Evaluation of the Zinc Sulfate Catalytic Effect in Empty Fruit Bunches Pyrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation and Characterization
2.2. TGA-FTIR Analysis
2.3. Kinetic Modeling
2.3.1. N-Reaction Order Model
2.3.2. Pre-Exponential Factor Model
2.3.3. Distributed Activation Energy Model
2.4. Standard Deviation
2.5. Experimental Design
2.6. FTIR Analysis of Evolved Gases
3. Results
3.1. Samples Characterization
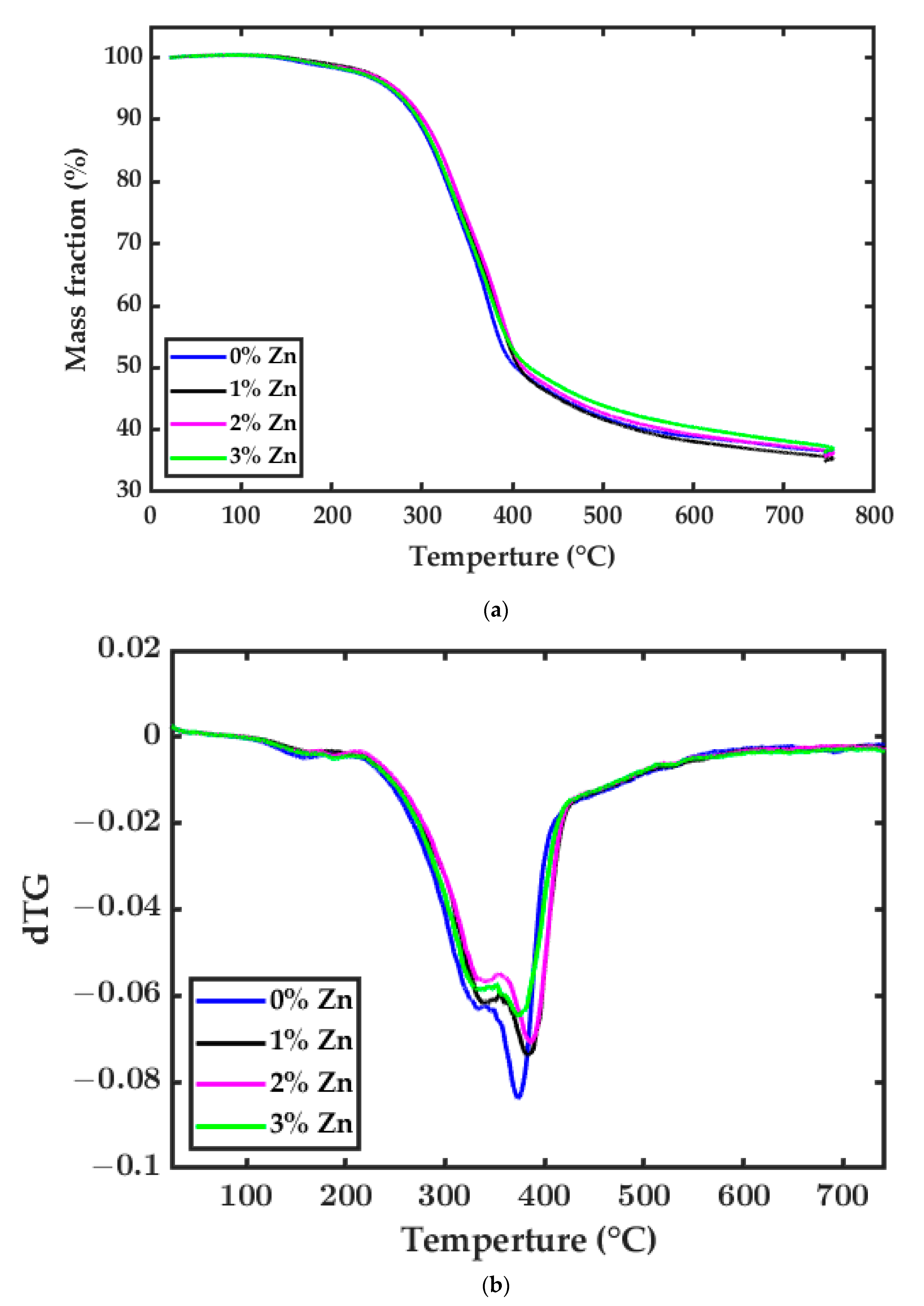
3.2. Thermogravimetric Analysis
3.3. Kinetic Parameters
3.3.1. N-Reaction Order Model Assessment
3.3.2. Pre-Exponential Factor Model Assessment
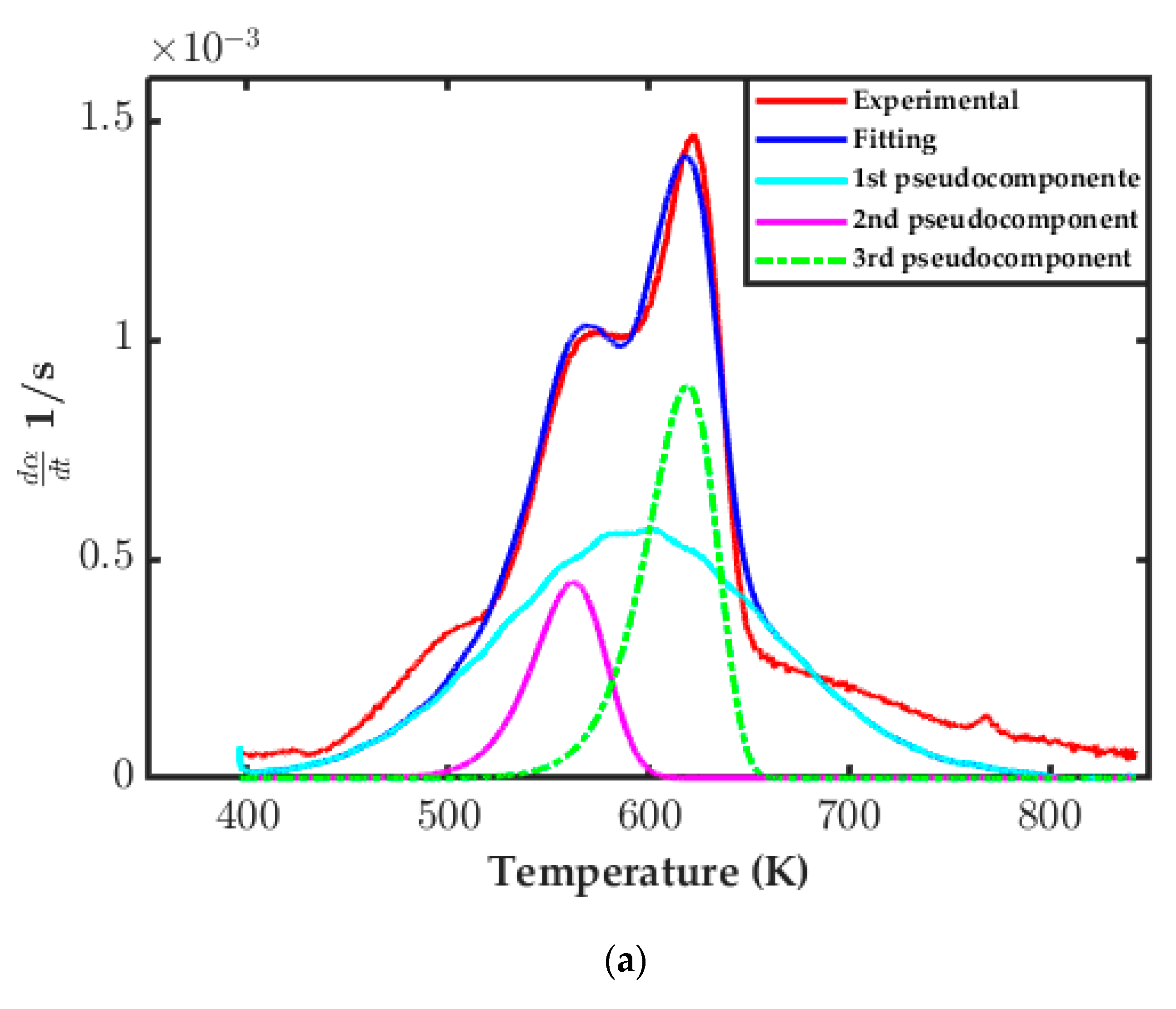
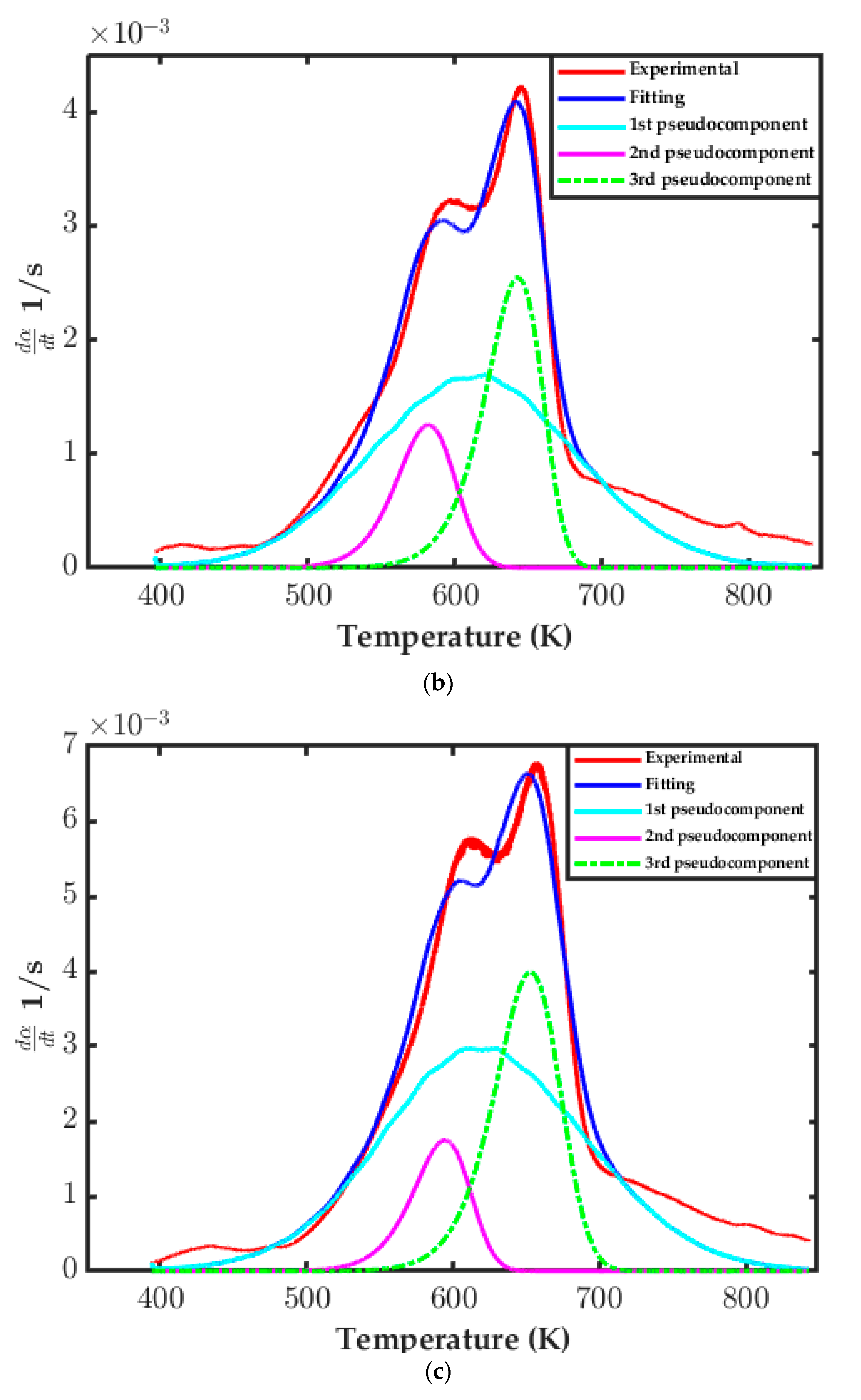
3.3.3. Distributed Activation Energy Model (DAEM) Assessment
3.4. FTIR Analysis of Evolved Gases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| CPO | Crude palm oil |
| POM | Palm oil mill |
| EFB | Empty fruit bunches |
| FFB | Fresh fruit bunches |
| TGA | Thermogravimetric analysis |
| FTIR | Fourier transform infrared spectrometry |
| DAEM | Distributed activation energy model |
| ZSM-5 | Zeolita socony mobil-5 |
| AHs | Aromatic hydrocarbons |
| HV | Heating value |
| dTG | The first derivative of the TGA curve respect to time |
| Ea | Activation energy |
| A | Pre-exponential factor |
| N | Reaction order |
| R2 | Determination coefficient |
| IR | Infrared spectra |
References
- Pareek, A.; Dom, R.; Gupta, J.; Chandran, J.; Adepu, V.; Borse, P.H. Insights into renewable hydrogen energy: Recent advances and prospects. Mater. Sci. Energy Technol. 2020, 3, 319–327. [Google Scholar] [CrossRef]
- Loh, S.K. The potential of the Malaysian oil palm biomass as a renewable energy source. Energy Convers. Manag. 2017, 141, 285–298. [Google Scholar] [CrossRef]
- Hoe, B.C.; Chan, E.S.; Ramanan, R.N.; Ooi, C.W. Direct recovery of palm carotene by liquid-liquid extraction. J. Food Eng. 2022, 313, 110755. [Google Scholar] [CrossRef]
