Review on the Mechanism of CO2 Storage and Enhanced Gas Recovery in Carbonate Sour Gas Reservoir
Abstract
1. Introduction
- (1)
- The phase state characteristics of CO2 multi-component mixed system and natural gas multi-component coexistence systems are complex.
- (2)
- The law of percolation, migration, and storage of CO2 mixed system in reservoir is still unclear.
- (3)
- The mechanism of underground CO2 storage to form “cushion gas” to prevent edge/bottom water intrusion and CO2 displacement to drive natural gas is unclear.
- (4)
- The evaluation system of CO2 storage potential and EOR potential of heterogeneous gas reservoirs with different geological characteristics has not been formed, so it is difficult to carry out large-scale field test and application.
2. Review on the Mechanism of CO2 Storage and Enhanced Gas Recovery
2.1. Current State of Thermodynamics Research on Solubility and Phase Equilibrium of Supercritical CO2 Multi-Component Coexistence Systems
2.2. Supercritical CO2-Water–Rock Reaction Experiment and Coupled Kinetic Model
2.3. Recent Research on Diffusion, Transport, and Flow Law of Supercritical CO2
2.4. Recent Research on Mathematical Model of CO2 Embedded Coupled Percolation in Carbonate Gas Reservoirs
2.5. Rencent Research on CO2 Injection to Enhance Oil Recovery Mechanism
2.6. Potential Assessment of CO2 Leakage
3. Suggestions on Future Research Directions
3.1. Supercritical CO2-Brine-Sour Gas Multi-Component Coexistence of Complex Fluid Phase Characteristics
3.2. The Intrinsic Mechanism of Supercritical CO2-Brine-Rock Reaction
3.3. Diffusion Flow and Displacement Characteristics of Supercritical CO2-Sour Gas
3.4. Machnism of Supercritical CO2 Cushion Gas Prevents the Intrusion of Edge/Bottom Water
3.5. Evaluation of Injecting CO2 Storage in Carbonate Sour Gas Reservoirs and Mechanism of Enhanced Recovery
4. Conclusions
- Study on phase characteristics of complex fluid with multi-component coexistence of supercritical CO2, brine, and sour gas.
- Study on the internal mechanism of supercritical CO2–brine–rock reaction.
- Diffusion flow and displacement characteristics of supercritical CO2-sour gas.
- Mechanism of supercritical CO2 cushion to prevent edge/bottom water intrusion.
- CO2 injection storage evaluation and enhanced oil recovery mechanism of carbonate sour gas reservoirs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diamond, L.W.; Akinfiev, N.N. Solubility of CO2 in water from −1.5 to 100 °C and from 0.1 to 100 MPa: Evaluation of literature data and thermodynamic modelling. Fluid Phase Equilibria 2003, 208, 265–290. [Google Scholar] [CrossRef]
- Tong, D.; Trusler, J.; Vega-Maza, D. Solubility of CO2 in Aqueous Solutions of CaCl2 or MgCl2 and in a Synthetic Formation Brine at Temperatures up to 423 K and Pressures up to 40 MPa. J. Chem. Eng. Data 2013, 58, 2116–2124. [Google Scholar] [CrossRef]
- Portier, S.; Rochelle, C. Modelling CO2 solubility in pure water and NaCl-type waters from 0 to 300 °C and from 1 to 300 bar application to the Utsira formation at Sleipner. Chem. Geol. 2005, 217, 187–199. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, M.; Yang, G.; Han, B. Solubility of CO2 in aqueous solutions of NaCl, KCl, CaCl2 and their mixed salts at different temperatures and pressures. J. Supercrit. Fluids 2011, 56, 125–129. [Google Scholar] [CrossRef]
