Essential Oils of Ocimum basilicum L. and Ocimum americanum L. from Djibouti: Chemical Composition, Antimicrobial and Cytotoxicity Evaluations
Abstract
:1. Introduction
- first: evaluate the chemical composition of Djiboutian essential oils including Ocimum basilicum L. (EOOB) and Ocimum americanum L. (EOOA) by GC-MS analysis;
- second: determine the possible cytotoxicity of the two essential oils against 13 cell lines: K562, A549, HCT116, PC3, U87-MG, MIA-Paca2, HEK293, NCI-N87, RT4, U2OS, A2780, MRC -5 and JIMT-T1;
- third: conduct microbial investigations of the two essential oils against 12 bacteria: Gram + (Staphylococcus aureus, Enterococcus faecalis, Streptococcus agalactiae, Staphylococcus epidermidis and Corynebacterium sp.) and Gram—(Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, Shigella sonnei, Salmonella enterica sv., Typhimurium and Enterobacter cloacae).
2. Materials and Methods
2.1. Plant Material and Essential Oils Preparation
2.2. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.3. Antibacterial Test
2.4. Cytotoxicity Tests
2.5. Statistical Analysis
3. Results
3.1. Chemical Composition
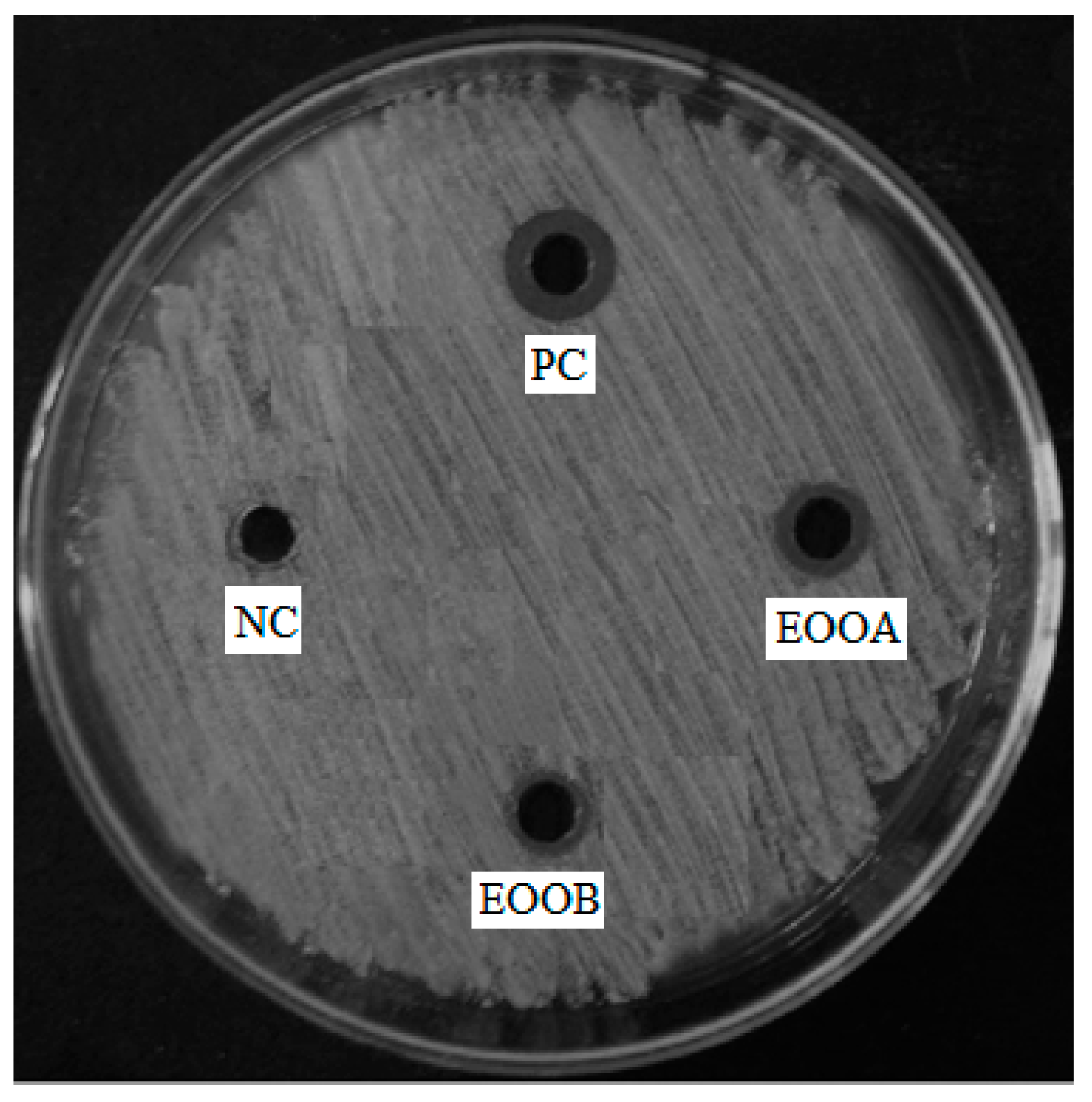
3.2. Antibacterial Activity
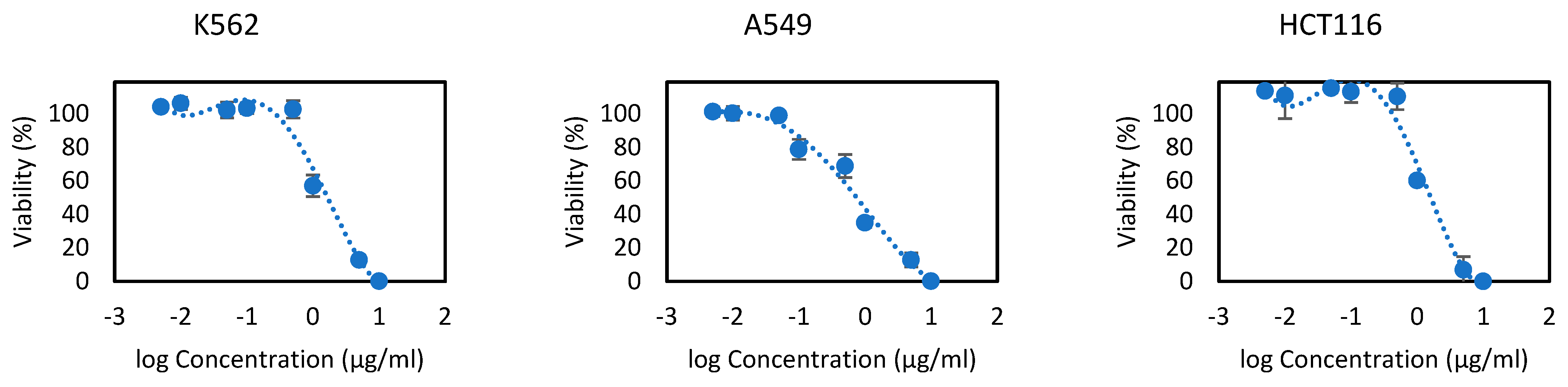
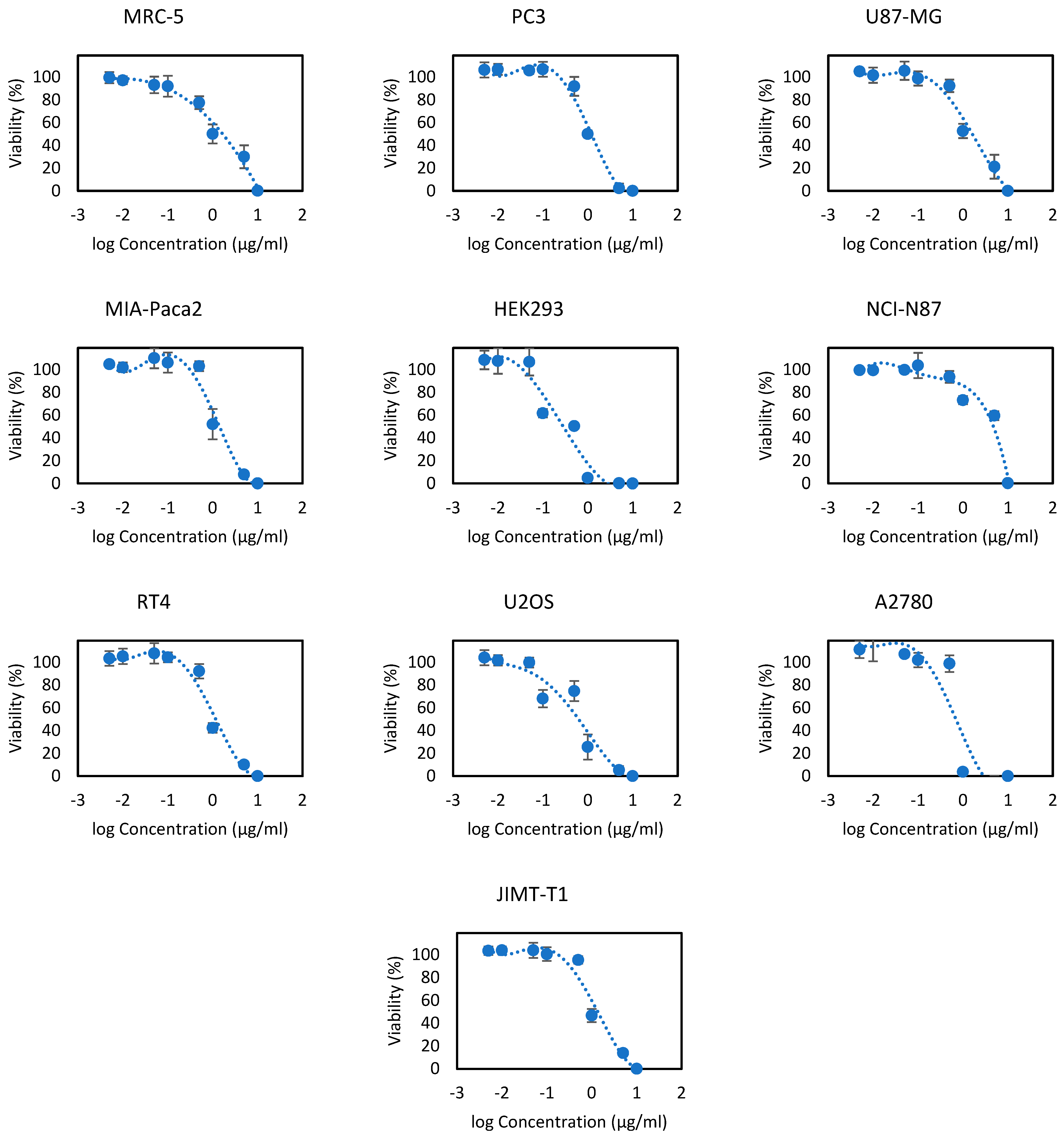
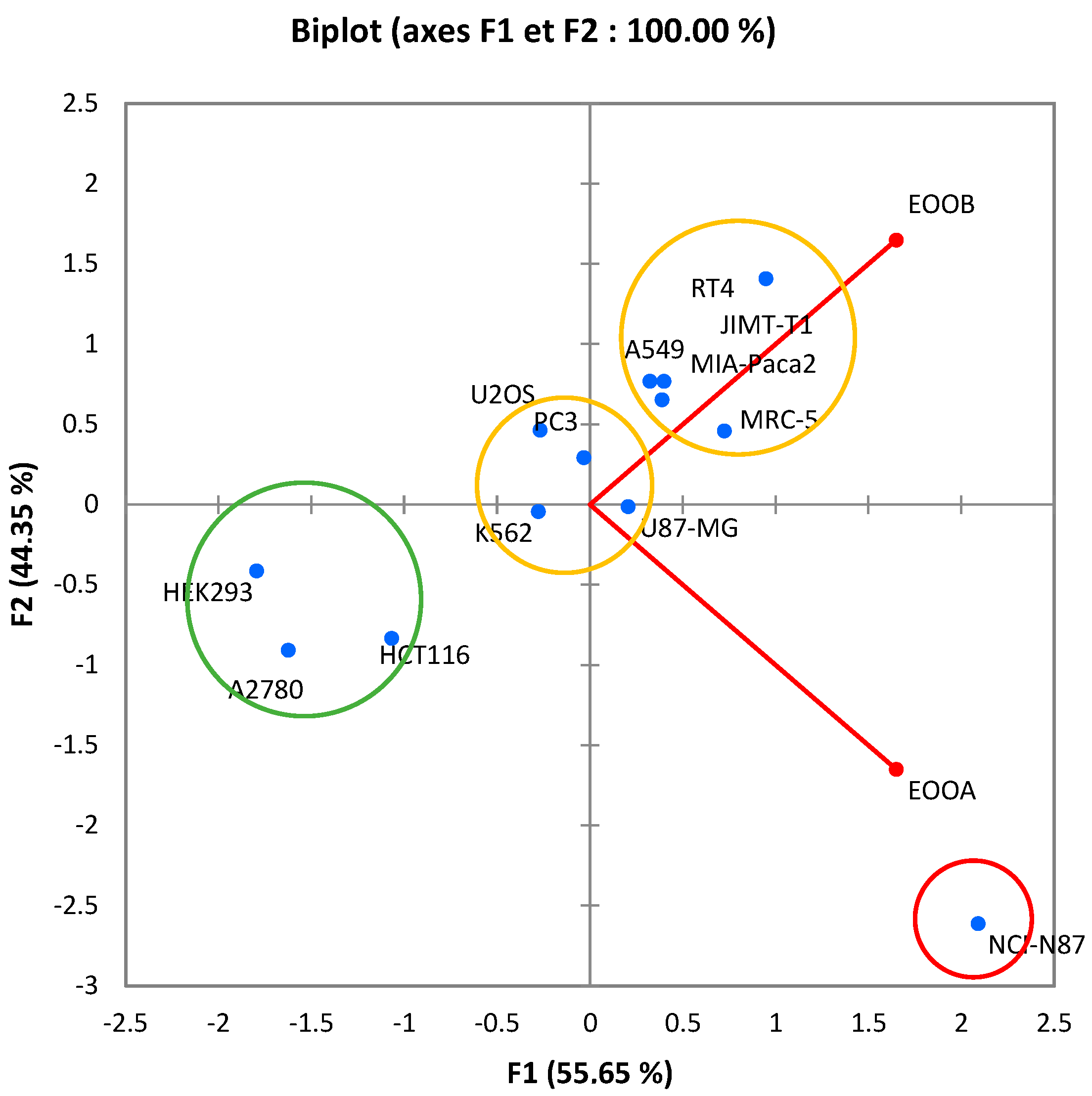
3.3. Cytotoxicity Activity
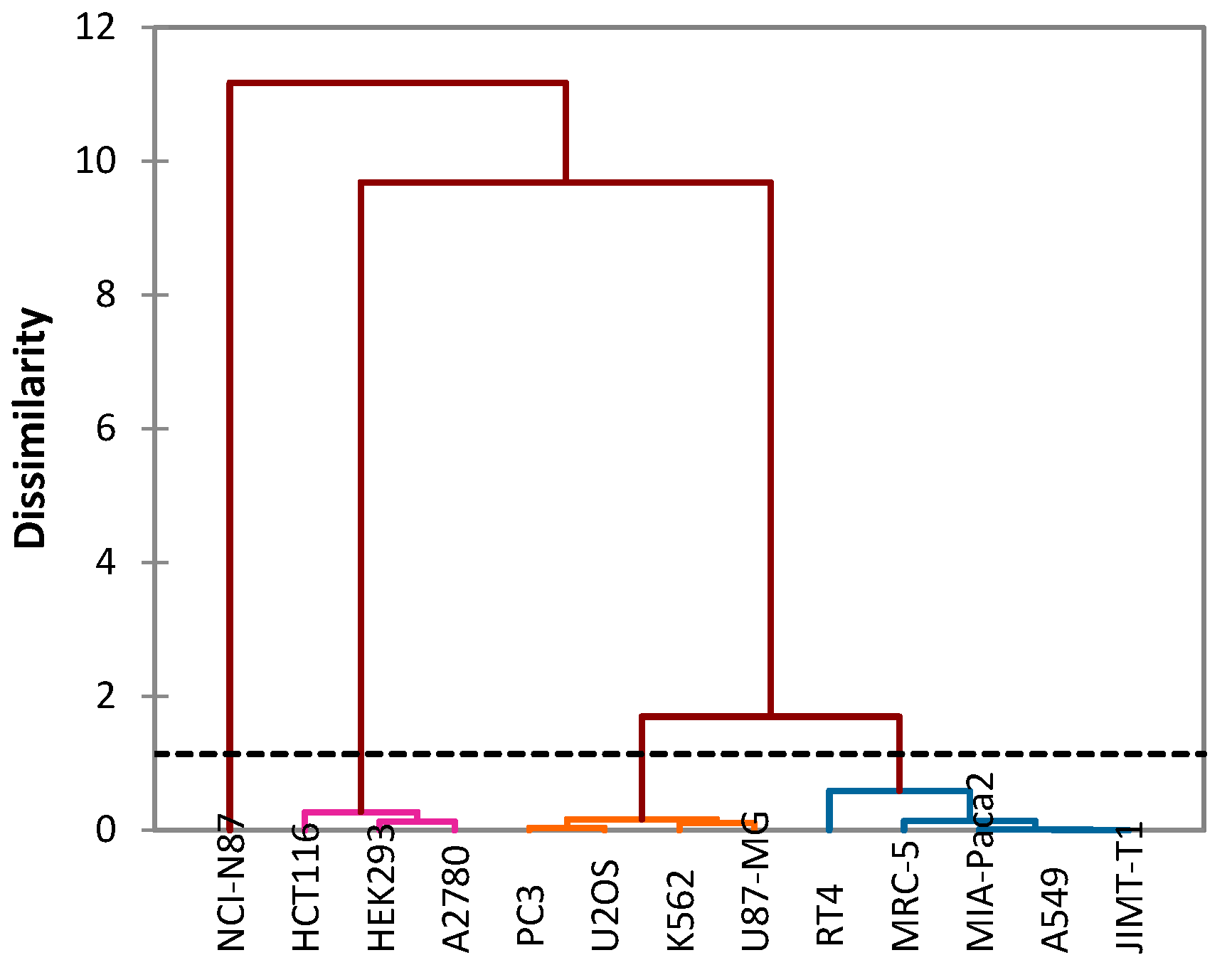
- Group I: Includes a single cancer cell line NCI-N87, from which essential oils show moderate IC50 values.