- Ramirez-Contreras, N.E.; Munar-Florez, D.A.; Garcia-Nuñez, J.A.; Mosquera-Montoya, M.; Faaij, A.P.C. The GHG emissions and economic performance of the Colombian palm oil sector; current status and long-term perspectives. J. Clean. Prod. 2020, 258, 120757. [Google Scholar] [CrossRef]
- González-Delgado, A.D.; Barajas-Solano, A.F.; Leon-Pulido, J. Evaluating the Sustainability and Inherent Safety of a Crude Palm Oil Production Process in North-Colombia. Appl. Sci. 2021, 11, 1046. [Google Scholar] [CrossRef]
- Fedepalma. The Oil Palm Agribusiness in Colombia; Icolgraf: Bogotá, Colombia, 2019. [Google Scholar]
- Khatun, R.; Reza, M.I.H.; Moniruzzaman, M.; Yaakob, Z. Sustainable oil palm industry: The possibilities. Renew. Sustain. Energy Rev. 2017, 76, 608–619. [Google Scholar] [CrossRef]
- Furumo, P.R.; Aide, T.M. Characterizing commercial oil palm expansion in Latin America: Land use change and trade. Environ. Res. Lett. 2017, 12, 24008. [Google Scholar] [CrossRef]
- Castiblanco, C.; Etter, A.; Aide, T.M. Oil palm plantations in Colombia: A model of future expansion. Environ. Sci. Policy 2013, 27, 172–183. [Google Scholar] [CrossRef]
- Castanheira, É.G.; Acevedo, H.; Freire, F. Greenhouse gas intensity of palm oil produced in Colombia addressing alternative land use change and fertilization scenarios. Appl. Energy 2014, 114, 958–967. [Google Scholar] [CrossRef]
- Batlle, E.A.O.; Santiago, Y.C.; Venturini, O.J.; Palacio, J.C.E.; Lora, E.E.S.; Maya, D.M.Y.; Arrieta, A.R.A. Thermodynamic and environmental assessment of different scenarios for the insertion of pyrolysis technology in palm oil biorefineries. J. Clean. Prod. 2020, 250, 119544. [Google Scholar] [CrossRef]
- Yan, M.; Hantoko, D.; Susanto, H.; Ardy, A.; Waluyo, J.; Weng, Z.; Lin, J. Hydrothermal treatment of empty fruit bunch and its pyrolysis characteristics. Biomass Convers. Biorefinery 2019, 9, 709–717. [Google Scholar] [CrossRef]