- Hu, L.; Chang, C.; Yu, Q. CO2 dissolution capacity of underground brackish water of Shanxi Formation, Ordos Basin. Earth Sci. J. China Univ. Geosci. 2012, 37, 6. [Google Scholar]
- Wang, L. Study on the Solubility of CO2 in Underground Brackish Water; China University of Geosciences (Beijing): Beijing, China, 2014. [Google Scholar]
- Jin, Y. Study on the Solubility of CO2 in Simulated Deep Saline Solution under Geological Storage Condition; Zhejiang University of Technology: Zhejiang, China, 2019. [Google Scholar]
- Wang, Q.; Wu, X. Calculation model of phase transitivity of gas, liquid and supercritical CO2. J. Shengli Coll. China Univ. Pet. 2012, 26, 11–14. [Google Scholar]
- Tomita, S.; Akatsu, S.; Ohmura, R. Experiments and thermodynamic simulations for continuous separation of CO2 from CH4+ CO2 gas mixture utilizing hydrate formation. Appl. Energy 2015, 146, 104–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Y.; Liu, S.; Zhao, C. Supercritical CO2 Displacement CH4 Phase Behavior and Percolation Characteristics Research in Porous Medium. In Proceedings of the AGU Fall Meeting Abstracts, New Orleans, LO, USA, 11–15 December 2017. H11G-1289. [Google Scholar]
- Carroll, J.J. Phase Equilibria Relevant to Acid Gas Injection: Part 1-Non-Aqueous Phase Behavior. J. Can. Pet. Technol. 2002, 41. [Google Scholar] [CrossRef]
- Carroll, J.J. Phase Equilibria Relevant to Acid Gas Injection: Part 2-Aqueous Phase Behavior. J. Can. Pet. Technol. 2002, 41. [Google Scholar] [CrossRef]
- Dubessy, J.; Tarantola, A.; Sterpenich, J. Modelling of liquid-vapor equilibria in the H2O-CO2-NaCl and H2O-H2S-NaCl systems to 270 °C. Oil Gas Sci. Technol. 2005, 60, 339–355. [Google Scholar] [CrossRef][Green Version]
- Mireault, R.A.; Stocker, R.; Dunn, D.W.; Pooladi-Darvish, M. Wellbore Dynamics of Acid Gas Injection Well Operation. In Proceedings of the Canadian Unconventional Resources and International Petroleum Conference, Calgary, AB, Canada, 19–21 October 2010. [Google Scholar]
- Guo, X.; Zhou, X.; Zhou, B. Prediction model of sulfur saturation considering the effects of non-Darcy flow and reservoir compaction. J. Nat. Gas Sci. Eng. 2015, 22, 371–376. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Y.; Hui, D.; Wang, S.; He, D.L. Study on New Evaluation Model for the Solubility of Element Sulfur and Deposition Prediction in High sour Gas Reservoir. Sci. Technol. Eng. 2016, 16, 48–52. [Google Scholar]
- Zhao, H.; Lvov, S.N. Phase behavior of the CO2–H2O system at temperatures of 273–623 K and pressures of 0.1–200 MPa using Peng-Robinson-Stryjek-Vera equation of state with a modified Wong-Sandler mixing rule: An extension to the CO2–CH4–H2O system. Fluid Phase Equilibria 2016, 417, 96–108. [Google Scholar] [CrossRef]
- Ababneh, H.; Al-Muhtaseb, S.A. An empirical correlation-based model to predict solid-fluid phase equilibria and phase separation of the ternary system CH4-CO2-H2S. J. Nat. Gas Sci. Eng. 2021, 94, 104–120. [Google Scholar] [CrossRef]
- Wang, S.; Colroll, J.J.; Tang, L. Wellbore flow model and phase distribution of acid gas injection. Nat. Gas Ind. 2010, 30, 95–100+137. [Google Scholar]
- Zhang, S. Experimental and Simulation Study on CO2 and H2S Gas-Brine Miscible; Southwest Petroleum University: Chengdu, China, 2018. [Google Scholar]