- Group II: Includes three cancer cell lines: HCT116, HEK293 and A2780. This group contains significant IC50 values of both essential oils.
- Group III: Includes four cancer cell lines: K562, PC3, U87-MG and U2OS.
- GROUP IV: Includes five cancer cell lines: A549, MIA-Paca2, RT4, MRC-5 and JIMT-T1.
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obame-Engonga, L.C.; Abdoul-Latif, F.M.; Ondo, J.P.; Sima-Obiang, C.; Ngoua-Meye-Misso, R.L.; Traoré, A. Phytochemical screening, antioxidant and antibacterial activities of Guibourtia ehie and Syzygium rowlandii medicinal plants from Gabon. Int. J. Curr. Res. 2017, 9, 56354–56360. [Google Scholar]
- Gharby, S.; Asdadi, A.; Ibourki, M.; Hamdouch, A.; Ainane, T.; Hassani, L.A.I. Chemical characterization of the essential oil from aerial parts of Lavandula rejdalii Upson & Jury, a medicinal plant endemic to Morocco. J. Essent. Oil Bear. Plants 2020, 23, 1422–1427. [Google Scholar]
- Abdoul-Latif, F.M.; Ainane, A.; Merito, A.; Ainane, T. Chemical composition and biological activities of essential oils from Djibouti. J. Anal. Sci. Appl. Biotechnol. 2022, 4, 1–9. [Google Scholar] [CrossRef]
- Ainane, A.; Abdoul-Latif, F.M.; Mohamed, J.; Attahar, W.; Ouassil, M.; Shybat, Z.L.; El Yaacoubi, A.; Ainane, T. Behaviour desorption study of the essential oil of Cedrus atlantica in a porous clay versus insecticidal activity against Sitophilus granarius: Explanation of the phenomenon by statistical studies. Int. J. Metrol. Qual. Eng. 2021, 12, 12. [Google Scholar] [CrossRef]
- Ainane, A.; Abdoul-Latif, F.M.; Abdoul-Latif, T.M.; Ainane, T. Evaluation of biological activities of two essential oils as a safe environmental bioinsecticides: Case of Eucalyptus globulus and Rosmarinus officinalis. Przegląd Nauk. Inżynieria i Kształtowanie Środowiska 2020, 29, 544–556. [Google Scholar] [CrossRef]
- Ainane, T.; Elkouali, M.; Ainane, A.; Talbi, M. Moroccan traditional fragrance based essential oils: Preparation, composition and chemical identification. Pharma Chem. 2014, 6, 84–89. [Google Scholar]
- Gali-Muhtasib, H.; Hilan, C.; Khater, C. Traditional uses of Salvia libanotica (East Mediterranean sage) and the effects of its essential oils. J. Ethnopharmacol. 2000, 71, 513–520. [Google Scholar] [CrossRef]
- Ouassil, M.; Abdoul-Latif, F.M.; Am, A.; Attahar, W.; Ainane, A.; Ainane, T. Chemical composition of bay laurel and rosemary essential oils from Morocco and their antifungal activity against fusarium strains. Pharmacologyonline 2021, 2, 426–433. [Google Scholar]
- Elabboubi, M.; Bennani, L.; Ainane, A.; Charaf, S.; Bouhadi, M.; Elkouali, M.; Talbi, M.; Cherroud, S.; Ainane, T. Treatment of mycoses by essential oils: Mini Review. J. Anal. Sci. Appl. Biotechnol. 2019, 1, 35–40. [Google Scholar]
- Abdoul-Latif, F.M.; Elmi, A.; Merito, A.; Nour, M.; Risler, A.; Ainane, A.; Bignon, J.; Ainane, T. Essential Oils of Tagetes minuta and Lavandula coronopifolia from Djibouti: Chemical Composition, Antibacterial Activity and Cytotoxic Activity against Various Human Cancer Cell Lines. Int. J. Plant Biol. 2022, 13, 315–329. [Google Scholar] [CrossRef]
- Shybat, Z.L.; Abdoul-Latif, F.M.; Mohamed, J.; Ainane, A.; Ainane, T. Antifungal activity of the essential oil of morrocan myrtle (Myrtus communis L.): Application in agriculture. Pharmacologyonline 2021, 2, 485–491. [Google Scholar]
- Abdoul-latif, F.M.; Ainane, A.; Abdoul-latif, T.M.; Ainane, T. Chemical study and evaluation of insectical properties of African Lippia citriodora essential oil. J. Biopestic. 2020, 13, 119–126. [Google Scholar]
- Campana, R.; Tiboni, M.; Maggi, F.; Cappellacci, L.; Cianfaglione, K.; Morshedloo, M.R.; Frangipani, E.; Casettari, L. Comparative Analysis of the Antimicrobial Activity of Essential Oils and Their Formulated Microemulsions against Foodborne Pathogens and Spoilage Bacteria. Antibiotics 2022, 11, 447. [Google Scholar] [CrossRef] [PubMed]
- Eddabbeh, F.-E.; Mohamed Abdoul-Latif, F.; Ainane, A.; Ejjabraoui, M.; Ainane, T. The composition of the essential oil and the antimicrobialand antifunqal activities of Tetraclinis articulata (Vahl) masters from the Moroccan central plateau (Morocco). Pharmacologyonline 2021, 2, 458–464. [Google Scholar]