- Garcia-Nunez, J.A.; Ramirez-Contreras, N.E.; Rodriguez, D.T.; Silva-Lora, E.; Frear, C.S.; Stockle, C.; Garcia-Perez, M. Evolution of palm oil mills into bio-refineries: Literature review on current and potential uses of residual biomass and effluents. Resour. Conserv. Recycl. 2016, 110, 99–114. [Google Scholar] [CrossRef]
- Marques, T.E.; Santiago, Y.C.; Renó, M.L.; Maya, D.M.Y.; Sphaier, L.A.; Shi, Y.; Ratner, A. Environmental and Energetic Evaluation of Refuse-Derived Fuel Gasification for Electricity Generation. Processes 2021, 9, 2255. [Google Scholar] [CrossRef]
- Santiago, Y.C.; González, A.M.; Venturini, O.J.; Sphaier, L.A.; Batlle, E.A.O. Energetic and environmental assessment of oil sludge use in a gasifier/gas microturbine system. Energy 2022, 244, 123103. [Google Scholar] [CrossRef]
- Branca, C.; Di Blasi, C. Thermal Devolatilization Kinetics of Dry Distiller’s Grains with Solubles (DDGS). Processes 2021, 9, 1907. [Google Scholar] [CrossRef]
- Kalargaris, I.; Tian, G.; Gu, S. Combustion, performance and emission analysis of a DI diesel engine using plastic pyrolysis oil. Fuel Process. Technol. 2017, 157, 108–115. [Google Scholar] [CrossRef]
- Al Arni, S. Comparison of slow and fast pyrolysis for converting biomass into fuel. Renew. Energy 2018, 124, 197–201. [Google Scholar] [CrossRef]
- Altantzis, A.-I.; Kallistridis, N.-C.; Stavropoulos, G.; Zabaniotou, A. Apparent Pyrolysis Kinetics and Index-Based Assessment of Pretreated Peach Seeds. Processes 2021, 9, 905. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis, and Torrefaction: Practical Design and Theory, 2nd ed.; Basu, P., Ed.; Elsevier Inc.: San Diego, CA, USA, 2013; ISBN 978-0-12-396488-5. [Google Scholar]
- Fateh, T.; Richard, F.; Rogaume, T.; Joseph, P. Experimental and modelling studies on the kinetics and mechanisms of thermal degradation of polymethyl methacrylate in nitrogen and air. J. Anal. Appl. Pyrolysis 2016, 120, 423–433. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, D.; Gu, J.; Bao, B.; Zhang, Q. Determination of pyrolysis characteristics and kinetics of palm kernel shell using TGA-FTIR and model-free integral methods. Energy Convers. Manag. 2015, 89, 251–259. [Google Scholar] [CrossRef]