- Théveneau, P.; Xu, X.; Baudouin, O.; Jaubert, J.N.; Ceragioli, P.; Coquelet, C. Vapor–liquid equilibria of the CH4+CO2+H2S ternary system with two different global compositions: Experiments and modeling. J. Chem. Eng. Data 2020, 65, 1802–1813. [Google Scholar] [CrossRef]
- Souza, L.F.; Al Ghafri, S.Z.; Fandiño, O.; Trusler, M. Vapor-liquid equilibria, solid-vapor-liquid equilibria and H2S partition coefficient in (CO2+ CH4) at temperatures between 203.96 and 303.15K at pressures up to 9 MPa. Fluid Phase Equilibria 2020, 522, 112762. [Google Scholar] [CrossRef]
- Zhang, C.; Fan, Z.; Xu, A.; Zhao, L.; Zhao, D.; Luo, E. Miscible mechanism of dissolved gas reinjection in volatile reservoir containing acid gas. Reserv. Eval. Dev. 2017, 7, 41–46. [Google Scholar]
- Li, K.; Firoozabadi, A. Experimental study of wettability alteration to preferential gas-wetting in porous media and its effects. SPE Reserv. Eval. Eng. 2000, 3, 139–149. [Google Scholar] [CrossRef]
- Xiao, L.; Mao, Z.; Liu, T.; Yang, H.; Zhang, H.; Shi, Y.; Zhao, T. Comparisons of Pore Structure for Unconventional Tight Gas, Coalbed Methane and Shale Gas Reservoir. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, SPE 165774, Jakarta, Indonesia, 23–25 October 2013. [Google Scholar]
- Shao, H.; Ray, J.R.; Jun, Y. Dissolution and precipitation of clay minerals under geologic CO2 sequestration conditions: CO2-brine-phlogopite interactions. Environ. Sci. Technol. 2010, 44, 5999–6005. [Google Scholar] [CrossRef]
- Zou, F.; Guo, X.; Zhu, Z.; Su, M.; Zhang, Y.; Gao, T. High temperature and high-pressure water-rock reaction experiment of Marine carbonate rocks in sour gas reservoir. Pet. Geol. Oilfield Dev. Daqing 2019, 38, 34–41. [Google Scholar]
- Tang, Y.; Hu, S.; He, Y.; Wang, Y.; Wan, X.; Cui, S.; Long, K. Experiment on CO2-brine-rock interaction during CO2 injection and storage in gas reservoirs with aquifer. Chem. Eng. J. 2021, 413, 127567. [Google Scholar] [CrossRef]
- Guo, X. Effect of Sulfur Adsorption on Reservoir Damage in Sour Gas Reservoir; Science Press: Beijing, China, 2021; ISBN 978-7-03-067769-3. [Google Scholar]
- Liu, F. Study on Water-Rock Interaction Using Geochemical Reaction Modelling—Taking Simulation Software Phreeqc for Example; China University of Geosciences (Beijing): Beijing, China, 2010. [Google Scholar]
- Dong, J.; Li, Y.; Yang, G.; Ke, Y.; Wu, R. Numerical Simulation of CO2-Water-Rock Interaction lmpact on Caprock Permeability. Geol. Sci. Technol. Inf. 2012, 31, 115–121. [Google Scholar]
- Pearce, J.; Dawson, G.; Sommacal, S.; Golding, D.S. Experimental and Modelled Reactions of CO2 and SO2 with Core from a Low Salinity Aquifer Overlying a Target CO2 Storage Complex. Geosciences 2019, 9, 513. [Google Scholar] [CrossRef]
- Ahmat, K.; Cheng, J.; Yu, Y.; Zhao, R.; Li, J. CO2-Water-Rock Interactions in Carbonate Formations at the Tazhong Uplift, Tarim Basin, China. Minerals 2022, 12, 635. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; An, Z.; Shabani, A. CO2 storage in carbonate rocks: An experimental and geochemical modeling study. J. Geochem. Explor. J. Assoc. Explor. Geochem. 2022, 234, 106942. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, P.; Wang, Y. On Fracture Strength of Rocks with Cracks Under Water Pressure and Chemical Damage. Chin. J. Rock Mech. Eng. 2003, 22, 2154–2158. [Google Scholar]
