Dissolvable Film-Controlled Buoyancy Pumping and Aliquoting on a Lab-On-A-Disc
Abstract
1. Introduction
2. Materials and Methods
2.1. Disc Manufacture and Assembly
2.2. Experimental Test Stand
2.3. Absorbance Measurements
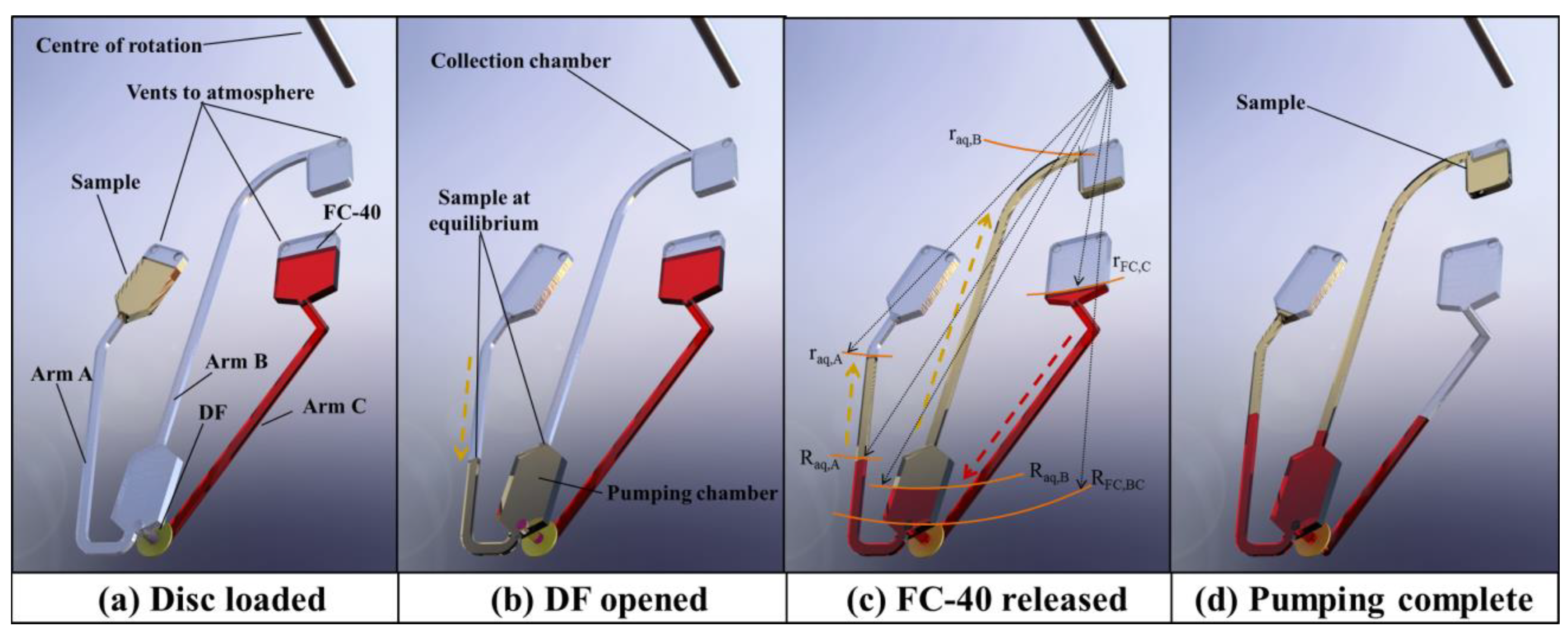
3. Pumping Concepts
3.1. Fundamental Description of Pumping Mechanism
- The sample volume Vaq should slightly exceed the volume of the pumping chamber. The sample will be metered by a pumping chamber and overflow will be returned to the sample reservoir. For example, in the reciprocal pumping design shown in Figure 4, a 90 µL volume is loaded and pumping chamber #1 is designed to be filled with 80 µL, and it is estimated that 80 µL is pumped to the next chamber (pumping chamber #2). In the case of the design shown in Figure 4, pumping chamber #2 and subsequent chambers, are sequentially smaller in size to ensure that they are filled by the sample prior to pumping.
- Channel/reservoir volumes in the transfer zone (see Figure 2) should be as small as possible to minimise the volume of the pumping liquid required.
- Before DF opening, the volume of pumping liquid VFC located radially inwards of should slightly exceed the total dead volume of the reservoirs/channels located in the transfer and pumping zones. When pumping completes, the inner location of the pumping liquid should be at or radially inwards of .
- No air vents should be located in the transfer zone in order to prevent leakage of the sample or pumping liquid during operation.