- Ordonez-Loza, J.; Chejne, F.; Jameel, A.G.A.; Telalovic, S.; Arrieta, A.A.; Sarathy, S.M. An investigation into the pyrolysis and oxidation of bio-oil from sugarcane bagasse: Kinetics and evolved gases using TGA-FTIR. J. Environ. Chem. Eng. 2021, 9, 106144. [Google Scholar] [CrossRef]
- Wang, Y.; Akbarzadeh, A.; Chong, L.; Du, J.; Tahir, N.; Awasthi, M.K. Catalytic pyrolysis of lignocellulosic biomass for bio-oil production: A review. Chemosphere 2022, 297, 134181. [Google Scholar] [CrossRef] [PubMed]
- Albis, A.; Ortiz, E.; Piñeres, I.; Osorio, J.; Monsalvo, J. Pirólisis de hemicelulosa catalizada por sulfato de zinc y sulfato férrico. Rev. ION 2018, 31, 37–49. [Google Scholar] [CrossRef]
- Chen, W.-H.; Cheng, C.-L.; Lee, K.-T.; Lam, S.S.; Ong, H.C.; Ok, Y.S.; Saeidi, S.; Sharma, A.K.; Hsieh, T.-H. Catalytic level identification of ZSM-5 on biomass pyrolysis and aromatic hydrocarbon formation. Chemosphere 2021, 271, 129510. [Google Scholar] [CrossRef]
- Rachel-Tang, D.Y.; Islam, A.; Taufiq-Yap, Y.H. Bio-oil production via catalytic solvolysis of biomass. RSC Adv. 2017, 7, 7820–7830. [Google Scholar] [CrossRef]
- Li, C.; Ji, G.; Qu, Y.; Irfan, M.; Zhu, K.; Wang, X.; Li, A. Influencing mechanism of zinc mineral contamination on pyrolysis kinetic and product characteristics of corn biomass. J. Environ. Manage. 2021, 281, 111837. [Google Scholar] [CrossRef]
- Mayer, Z.A.; Apfelbacher, A.; Hornung, A. Effect of sample preparation on the thermal degradation of metal-added biomass. J. Anal. Appl. Pyrolysis 2012, 94, 170–176. [Google Scholar] [CrossRef]
- Ona, T.; Sonoda, T.; Shibata, M.; Fukazawa, K. Small-scale method to determine the content of wood components from multiple eucalypt samples. Tappi J. 1995, 78, 121–126. [Google Scholar]
- ASTM D3172–07a; Standard Practice for Proximate Analysis of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2007.