- Huang, W.; Zhou, W.; Chen, P. The Study on Mechanical Effect of the Chemical Action of Water-Rock on Rocks. West China Prospect. Eng. 2006, 18, 122–125. [Google Scholar]
- Nguyen, M.T.; Bemer, E.; Dormieux, L. Micromechanical Modeling of Carbonate Geomechanical Properties Evolution During Acid Gas Injection. In Proceedings of the 45th US Rock Mechanics/Geomechanics Symposium, San Francisco, CA, USA, 26–29 June 2011. [Google Scholar]
- He, C.; Guo, J. Mechanism Study of Acid on Mechanical Properties of Limestone. Chin. J. Rock Mech. Eng. 2013, 32, 3016–3021. [Google Scholar]
- Wang, Y.; Tang, J.; Jiang, J.; Dai, Z.; Shu, G. Mechanical properties and parameter damage effects of grey sandstone under water-rock chemistry. J. China Coal Soc. 2017, 42, 227–235. [Google Scholar]
- Feng, X.; Li, S.; Chen, S. Effect of Water Chemical Corrosion on Strength and Cracking Characteristics of Rocks: A Review. Key Eng. Mater. 2004, 261, 1355–1360. [Google Scholar] [CrossRef]
- Lu, Z. Experimental Study and Theoretical Analysis of Mechanical Properties of Water-Rock Action in Fractured Rocks; Graduate School of Chinese Academy of Sciences (Wuhan Institute of Rock and Soil Mechanics): Wuhan, China, 2010. [Google Scholar]
- Smith, S.A.; Sorensen, J.A.; Steadman, E.N.; Harju, J.A. Acid gas injection and monitoring at the Zama oil field in Alberta, Canada: A case study in demonstration-scale carbon dioxide sequestration. Energy Procedia 2009, 1, 1981–1988. [Google Scholar] [CrossRef]
- Guo, X. Mechanism of Sulfur Deposition and Water-Rock Reaction in Sour Gas Reservoirs; Science Press: Beijing, China, 2020; ISBN 978-7-03-065011-5. [Google Scholar]
- Tan, K.; Xie, Y.; Zhao, Z.; Wang, Y. A Coupled Reaction-Transport-Mechanical Model for Tectonic-Fluid-Mineralization System and Dynamic Simulation. Earth Sci. Front. 2001, 8, 311–321. [Google Scholar]
- Li, X. Study on Hydrocarbon Migration Dynamics and Dynamic Coupling Mechanism; Institute of Geology, China Earthquake Administration: Beijing, China, 2007. [Google Scholar]
- Wang, Y. Study on the Near-Field Dynamic Model And Numerical Simulation of Rock Mass Coupled with Thermal-Water-Chemical-Mechanical; Chongqing University: Chongqing, China, 2019. [Google Scholar]
- Woessner, D.E.; Snowden, B.S., Jr.; George, R.A.; Melrose, J.C. Dense gas diffusion coefficients for the methane-propane system. Industrial & Eng. Chem. Fundam. 1969, 8, 779–786. [Google Scholar]
- Wen, Y.; Bryan, J.; Kantzas, A. Estimation of diffusion coefficients in bitumen solvent mixtures as derived from low field NMR spectra. J. Can. Pet. Technol. 2005, 44, 29–35. [Google Scholar] [CrossRef]
- Renner, T.A. Measurement and correlation of diffusion coefficients for CO2 and rich-gas applications. SPE Reserv. Eng. 1988, 3, 517–523. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, L.; Chen, G.; Cai, G.; Wang, Q. Evaluation of concentration-dependent diffusivity with uptake curve. J. Chem. Soc. Faraday Trans. 1994, 90, 3007–3010. [Google Scholar]
- Guo, B.; Hou, J.; Yu, C.; Li, D.; Lin, Y. Determination of Diffusion Coefficient for Carbon Dioxide in the Porous Media. J. Petrochem. Univ. 2009, 22, 38–40. [Google Scholar]
- Zhang, W.; Wu, S.; Ren, S.; Zhang, L.; Li, J. The modeling and experimental studies on the diffusion coefficient of CO2 in saline water. J. CO2 Util. 2015, 11, 49–53. [Google Scholar] [CrossRef]