- Morshedloo, M.R.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Maggi, F. Chemical composition, antioxidant activity and cytotoxicity on tumour cells of the essential oil from flowers of Magnolia grandiflora cultivated in Iran. Nat. Prod. Res. 2017, 31, 2857–2864. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Adholeya, A.; Conlan, X.A.; Cahill, D.M. Acidic potassium permanganate chemiluminescence for the determination of antioxidant potential in three cultivars of Ocimum basilicum. Plant Foods Hum. Nutr. 2016, 71, 72–80. [Google Scholar] [CrossRef]
- Wossa, S.W.; Rali, T.; Leach, D.N. Atile chemical constituents of three Ocimum species (Lamiaceae) from Papua New Guinea. South Pac. J. Nat. Appl. Sci. 2008, 26, 25–27. [Google Scholar] [CrossRef]
- Abdoul-Latif, F.M.; Ainane, A.; Oumaskour, K.; Boujaber, N.; Mohamed, J.; Ainane, T. In vitro antidiabetic activity of essential oil of two species of Artemisia: Artemisia heba-alba asso and Artemisia ifranensis. Pharmacologyonline 2021, 3, 812–820. [Google Scholar]
- Ainane, A.; Cherroud, S.; El Kouali, M.; Abba, E.H.; Ainane, T. Chemical compositions, insecticidal and antimicrobial activities of two moroccan essential oils of Citrus limonum and Syzygium aromaticum. Pharmacol. J. 2020, 30, 190–199. [Google Scholar]
- Ainane, T.; Mohamed Abdoul-Latif, F.; Ouassil, M.; Am, A.; Ainane, A. Antagonistic antifungal activities of Mentha suaveolens and Artemisia absinthium essential oils from Morocco. Pharmacologyonline 2021, 2, 470–478. [Google Scholar]
- Elmi, A.; Spina, R.; Risler, A.; Philippot, S.; Mérito, A.; Duval, R.E.; Abdoul-Latif, F.M.; Laurain-Mattar, D. Evaluation of antioxidant and antibacterial activities, cytotoxicity of Acacia seyal Del bark extracts and isolated compounds. Molecules 2020, 25, 2392. [Google Scholar] [CrossRef] [PubMed]
- Saviano, A.M.; Lourenço, F.R. Measurement uncertainty estimation based on multiple regression analysis (MRA) and Monte Carlo (MC) simulations—Application to agar diffusion method. Measurement 2018, 115, 269–278. [Google Scholar] [CrossRef]
- Elmi, A.; Spina, R.; Abdoul-Latif, F.M.; Yagi, S.; Fontanay, S.; Risler, A.; Laurain-Mattar, D. Rapid screening for bioactive natural compounds in Indigofera caerulea Rox fruits. Ind. Crops Prod. 2018, 125, 123–130. [Google Scholar] [CrossRef]
- Aborehab, N.M.; Elnagar, M.R.; Waly, N.E. Gallic acid potentiates the apoptotic effect of paclitaxel and carboplatin via overexpression of Bax and P53 on the MCF-7 human breast cancer cell line. J. Biochem. Mol. Toxicol. 2021, 35, e22638. [Google Scholar] [CrossRef] [PubMed]
- Pecnard, S.; Provot, O.; Levaique, H.; Bignon, J.; Askenatzis, L.; Saller, F.; Hamze, A. Cyclic bridged analogs of isoCA-4: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2021, 209, 112873. [Google Scholar] [CrossRef]
- Gurav, T.P.; Dholakia, B.B.; Giri, A.P. A glance at the chemodiversity of Ocimum species: Trends, implications, and strategies for the quality and yield improvement of essential oil. Phytochem. Rev. 2021, 21, 879–913. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Adetunji, C.O.; Olaniyan, O.T.; Ojo, S.K.; Samuel, M.O.; Temitayo, B.T.; Contreras, M.d. Antimicrobial, Antioxidant and Other Pharmacological Activities of Ocimum Species: Potential to Be Used as Food Preservatives and Functional Ingredients. Food Rev. Int. 2021, 1–31. [Google Scholar] [CrossRef]
- Kumar, R.; Saha, P.; Lokare, P.; Datta, K.; Selvakumar, P.; Chourasia, A. A Systemic Review of Ocimum sanctum (Tulsi): Morphological Characteristics, Phytoconstituents and Therapeutic Applications. Int. J. Res. Appl. Sci. Biotechnol. 2022, 9, 221–226. [Google Scholar] [CrossRef]
- Hassan-Abdallah, A.; Merito, A.; Hassan, S.; Aboubaker, D.; Djama, M.; Asfaw, Z.; Kelbessa, E. Medicinal plants and their uses by the people in the Region of Randa, Djibouti. J. Ethnopharmacol. 2013, 148, 701–713. [Google Scholar] [CrossRef]
- Vieira, R.F.; Simon, J.E. Chemical characterization of basil (Ocimum spp.) found in the markets and used in traditional medicine in Brazil. Econ. Bot. 2000, 54, 207–216. [Google Scholar] [CrossRef]
- Farouk, A.; Fikry, R.; Mohsen, M. Chemical composition and antioxidant activity of Ocimum basilicum L. essential oil cultivated in Madinah Monawara, Saudi Arabia and its comparison to the Egyptian chemotype. J. Essent. Oil Bear. Plants 2016, 19, 1119–1128. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Attia, F.A.; Liu, Z.; Li, C.; Wei, J.; Kang, W. Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants. Food Sci. Hum. Wellness 2019, 8, 299–305. [Google Scholar] [CrossRef]