- Barbosa, K.P.; Chamorro, M.V.; Arrieta, A.A.; Ariza, I.P.; Ortíz, E.V. Evaluation of the effect of silicon on the carbonization process of Colombian semi-anthracites. J. Therm. Anal. Calorim. 2022. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Hu, G.; Li, J.; Zhang, X.; Li, Y. Investigation of waste biomass co-pyrolysis with petroleum sludge using a response surface methodology. J. Environ. Manage. 2017, 192, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zhang, H.; Chang, F.; Zhang, F.; Wang, K.; Qin, Y.; Huang, T. Combustion behavior and thermochemical treatment scheme analysis of oil sludges and oil sludge semicokes. Energy 2019, 167, 575–587. [Google Scholar] [CrossRef]
- Chang, G.; Huang, Y.; Xie, J.; Yang, H.; Liu, H.; Yin, X.; Wu, C. The lignin pyrolysis composition and pyrolysis products of palm kernel shell, wheat straw, and pine sawdust. Energy Convers. Manag. 2016, 124, 587–597. [Google Scholar] [CrossRef]
- Blaine, R.L.; Kissinger, H.E. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Z.; Wei, M.; Lu, T.; Nong, D.; Zhao, J.; Gao, X.; Teng, L. Catalytic effect of nanosized ZnO and TiO2 on thermal degradation of poly(lactic acid) and isoconversional kinetic analysis. Thermochim. Acta 2019, 672, 14–24. [Google Scholar] [CrossRef]
- Chen, N.; Ren, J.; Ye, Z.; Xu, Q.; Liu, J.; Sun, S. Kinetics of coffee industrial residue pyrolysis using distributed activation energy model and components separation of bio-oil by sequencing temperature-raising pyrolysis. Bioresour. Technol. 2016, 221, 534–540. [Google Scholar] [CrossRef]
- Martín-Lara, M.A.; Blázquez, G.; Zamora, M.C.; Calero, M. Kinetic modelling of torrefaction of olive tree pruning. Appl. Therm. Eng. 2017, 113, 1410–1418. [Google Scholar] [CrossRef]
- Albis, A.; Ortiz, E.; Suárez, A.; Piñeres, I. TG/MS study of the thermal devolatization of Copoazú peels (Theobroma grandiflorum). J. Therm. Anal. Calorim. 2014, 115, 275–283. [Google Scholar] [CrossRef]
- Yu, C.; Ren, S.; Wang, G.; Xu, J.; Teng, H.; Li, T.; Huang, C.; Wang, C. Kinetic analysis and modeling of maize straw hydrochar combustion using a multi-Gaussian-distributed activation energy model. Int. J. Miner. Metall. Mater. 2022, 29, 464–472. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Characterization of non-edible lignocellulosic biomass in terms of their candidacy towards alternative renewable fuels. Biomass Convers. Biorefinery 2018, 8, 799–812. [Google Scholar] [CrossRef]
- Yu, J.; Paterson, N.; Blamey, J.; Millan, M. Cellulose, xylan and lignin interactions during pyrolysis of lignocellulosic biomass. Fuel 2017, 191, 140–149. [Google Scholar] [CrossRef]
- Meng, A.; Zhou, H.; Qin, L.; Zhang, Y.; Li, Q. Quantitative and kinetic TG-FTIR investigation on three kinds of biomass pyrolysis. J. Anal. Appl. Pyrolysis 2013, 104, 28–37. [Google Scholar] [CrossRef]
- Chen, W.-H.; Eng, C.F.; Lin, Y.-Y.; Bach, Q.-V. Independent parallel pyrolysis kinetics of cellulose, hemicelluloses and lignin at various heating rates analyzed by evolutionary computation. Energy Convers. Manag. 2020, 221, 113165. [Google Scholar] [CrossRef]
