Food Quality, Drug Safety, and Increasing Public Health Measures in Supply Chain Management
Abstract
:1. Introduction
1.1. Purpose of This Review
1.2. State of the Review
1.2.1. Food Quality
1.2.2. Drug Safety
1.3. Review Objectives
- Identify the factors affecting food quality and possible solutions to improve results.
- Analyze the factors that affect drug safety and identify ways to mitigate them through proper supply chain management.
- Establish integrated supply chains for food and drugs by implementing modern technologies, followed by one another, to ensure a multi-layered, cross-verification cascade and resource management at the different phases to ensure quality, safety, and sustainability for the benefits of public health.
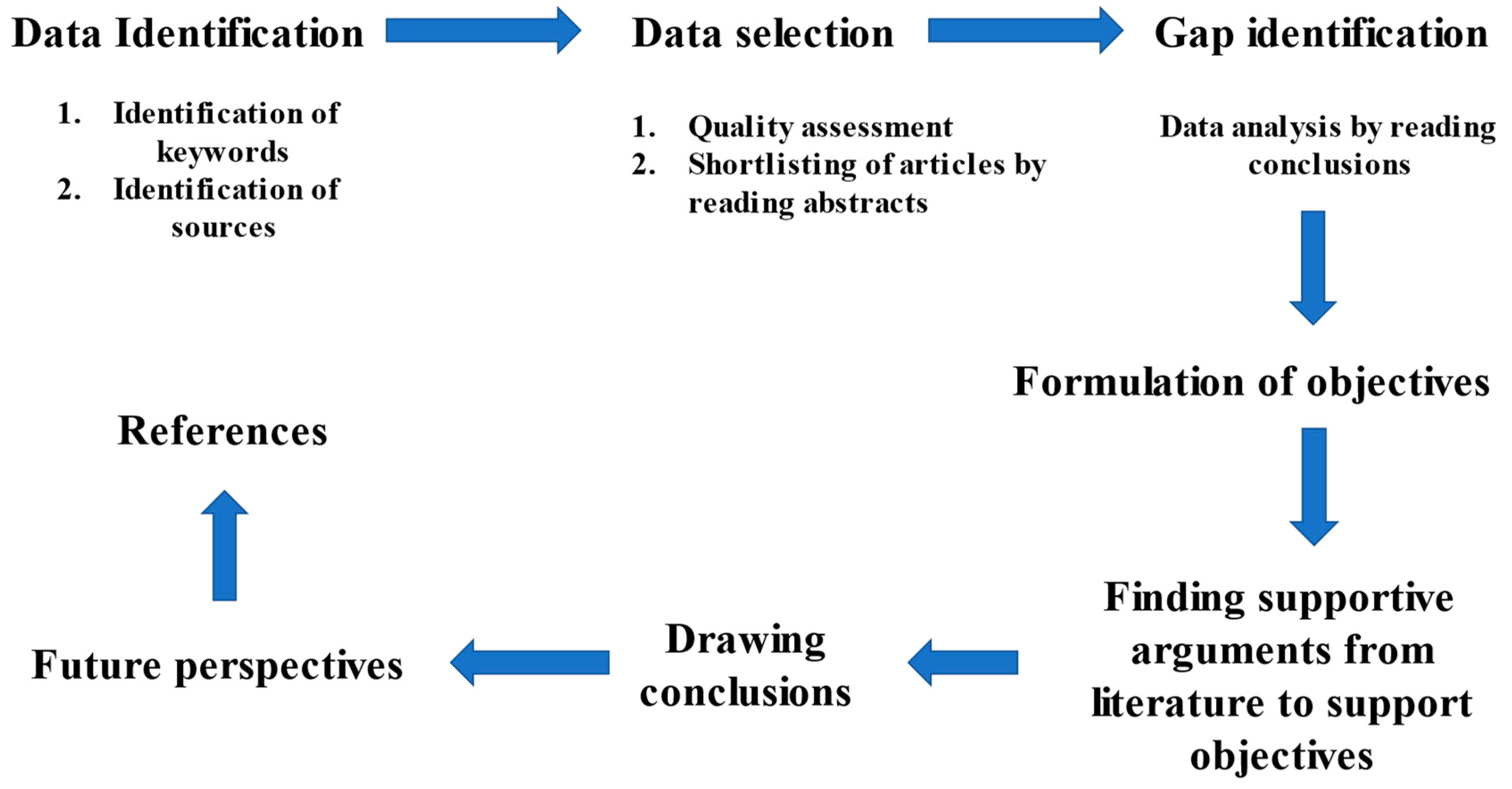
2. Review Methodology
2.1. Data Identification
2.2. Data Selection
2.3. Gap Identification
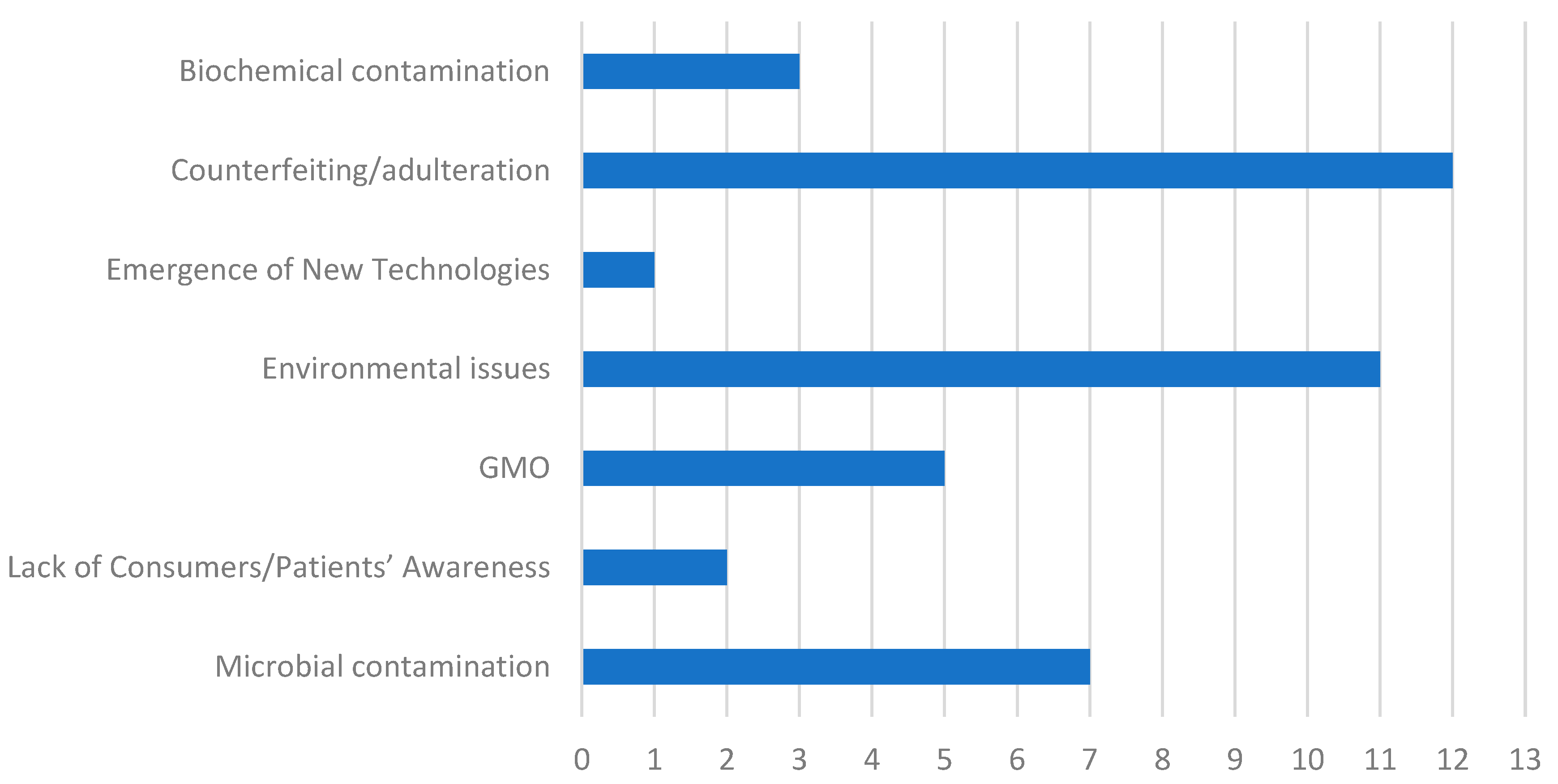
3. Issues Affecting Food Quality and Drug Safety
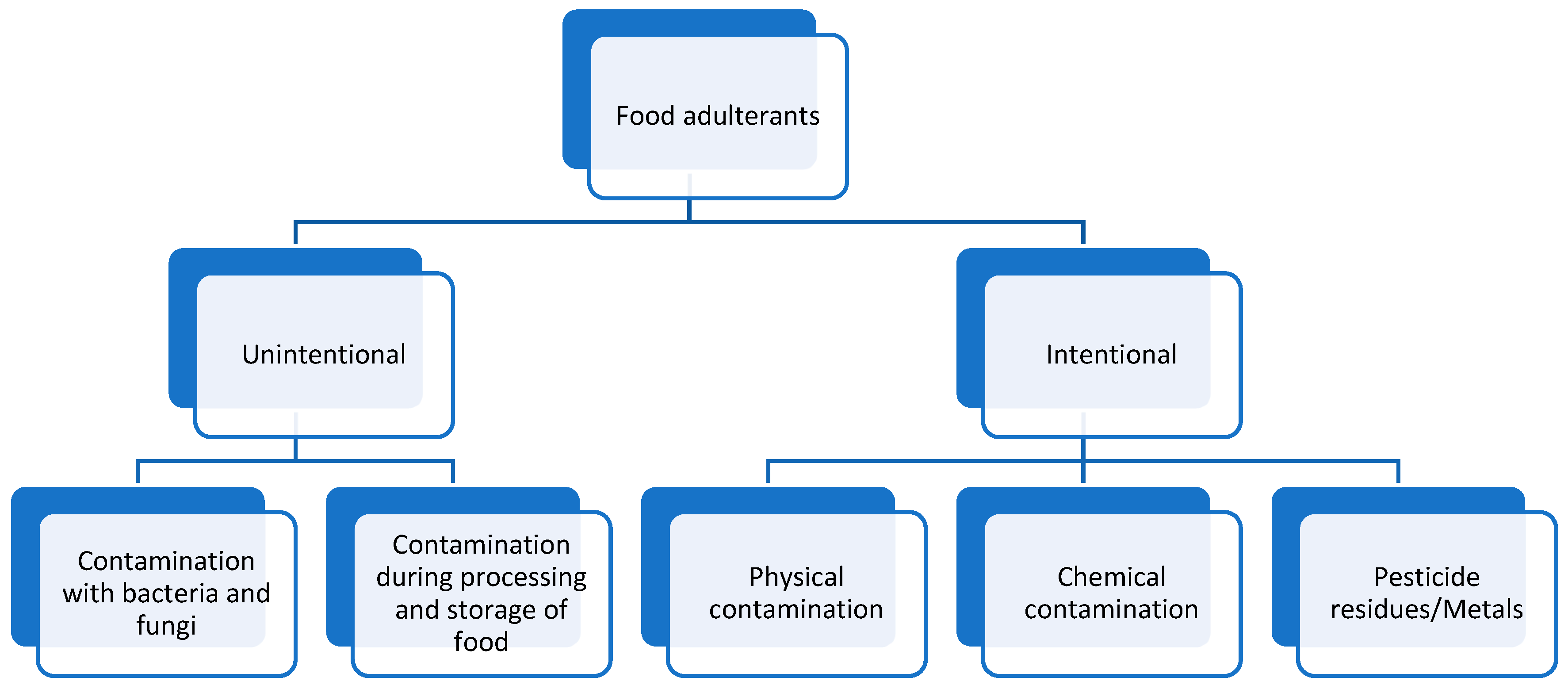
3.1. Counterfeiting/Adulteration
- Foods
- Food prepared with expired ingredients. Many manufacturers use outdated components to escape the loss, which poses a serious threat to human health as was observed in Pakistan, where expired white flour was used in making pasta.
- Selling cheaper food at the same price as expensive food and fake labelling and packaging. For example, selling soya oil as extra virgin olive oil after being dyed.
- The use of unauthorized additives such as preservatives, sweeteners, or dyes.
- Marketing non-organic goods as organic products. For example, mixing milk with vegetable oil to produce high-fat cheese. The production of artificial honey uses sugar syrups, vitamin C, and different enzymes that are not following the quality required by law.