- The receiving reservoir radius, , should be radially inwards of the pumping reservoir . This prevents the pumping liquid from being displaced into the sample collection reservoir (which might cause issues with later downstream processing).
- The length and cross-section of the connecting microchannels should be minimised as much as possible to reduce sample loss.
- Variations in the microchannel parameters due to manufacturing tolerances should be taken into account in the designing phase.
3.2. Simulation of Manufacturing Tolerances
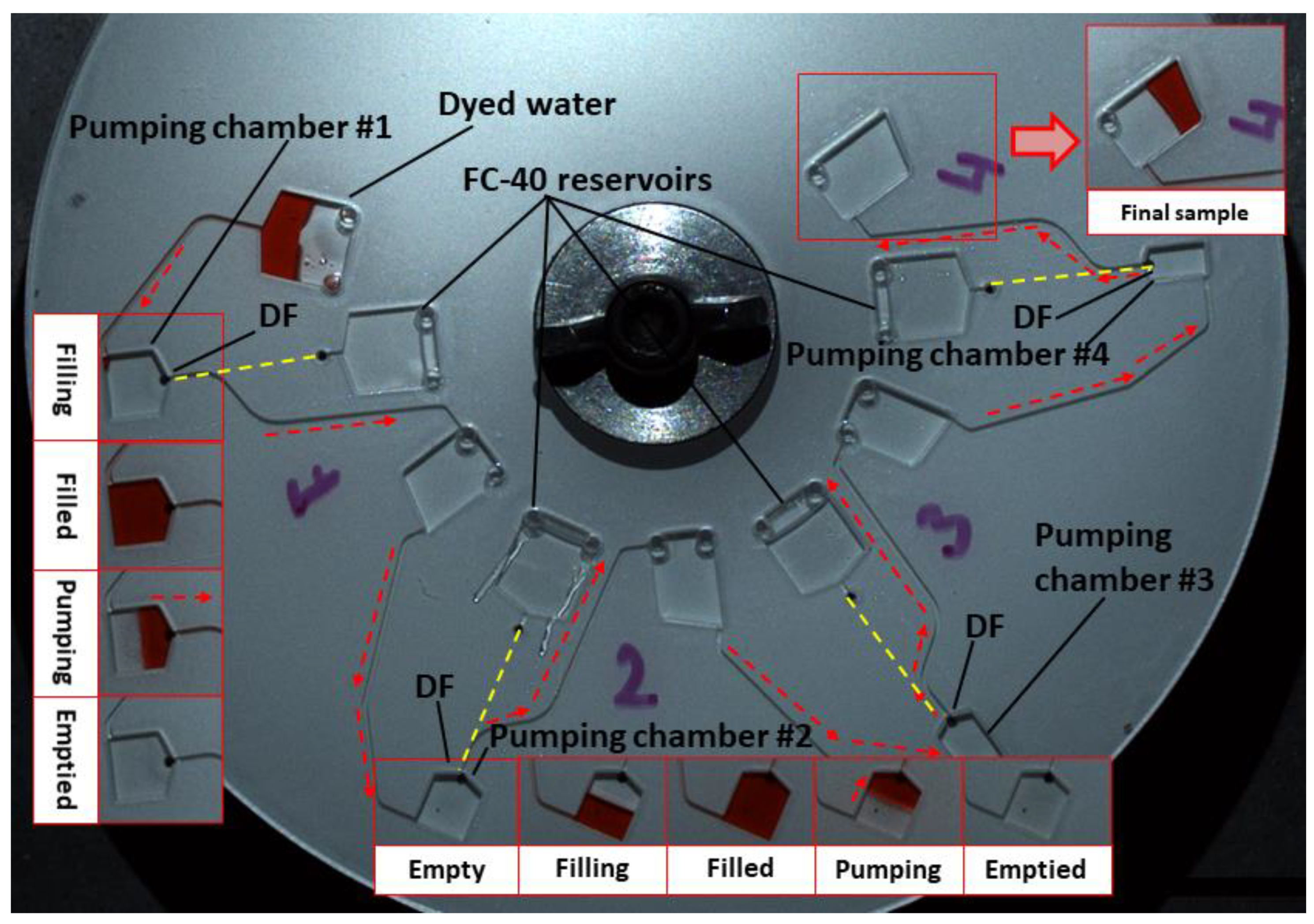
3.3. Multi-Step Pumping
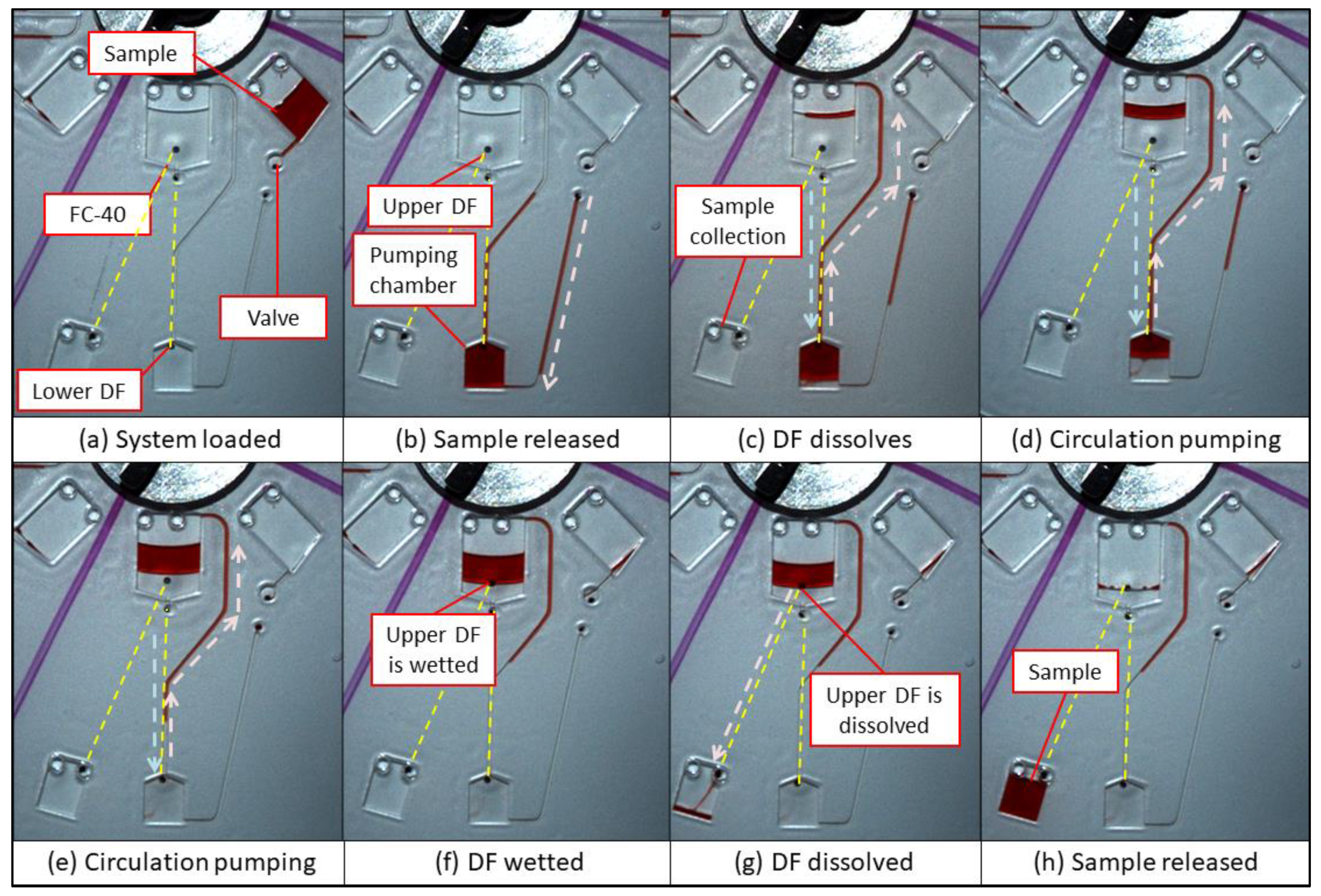
3.4. Recirculating Multi-Step Pumping
3.5. Sample Aliquoting
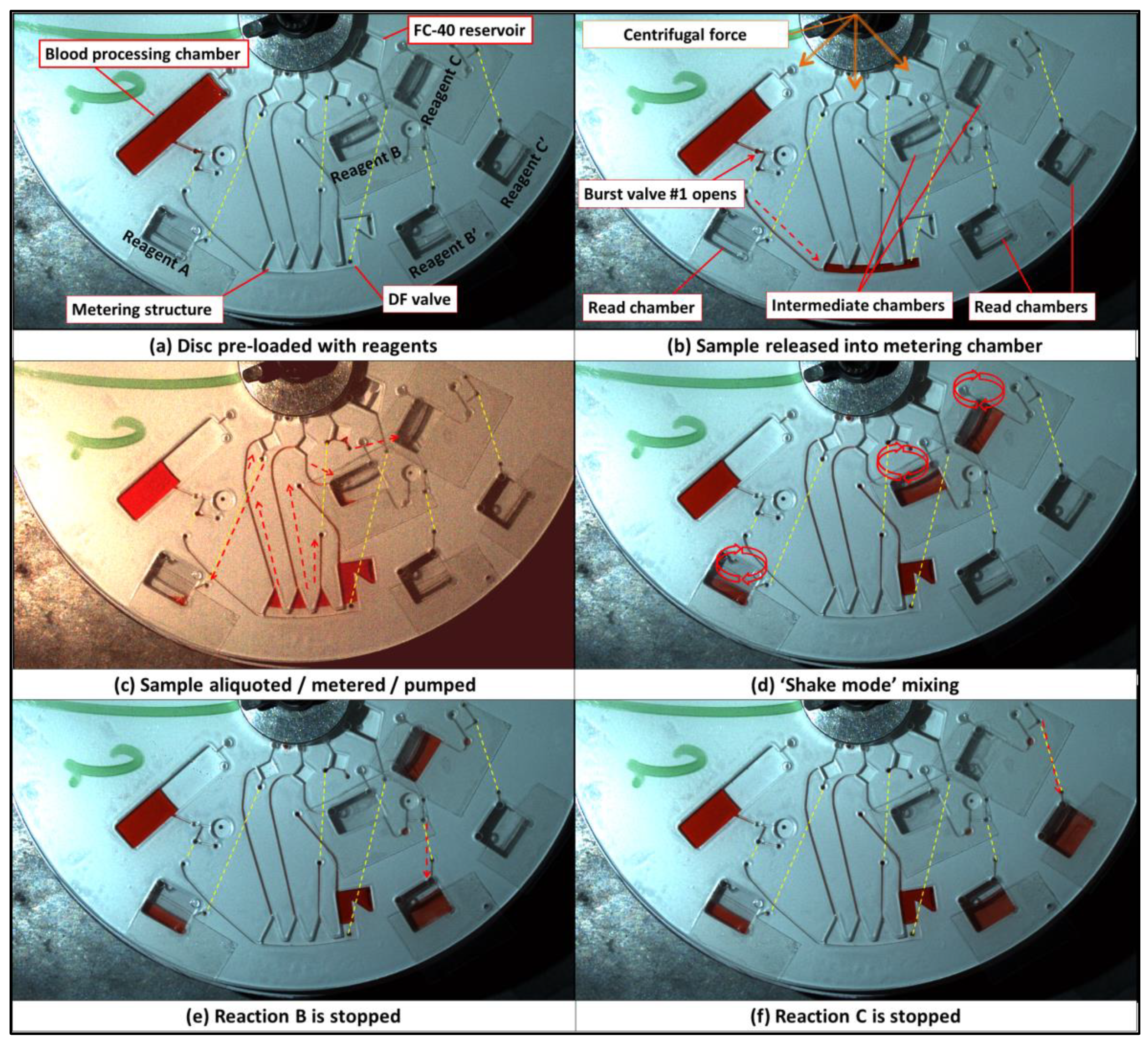
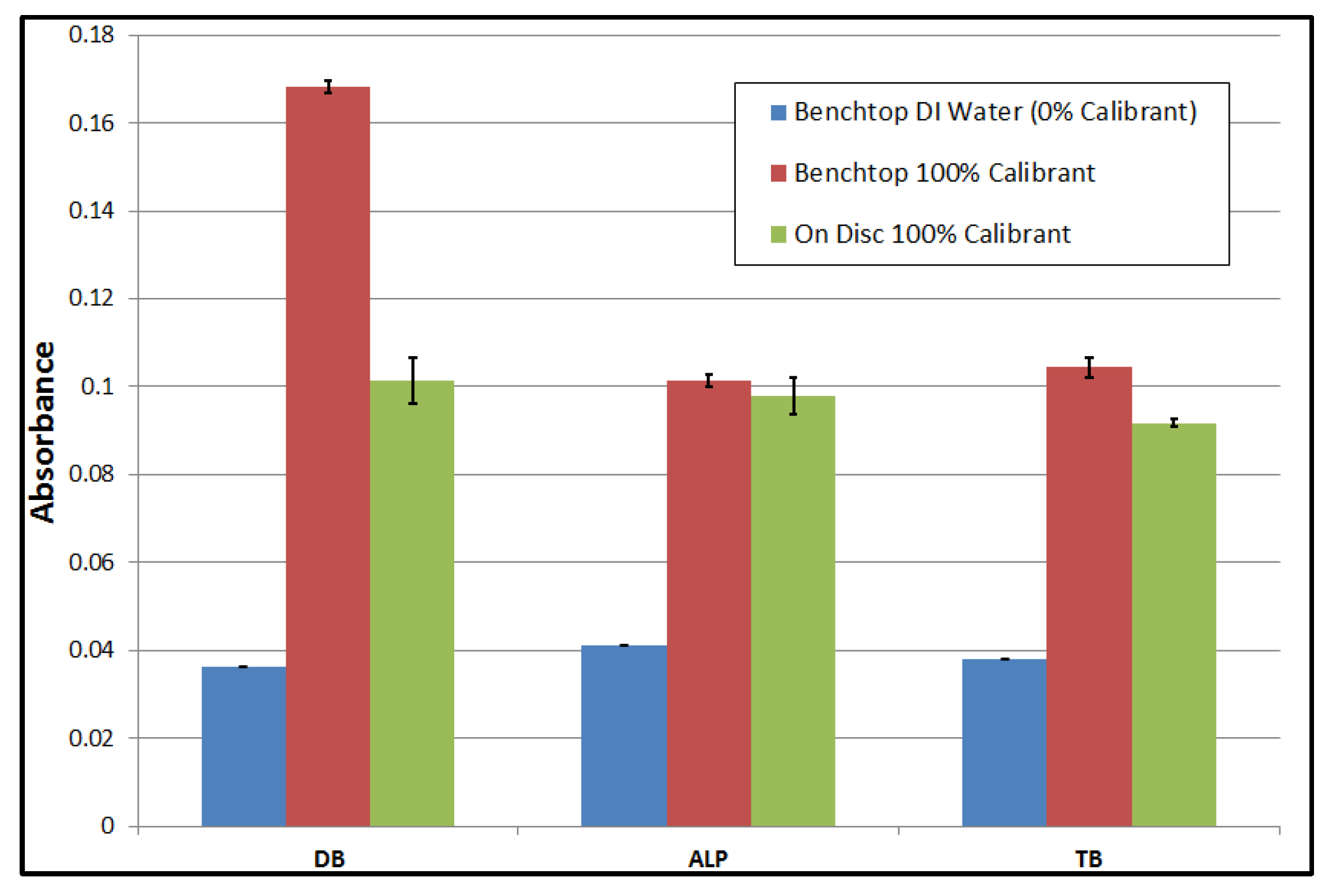
4. Liver Assay Panel
5. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madou, M.; Zoval, J.; Jia, G.; Kido, H.; Kim, J.; Kim, N. Lab on a CD. Annu. Rev. Biomed. Eng. 2006, 8, 601–628. [Google Scholar] [CrossRef] [PubMed]
- Ducrée, J.; Haeberle, S.; Lutz, S.; Pausch, S.; Von Stetten, F.; Zengerle, R. The Centrifugal Microfluidic Bio-Disk Platform. J. Micromechanics Microengineering 2007, 17, S103. [Google Scholar] [CrossRef]
- Gorkin, R.; Park, J.; Siegrist, J.; Amasia, M.; Lee, B.S.; Park, J.-M.; Kim, J.; Kim, H.; Madou, M.; Cho, Y.-K. Centrifugal Microfluidics for Biomedical Applications. Lab A Chip 2010, 10, 1758–1773. [Google Scholar] [CrossRef] [PubMed]
- Strohmeier, O.; Keller, M.; Schwemmer, F.; Zehnle, S.; Mark, D.; von Stetten, F.; Zengerle, R.; Paust, N. Centrifugal Microfluidic Platforms: Advanced Unit Operations and Applications. Chem. Soc. Rev. 2015, 44, 6187–6229. [Google Scholar] [CrossRef]
- Chen, Q.; Cheung, K.; Kong, S.; Zhou, J.; Kwan, Y.; Wong, C.; Ho, H. An Integrated Lab-on-a-Disc for Automated Cell-Based Allergen Screening Bioassays. Talanta 2012, 97, 48–54. [Google Scholar] [CrossRef]