- Salem, I.B.; Saleh, M.B.; Iqbal, J.; El Gamal, M.; Hameed, S. Date palm waste pyrolysis into biochar for carbon dioxide adsorption. Energy Rep. 2021, 7, 152–159. [Google Scholar] [CrossRef]
- Yeo, J.Y.; Chin, B.L.F.; Tan, J.K.; Loh, Y.S. Comparative studies on the pyrolysis of cellulose, hemicellulose, and lignin based on combined kinetics. J. Energy Inst. 2019, 92, 27–37. [Google Scholar] [CrossRef]
- Xing, D.; Li, J. Effects of Heat Treatment on Thermal Decomposition and Combustion Performance of Larix spp. Wood. BioResources 2014, 9, 4274–4287. [Google Scholar] [CrossRef]
- Yu, S.; Yang, X.; Zhou, H.; Tan, Z.; Cong, K.; Zhang, Y.; Li, Q. Thermal and Kinetic Behaviors during Co-Pyrolysis of Microcrystalline Cellulose and Styrene–Butadiene–Styrene Triblock Copolymer. Processes 2021, 9, 1335. [Google Scholar] [CrossRef]
- Arrieta, A.A.; Muñoz, E.O.; García, V.B.; Cantillo, A.G.; Chamorro, M.V.; Ochoa, G.V. Catalytic effect of CaCl2 and ZnSO4 on the pyrolysis of cedar sawdust. Chem. Eng. Trans. 2018, 65, 673–678. [Google Scholar] [CrossRef]
- Sonobe, T.; Worasuwannarak, N. Kinetic analyses of biomass pyrolysis using the distributed activation energy model. Fuel 2008, 87, 414–421. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C.; Du, L.; Duan, Y. TG–FTIR and Py–GC/MS analysis on pyrolysis and combustion of pine sawdust. J. Anal. Appl. Pyrolysis 2013, 100, 26–32. [Google Scholar] [CrossRef]
- Sheng, J.; Ji, D.; Yu, F.; Cui, L.; Zeng, Q.; Ai, N.; Ji, J. Influence of Chemical Treatment on Rice Straw Pyrolysis by TG-FTIR. IERI Procedia 2014, 8, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Shen, D.K.; Gu, S.; Luo, K.H.; Wang, S.R.; Fang, M.X. The pyrolytic degradation of wood-derived lignin from pulping process. Bioresour. Technol. 2010, 101, 6136–6146. [Google Scholar] [CrossRef] [PubMed]




| Structural Analysis, Dry Basis (wt.%) | |
|---|---|
| Extractives | 20.0 |
| Lignin | 17.1 |
| Cellulose | 50.2 |
| Hemicellulose | 12.8 |
| Ultimate analysis, dry basis (wt.%) | |
| Carbon | 42.9 |
| Sulfur | 0.2 |
| Nitrogen | 0.8 |
| Hydrogen | 6.0 |
| Oxygen 1 | 50.1 |
| Proximate analysis, dry basis (wt.%) | |
| Moisture content | 8.0 |
| Ash | 17.6 |
| Volatiles | 65.6 |
| Fixed carbon | 16.8 |
| Higher heating value (kJ/kg) | 17953.5 |
| Heating Rates | EFB | EFB + 1% Zn | EFB + 2% Zn | EFB + 3% Zn | |
|---|---|---|---|---|---|
| 10 K/min | Max. temperature (°C) | 750.3 | 750.4 | 750.3 | 750.3 |
| % mass loss | 67.9 | 69.0 | 68.9 | 63.6 | |
| % Char/carbonized | 32.1 | 31.0 | 31.1 | 36.4 | |
| 30 K/min | Max temperature (°C) | 799.8 | 749.5 | 749.5 | 749.6 |
| % mass loss | 68.2 | 65.2 | 68.5 | 67.2 | |
| % Char/carbonized | 31.8 | 34.8 | 31.5 | 32.8 | |
| 50 K/min | Max. temperature (°C) | 746.6 | 746.6 | 746.7 | 746.7 |
| % mass loss | 66.3 | 66.7 | 68.8 | 66.9 | |
| % Char/carbonized | 33.7 | 33.3 | 31.2 | 33.1 |