- Products are being imported and sold as homegrown. For instance, strawberries and cherries are marketed in March and April as being locally produced.
- Drugs
- Drugs without the correct amount of active ingredients do not provide any therapeutic benefit to the patient and worsen their condition. An example is the development of antibiotic resistance due to counterfeit antibiotic therapy.
- A patient’s health can also be affected by drugs with fake ingredients or incorrect concentrations or doses for some ingredients. For example, a botulinum toxin, A (Botox), was more concentrated than the real version when given to a physician, resulting in respiratory paralysis and mortality for both the patient and the physician.
- Drugs with completely wrong and counterfeit ingredients can also contain harmful substances such as floor wax, boric acid, powdered cement, or other harmful chemicals. For example, counterfeit cough syrups with antifreeze chemicals such as ethylene glycol have caused more than 500 deaths among children worldwide. Erythropoietin is another example of a counterfeit IV drug diluted with contaminated water and directly injected into cancer patients. Drug products containing incorrect medications cause several issues. Anti-obesity medicine orlistat (Alli) by GSK was distributed in the United States, containing sibutramine instead of orlistat, which may interact negatively with other medications taken by patients and cause a negative reaction in their bodies.
- A lack of constitutional protection.
- A failure to enforce the existing constitution.
- Insufficient national drug regulations.
- Ineffective punishment.
- Corruption.
- Excessive demand over supply.
- Inadequate coordination between stakeholders.