- Tang, M.; Wang, G.; Kong, S.-K.; Ho, H.-P. A Review of Biomedical Centrifugal Microfluidic Platforms. Micromachines 2016, 7, 26. [Google Scholar] [CrossRef]
- Smith, S.; Mager, D.; Perebikovsky, A.; Shamloo, E.; Kinahan, D.; Mishra, R.; Torres Delgado, S.M.; Kido, H.; Saha, S.; Ducrée, J. CD-Based Microfluidics for Primary Care in Extreme Point-of-Care Settings. Micromachines 2016, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.C.; Salin, E.D. Spectrophotometric Determination of Aqueous Sulfide on a Pneumatically Enhanced Centrifugal Microfluidic Platform. Anal. Chem. 2012, 84, 10038–10043. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, Y.; Cho, J.; Lee, J.; Choi, M.-S.; Cho, Y.-K. Lab-on-a-Disc for Simultaneous Determination of Nutrients in Water. Anal. Chem. 2013, 85, 2954–2960. [Google Scholar] [CrossRef]
- Czugala, M.; Maher, D.; Collins, F.; Burger, R.; Hopfgartner, F.; Yang, Y.; Zhaou, J.; Ducrée, J.; Smeaton, A.; Fraser, K.J. CMAS: Fully Integrated Portable Centrifugal Microfluidic Analysis System for on-Site Colorimetric Analysis. RSC Adv. 2013, 3, 15928–15938. [Google Scholar] [CrossRef]
- Nwankire, C.E.; Donohoe, G.G.; Zhang, X.; Siegrist, J.; Somers, M.; Kurzbuch, D.; Monaghan, R.; Kitsara, M.; Burger, R.; Hearty, S. At-Line Bioprocess Monitoring by Immunoassay with Rotationally Controlled Serial Siphoning and Integrated Supercritical Angle Fluorescence Optics. Anal. Chim. Acta 2013, 781, 54–62. [Google Scholar] [CrossRef]
- Glynn, M.; Kirby, D.; Chung, D.; Kinahan, D.J.; Kijanka, G.; Ducrée, J. Centrifugo-Magnetophoretic Purification of CD4+ Cells from Whole Blood toward Future HIV/AIDS Point-of-Care Applications. J. Lab. Autom. 2014, 19, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Kinahan, D.J.; Kearney, S.M.; Kilcawley, N.A.; Early, P.L.; Glynn, M.T.; Ducree, J. Density-Gradient Mediated Band Extraction of Leukocytes from Whole Blood Using Centrifugo-Pneumatic Siphon Valving on Centrifugal Microfluidic Discs. PLoS ONE 2016, 11, e0155545. [Google Scholar] [CrossRef]