| Heating Rates | EFB Temperature (°C) | EFB + 1% Zn Temperature (°C) | EFB + 2% Zn Temperature (°C) | EFB + 3% Zn Temperature (°C) |
|---|---|---|---|---|
| 10 K/min | 311.7 | 309.3 | 313.8 | 313.6 |
| 30 K/min | 328.4 | 350.7 | 339.6 | 342.6 |
| 50 K/min | 350.3 | 358.7 | 347.8 | 353.0 |
| % Catalyst | Heating Rate (K/min) | Ea (J/mol) | A | N | R2 |
|---|---|---|---|---|---|
| 0 | 10 | 2.37 × 107 | 298.12 | 0.10 | 0.88 |
| 0 | 30 | 2.38 × 107 | 734.80 | 0.08 | 0.86 |
| 0 | 50 | 2.65 × 107 | 1665.90 | 0.07 | 0.85 |
| 1 | 10 | 2.51 × 107 | 363.83 | 0.10 | 0.87 |
| 1 | 30 | 2.80 × 107 | 1347.35 | 0.09 | 0.84 |
| 1 | 50 | 2.85 × 107 | 2133.51 | 0.11 | 0.84 |
| 2 | 10 | 2.55 × 107 | 381.53 | 0.10 | 0.88 |
| 2 | 30 | 2.69 × 107 | 1136.15 | 0.09 | 0.87 |
| 2 | 50 | 2.81 × 107 | 2008.06 | 0.08 | 0.85 |
| 3 | 10 | 1.79 × 107 | 117.95 | 0.05 | 0.78 |
| 3 | 30 | 2.64 × 107 | 1082.68 | 0.09 | 0.85 |
| 3 | 50 | 2.71 × 107 | 1760.98 | 0.08 | 0.85 |
| Conversion | EFB | EFB + 1% Zn | EFB + 2% Zn | EFB + 3% Zn | ||||
|---|---|---|---|---|---|---|---|---|
| Ea (J/mol) | R2 | Ea (J/mol) | R2 | Ea (J/mol) | R2 | Ea (J/mol) | R2 | |
| 0.1 | 7.74 × 104 | 0.98 | 7.57 × 104 | 1.0 | 8.52 × 105 | 1.00 | 8.49 × 104 | 0.99 |
| 0.2 | 1.03 × 105 | 0.98 | 1.05 × 105 | 0.99 | 1.10 × 105 | 0.99 | 1.12 × 105 | 0.99 |
| 0.3 | 1.17 × 105 | 0.97 | 1.17 × 105 | 0.99 | 1.24 × 105 | 0.99 | 1.23 × 105 | 0.99 |
| 0.4 | 1.30 × 105 | 0.97 | 1.29 × 105 | 0.99 | 1.37 × 105 | 0.99 | 1.37 × 105 | 0.99 |
| 0.5 | 1.41 × 105 | 0.98 | 1.40 × 105 | 0.99 | 1.48 × 105 | 0.99 | 1.50 × 105 | 0.99 |
| 0.6 | 1.45 × 105 | 0.98 | 1.46 × 105 | 0.99 | 1.51 × 105 | 0.99 | 1.56 × 105 | 0.99 |
| 0.7 | 1.48 × 105 | 0.99 | 1.46 × 105 | 0.99 | 1.49 × 105 | 0.99 | 1.59 × 105 | 0.99 |
| 0.8 | 1.82 × 105 | 0.99 | 1.61 × 105 | 0.99 | 1.66 × 105 | 0.99 | 1.89 × 105 | 0.99 |
| 0.9 | 2.30 × 105 | 0.99 | 2.08 × 105 | 0.99 | 2.10 × 105 | 0.98 | 2.54 × 105 | 0.99 |
| 1.0 | 9.36 × 104 | 0.40 | 2.40 × 105 | 0.85 | 2.02 × 105 | 0.86 | 2.12 × 105 | 0.86 |
| Samples | Average Ea (J/mol) | Standard Deviation |
|---|---|---|
| EFB | 1.44 × 103 | 21,885 |
| EFB + 1% Zn | 1.40 × 103 | 15,250 |
| EFB + 2% Zn | 1.46 × 103 | 14,162 |
| EFB + 3% Zn | 1.52 × 103 | 22,376 |
| Parameters | Heating Rate (K/min) | ||
|---|---|---|---|
| 10 | 30 | 50 | |
| C1 | 0.48 | 0.47 | 0.49 |
| A1 (s−1) | 2.01 × 1013 | 2.01 × 1013 | 2.01 × 1013 |
| Ea1 (Kj/mol) | 1.79 × 105 | 1.79 × 105 | 1.81 × 105 |
| s1 | 2.98 × 102 | 1.92 × 103 | 1.75 × 103 |
| C2 | 0.28 | 0.26 | 0.23 |
| A2 (s−1) | 2.09 × 1013 | 2.09 × 1013 | 2.09 × 1013 |
| Ea2 (Kj/mol) | 1.65 × 105 | 1.65 × 105 | 1.66 × 105 |
| s2 | 5.01 × 103 | 4.80 × 103 | 4.28 × 103 |
| C3 | 0.94 | 1.08 | 1.11 |
| A3 (s−1) | 2.09 × 1013 | 2.09 × 1013 | 2.09 × 1013 |
| Ea3 (Kj/mol) | 1.77 × 105 | 1.77 × 105 | 1.77 × 105 |
| s3 | 3.97 × 104 | 3.97 × 104 | 3.97 × 104 |
| SE | 3.36 × 10−15 | 1.77 × 10−15 | 8.38 × 10−16 |
| Parameters | Heating Rate (K/min) | ||
|---|---|---|---|
| 10 | 30 | 50 | |
| C1 | 0.47 | 0.46 | 0.43 |
| A1 (s−1) | 2.01 × 1013 | 2.01 × 1013 | 2.01 × 1013 |