- Guedri, M.M.; Ahlem, Z.; Mariem, B.A.; Romdhane, M.; Okla, M.; Al-Hashimi, A.; Elgawad, H.A. Essential Oil Composition and Antioxidant and Antifungal Activities of Two Varieties of Ocimum basilicum L. (Lamiaceae) at Two Phenological Stages. Agronomy 2022, 12, 825. [Google Scholar]
- Mondello, L.; Zappia, G.; Cotroneo, A.; Bonaccorsi, I.; Chowdhury, J.U.; Yusuf, M.; Dugo, G. Studies on the essential oil-bearing plants of Bangladesh. Part VIII. Composition of some Ocimum oils O. basilicum L. var. purpurascens, O. sanctum L. green, O. sanctum L. purple, O. americanum L., citral type, O. americanum L., camphor type. Flavour Fragr. J. 2002, 17, 335–340. [Google Scholar] [CrossRef]
- de Almeida, I.; Alviano, D.S.; Vieira, D.O.; Alves, P.B.; Blank, A.F.; Lopes, A.H.; Rosa, M.D.S.S. Antigiardial activity of Ocimum basilicum essential oil. Parasitol. Res. 2007, 101, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Sabo, V.A.; Knezevic, P. 3Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind. Crops Prod. 2019, 132, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Ainane, T.; Abdoul-Latif, F.M.; Shybat, Z.L.; Mohamed, J.; Ainane, A. Antifungal activity of essential oil of Pistacia atlantica against Ascochyta rabiei and its correlation with antioxidant activity. Pharmacologyonline 2021, 3, 829–837. [Google Scholar]
- Mohamed Abdoul-Latif, F.; Elmi, A.; Merito, A.; Nour, M.; Risler, A.; Ainane, A.; Bignon, J.; Ainane, T. Chemical Analysis of Essential Oils of Cymbopogon schoenanthus (L.) Spreng. and Nepeta azurea R.Br. ex Benth from Djbouti, In-Vitro Cytotoxicity against Cancer Cell Lines and Antibacterial Activities. Appl. Sci. 2022, 12, 8699. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singh, P.; Tripathi, N.N. Chemistry and bioactivities of essential oils of some Ocimum species: An overview. Asian Pac. J. Trop. Biomed. 2014, 4, 682–694. [Google Scholar] [CrossRef]
- Avetisyan, A.; Markosian, A.; Petrosyan, M.; Sahakyan, N.; Babayan, A.; Aloyan, S.; Trchounian, A. Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complementary Altern. Med. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Jakovljević, D.; Stanković, M.; Warchoł, M.; Skrzypek, E. Basil (Ocimum L.) cell and organ culture for the secondary metabolites production: A review. Plant Cell Tissue Organ Cult. 2022, 149, 61–79. [Google Scholar] [CrossRef]
- Bharati, A.C.; Yadav, P.K.; Pandey, S.; Wal, P.; Sagar, M.K.; Kumar, A. Chemistry, Biological Activities, and Uses of Basil Seed Gum. In Gums, Resins and Latexes of Plant Origin: Chemistry, Biological Activities and Uses; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–17. [Google Scholar]
- Habyarimana, T.; Cyuzuzo, P.; Yamukujije, C.; Yadufashije, C.; Niyonzima, F.N. Phytochemical and Antimicrobial Activity of Ocimum suave Against Selected Human Pathogenic Bacteria. J. Drug Deliv. Ther. 2022, 12, 123–128. [Google Scholar] [CrossRef]
- Sneha, K.; Narayanankutty, A.; Job, J.T.; Olatunji, O.J.; Alfarhan, A.; Famurewa, A.C.; Ramesh, V. Antimicrobial and larvicidal activities of different ocimum essential oils extracted by ultrasound-assisted hydrodistillation. Molecules 2022, 27, 1456. [Google Scholar] [CrossRef] [PubMed]
- Talbi, M.; Saadali, B.; Boriky, D.; Bennani, L.; Elkouali, M.; Ainane, T. Two natural compounds–a benzofuran and a phenylpropane–from Artemisia dracunculus. J. Asian Nat. Prod. Res. 2016, 18, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Levorato, S.; Dominici, L.; Fatigoni, C.; Zadra, C.; Pagiotti, R.; Moretti, M.; Villarini, M. In vitro toxicity evaluation of estragole-containing preparations derived from Foeniculum vulgare Mill. (fennel) on HepG2 cells. Food Chem. Toxicol. 2018, 111, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Monien, B.H.; Sachse, B.; Niederwieser, B.; Abraham, K. Detection of N-acetyl-S-[3′-(4-methoxyphenyl) allyl]-l-Cys (AMPAC) in human urine samples after controlled exposure to fennel tea: A new metabolite of estragole and trans-anethole. Chem. Res. Toxicol. 2019, 32, 2260–2267. [Google Scholar] [CrossRef]