- Ahmadi, H.; Jamialahmadi, M.; Soulgani, B.S.; Dinarvand, N.; Sharafi, M.S. Experimental study and modelling on diffusion coefficient of CO2 in water. Fluid Phase Equilibria 2020, 523, 112584. [Google Scholar] [CrossRef]
- Wu, D. Fundamentals of Chemical Engineering (Volume 1); Higher Education Press: Beijing, China, 2000; ISBN 978-7-04-007954-8. [Google Scholar]
- Unver, A.A.; Himmelblau, D.M. Diffusion Coefficients of CO2, C2H4, C3H6 and C4H8 in Water from 6 to 65 °C. J. Chem. Eng. Data 1964, 9, 428–431. [Google Scholar] [CrossRef]
- Xu, H.; Sun, L.; Chang, Z. CO2 Injection to Improve Recovery Factor of Gas Reservoir. Nat. Gas Explor. Dev. 2006, 29, 40–41+50+72. [Google Scholar]
- He, Y. Study on Diffusion Properties and Microstructure of Supercritical CO2 System by MOLECULAR Dynamics; Tianjin University: Tianjin, China, 2007. [Google Scholar]
- Du, L. Study on the Law of CO2 Diffusion and Mass Transfer in Multiphase Fluid; Chengdu University of Technology: Chengdu, China, 2020. [Google Scholar]
- Kivi, I.R.; Makhnenko, R.Y.; Vilarrasa, V. Two-Phase Flow Mechanisms Controlling CO2 Intrusion into Shaly Caprock. Transp. Porous Media 2022, 141, 771–798. [Google Scholar] [CrossRef]
- Liu, B.; Wang, B.; Li, Z.; Lv, Y. The diffusion of CO2-brine storage based on stochastic partial differential equations. EDP Sci. 2020, 206, 03031. [Google Scholar]
- Wang, F.; Dai, S.; Zhu, W.; Hu, P.; Gong, Y. Migration law of CO2 storage in low-porosity and low-permeability reservoirs—Taking Ordos Basin as an example. China Geol. 2020, 1–18. [Google Scholar]
- Basbug, B.; Gumrah, F.; Oz, B. Simulating the Effects of Deep Saline Aquifer Properties for CO Sequestration. J. Can. Pet. Technol. 2007, 46, 30–38. [Google Scholar] [CrossRef]
- Al-Khdheeawi, E.A.; Vialle, S.; Barifcani, A.; Sarmadivaleh, M.; Iglauer, S. Impact of reservoir wettability and heterogeneity on CO2-plume migration and trapping capacity. Int. J. Greenh. Gas Control 2017, 58, 142–158. [Google Scholar] [CrossRef]
- Nordbotten, J.M.; Dahle, H.K. Impact of the capillary fringe in vertically integrated models for CO2 storage. Water Resour. Res. 2011, 47. [Google Scholar] [CrossRef]
- Wang, B.; Yan, X.; Yang, X.; Feng, Y. Study the Thermo-hydro-mechanical Coupling Model of Carbon Dioxide Migration in Deep Aquifer. Sci. Technol. Eng. 2012, 12, 2919–2922. [Google Scholar]
- Ao, X. Study on Shale Deformation and CO2 Migration under Supercritical CO2; Chongqing University: Chongqing, China, 2017. [Google Scholar]
- Wu, D. Mechanism and Experimental Study of Carbon Dioxide Gas Displacement in Coal Seam; Taiyuan University of Technology: Taiyuan, China, 2010. [Google Scholar]
- Liu, S. Study on Dispersion Characteristics Of supercritical CO2-CH4 Miscible Flooding in Porous Media; Dalian University of Technology: Dalian, China, 2018. [Google Scholar]
- Kuo, C.H. On the production of hydrogen sulfide-sulfur mixtures from deep formations. J. Pet. Technol. 1972, 24, 1142–1146. [Google Scholar] [CrossRef]
- Roberts, B.E. The effect of sulfur deposition on gas well inflow performance. SPE Reserv. Eng. 1997, 12, 118–123. [Google Scholar] [CrossRef]
- Abou-Kassem, J.H. Experimental and numerical model of sulfur plugging in carbonate reservoirs. J. Pet. Sci. Eng. 2000, 26, 91–103. [Google Scholar] [CrossRef]