- Absence of regulation by exporting countries.
3.2. Genetically Modified (GMO)
- Foods
- Drugs
3.3. Microbial Contamination
- Foods
- Drugs
3.4. Environmental Issues
- Foods
- Drugs
3.5. Biochemical Contamination
- Foods
3.6. Emergence of New Technologies
- Drugs
3.7. Lack of Consumers/Patients’ Awareness
- Foods
- Drugs
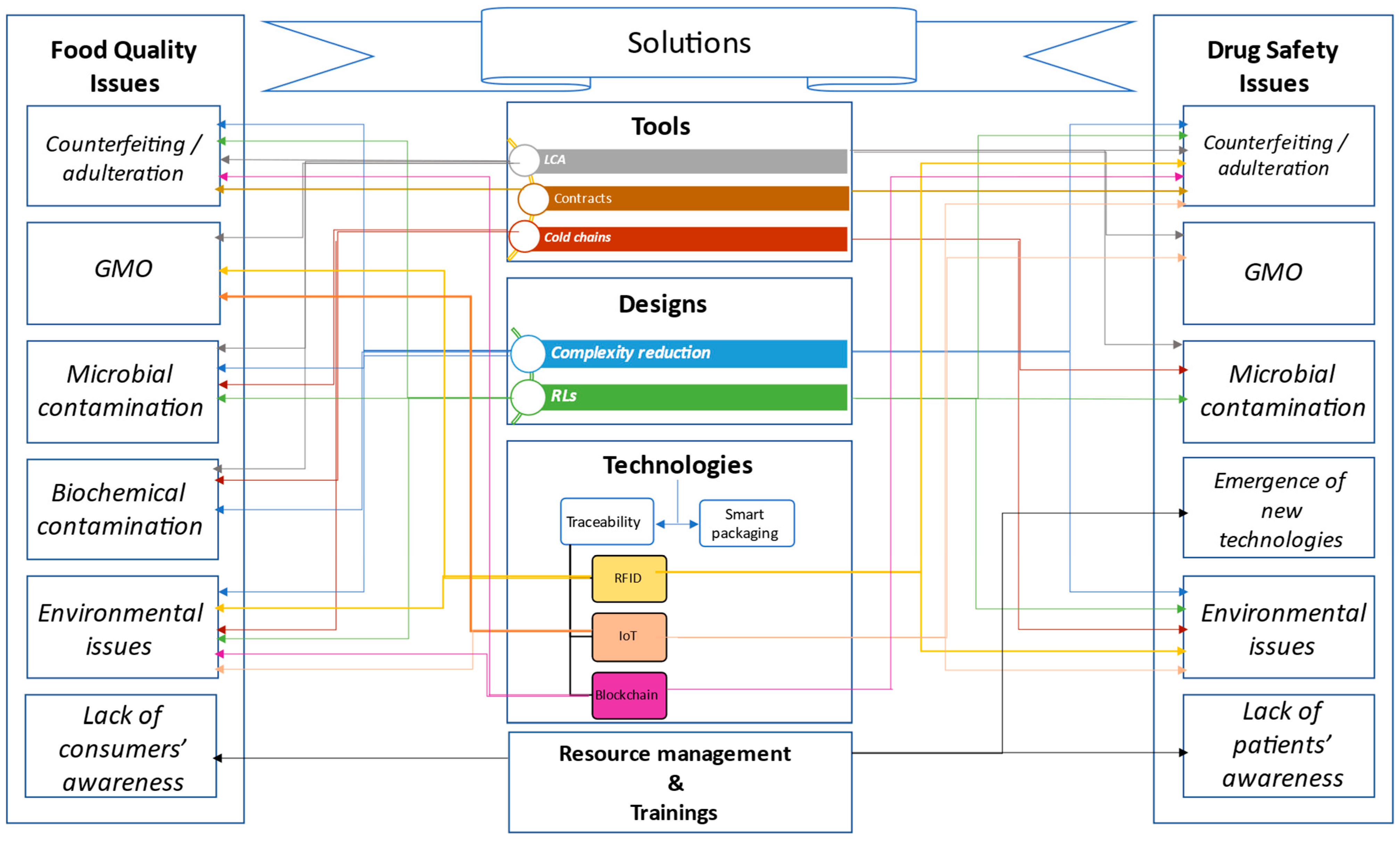
4. Solutions for Food Quality and Drug Safety
4.1. Tools
4.1.1. Life Cycle Assessment (LCA)
4.1.2. Contracts
4.1.3. Cold Chains
4.2. Design
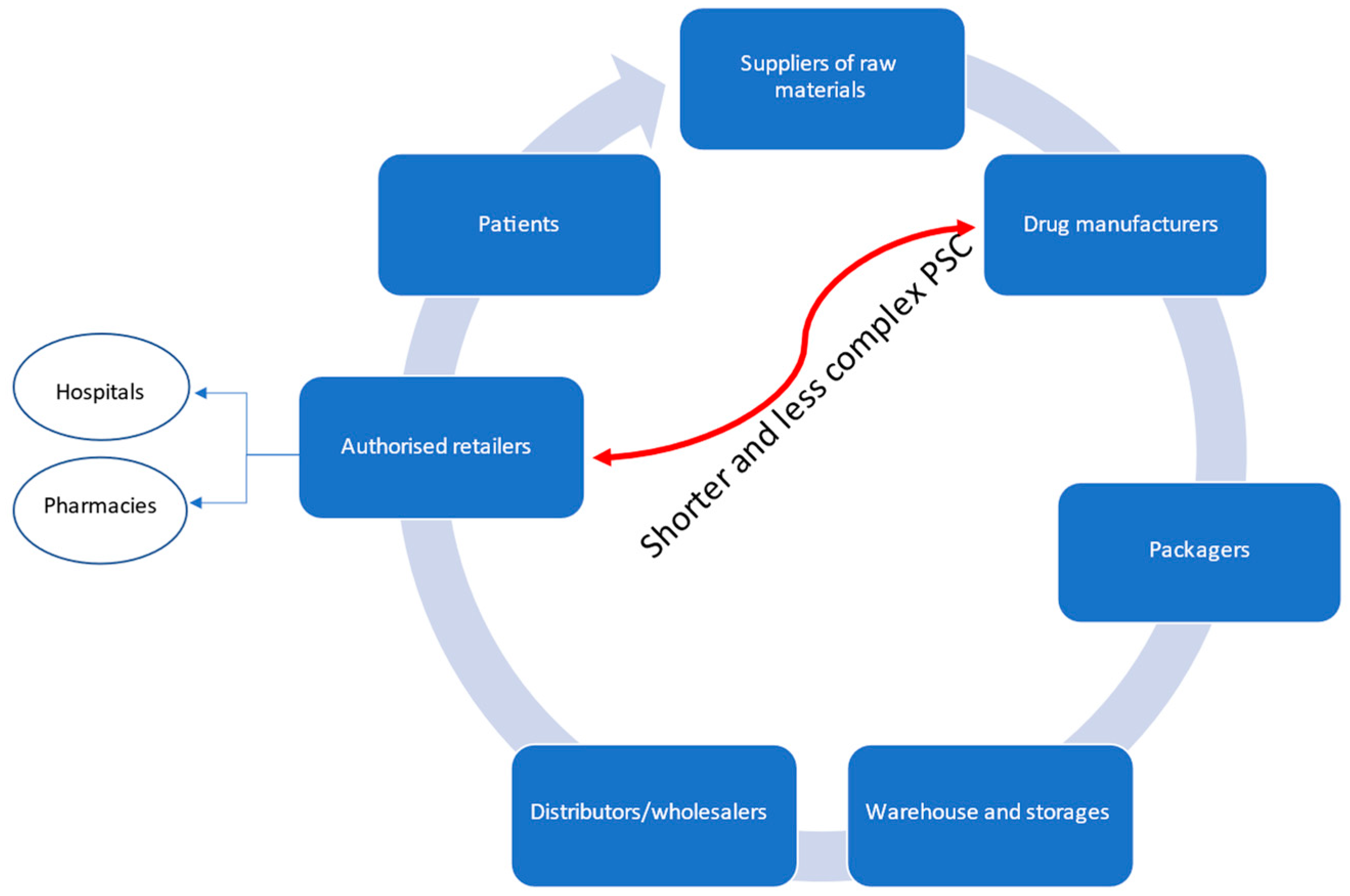
4.2.1. Complexity Reduction
4.2.2. Reverse Logistics (RLs)
4.3. Technologies
4.3.1. Anti-Counterfeiting Technologies
- Overt anti-counterfeiting technologies (OACTS):
- Covert anti-counterfeiting technologies:
- Forensic anti-counterfeiting technologies:
- Traceability technologies:
- RFID
- 2.
- IOT
- 3.
- Blockchain
4.3.2. Smart Packaging
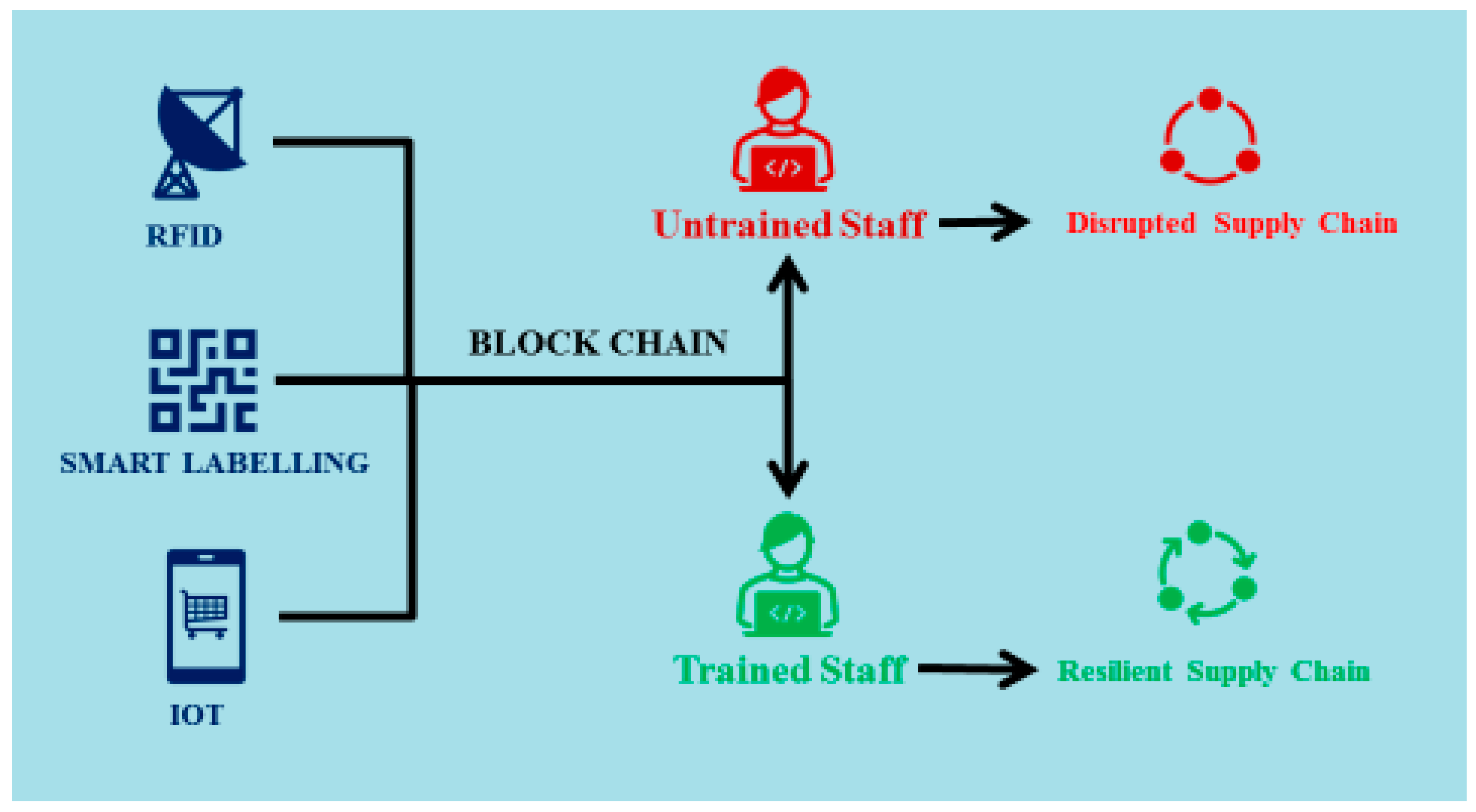
4.4. Resource Management and Training
5. Overall Review Findings
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barbosa, M.W. Uncovering research streams on agri-food supply chain management: A bibliometric study. Glob. Food Secur. 2021, 28, 100517. [Google Scholar] [CrossRef]
- Behzadi, G.; O’Sullivan, M.J.; Olsen, T.L.; Zhang, A. Agribusiness supply chain risk management: A review of quantitative decision models. Omega 2018, 79, 21–42. [Google Scholar] [CrossRef]
- Onggo, B.S.; Panadero, J.; Corlu, C.G.; Juan, A.A. Agri-food supply chains with stochastic demands: A multi-period inventory routing problem with perishable products. Simul. Model. Pract. Theory 2019, 97, 101970. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Mission. Available online: https://www.fda.gov/about-fda/what-we-do#mission (accessed on 19 March 2022).