- Kalantari, H.; Galehdari, H.; Zaree, Z.; Gesztelyi, R.; Varga, B.; Haines, D.; Juhasz, B. Toxicological and mutagenic analysis of Artemisia dracunculus (tarragon) extract. Food Chem. Toxicol. 2013, 51, 26–32. [Google Scholar] [CrossRef]
- Zeni, V.; Benelli, G.; Campolo, O.; Giunti, G.; Palmeri, V.; Maggi, F.; Canale, A. Toxics or lures? Biological and behavioral effects of plant essential oils on tephritidae fruit flies. Molecules 2021, 26, 5898. [Google Scholar] [CrossRef]


| Column Type | Agilent Technologies HP-5 MS poly-5% diphenyl-95% dimethyl polysiloxane cross-linked capillary column |
| Column dimension | 30 m × 0.32 mm × 0.25 mm. |
| Injection volume | 0.1 μL in 1:20 split mode. |
| Carrier gas | Helium. |
| Gas flow | 1.0 mL/min. |
| Injector temperature | 250 °C |
| Detector temperature | 300 °C |
| Sample preparation | The EOOB and EOOA were diluted in n-hexane at a concentration of 1:20 (v/v). |
| Gram-Positive | Gram-Negative | ||
|---|---|---|---|
| Strains | Code/Source | Strains | Code/Source |
| Staphylococcus aureus Enterococcus faecalis Streptococcus agalactiae Staphylococcus epidermidis Corynebacterium sp. | ATCC 29213 ATCC 29212 ATCC 27956 Clinical isolate Clinical isolate | Pseudomonas aeruginosa Escherichia coli Klebsiella pneumoniae Acinetobacter baumannii Shigella sonnei Salmonella enterica Enterobacter cloacae | ATCC 27853 ATCC 25922 ATCC 70603 ATCC 19606 ATCC 9290 ATCC 13311 Clinical isolate |
| Cell Lines | Origin | Source | Growth Medium |
|---|---|---|---|
| A549 | Lung carcinoma | ATCC®®-CCL-185TM | Gibco RPMI 1640 + 10% FCS + 1% Glutamine |
| HCT116 | Colorectalcarcinoma | ATCC®®-CCL-247TM | Gibco McCoy’s 5A + 10% FCS + 1% Glutamine |
| K562 | Myelogenous leukemia | ATCC®®-CCL-243TM | Gibco RPMI 1640 + 10% FCS + 1% Glutamine |
| MIA-Paca2 | Pancreas carcinoma | ATCC®®-CRL-1420TM | Gibco DMEM + 10% FCS + 1% Glutamine |
| NCI-N87 | Gastric carcinoma | ATCC®®-CRL-5822TM | Gibco RPMI 1640 + 10% FCS + 1% Glutamine |
| PC3 | Prostate carcinoma | ATCC®®-CRL-1435TM | Gibco RPMI 1640 + 10% FCS + 1% Glutamine |
| RT4 | Urinary bladder | ATCC®®-HTB-2TM | Gibco McCoy’s 5A + 10% FCS + 1% Glutamine |
| MRC5 | Lung normal | ATCC®®-CCL-171TM | Gibco DMEM + 10% FCS + 1% Glutamine |
| HEK-293 | Embryonic kidney | ATCC®®-CRL-1573TM | Gibco RPMI 1640 + 10% FCS + 1% Glutamine |
| JIMT-T1 | Breast carcinoma | DSMZ-ACC 589 | Gibco DMEM + 10% FCS + 1% Glutamine |
| U87-MG | Brain glioblastoma | ATCC®®-HTB-14TM | Gibco DMEM + 10% FCS + 1% Glutamine |
| A2780 | Ovarian carcinoma | ECACC-93112517 | Gibco RPMI 1640 + 10% FCS + 1% Glutamine |
| U2OS | Bone osteosarcoma | ATCC®®-HTB-96TM | Gibco McCoy’s 5A + 10% FCS + 1% Glutamine |
| Pic | RT * | Compounds | EOOB | EOOA |
|---|---|---|---|---|
| 1 | 8.1 | Linalol oxide | 0.4 | 0.4 |
| 2 | 8.75 | Linalol | 41.2 | - |
| 3 | 8.85 | Carvotanacetol | - | 38.4 |
| 4 | 8.93 | Octen-1-ol, acetate | - | 0.1 |
| 5 | 9.54 | Oxirane, 2-(hexyn-1-yl)-3-methoxymethylene- | - | 0.2 |
| 6 | 9.67 | Camphor | 0.9 | 1 |
| 7 | 10.47 | Cis-3-hexenyl isobutyrate | 0.1 | - |
| 8 | 10.52 | 3,7-octadiene-2,6-diol, 2,6-dimethyl- | - | 0.3 |
| 9 | 10.62 | Estragole | 30.1 | 27.5 |
| 10 | 10.82 | Octyl acetate | 0.2 | 0.3 |
| 11 | 11.43 | Bergamol | - | 0.1 |
| 12 | 11.55 | Piperitone | - | 0.3 |
| 13 | 11.99 | Bornyl acetate | 1 | 1 |
| 14 | 13.32 | Copaene | 0.1 | 0.2 |
| 15 | 13.4 | 1,5,9-trimethyl cyclododecatriene | - | 0.2 |
| 16 | 13.43 | Beta-bourbonene | - | 0.2 |
| 17 | 13.5 | Elemene | 1 | 2.1 |
| 18 | 13.69 | Methyl isoeugenol | 0.1 | 0.2 |
| 19 | 13.82 | Bergamotene trans | 0.1 | 0.1 |
| 20 | 13.93 | Cis-caryophyllene | 1.1 | 0.2 |
| 21 | 14.09 | α-bergamotene | 7.5 | 9 |
| 22 | 14.36 | β-sesquiphellandrene | 0.3 | 0.1 |
| 23 | 14.41 | Humulene | 0.6 | 0.5 |
| 24 | 14.49 | Bicyclosesquiphellandrene | - | 0.2 |
| 25 | 14.74 | Germacrene d | - | 1.7 |
| 26 | 14.86 | β-eudesmene | 0.1 | 0.3 |
| 27 | 14.93 | inknown | 0.3 | - |
| 28 | 15 | α-bulnesene | 0.4 | 0.7 |
| 29 | 15.06 | Bisabolene | 0.2 | 0.2 |
| 30 | 15.2 | δ-cadinene | 2.3 | 2.5 |
| 31 | 15.27 | β-cedrene | - | 0.5 |
| 32 | 15.28 | α-farnesene | 0.2 | - |
| 33 | 15.37 | τ-cadinol | - | 0.2 |
| 34 | 15.46 | α-caryophyllene | - | 0.2 |
| 35 | 15.51 | Epoxide farnesene | 0.4 | - |
| 36 | 15.59 | β-elemol | 0.4 | 1.3 |
| 37 | 15.65 | Epiglobulol | 0.9 | - |