- Hands, N.; Oz, B.; Roberts, B.; Davis, P.; Minchau, M. Advances in the prediction and management of elemental sulfur deposition associated with sour gas production from fractured carbonate reservoirs. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 29 September–2 October 2002. [Google Scholar]
- Faruk, C. Formation Damage Mechanisms and Their Phenomenological Modeling—An Overview. In Proceedings of the European Formation Damage Conference, Scheveningen, The Netherlands, 30 May–1 June 2007. [Google Scholar]
- Hu, J.; He, S.; Zhao, J.; Li, Y. Modeling of sulfur plugging in a sour gas reservoir. J. Nat. Gas Sci. Eng. 2013, 11, 18–22. [Google Scholar] [CrossRef]
- Mahmoud, M.A. New Numerical and Analytical Models to Quantify the Near-Wellbore Damage due to Sulfur Deposition in Sour Gas Reservoirs. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 10–13 March 2013. [Google Scholar]
- He, L.; Guo, X. Study on sulfur deposition damage model of fractured gas reservoirs with high-content H2S. Petroleum 2017, 3, 321–325. [Google Scholar] [CrossRef]
- Guo, X. Integrated Reservoir-Wellbore Simulation for Sour Gas Reservoirs; Science Press: Beijing, China, 2021; ISBN 978-7-03-067770-9. [Google Scholar]
- Wang, R.; Zhang, C.; Chen, D.; Yang, F.; Li, H.; Li, M. Microscopic Seepage Mechanism of Gas and Water in Ultra-Deep Fractured Sandstone Gas Reservoirs of Low Porosity: A Case Study of Keshen Gas Field in Kuqa Depression of Tarim Basin, China. Front. Earth Sci. 2022, 10, 893701. [Google Scholar] [CrossRef]
- Zhang, G. Study on Features of Phase Behaviors and Seepage Mechanism in High Sour Gas Reservoir—Take Samples from Changxing Gas reservoir in Yuanba as Study Objects; Chengdu University of Technology: Chengdu, China, 2014. [Google Scholar]
- Zhang, Y. Study on Sulfur Deposition Model and Productivity Prediction of Horizontal Well in Sour Gas Reservoir; Southwest Petroleum University: Chengdu, China, 2016. [Google Scholar]
- Bachu, S.; Bonijoly, D.; Bradshaw, J.; Burruss, R.; Holloway, S.; Christensen, N.P.; Mathiassen, O.M. CO2 storage capacity estimation: Methodology and gaps. Int. J. Greenh. Gas Control 2007, 1, 430–443. [Google Scholar] [CrossRef]
- Zhao, X.; Liao, X.; Wang, W.; Chen, C. Evaluative model of CO2 geological sequestration and determination of key parameters. Spec. Oil Gas Reserv. 2013, 20, 72–74+145. [Google Scholar]
- Li, B.; Chen, X.; Wang, Z.; Zhai, W.; Li, M.; Liu, D.; Kang, J. Study on the effect of CO2 oil displacement in sandstone reservoir. West. Explor. Eng. 2021, 33, 74–76+79. [Google Scholar]
- Jiang, B.; Li, Z.; Lou, W.; Wang, J.; Zhang, C.; Liu, R. Model of Carbon Dioxide Miscible Flooding Based on Method of Characteristics. Resour. Ind. 2013, 15, 60–63. [Google Scholar]
- Cheng, J.; Liu, C.; Wang, Y.; Bai, W.; Mou, G. Near-Miscible CO2 Flooding Test in Ultra-Low Permeability Oil Reservoir. Spec. Oil Gas Reserv. 2016, 23, 64–67+144. [Google Scholar]
- Jiang, J.; Shao, Y.; Younis, R.M. Development of a multi-continuum multi-component model for enhanced gas recovery and CO2 storage in fractured shale gas reservoirs. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Xu, R.; Zeng, K.; Zhang, C.; Jiang, P. Assessing the feasibility and CO2 storage capacity of CO2 enhanced shale gas recovery using Triple-Porosity reservoir model. Appl. Therm. Eng. 2017, 115, 1306–1314. [Google Scholar] [CrossRef]