- Ali, S.M.; Moktadir, A.; Kabir, G.; Chakma, J.; Rumi, J.U.; Islam, T. Framework for evaluating risks in food supply chain: Implications in food wastage reduction. J. Clean. Prod. 2019, 228, 786–800. [Google Scholar] [CrossRef]
- Ghadge, A.; Kara, M.E.; Mogale, D.G.; Choudhary, S.; Dani, S. Sustainability implementation challenges in food supply chains: A case of UK artisan cheese producers. Prod. Plan. Control 2021, 32, 1191–1206. [Google Scholar] [CrossRef]
- Zhao, G.; Lis, S.; Lopez, C.; Lu, H.; Elgueta, S.; Chen, H.; Boshkoska, B.M. Blockchain technology in agri-food value chain management: A synthesis of applications, challenges and future research directions. Comput. Ind. 2019, 109, 83–99. [Google Scholar] [CrossRef]
- US Centers for Disease Control and Prevention. Food Security. Available online: https://www.cdc.gov/climateandhealth/effects/food_security.htm (accessed on 19 March 2022).
- Blando, G.M. Our Worst Enemy: Unhealthy Food. In English Department: Research for Change—Wicked Problems in Our World. 12; Kutztown University: Kutztown, PA, USA, 2020; Available online: https://research.library.kutztown.edu/wickedproblems/12 (accessed on 1 June 2022).
- Banerjee, S.; Radak, T.; Khubchandani, J.; Dunn, P. Food Insecurity and Mortality in American Adults: Results From the NHANES-Linked Mortality Study. Health Promot. Pract. 2021, 22, 204–214. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, Y. Etiological agents implicated in foodborne illness world wide. Food Sci. Anim. Resour. 2021, 41, 1–7. [Google Scholar] [CrossRef]
- Jung, J.; Bir, C.; Widmar, N.O.; Sayal, P. Initial Reports of Foodborne Illness Drive More Public Attention Than Do Food Recall Announcements. J. Food Prot. 2021, 84, 1150–1159. [Google Scholar] [CrossRef]
- Onwujekwe, E.C.; Ezemba, C.C. Food Security and Safety: Africans Perspectives A Review. Arch. Curr. Res. Int. 2021, 21, 14–20. [Google Scholar] [CrossRef]
- Dada, A.C.; Somorin, Y.M.; Ateba, C.N.; Onyeaka, H.; Anyogu, A.; Kasan, N.A.; Odeyemi, O.A. Microbiological hazards associated with food products imported from the Asia-Pacific region based on analysis of the rapid alert system for food and feed (RASFF) notifications. Food Control 2021, 129, 108243. [Google Scholar] [CrossRef]
- Kerr, E.J.; Stafford, R.; Rathnayake, I.U.; Graham, R.M.; Fearnley, E.; Gregory, J.; Glasgow, K.; Wright, R.; Sintchenko, V.; Wang, Q.; et al. Multistate Outbreak of Salmonella enterica Serovar Heidelberg with Unidentified Source, Australia, 2018–2019. Emerg. Infect. Dis. 2022, 28, 238–241. [Google Scholar] [CrossRef]
- Agbaraji, E.C.; Ochlor, D.O.; Ezeh, G.N. Food and drug counterfeiting in the developing nations; The implications and way-out. Acad. Res. Int. 2012, 3, 24–31. Available online: https://www.researchgate.net/profile/Chukwudi-Agbaraji/publication/336717853_FOOD_AND_DRUG_COUNTERFEITING_IN_THE_DEVELOPING_NATIONS_THE_IMPLICATIONS_AND_WAY-OUT/links/61c4d1b50ae6751c882fc6b6/FOOD-AND-DRUG-COUNTERFEITING-IN-THE-DEVELOPING-NATIONS-THE-IMPLICATIONS-AND-WAY-OUT.pdf (accessed on 1 June 2022).
- Blackstone, E.A.; Fuhr, J.P., Jr.; Pociask, S. The health and economic effects of counterfeit drugs. Am. Health Drug Benefits 2014, 7, 216–224. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4105729/ (accessed on 1 June 2022). [PubMed]
- Dania, W.A.P.; Xing, K.; Amer, Y. Collaboration behavioural factors for sustainable agri-food supply chains: A systematic review. J. Clean. Prod. 2018, 186, 851–864. [Google Scholar] [CrossRef]
- Bartolini, M.; Bottani, E.; Grosse, E.H. Green warehousing: Systematic literature review and bibliometric analysis. J. Clean. Prod. 2019, 226, 242–258. [Google Scholar] [CrossRef]
- George, A. Antimicrobial resistance (AMR) in the food chain: Trade, one health and codex. Trop. Med. Infect. Dis. 2019, 4, 54. [Google Scholar] [CrossRef]
- Aamer, A.M.; Al-Awlaqi, M.A.; Affia, I.; Arumsari, S.; Mandahawi, N. The internet of things in the food supply chain: Adoption challenges. Benchmarking Int. J. 2021, 28, 2521–2541. [Google Scholar] [CrossRef]
- Tan, B.; Yan, J.; Chen, S.; Liu, X. The Impact of Blockchain on Food Supply Chain: The Case of Walmart. In Smart Blockchain; Qiu, M., Ed.; Springer: Cham, Switzerland, 2018; Volume 11373. [Google Scholar] [CrossRef]
- Bernstad, A.K.; Cánovas, A.; Valle, R. Consideration of food wastage along the supply chain in lifecycle assessments: A mini-review based on the case of tomatoes. Waste Manag. Res. J. Sustain. Circ. Econ. 2016, 35, 29–39. [Google Scholar] [CrossRef]
- Zhu, Z.; Chu, F.; Dolgui, A.; Chu, C.; Zhou, W.; Piramuthu, S. Recent advances and opportunities in sustainable food supply chain: A model-oriented review. Int. J. Prod. Res. 2018, 56, 5700–5722. [Google Scholar] [CrossRef]
- Sohail, M.; Sun, D.W.; Zhu, Z. Recent developments in intelligent packaging for enhancing food quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 2650–2662. [Google Scholar] [CrossRef] [PubMed]
- Scholten, K.; Scott, P.S.; Fynes, B. Building routines for non-routine events: Supply chain resilience learning mechanisms and their antecedents. Supply Chain. Manag. Int. J. 2019, 24, 430–442. [Google Scholar] [CrossRef]
- Kumar, R.; Tripathi, R. Traceability of counterfeit medicine supply chain through Blockchain. In Proceedings of the 2019 11th International Conference on Communication Systems & Networks (COMSNETS), Bengaluru, India, 7–11 January 2019; pp. 568–570. [Google Scholar] [CrossRef]
- Jamil, F.; Hang, L.; Kim, K.; Kim, D. A Novel Medical Blockchain Model for Drug Supply Chain Integrity Management in a Smart Hospital. Electronics 2019, 8, 505. [Google Scholar] [CrossRef]
- Marques, C.M.; Moniz, S.; de Sousa, J.P.; Barbosa-Povoa, A.P.; Reklaitis, G. Decision-support challenges in the chemical-pharmaceutical industry: Findings and future research directions. Comput. Chem. Eng. 2020, 134, 106672. [Google Scholar] [CrossRef]
- Krämer, I.; Thiesen, J.; Astier, A. Formulation and Administration of Biological Medicinal Products. Pharm. Res. 2020, 37, 1–18. [Google Scholar] [CrossRef]
- Gunnarsson, L.; Snape, J.R.; Verbruggen, B.; Owen, S.F.; Kristiansson, E.; Margiotta-Casaluci, L.; Österlund, T.; Hutchinson, K.; Leverett, D.; Marks, B.; et al. Pharmacology beyond the patient – The environmental risks of human drugs. Environ. Int. 2019, 129, 320–332. [Google Scholar] [CrossRef]
- Apostolopoulos, N.; Ratten, V.; Petropoulos, D.; Liargovas, P.; Anastasopoulou, E. Agri-food sector and entrepreneurship during the COVID-19 crisis: A systematic literature review and research agenda. Strat. Chang. 2021, 30, 159–167. [Google Scholar] [CrossRef]
- Jones, M.V.; Coviello, N.; Tang, Y.K. International Entrepreneurship research (1989–2009): A domain ontology and thematic analysis. J. Bus. Ventur. 2011, 26, 632–659. [Google Scholar] [CrossRef]
- Phillips, W.; Lee, H.; Ghobadian, A.; O’Regan, N.; James, P. Social innovation and social entrepreneurship: A systematic review. Group Organ. Manag. 2015, 40, 428–461. [Google Scholar] [CrossRef]
- Phillipson, J.; Gorton, M.; Turner, R.; Shucksmith, M.; Aitken-McDermott, K.; Areal, F.; Cowie, P.; Hubbard, C.; Maioli, S.; McAreavey, R.; et al. The COVID-19 Pandemic and Its Implications for Rural Economies. Sustainability 2020, 12, 3973. [Google Scholar] [CrossRef]
- Aday, S.; Aday, M.S. Impact of COVID-19 on the food supply chain. Food Qual. Saf. 2020, 4, 167–180. [Google Scholar] [CrossRef]
- Lakuma, P.C.; Sunday, N.; Sserunjogi, B.; Kanunde, R.; Munyambonera, E.F. How Has the COVID-19 Pandemic Impacted Ugandan Businesses? Results from a Business Climate Survey; Economic Policy Research Centre: Kampala, Uganda, 2020; Available online: https://www.africaportal.org/publications/how-has-covid-19-pandemic-impacted-ugandan-businesses-results-business-climate-survey/ (accessed on 1 June 2022).