- Kinahan, D.J.; Kearney, S.M.; Glynn, M.T.; Ducrée, J. Spira Mirabilis Enhanced Whole Blood Processing in a Lab-on-a-Disk. Sens. Actuators A Phys. 2014, 215, 71–76. [Google Scholar] [CrossRef]
- Clime, L.; Brassard, D.; Geissler, M.; Veres, T. Active Pneumatic Control of Centrifugal Microfluidic Flows for Lab-on-a-Chip Applications. Lab A Chip 2015, 15, 2400–2411. [Google Scholar] [CrossRef] [PubMed]
- Abi-Samra, K.; Clime, L.; Kong, L.; Gorkin, R., III; Kim, T.-H.; Cho, Y.-K.; Madou, M. Thermo-Pneumatic Pumping in Centrifugal Microfluidic Platforms. Microfluid. Nanofluidics 2011, 11, 643–652. [Google Scholar] [CrossRef]
- Noroozi, Z.; Kido, H.; Madou, M.J. Electrolysis-Induced Pneumatic Pressure for Control of Liquids in a Centrifugal System. J. Electrochem. Soc. 2011, 158, P130. [Google Scholar] [CrossRef]
- Garcia-Cordero, J.L.; Basabe-Desmonts, L.; Ducrée, J.; Ricco, A.J. Liquid Recirculation in Microfluidic Channels by the Interplay of Capillary and Centrifugal Forces. Microfluid. Nanofluidics 2010, 9, 695–703. [Google Scholar] [CrossRef]
- Godino, N.; Gorkin, R., III; Linares, A.V.; Burger, R.; Ducrée, J. Comprehensive Integration of Homogeneous Bioassays via Centrifugo-Pneumatic Cascading. Lab A Chip 2013, 13, 685–694. [Google Scholar] [CrossRef]
- Zehnle, S.; Schwemmer, F.; Roth, G.; von Stetten, F.; Zengerle, R.; Paust, N. Centrifugo-Dynamic Inward Pumping of Liquids on a Centrifugal Microfluidic Platform. Lab A Chip 2012, 12, 5142–5145. [Google Scholar] [CrossRef]
- Schwemmer, F.; Zehnle, S.; Mark, D.; von Stetten, F.; Zengerle, R.; Paust, N. A Microfluidic Timer for Timed Valving and Pumping in Centrifugal Microfluidics. Lab A Chip 2015, 15, 1545–1553. [Google Scholar] [CrossRef]