- Tang, L.; Jia, Y.; Yan, J.; Li, G.; Wang, Y.; He, Y.; Qing, J.; Tang, Y. Study on calculation method of CO2 storage potential in depleted gas reservoir. Pet. Reserv. Eval. Dev. 2021, 11, 858–863. [Google Scholar]
- Gao, R.; Lyu, C.; Zhou, K.; Lun, Z.; Zhou, B. A CO2 flooding dynamic storage potential calculation method based on compositional flash calculation. Oil Drill. Prod. Technol. 2021, 43, 70–75. [Google Scholar]
- Zhao, F.; Xin, C. Study on CO2 storage capacity of reservoirs. Unconv. Oil Gas 2020, 7, 72–76+54. [Google Scholar]
- Dai, S.; Dong, Y.; Wang, F.; Xing, Z.; Hu, P.; Yang, F. A sensitivity analysis of factors affecting in geologic CO2 storage in the Ordos Basin and its contribution to carbon neutrality. China Geol. 2022, 5, 359–371. [Google Scholar]
- Cao, C.; Hou, Z.; Li, Z.; Pu, X.; Liao, J.; Wang, G. Numerical modeling for CO2 storage with impurities associated with enhanced gas recovery in depleted gas reservoirs. J. Nat. Gas Sci. Eng. 2022, 102, 104554. [Google Scholar] [CrossRef]
- Zhao, M.; Li, J.; Wang, Z. Study on mechanism of CO2 immiscible oil flooding in ultra-low permeability reservoir. Sci. Technol. Eng. 2011, 11, 1438–1440+1446. [Google Scholar]
- Liang, T. Application of CO2 Flooding Technology in Enhanced Oil Recovery of Low Permeability Reservoir; China University of Geosciences (Beijing): Beijing, China, 2013. [Google Scholar]
- Xiao, G.; Du, Z.; Lei, S.; Huang, W.; Cai, Z. Optimization of Tertiary Water-Alternate-CO2 Flood in Jilin Oil Field of China: Laboratory and Simulation Studies. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 22–26 April 2006. [Google Scholar]
- Vidiuk, K.E. Management of Sulfur Disposal by Acid Gas Underground Injection. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 24–27 September 2006. [Google Scholar]
- Yonebayashi, H.; Takabayashi, K.; Iizuka, R.; Tosic, S. Managing Experimental-Data Shortfalls for Fair Screening at Concept Selection: Case Study to Estimate How Acid-Gas Injection Affects Asphaltene-Precipitation Behavior. Oil Gas Facil. 2016, 5. [Google Scholar] [CrossRef]
- Luo, E.; Fan, Z.; Hu, Y.; Zhao, L.; Zhao, H.; Wang, J.; He, C.; Zeng, X. A dual-porosity dual-permeability model for acid gas injection process evaluation in hydrogen-carbonate reservoirs. Int. J. Hydrogen Energy 2019, 44, 25169–25179. [Google Scholar] [CrossRef]
- Heller, R.; Zoback, M. Adsorption of methane and carbon dioxide on gas shale and pure mineral samples. J. Unconv. Oil Gas Resour. 2014, 8, 14–24. [Google Scholar] [CrossRef]
- Ding, J.; Cao, T.; Wu, J. Experimental Investigation of Supercritical CO2 Injection for Enhanced Gas Recovery in Tight Gas Reservoir. In Proceedings of the Carbon Management Technology Conference, Houston, TX, USA, 15–18 July 2019. [Google Scholar]
- Jia, L.; Liu, P.; Yuan, D.; Lei, T.; Ran, J.; Wang, G. Experiment of enhancing the recovery of the shale adsorbed gas by CO2 injection: Taking Yanchang-Formation Chang-7 shale gas in Ordos Basin as an example. Pet. Geol. Oilfield Dev. Daqing 2021, 40, 153–159. [Google Scholar]
- Lu, D.; Zhang, J.; Zhang, Y.; Chi, Y.; Zhao, C. Experimental study on improving oil recovery of shale gas by injecting CO2. J. Dalian Univ. Technol. 2021, 61, 464–470. [Google Scholar]