- Dai, R.; Feng, H.; Hu, J.; Jin, Q.; Li, H.; Wang, R.; Wang, R.; Xu, L.; Zhang, X. The impact of COVID-19 on small and medium-sized enterprises (SMEs): Evidence from two-wave phone surveys in China. China Econ. Rev. 2021, 67, 101607. [Google Scholar] [CrossRef]
- Udmale, P.; Pal, I.; Szabo, S.; Pramanik, M.; Large, A. Global food security in the context of COVID-19: A scenario-based exploratory analysis. Prog. Disaster Sci. 2020, 7, 100120. [Google Scholar] [CrossRef] [PubMed]
- Tipmontian, J.; Alcover, J.C.; Rajmohan, M. Impact of blockchain adoption for safe food supply chain management through system dynamics approach from management perspectives in Thailand. Proceedings 2020, 39, 14. [Google Scholar] [CrossRef]
- Kamble, S.S.; Gunasekaran, A.; Gawankar, S.A. Achieving sustainable performance in a data-driven agriculture supply chain: A review for research and applications. Int. J. Prod. Econ. 2019, 219, 179–194. [Google Scholar] [CrossRef]
- Jouzdani, J.; Govindan, K. On the sustainable perishable food supply chain network design: A dairy products case to achieve sustainable development goals. J. Clean. Prod. 2020, 278, 123060. [Google Scholar] [CrossRef]
- Fung, F.; Wang, H.-S.; Menon, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef]
- Yin, S.; Hu, W.; Chen, Y.; Han, F.; Wang, Y.; Chen, M. Chinese consumer preferences for fresh produce: Interaction between food safety labels and brands. Agribusiness 2019, 35, 53–68. [Google Scholar] [CrossRef]
- Sterniša, M.; Možina, S.S.; Levstek, S.; Kukec, A.; Raspor, P.; Jevšnik, M. Food safety knowledge, self-reported practices and attitude of poultry meat handling among Slovenian consumers. Br. Food J. 2018, 120, 1344–1357. [Google Scholar] [CrossRef]
- Li, S.; Tian, Y.; Jiang, P.; Lin, Y.; Liu, X.; Yang, H. Recent advances in the application of metabolomics for food safety control and food quality analyses. Crit. Rev. Food Sci. Nutr. 2020, 61, 1448–1469. [Google Scholar] [CrossRef]
- Rao, M.; Bast, A.; de Boer, A. European private food safety standards in global agri-food supply chains: A systematic review. Int. Food Agribus. Manag. Rev. 2021, 24, 739–754. [Google Scholar] [CrossRef]
- Haji, M.; Kerbache, L.; Sheriff, K.M.; Al-Ansari, T. Critical Success Factors and Traceability Technologies for Establishing a Safe Pharmaceutical Supply Chain. Methods Protoc. 2021, 4, 85. [Google Scholar] [CrossRef] [PubMed]
- Haji, M.; Kerbache, L.; Muhammad, M.; Al-Ansari, T. Roles of Technology in Improving Perishable Food Supply Chains. Logistics 2020, 4, 33. [Google Scholar] [CrossRef]
- Csapó, J.; Némethy, S.; Albert, C. Food counterfeiting in general; counterfeiting of milk and dairy products. Ecocycles 2019, 5, 26–41. [Google Scholar] [CrossRef]
- Spink, J.; Moyer, D.C.; Park, H.; A Heinonen, J. Defining the types of counterfeiters, counterfeiting, and offender organizations. Crime Sci. 2013, 2, 8. [Google Scholar] [CrossRef]
- Huck, C.W.; Pezzei, C.K.; Huck-Pezzei, V.A. An industry perspective of food fraud. Curr. Opin. Food Sci. 2016, 10, 32–37. [Google Scholar] [CrossRef]
- Kang’ethe, E.K.; Grace, D.; Alonso, S.; Lindahl, J.F.; Mutua, F.; Haggblade, S. Food safety and public health implications of growing urban food markets. In AGRA, Africa Agriculture Status Report. Feeding Africa’s Cities: Opportunities, Challenges, and Policies for Linking African Farmers with Growing Urban Food Markets; Alliance for a Green Revolution in Africa: Nairobi, Kenya, 2020; pp. 101–119. [Google Scholar]
- O’Brien, S.J. The public health impact of food-related illness. Curr. Opin. Infect. Dis. 2012, 25, 537–545. [Google Scholar] [CrossRef]
- Oluwafemi, R.; Edugbo, O.; Solanke, E.; Akinyeye, A. Meat quality, nutrition, security and public health. A review of beef processing practices in Nigeria. Afr. J. Food Sci. Technol. 2013, 4, 96–99. Available online: https://d1wqtxts1xzle7.cloudfront.net/33000089/Oluwafemi_et_al-with-cover-page-v2.pdf?Expires=1654078340&Signature=APZR4AQlQ93VWrIGnRJapfRzkz5SVI18NJUdHu4~PJzJROv731PJqg2vJ3b2q3eS~YyWHPIsIGWrtKKcXwiimLNKJTLzPieMDVk-M0gui2BFswzIlAUdLgkkAXru3Kajkvkqsd2cTsW3rXtwFYVj1h2VtQd5294Zl5ZKGLAUH-xMPWfYwtsmN0W8nwL3~oQeczVxI7xUTokkQYnwFqOW6wj9y7RO4JcU5K6XBEEfGYauYnDxh2rP-Hc5Lbm4ZU4VCWGl2qWEQ80MPp8TKpxJqnH72SdkDyxDLCKuWRlLALKKhWfAcGWD2gLPad6eJ63yLSJtLtCAfoTNlj7CK-ae~A__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA (accessed on 1 June 2022).