- Gorkin, R., III; Clime, L.; Madou, M.; Kido, H. Pneumatic Pumping in Centrifugal Microfluidic Platforms. Microfluid. Nanofluidics 2010, 9, 541–549. [Google Scholar] [CrossRef]
- Schwemmer, F.; Hutzenlaub, T.; Buselmeier, D.; Paust, N.; von Stetten, F.; Mark, D.; Zengerle, R.; Kosse, D. Centrifugo-Pneumatic Multi-Liquid Aliquoting—Parallel Aliquoting and Combination of Multiple Liquids in Centrifugal Microfluidics. Lab A Chip 2015, 15, 3250–3258. [Google Scholar] [CrossRef]
- Kinahan, D.J.; Burger, R.; Lawlor, D.; Early, P.L.; Vembadi, A.; McArdle, N.A.; Kilcawley, N.A.; Glynn, M.T.; Ducrée, J. Centrifugally Automated Solid-Phase Extraction of DNA by Immiscible Liquid Valving and Chemically Powered Centripetal Pumping of Peripherally Stored Reagents. Biosens. Bioelectron. X 2021, 9, 100085. [Google Scholar] [CrossRef]
- Dignan, L.M.; Karas, S.M.; Mighell, I.K.; Treene, W.R.; Landers, J.P.; Woolf, M.S. A Novel Method for Inward Fluid Displacement in Centrifugal Microdevices for Highly Integrated Nucleic Acid Processing with Long-Term Reagent Storage. Anal. Chim. Acta 2022, 1221, 340063. [Google Scholar] [CrossRef]
- Krauss, S.T.; Woolf, M.S.; Hadley, K.C.; Collins, N.M.; Nauman, A.Q.; Landers, J.P. Centrifugal Microfluidic Devices Using Low-Volume Reagent Storage and Inward Fluid Displacement for Presumptive Drug Detection. Sens. Actuators B Chem. 2019, 284, 704–710. [Google Scholar] [CrossRef]
- Kong, M.C.; Bouchard, A.P.; Salin, E.D. Displacement Pumping of Liquids Radially Inward on Centrifugal Microfluidic Platforms in Motion. Micromachines 2011, 3, 1–9. [Google Scholar] [CrossRef]
- Soroori, S.; Kulinsky, L.; Kido, H.; Madou, M. Design and Implementation of Fluidic Micro-Pulleys for Flow Control on Centrifugal Microfluidic Platforms. Microfluid. Nanofluidics 2014, 16, 1117–1129. [Google Scholar] [CrossRef]