| 38 | 15.71 | Nerolidol | 0.3 | 0.4 |
| 39 | 15.89 | Spiro [4.5]decane, 6-methylene- | 0.1 | - |
| 40 | 15.91 | Trans-4-methoxycinnamaldehyde | - | 0.7 |
| 41 | 15.95 | (-)-Spathulenol | 0.5 | 0.1 |
| 42 | 16.03 | Caryophyllene oxide | 0.7 | 0.3 |
| 43 | 16.31 | Cis-carveol | 0.2 | - |
| 44 | 16.37 | Humulene epoxide | 0.3 | - |
| 45 | 16.41 | Cadinol | 0.6 | 0.8 |
| 46 | 16.61 | γ-eudesmol | - | 0.1 |
| 47 | 16.72 | τ-cadinol | 3.7 | 5.1 |
| 48 | 16.79 | Cyclopentanone, 3-[3,5-decadienyl]-, | 0.2 | 0.1 |
| 49 | 16.89 | 10-epi-β-eudesmol | 0.8 | 1.2 |
| 50 | 16.97 | Viridiflorol | 0.9 | - |
| 51 | 17.25 | Jasmone | 0.4 | 0.4 |
| 52 | 17.49 | Eudesma-4,11-dien-2-ol | - | 0.1 |
| 53 | 17.57 | Isolongifolanone | 0.2 | 0.2 |
| 54 | 17.68 | Longipinocarveol | - | 0.1 |
| 55 | 18.83 | Methyl crotonate | - | 0.2 |
| Total (%) | 98.8 | 100 | ||
| Bacterial Strain | EOOB | EOOA |
| Staphylococcus aureus | − | − |
| Enterococcus faecalis | − | − |
| Streptococcus agalactiae | + | + |
| Staphylococcus epidermidis | − | − |
| Corynebacterium sp. | − | − |
| Pseudomonas aeruginosa | + | − |
| Escherichia coli | − | − |
| Klebsiella pneumoniae | − | − |
| Acinetobacter baumannii | - | − |
| Shigella sonnei | − | − |
| Salmonella enterica sv. Typhimurium | − | − |
| Enterobacter cloacae | − | + |
| Cell Line | EOOB | EOOA | Vinblastine | Doxorubicine | Combrestatin A4 | Monomethyl Auristatin E |
|---|---|---|---|---|---|---|
| K562 | 3.67 ± 0.65 | 1.01 ± 0.01 | 20.00 ± 0.12 | - | 5.00 ± 0.30 | 3.12 ± 0.20 |
| A549 | 5.37 ± 0.16 | 0.87 ± 0.06 | - | 56.6 ± 0.84 | 20.00 ± 0.10 | 0.46 ± 0.05 |
| HCT116 | 1.77 ± 0.07 | 1.01 ± 0.01 | 35.00 ± 0.84 | - | 2.00 ± 0.10 | 2.07 ± 0.02 |
| PC3 | 4.37 ± 1.02 | 0.95 ± 0.02 | - | 2.09 ± 0.03 | 0.36 ± 0.03 | |
| U87-MG | 4.29 ± 0.65 | 1.31 ± 0.62 | 2.00 ± 0.04 | 99.61 ± 2.34 | 9.00 ± 0.50 | 0.21 ± 0.03 |
| MIA-Paca2 | 5.31 ± 0.17 | 0.99 ± 0.03 | - | - | - | 4.36 ± 0.20 |
| HEK293 | 1.40 ± 0.11 | 0.25 ± 0.03 | - | - | - | - |
| NCI-N87 | 3.43 ± 0.12 | 4.28 ± 0.83 | - | - | - | 1.65 ± 0.07 |
| RT4 | 6.89 ± 1.52 | 0.86 ± 0.02 | - | 36.29 ± 1.20 | - | 0.5 ± 0.01 |
| U2OS | 4.29 ± 0.97 | 0.68 ± 0.05 | - | - | - | - |
| A2780 | 1.01 ± 0.11 | 0.69 ± 0.02 | - | - | - | 0.45 ± 0.01 |
| MRC-5 | 5.48 ± 0.182 | 1.34 ± 0.16 | - | 39.88 ± 1.22 | - | - |
| JIMT-T1 | 5.46 ± 0.051 | 0.92 ± 0.03 | - | - | - | - |
| Essential Oils | Investigator | Place | Major Constituents |
|---|---|---|---|
| EOOB | Farouk et al. (2016) [31] | Al Madinah Al Munwara (Saudi Arabia) | Eugenol and linalool |
| Ahmed et al. (2019) [32] | Three locations: Assiut, Minia and BeniSuef (Egypt) | Linalool, estragole, methyl cinnamate | |
| Guedri et al. (2022) [33] | Gabés (Tunisia) | linalool and 1.8 cineole | |
| EOOA | Mondello et al. (2002) [34] | Chittagong (Bangladesh) | Citral type: Geranial, Neral Camphor type: Linalool, Geranial, Geranyl acetate |
| De Almeida et al. (2007) [35] | São Cristóvão (Brazil) | Linalool, Eugenol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed Abdoul-Latif, F.; Elmi, A.; Merito, A.; Nour, M.; Risler, A.; Ainane, A.; Bignon, J.; Ainane, T. Essential Oils of Ocimum basilicum L. and Ocimum americanum L. from Djibouti: Chemical Composition, Antimicrobial and Cytotoxicity Evaluations. Processes 2022, 10, 1785. https://doi.org/10.3390/pr10091785
Mohamed Abdoul-Latif F, Elmi A, Merito A, Nour M, Risler A, Ainane A, Bignon J, Ainane T. Essential Oils of Ocimum basilicum L. and Ocimum americanum L. from Djibouti: Chemical Composition, Antimicrobial and Cytotoxicity Evaluations. Processes. 2022; 10(9):1785. https://doi.org/10.3390/pr10091785
Chicago/Turabian StyleMohamed Abdoul-Latif, Fatouma, Abdirahman Elmi, Ali Merito, Moustapha Nour, Arnaud Risler, Ayoub Ainane, Jérôme Bignon, and Tarik Ainane. 2022. "Essential Oils of Ocimum basilicum L. and Ocimum americanum L. from Djibouti: Chemical Composition, Antimicrobial and Cytotoxicity Evaluations" Processes 10, no. 9: 1785. https://doi.org/10.3390/pr10091785
APA StyleMohamed Abdoul-Latif, F., Elmi, A., Merito, A., Nour, M., Risler, A., Ainane, A., Bignon, J., & Ainane, T. (2022). Essential Oils of Ocimum basilicum L. and Ocimum americanum L. from Djibouti: Chemical Composition, Antimicrobial and Cytotoxicity Evaluations. Processes, 10(9), 1785. https://doi.org/10.3390/pr10091785