- Argiyantari, B.; Simatupang, T.M.; Basri, M.H. Pharmaceutical supply chain transformation through application of the Lean principle: A literature review. J. Ind. Eng. Manag. 2020, 13, 475–494. [Google Scholar] [CrossRef]
- Glass, B.D. Counterfeit drugs and medical devices in developing countries. Res. Rep. Trop. Med. 2014, 5, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.M. Is the Drugstore Safe? Counterfeit Diabetes Products on the Shelves. J. Diabetes Sci. Technol. 2009, 3, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Mamtashanti, M.; Rahul, J.; Kashyap, T. A Review On Regulatory Requirements To Prevent Counterfeit Drugs In India. Int. J. Pharm. Investig. 2020, 10, 257–262. [Google Scholar] [CrossRef]
- Oye, N.; Vivian, N.; Jemimah, N. Drugs verification and management system. Int. J. Innov. Res. Eng. Manag. 2018, 5, 35–41. [Google Scholar] [CrossRef]
- Bawa, A.S.; Anilakumar, K.R. Genetically modified foods: Safety, risks and public concerns—A review. J. Food Sci. Technol. 2013, 50, 1035–1046. [Google Scholar] [CrossRef]
- Zhang, C.; Wohlhueter, R.; Zhang, H. Genetically modified foods: A critical review of their promise and problems. Food Sci. Hum. Wellness 2016, 5, 116–123. [Google Scholar] [CrossRef]
- Atalan-Helicke, N. The halal paradox: Negotiating identity, religious values, and genetically engineered food in Turkey. Agric. Hum. Values 2015, 32, 663–674. [Google Scholar] [CrossRef]
- Lusk, J.L.; McFadden, B.R.; Rickard, B.J. Which biotech foods are most acceptable to the public? Biotechnol. J. 2014, 10, 13–16. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. New Horizons for Old Drugs and Drug Leads. J. Nat. Prod. 2014, 77, 703–723. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Benbrook, C. GMOs, herbicides, and public health. N. Engl. J. Med. 2015, 373, 693–695. Available online: https://www.researchgate.net/profile/Charles-Benbrook/publication/281172542_GMOs_Herbicides_and_Public_Health/links/55e9d0a008ae3e121844610a/GMOs-Herbicides-and-Public-Health.pdf (accessed on 4 June 2022). [CrossRef]
- Amin, L.; Jahi, J.; Nor, A.R. Stakeholders’ attitude to genetically modified foods and medicine. Sci. World J. 2013, 2013, 516742. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Ullah, M.W.; Siddique, R.; Nabi, G.; Manan, S.; Yousaf, M.; Hou, H. Role of Recombinant DNA Technology to Improve Life. Int. J. Genom. 2016, 2016, 2405954. [Google Scholar] [CrossRef] [PubMed]
- Teena, M.; Manickavasagan, A.; Mothershaw, A.; El Hadi, S.; Jayas, D.S. Potential of Machine Vision Techniques for Detecting Fecal and Microbial Contamination of Food Products: A Review. Food Bioprocess Technol. 2013, 6, 1621–1634. [Google Scholar] [CrossRef]
- Chatterjee, A.; Abraham, J. Microbial contamination, prevention, and early detection in food industry. In Microbial Contamination and Food Degradation; Academic Press: Cambridge, MA, USA, 2018; pp. 21–47. [Google Scholar] [CrossRef]
- Al-Qadiri, H.M.; Ovissipour, M.; Al-Alami, N.; Govindan, B.N.; Shiroodi, S.G.; Rasco, B. Efficacy of Neutral Electrolyzed Water, Quaternary Ammonium and Lactic Acid-Based Solutions in Controlling Microbial Contamination of Food Cutting Boards Using a Manual Spraying Technique. J. Food Sci. 2016, 81, M1177–M1183. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, M.; Pamuk, Ş. Microbial contamination in food, food-handlers’ hands and surfaces and evaluation of contamination sources by the similarity between isolates. Ank. Üniversitesi Vet. Fakültesi Derg. 2019, 67, 73–80. Available online: http://vetjournal.ankara.edu.tr/en/download/article-file/901749 (accessed on 1 June 2022).
- Ratajczak, M.; Kubicka, M.; Kamińska, D.; Sawicka, P.; Długaszewska, J. Microbiological quality of non-sterile pharmaceutical products. Saudi Pharm. J. 2014, 23, 303–307. [Google Scholar] [CrossRef]
- Schatz, S.; Weber, R.J. Adverse drug reactions. Pharm. Pract. 2015, 1, 5–26. Available online: https://www.accp.com/docs/bookstore/psap/2015B2.SampleChapter.pdf (accessed on 4 June 2022).
- Giardina, C.; Cutroneo, P.M.; Mocciaro, E.; Russo, G.T.; Mandraffino, G.; Basile, G.; Rapisarda, F.; Ferrara, R.; Spina, E.; Arcoraci, V. Adverse Drug Reactions in Hospitalized Patients: Results of the FORWARD (Facilitation of Reporting in Hospital Ward) Study. Front. Pharmacol. 2018, 9, 350. [Google Scholar] [CrossRef]
- Pojić, M.; Mišan, A.; Tiwari, B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends Food Sci. Technol. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- Loveday, S.M. Food Proteins: Technological, Nutritional, and Sustainability Attributes of Traditional and Emerging Proteins. Annu. Rev. Food Sci. Technol. 2019, 10, 311–339. [Google Scholar] [CrossRef]
- Dong, S.-J.; Lin, X.-H.; Li, H. Regulation of Lactobacillus plantarum contamination on the carbohydrate and energy related metabolisms of Saccharomyces cerevisiae during bioethanol fermentation. Int. J. Biochem. Cell Biol. 2015, 68, 33–41. [Google Scholar] [CrossRef]
- Shahid, S.; Hasan, I.; Ahmad, F.; Hassan, I.; Islam, A. Carbohydrate-Based Macromolecular Crowding-Induced Stabilization of Proteins: Towards Understanding the Significance of the Size of the Crowder. Biomolecules 2019, 9, 477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, M.A.; Adhikari, B. Drying and denaturation of proteins in spray drying process. Handb. Ind. Dry. 2015, 33, 971–985. Available online: https://www.researchgate.net/profile/Md-Amdadul-Haque/publication/275100415_Drying_and_Denaturation_of_Proteins_in_Spray_Drying_Process/links/5b457dfea6fdcc6619171c1e/Drying-and-Denaturation-of-Proteins-in-Spray-Drying-Process.pdf (accessed on 1 June 2022).
- Han, J.-W.; Ruiz-Garcia, L.; Qian, J.-P.; Yang, X.-T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef]
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale Drug Delivery Systems: From Medicine to Agriculture. Front. Bioeng. Biotechnol. 2020, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Jaberidoost, M.; Nikfar, S.; Abdollahiasl, A.; Dinarvand, R. Pharmaceutical supply chain risks: A systematic review. DARU J. Pharm. Sci. 2013, 21, 69. [Google Scholar] [CrossRef] [PubMed]
- Punniyamoorthy, M.; Thamaraiselvan, N.; Manikandan, L. Assessment of supply chain risk: Scale development and validation. Benchmarking Int. J. 2013, 20, 79–105. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, D.; Raju, M.S.; Chaudhary, D.K.; Sagar, R.K. Combating counterfeit drugs: A quantitative analysis on cracking down the fake drug industry by using blockchain technology. In Proceedings of the 2019 9th International Conference on Cloud Computing, Data Science & Engineering (Confluence), Noida, India, 10–11 January 2019; pp. 174–178. [Google Scholar] [CrossRef]
- Caracciolo, A.B.; Topp, E.; Grenni, P. Pharmaceuticals in the environment: Biodegradation and effects on natural microbial communities. A review. J. Pharm. Biomed. Anal. 2015, 106, 25–36. [Google Scholar] [CrossRef]
- Amorim, C.L.; Alves, M.; Castro, P.M.; Henriques, I. Bacterial community dynamics within an aerobic granular sludge reactor treating wastewater loaded with pharmaceuticals. Ecotoxicol. Environ. Saf. 2018, 147, 905–912. [Google Scholar] [CrossRef]
- Li, D.; Baert, L.; Uyttendaele, M. Inactivation of food-borne viruses using natural biochemical substances. Food Microbiol. 2013, 35, 1–9. [Google Scholar] [CrossRef]
- Virendra, S.; Rita, M.; Pramod, U.; Vinayak, S. Study on the biochemical, sensory and microbial contamination of custard apple RTS beverage. HortFlora Res. Spectr. 2013, 2, 202–207. Available online: https://www.cabdirect.org/cabdirect/abstract/20143066888 (accessed on 1 June 2022).
- Susanna, S.; Prabhasankar, P. A study on development of Gluten free pasta and its biochemical and immunological validation. LWT-Food Sci. Technol. 2013, 50, 613–621. [Google Scholar] [CrossRef]
- Rosell, C.M.; Barro, F.; Sousa, C.; Mena, M.C. Cereals for developing gluten-free products and analytical tools for gluten detection. J. Cereal Sci. 2014, 59, 354–364. [Google Scholar] [CrossRef]
- Narayana, S.A.; Elias, A.A.; Pati, R.K. Reverse logistics in the pharmaceuticals industry: A systemic analysis. Int. J. Logist. Manag. 2014, 25, 379–398. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, R.; Kumar, P. Strategic issues in pharmaceutical supply chains: A review. Int. J. Pharm. Heal. Mark. 2016, 10, 234–257. [Google Scholar] [CrossRef]
- Ding, B. Pharma Industry 4.0: Literature review and research opportunities in sustainable pharmaceutical supply chains. Process Saf. Environ. Prot. 2018, 119, 115–130. [Google Scholar] [CrossRef]
- Kneafsey, M.; Dowler, E.; Lambie-Mumford, H.; Inman, A.; Collier, R. Consumers and food security: Uncertain or empowered? J. Rural. Stud. 2013, 29, 101–112. [Google Scholar] [CrossRef]
- Nagyová, Ľ.; Andocsová, A.; Géci, A.; Zajác, P.; Palkovič, J.; Košičiarová, I.; Golian, J. Consumers’ awareness of food safety. Potravinarstvo Slovak J. Food Sci. 2019, 13, 8–17. [Google Scholar] [CrossRef]
- Teuber, R. Consumers’ and producers’ expectations towards geographical indications: Empirical evidence for a German case study. Br. Food J. 2011, 113, 900–918. [Google Scholar] [CrossRef]
- Kocetkovs, V.; Muizniece-Brasava, S.; Kirse-Ozolina, A. Consumer awareness and attitudes towards active and intelligent packaging systems in the Latvian market. In Baltic Conference on Food Science and Technology: Conference Proceedings, LLU; Department of Food Technology, Faculty of Food Technology, Latvia University of Life Sciences and Technologies: Jelgava, Latvia, 2019; pp. 222–226. Available online: https://www.researchgate.net/profile/Vjaceslavs--Kocetkovs/publication/333084312_Consumer_awareness_and_attitudes_towards_active_and_intelligent_packaging_systems_in_the_Latvian_market/links/5d6bff60a6fdcc547d7200a8/Consumer-awareness-and-attitudes-towards-active-and-intelligent-packaging-systems-in-the-Latvian-market.pdf (accessed on 4 June 2022).