| Ea1 (Kj/mol) | 1.82 × 105 | 1.82 × 105 | 1.83 × 105 |
| s1 | 7.12 × 102 | 3.20 × 103 | 2.37 × 103 |
| C2 | 0.24 | 0.21 | 0.14 |
| A2 (s−1) | 2.09 × 1013 | 2.09 × 1013 | 2.09 × 1013 |
| Ea2 (Kj/mol) | 1.65 × 105 | 1.65 × 105 | 1.66 × 105 |
| s2 | 5.07 × 103 | 5.41 × 103 | 4.96 × 103 |
| C3 | 1.09 | 1.14 | 1.34 |
| A3 (s−1) | 2.09 × 1013 | 2.09 × 1013 | 2.09 × 1013 |
| Ea3 (Kj/mol) | 1.77 × 105 | 1.77 × 105 | 1.77 × 105 |
| s3 | 3.97 × 104 | 3.97 × 104 | 3.97 × 104 |
| SE | 1.12 × 10−16 | 8.92 × 10−16 | 5.61 × 10−15 |
| Parameters | Heating Rate (K/min) | ||
|---|---|---|---|
| 10 | 30 | 50 | |
| C1 | 0.50 | 0.49 | 0.42 |
| A1 (s−1) | 2.01 × 1013 | 2.01 × 1013 | 2.01 × 1013 |
| Ea1 (Kj/mol) | 1.82 × 105 | 1.83 × 105 | 1.84 × 105 |
| s1 | 6.69 × 102 | 7.75 × 103 | 1.54 × 104 |
| C2 | 0.19 | 0.24 | 0.17 |
| A2 (s−1) | 2.09 × 1013 | 2.09 × 1013 | 2.09 × 1013 |
| Ea2 (Kj/mol) | 1.65 × 105 | 1.65 × 105 | 1.66 × 105 |
| s2 | 5.17 × 103 | 5.39 × 104 | 1.12 × 104 |
| C3 | 1.06 | 1.10 | 1.32 |
| A3 (s−1) | 2.09 × 1013 | 2.09 × 1013 | 2.09 × 1013 |
| Ea3 (Kj/mol) | 1.77 × 105 | 1.77 × 105 | 1.77 × 105 |
| s3 | 3.97 × 104 | 3.97 × 104 | 3.96 × 104 |
| SE | 4.94 × 10−16 | 7.08 × 10−16 | 9.11 × 10−15 |
| Parameters | Heating Rate (K/min) | ||
|---|---|---|---|
| 10 | 30 | 50 | |
| C1 | 0.37 | 0.36 | 0.35 |
| A1 (s−1) | 2.01 × 1013 | 2.01 × 1013 | 2.01 × 1013 |
| Ea1 (Kj/mol) | 1.81 × 105 | 1.81 × 105 | 1.81 × 105 |
| s1 | 7.69 × 103 | 70,58 | 1.63 × 102 |
| C2 | 0.27 | 0.28 | 0.20 |
| A2 (s−1) | 2.09 × 1013 | 2.09 × 1013 | 2.09 × 1013 |
| Ea2 (Kj/mol) | 1.65 × 105 | 1.65 × 105 | 1.66 × 105 |
| s2 | 5.09 × 104 | 6.86 × 103 | 8.72 × 103 |
| C3 | 1.10 | 1.17 | 1.36 |
| A3 (s−1) | 2.09 × 1013 | 2.09 × 1013 | 2.09 × 1013 |
| Ea3 (Kj/mol) | 1.77 × 105 | 1.77 × 105 | 1.77 × 105 |
| s3 | 3.97 × 104 | 3.97 × 104 | 3.97 × 104 |
| SE | 2.26 × 10−16 | 5.46 × 10−16 | 2.54 × 10−15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez Useche, M.A.; Castillo Santiago, Y.; Restrepo, J.B.; Albis Arrieta, A.R.; Agámez Salgado, K.P. Evaluation of the Zinc Sulfate Catalytic Effect in Empty Fruit Bunches Pyrolysis. Processes 2022, 10, 1748. https://doi.org/10.3390/pr10091748
Suárez Useche MA, Castillo Santiago Y, Restrepo JB, Albis Arrieta AR, Agámez Salgado KP. Evaluation of the Zinc Sulfate Catalytic Effect in Empty Fruit Bunches Pyrolysis. Processes. 2022; 10(9):1748. https://doi.org/10.3390/pr10091748
Chicago/Turabian StyleSuárez Useche, María Alejandra, York Castillo Santiago, Juan B. Restrepo, Alberto Ricardo Albis Arrieta, and Karen Patricia Agámez Salgado. 2022. "Evaluation of the Zinc Sulfate Catalytic Effect in Empty Fruit Bunches Pyrolysis" Processes 10, no. 9: 1748. https://doi.org/10.3390/pr10091748
APA StyleSuárez Useche, M. A., Castillo Santiago, Y., Restrepo, J. B., Albis Arrieta, A. R., & Agámez Salgado, K. P. (2022). Evaluation of the Zinc Sulfate Catalytic Effect in Empty Fruit Bunches Pyrolysis. Processes, 10(9), 1748. https://doi.org/10.3390/pr10091748