- Jikich, S.A.; Smith, D.H.; Sams, W.N.; Bromhal, G.S. Enhanced Gas Recovery (EGR) with Carbon Dioxide Sequestration: A Simulation Study of Effects of Injection Strategy and Operational Parameters. In Proceedings of the SPE Eastern Regional Meeting, Pittsburgh, PA, USA, 6–10 September 2003. [Google Scholar]
- Sun, Y. Mechanism of Supercritical CO2 Storage and Enhanced Gas Recovery; Southwest Petroleum University: Chengdu, China, 2012. [Google Scholar]
- Wang, H.; Rezaee, R. CO2 Storage with Enhanced Gas Recovery CS-EGR in Conventional and Unconventional Gas Reservoirs in Australia. In Proceedings of the SPE Asia Pacific Oil & Gas Conference and Exhibition, Virtual, 17–19 November 2020. [Google Scholar]
- Wu, J.; Ma, J. Discussion on the potential risk of CO2 geological sequestration. J. Environ. Sci. 2012, 31, 89–93. [Google Scholar]
- Pulchan, S.; Alexander, D.; Boodlal, D. Investigating the effects of fault reactivation and CO2 migration during combined CO2-EOR and sequestration within a mature oil reservoir. J. Pet. Explor. Prod. Technol. 2020, 10, 3827–3848. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J. Factors affecting the lower limit of the safe mud weight window for drilling operation in hydrate-bearing sediments in the Northern South China Sea. Geomech. Geophys. Geo-Energy Geo-Resour. 2022, 8, 1–21. [Google Scholar] [CrossRef]
- Duguid, A.; Glier, J.; Heinrichs, M.; Hawkins, J.; Peterson, R.; Mishra, S. Practical leakage risk assessment for CO2 assisted enhanced oil recovery and geologic storage in Ohio’s depleted oil fields. Int. J. Greenh. Gas Control 2021, 109, 103338. [Google Scholar] [CrossRef]
- Li, Q.; Han, Y.; Liu, X.; Ansari, U.; Cheng, Y.; Yan, C. Hydrate as a by-product in CO2 leakage during the long-term sub-seabed sequestration and its role in preventing further leakage. Environ. Sci. Pollut. Res. 2022, 29, 77737–77754. [Google Scholar] [CrossRef] [PubMed]
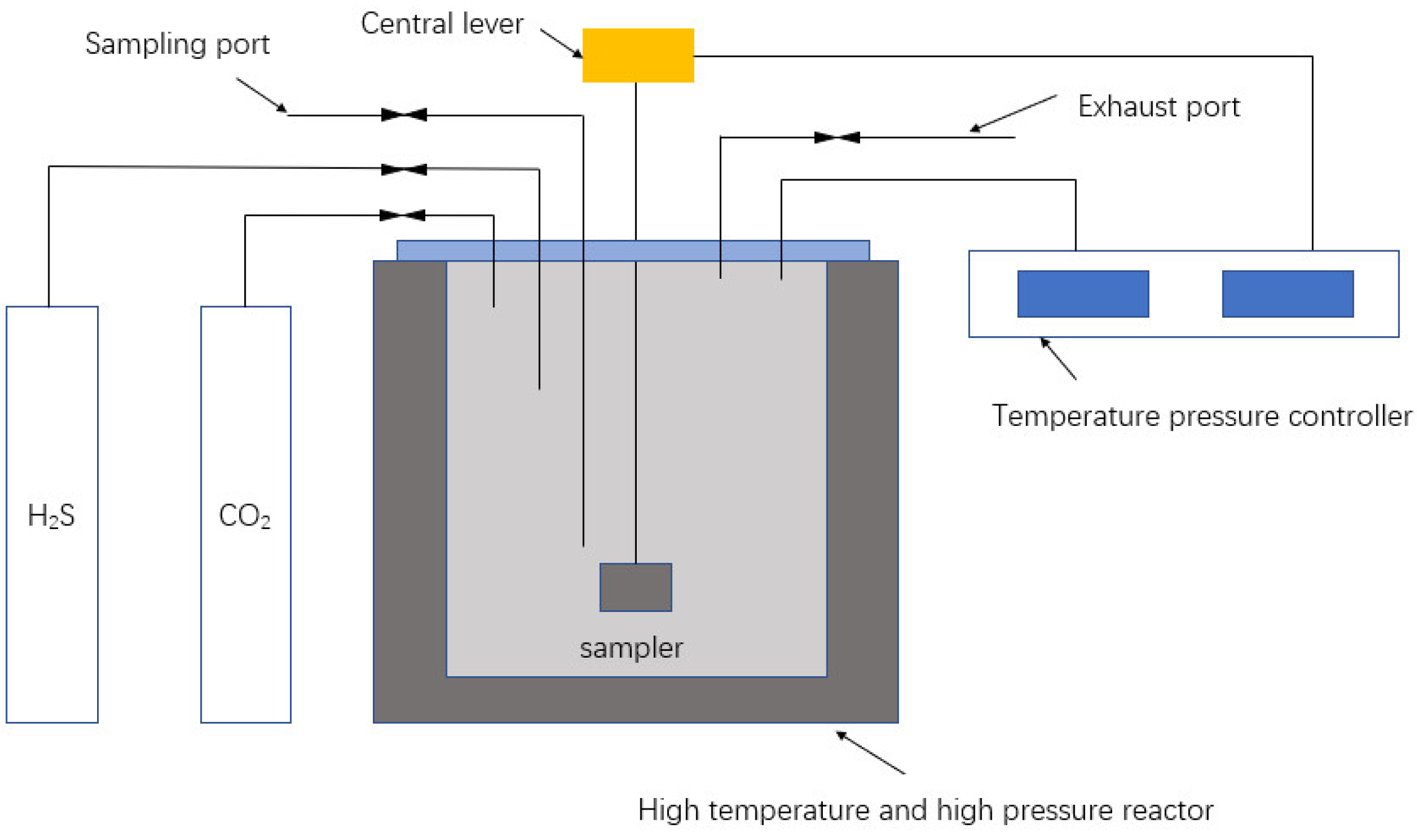
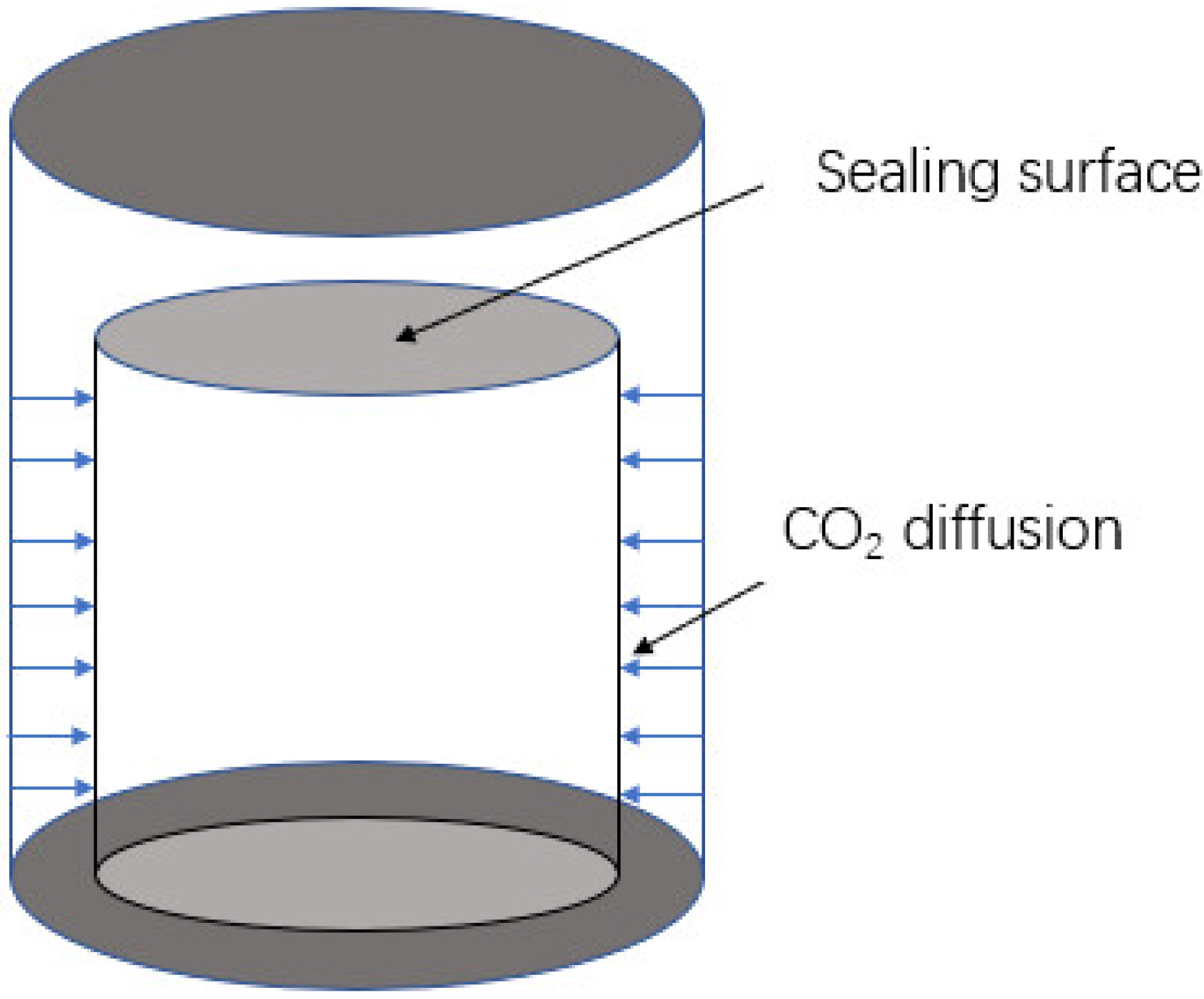
| Substance | Critical Temperature/°C | Critical Pressure/MPa |
|---|---|---|
| CH4 | −83.00 | 4.60 |
| C2H6 | 32.40 | 4.89 |
| CO2 | 31.06 | 7.39 |
| H2S | 100.20 | 8.94 |
| Ionic Reaction Formula |
|---|
| H2S = H+ + HS− |
| CO2 + H2O = H+ + HC |
| Mg•CaCO3 + 2H+ = Ca2+ +Mg2+ + CO2 + H2O |
| Ca2+ +CO2 + H2O = CaCO3↓ + 2H+ |
| HS− + Fe2+ = FeS↓ + 2H+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Feng, J.; Wang, P.; Kong, B.; Wang, L.; Dong, X.; Guo, S. Review on the Mechanism of CO2 Storage and Enhanced Gas Recovery in Carbonate Sour Gas Reservoir. Processes 2023, 11, 164. https://doi.org/10.3390/pr11010164
Guo X, Feng J, Wang P, Kong B, Wang L, Dong X, Guo S. Review on the Mechanism of CO2 Storage and Enhanced Gas Recovery in Carbonate Sour Gas Reservoir. Processes. 2023; 11(1):164. https://doi.org/10.3390/pr11010164
Chicago/Turabian StyleGuo, Xiao, Jin Feng, Pengkun Wang, Bing Kong, Lan Wang, Xu Dong, and Shanfeng Guo. 2023. "Review on the Mechanism of CO2 Storage and Enhanced Gas Recovery in Carbonate Sour Gas Reservoir" Processes 11, no. 1: 164. https://doi.org/10.3390/pr11010164
APA StyleGuo, X., Feng, J., Wang, P., Kong, B., Wang, L., Dong, X., & Guo, S. (2023). Review on the Mechanism of CO2 Storage and Enhanced Gas Recovery in Carbonate Sour Gas Reservoir. Processes, 11(1), 164. https://doi.org/10.3390/pr11010164