- Rasool, S.; Cerchione, R.; Salo, J.; Ferraris, A.; Abbate, S. Measurement of consumer awareness of food waste: Construct development with a confirmatory factor analysis. Br. Food J. 2021, 123, 337–361. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Structural Design Principles for Delivery of Bioactive Components in Nutraceuticals and Functional Foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 577–606. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Rodrigues, C.; Stojanović-Radić, Z.; Dimitrijević, M.; Aleksić, A.; Neffe-Skocińska, K.; Zielińska, D.; Kołożyn-Krajewska, D.; Salehi, B.; Prabu, S.M.; et al. Probiotics: Versatile Bioactive Components in Promoting Human Health. Medicina 2020, 56, 433. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.T.; Kasper, J.D.; Hauser, J.M.; Berdes, C.; Chang, C.-H.; Berman, R.L.; Masin-Peters, J.; Paice, J.; Emanuel, L. Family Caregiver Skills in Medication Management for Hospice Patients: A Qualitative Study to Define a Construct. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2009, 64, 799–807. [Google Scholar] [CrossRef]
- Sivasankaran, P.; Mohammed, E.B.; Ganesan, N.; Durai, R. Storage and Safe Disposal of Unwanted/Unused and Expired Medicines: A Descriptive Cross-Sectional Survey among Indian Rural Population. J. Young Pharm. 2019, 11, 97–100. [Google Scholar] [CrossRef]
- Clark, F. Rise in online pharmacies sees counterfeit drugs go global. Lancet 2015, 386, 1327–1328. [Google Scholar] [CrossRef]
- Klöpffer, W. Life cycle assessment. Environ. Sci. Pollut. Res. 1997, 4, 223–228. [Google Scholar] [CrossRef]
- Algren, M.; Fisher, W.; Landis, A.E. Machine learning in life cycle assessment. In Data Science Applied to Sustainability Analysis; Elsevier: Amsterdam, The Netherlands, 2021; pp. 167–190. [Google Scholar] [CrossRef]
- Muthu, S.S. Estimating the overall environmental impact of textile processing: Life cycle assessment (LCA) of textile products. In Assessing the Environmental Impact of Textiles and the Clothing Supply Chain; Elsevier B.V.: Sawston, UK, 2014; pp. 105–131. [Google Scholar]
- Cucurachi, S.; Scherer, L.; Guinée, J.; Tukker, A. Life Cycle Assessment of Food Systems. One Earth 2019, 1, 292–297. [Google Scholar] [CrossRef]
- Sonesson, U.; Davis, J.; Flysjö, A.; Gustavsson, J.; Witthöft, C. Protein quality as functional unit—A methodological framework for inclusion in life cycle assessment of food. J. Clean. Prod. 2017, 140, 470–478. [Google Scholar] [CrossRef]
- Molina-Besch, K.; Wikström, F.; Williams, H. The environmental impact of packaging in food supply chains—Does life cycle assessment of food provide the full picture? Int. J. Life Cycle Assess. 2019, 24, 37–50. [Google Scholar] [CrossRef]
- Choudhary, A.; Gupta, N.; Hameed, F.; Choton, S. An overview of food adulteration: Concept, sources, impact, challenges and detection. Int. J. Chem. Stud. 2020, 8, 2564–2573. Available online: https://www.researchgate.net/profile/Neeraj-Gupta-17/publication/339598102_An_overview_of_food_adulteration_Concept_sources_impact_challenges_and_detection/links/5e7f7bfb299bf1a91b866068/An-overview-of-food-adulteration-Concept-sources-impact-challenges-and-detection.pdf (accessed on 1 June 2022). [CrossRef]
- Modi, B.; Timilsina, H.; Bhandari, S.; Achhami, A.; Pakka, S.; Shrestha, P.; Kandel, D.; Bahadur, G.C.D.; Khatri, S.; Chhetri, P.M.; et al. Current trends of food analysis, safety, and packaging. Int. J. Food Sci. 2021, 2021, 9924667. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Qin, Z.; Han, J.; Wang, M.; Taheripour, F.; Tyner, W.; O’Connor, D.; Duffield, J. Life cycle energy and greenhouse gas emission effects of biodiesel in the United States with induced land use change impacts. Bioresour. Technol. 2018, 251, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Larroche, C.; Dussap, C.-G. Comprehensive assessment of 2G bioethanol production. Bioresour. Technol. 2020, 313, 123630. [Google Scholar] [CrossRef] [PubMed]
- Kakadellis, S.; Harris, Z.M. Don’t scrap the waste: The need for broader system boundaries in bioplastic food packaging life-cycle assessment—A critical review. J. Clean. Prod. 2020, 274, 122831. [Google Scholar] [CrossRef]
- Khoshnevisan, B.; Tabatabaei, M.; Tsapekos, P.; Rafiee, S.; Aghbashlo, M.; Lindeneg, S.; Angelidaki, I. Environmental life cycle assessment of different biorefinery platforms valorizing municipal solid waste to bioenergy, microbial protein, lactic and succinic acid. Renew. Sustain. Energy Rev. 2020, 117, 109493. [Google Scholar] [CrossRef]
- Prasad, S.; Singh, A.; Korres, N.E.; Rathore, D.; Sevda, S.; Pant, D. Sustainable utilization of crop residues for energy generation: A life cycle assessment (LCA) perspective. Bioresour. Technol. 2020, 303, 122964. [Google Scholar] [CrossRef]
- Van Der Werf, H.M.G.; Knudsen, M.T.; Cederberg, C. Towards better representation of organic agriculture in life cycle assessment. Nat. Sustain. 2020, 3, 419–425. [Google Scholar] [CrossRef]
- Erokhin, A.; Koshechkin, K.; Ryabkov, I. The distributed ledger technology as a measure to minimize risks of poor-quality pharmaceuticals circulation. PeerJ Comput. Sci. 2020, 6, e292. Available online: https://peerj.com/articles/cs-292.pdf (accessed on 4 June 2022). [CrossRef]
- Xia, M.C.; Zhan, Q.; Cai, L.; Wu, J.; Yang, L.; Sun, S.; Liang, H.; Li, Z. Investigation into the content change and distribution of active components in Cordyceps Sinensis with growth cycle by direct TOF-SIMS detection. Microchem. J. 2021, 164, 106026. [Google Scholar] [CrossRef]
- de Oliveira, R.A.; Komesu, A.; Rossell, C.E.V.; Filho, R.M. Challenges and opportunities in lactic acid bioprocess design—From economic to production aspects. Biochem. Eng. J. 2018, 133, 219–239. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Thanaraj, T.A.; Qaddoumi, M.G.; Hashem, A.; Abubaker, J.; Al-Mulla, F. The Role of Lipid Metabolism in COVID-19 Virus Infection and as a Drug Target. Int. J. Mol. Sci. 2020, 21, 3544. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, N.P.; Gopi, S. Overview of biopolymers: Resources, demands, sustainability, and life cycle assessment modeling and simulation. In Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–19. [Google Scholar] [CrossRef]
- Muiruri, J.K.; Yeo, J.C.C.; Zhu, Q.; Ye, E.; Loh, X.J.; Li, Z. Poly(hydroxyalkanoates): Production, Applications and End-of-Life Strategies–Life Cycle Assessment Nexus. ACS Sustain. Chem. Eng. 2022, 10, 3387–3406. [Google Scholar] [CrossRef]
- Rajesh, R. Flexible business strategies to enhance resilience in manufacturing supply chains: An empirical study. J. Manuf. Syst. 2021, 60, 903–919. [Google Scholar] [CrossRef]
- Grinberga-Zalite, G.; Pilvere, I.; Muska, A.; Kruzmetra, Z. Resilience of Meat Supply Chains during and after COVID-19 Crisis. Emerg. Sci. J. 2021, 5, 57–66. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.; Delaurentis, T. Quality control in food supply chain management: An analytical model and case study of the adulterated milk incident in China. Int. J. Prod. Econ. 2014, 152, 188–199. [Google Scholar] [CrossRef]
- Pearson, S.; May, D.; Leontidis, G.; Swainson, M.; Brewer, S.; Bidaut, L.; Frey, J.G.; Parr, G.; Maull, R.; Zisman, A. Are Distributed Ledger Technologies the panacea for food traceability? Glob. Food Secur. 2019, 20, 145–149. [Google Scholar] [CrossRef]
- Uddin, M. Blockchain Medledger: Hyperledger fabric enabled drug traceability system for counterfeit drugs in pharmaceutical industry. Int. J. Pharm. 2021, 597, 120235. [Google Scholar] [CrossRef]
- Chaudhri, R.; Borriello, G.; Anderson, R. Pervasive computing technologies to monitor vaccine cold chains in developing countries. IEEE Pervasive Comput. Spec. Issue Inf. Commun. Technol. Dev. 2012, 10. Available online: https://ucilnica.fri.uni-lj.si/pluginfile.php/8000/mod_page/content/12/CBA12.pdf (accessed on 1 June 2022).