- Gorkin, R., III; Nwankire, C.E.; Gaughran, J.; Zhang, X.; Donohoe, G.G.; Rook, M.; O’Kennedy, R.; Ducrée, J. Centrifugo-Pneumatic Valving Utilizing Dissolvable Films. Lab A Chip 2012, 12, 2894–2902. [Google Scholar] [CrossRef] [PubMed]
- Nwankire, C.E.; Chan, D.-S.S.; Gaughran, J.; Burger, R.; Gorkin, R., III; Ducrée, J. Fluidic Automation of Nitrate and Nitrite Bioassays in Whole Blood by Dissolvable-Film Based Centrifugo-Pneumatic Actuation. Sensors 2013, 13, 11336–11349. [Google Scholar] [CrossRef]
- Nwankire, C.E.; Czugala, M.; Burger, R.; Fraser, K.J.; Glennon, T.; Onwuliri, B.E.; Nduaguibe, I.E.; Diamond, D.; Ducrée, J. A Portable Centrifugal Analyser for Liver Function Screening. Biosens. Bioelectron. 2014, 56, 352–358. [Google Scholar] [CrossRef]
- Kinahan, D.J.; Kearney, S.M.; Dimov, N.; Glynn, M.T.; Ducrée, J. Event-Triggered Logical Flow Control for Comprehensive Process Integration of Multi-Step Assays on Centrifugal Microfluidic Platforms. Lab A Chip 2014, 14, 2249–2258. [Google Scholar] [CrossRef]
- Kinahan, D.J.; Kearney, S.M.; Faneuil, O.P.; Glynn, M.T.; Dimov, N.; Ducrée, J. Paper Imbibition for Timing of Multi-Step Liquid Handling Protocols on Event-Triggered Centrifugal Microfluidic Lab-on-a-Disc Platforms. RSC Adv. 2015, 5, 1818–1826. [Google Scholar] [CrossRef]
- Kinahan, D.J.; Early, P.L.; Vembadi, A.; MacNamara, E.; Kilcawley, N.A.; Glennon, T.; Diamond, D.; Brabazon, D.; Ducrée, J. Xurography Actuated Valving for Centrifugal Flow Control. Lab A Chip 2016, 16, 3454–3459. [Google Scholar] [CrossRef]
- Miyazaki, C.M.; Kinahan, D.J.; Mishra, R.; Mangwanya, F.; Kilcawley, N.; Ferreira, M.; Ducrée, J. Label-Free, Spatially Multiplexed SPR Detection of Immunoassays on a Highly Integrated Centrifugal Lab-on-a-Disc Platform. Biosens. Bioelectron. 2018, 119, 86–93. [Google Scholar] [CrossRef]
