Google Scholar]
- Hizarcioglu, S.Y.; Ay, Z.; Ozyazici, M. Bioavailability file: Ornidazole. FABAD J. Pharm. Sci. 2004, 29, 133–144. [Google Scholar]
- Bakshi, M.; Singh, S. Development of validated stability-indicating assay methods—Critical review. J. Pharm. Biomed. Anal. 2002, 28, 1011–1040. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).