- Bartholomeusz, D.A.; Boutté, R.W.; Andrade, J.D. Xurography: Rapid Prototyping of Microstructures Using a Cutting Plotter. J. Microelectromechanical Syst. 2005, 14, 1364–1374. [Google Scholar] [CrossRef]
- Grumann, M.; Brenner, T.; Beer, C.; Zengerle, R.; Ducrée, J. Visualization of Flow Patterning in High-Speed Centrifugal Microfluidics. Rev. Sci. Instrum. 2005, 76, 025101. [Google Scholar] [CrossRef]
- Brennan, D.; Coughlan, H.; Clancy, E.; Dimov, N.; Barry, T.; Kinahan, D.; Ducrée, J.; Smith, T.J.; Galvin, P. Development of an On-Disc Isothermal In Vitro Amplification and Detection of Bacterial RNA. Sens. Actuators B Chem. 2017, 239, 235–242. [Google Scholar] [CrossRef]
- Delgado, S.M.T.; Kinahan, D.J.; Julius, L.A.N.; Mallette, A.; Ardila, D.S.; Mishra, R.; Miyazaki, C.M.; Korvink, J.G.; Ducrée, J.; Mager, D. Wirelessly Powered and Remotely Controlled Valve-Array for Highly Multiplexed Analytical Assay Automation on a Centrifugal Microfluidic Platform. Biosens. Bioelectron. 2018, 109, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L.; Serioli, L.; Centorbi, F.A.; Jendresen, C.B.; Matteucci, M.; Ilchenko, O.; Demarchi, D.; Nielsen, A.T.; Zór, K.; Boisen, A. Injection Molded Lab-on-a-Disc Platform for Screening of Genetically Modified, E. Coli Using Liquid-Liquid Extraction and Surface Enhanced Raman Scattering. Lab A Chip 2018, 18, 869–877. [Google Scholar] [CrossRef]
- Mishra, R.; Alam, R.; McAuley, D.; Bharaj, T.; Chung, D.; Kinahan, D.J.; Nwankire, C.; Anderson, K.S.; Ducrée, J. Solvent Selective Membrane Routing and Microfluidic Architecture towards Centrifugal Automation of Customisable Bead Based Immunoassays. Sens. Actuators B Chem. 2022, 356, 131305. [Google Scholar] [CrossRef]
- Mishra, R.; Zapatero-Rodriguez, J.; Sharma, S.; Kelly, D.; McAuley, D.; Gilgunn, S.; O’Kennedy, R.; Ducree, J. Automation of Multi-Analyte Prostate Cancer Biomarker Immunoassay Panel from Whole Blood by Minimum-Instrumentation Rotational Flow Control. Sens. Actuators B Chem. 2018, 263, 668–675. [Google Scholar] [CrossRef]
- Grumann, M.; Geipel, A.; Riegger, L.; Zengerle, R.; Ducrée, J. Batch-Mode Mixing on Centrifugal Microfluidic Platforms. Lab A Chip 2005, 5, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Huang, P.-C.; Lin, M.-G. Analysis and Experiment of Capillary Valves for Microfluidics on a Rotating Disk. Microfluid. Nanofluidics 2008, 4, 427–437. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kilcawley, N.A.; Voebel, T.C.; Early, P.L.; McArdle, N.A.; Renou, M.; Rio, J.; Saint-Martin, G.; Glynn, M.T.; Zontar, D.; Brecher, C.; et al. Dissolvable Film-Controlled Buoyancy Pumping and Aliquoting on a Lab-On-A-Disc. Processes 2023, 11, 128. https://doi.org/10.3390/pr11010128
Kilcawley NA, Voebel TC, Early PL, McArdle NA, Renou M, Rio J, Saint-Martin G, Glynn MT, Zontar D, Brecher C, et al. Dissolvable Film-Controlled Buoyancy Pumping and Aliquoting on a Lab-On-A-Disc. Processes. 2023; 11(1):128. https://doi.org/10.3390/pr11010128
Chicago/Turabian StyleKilcawley, Niamh A., Toni C. Voebel, Philip L. Early, Niamh A. McArdle, Marine Renou, Jeanne Rio, Godefroi Saint-Martin, Macdara T. Glynn, Daniel Zontar, Christian Brecher, and et al. 2023. "Dissolvable Film-Controlled Buoyancy Pumping and Aliquoting on a Lab-On-A-Disc" Processes 11, no. 1: 128. https://doi.org/10.3390/pr11010128
APA StyleKilcawley, N. A., Voebel, T. C., Early, P. L., McArdle, N. A., Renou, M., Rio, J., Saint-Martin, G., Glynn, M. T., Zontar, D., Brecher, C., Ducrée, J., & Kinahan, D. J. (2023). Dissolvable Film-Controlled Buoyancy Pumping and Aliquoting on a Lab-On-A-Disc. Processes, 11(1), 128. https://doi.org/10.3390/pr11010128