- Kitinoja, L. Use of cold chains for reducing food losses in developing countries. Population 2013, 6, 5–60. Available online: http://postharvest.org/Use%20of%20cold%20chains%20PEF%20white%20paper%2013-03%20final.pdf (accessed on 1 June 2022).
- Badia-Melis, R.; Mc Carthy, U.; Ruiz-Garcia, L.; Garcia-Hierro, J.; Villalba, J.I.R. New trends in cold chain monitoring applications—A review. Food Control 2018, 86, 170–182. [Google Scholar] [CrossRef]
- Ndraha, N.; Hsiao, H.-I.; Vlajic, J.V.; Yang, M.-F.; Lin, H.-T.V. Time-temperature abuse in the food cold chain: Review of issues, challenges, and recommendations. Food Control 2018, 89, 12–21. [Google Scholar] [CrossRef]
- Aworh, O.C. Food safety issues in fresh produce supply chain with particular reference to sub-Saharan Africa. Food Control 2021, 123, 107737. [Google Scholar] [CrossRef]
- Cerchione, R.; Singh, R.; Centobelli, P.; Shabani, A. Food cold chain management: From a structured literature review to a conceptual framework and research agenda. Int. J. Logist. Manag. 2018, 29, 792–821. [Google Scholar] [CrossRef]
- Comes, T.; Sandvik, K.B.; Van de Walle, B. Cold chains, interrupted: The use of technology and information for decisions that keep humanitarian vaccines cool. J. Humanit. Logist. Supply Chain. Manag. 2018, 8, 49–69. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, S.; Tian, C.; Yan, G.; Wang, D. An overview of current status of cold chain in China. Int. J. Refrig. 2018, 88, 483–495. [Google Scholar] [CrossRef]
- BBremer, P. Towards a reference model for the cold chain. Int. J. Logist. Manag. 2018, 29, 822–838. [Google Scholar] [CrossRef]
- Gligor, D.; Tan, A.; Nguyen, T.N.T. The obstacles to cold chain implementation in developing countries: Insights from Vietnam. Int. J. Logist. Manag. 2018, 29, 942–958. [Google Scholar] [CrossRef]
- Hoang, V. Modern short food supply chain, good agricultural practices, and sustainability: A conceptual framework and case study in Vietnam. Agronomy 2021, 11, 2408. [Google Scholar] [CrossRef]
- Sellitto, M.A.; Vial, L.A.M.; Viegas, C.V. Critical success factors in short food supply chains: Case studies with milk and dairy producers from Italy and Brazil. J. Clean. Prod. 2018, 170, 1361–1368. [Google Scholar] [CrossRef]
- Kallas, Z.; Alba, M.F.; Casellas, K.; Berges, M.; Degreef, G.; Gil, J.M. The development of short food supply chain for locally produced honey: Understanding consumers’ opinions and willingness to pay in Argentina. Br. Food J. 2019, 123, 1664–1680. [Google Scholar] [CrossRef]
- Elghannam, A.; Mesias, F.J.; Escribano, M.; Fouad, L.; Horrillo, A.; Escribano, A.J. Consumers’ Perspectives on Alternative Short Food Supply Chains Based on Social Media: A Focus Group Study in Spain. Foods 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Wubshet Tesfaye, S.A.; Sinnollareddy, M.; Arnold, B.; Brown, A.; Matthew, C.; Oguoma, V.M.; Peterson, G.M.; Thomas, J. How do we combat bogus medicines in the age of the COVID-19 pandemic? Am. J. Trop. Med. Hyg. 2020, 103, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Balas, V.E.; Solanki, V.K.; Kumar, R. (Eds.) An Industrial IoT Approach for Pharmaceutical Industry Growth; Academic Press: Cambridge, MA, USA, 2020; Volume 2. [Google Scholar]
- Wahyuni, H.; Vanany, I.; Ciptomulyono, U. Food safety and halal food in the supply chain: Review and bibliometric analysis. J. Ind. Eng. Manag. 2019, 12, 373–391. [Google Scholar] [CrossRef]
- Sharma, R.; Shishodia, A.; Kamble, S.; Gunasekaran, A.; Belhadi, A. Agriculture supply chain risks and COVID-19: Mitigation strategies and implications for the practitioners. Logist. Res. Appl. 2020, 1–27. [Google Scholar] [CrossRef]
- Nandi, S.; Sarkis, J.; Hervani, A.A.; Helms, M.M. Redesigning Supply Chains using Blockchain-Enabled Circular Economy and COVID-19 Experiences. Sustain. Prod. Consum. 2021, 27, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.; Stevenson, M.; Aitken, J. Blockchain technology: Implications for operations and supply chain management. Supply Chain Manag. Int. J. 2019, 24, 469–483. [Google Scholar] [CrossRef]
- Akhtar, M.M.; Rizvi, D.R. Traceability and detection of counterfeit medicines in pharmaceutical supply chain using blockchain-based architectures. In Sustainable and Energy Efficient Computing Paradigms for Society; Springer: Cham, Switzerland, 2021; pp. 1–31. [Google Scholar] [CrossRef]
- Kazancoglu, Y.; Ekinci, E.; Mangla, S.K.; Sezer, M.D.; Kayikci, Y. Performance evaluation of reverse logistics in food supply chains in a circular economy using system dynamics. Bus. Strat. Environ. 2021, 30, 71–91. [Google Scholar] [CrossRef]
- Guide, V.D.R.; Harrison, T.P.; Van Wassenhove, L.N. The Challenge of Closed-Loop Supply Chains. Interfaces 2003, 33, 3–6. Available online: https://pubsonline.informs.org/doi/abs/10.1287/inte.33.6.3.25182 (accessed on 5 June 2022).
- Govindan, K.; Soleimani, H. A review of reverse logistics and closed-loop supply chains: A Journal of Cleaner Production focus. J. Clean. Prod. 2017, 142, 371–384. [Google Scholar] [CrossRef]
- Ali, S.M.; Arafin, A.; Moktadir, A.; Rahman, T.; Zahan, N. Barriers to Reverse Logistics in the Computer Supply Chain Using Interpretive Structural Model. Glob. J. Flex. Syst. Manag. 2018, 19, 53–68. [Google Scholar] [CrossRef]
- Moghaddam, S.T.; Javadi, M.; Molana, S.M.H. A reverse logistics chain mathematical model for a sustainable production system of perishable goods based on demand optimization. J. Ind. Eng. Int. 2019, 15, 709–721. [Google Scholar] [CrossRef]
- Shirzadi, S.; Ghezavati, V.; Tavakkoli-Moghaddam, R.; Ebrahimnejad, S. Developing a green and bipolar fuzzy inventory-routing model in agri-food reverse logistics with postharvest behavior. Environ. Sci. Pollut. Res. 2021, 28, 41071–41088. [Google Scholar] [CrossRef] [PubMed]
- Paduloh, P.; Djatna, T.; Sukardi, S.; Muslich, M. Uncertainty models in reverse supply chain: A review. Int. J. Supply Chain. Manag. 2020, 9, 139–149. Available online: https://core.ac.uk/download/pdf/322571562.pdf (accessed on 5 June 2022).
- Alshemari, A.; Breen, L.; Quinn, G.; Sivarajah, U. Can We Create a Circular Pharmaceutical Supply Chain (CPSC) to Reduce Medicines Waste? Pharmacy 2020, 8, 221. [Google Scholar] [CrossRef]
- Rebehy, P.C.P.W.; dos Santos Lima, S.A.; Novi, J.C.; Salgado, A.P., Jr. Reverse logistics systems in Brazil: Comparative study and interest of multistakeholders. J. Environ. Manag. 2019, 250, 109223. [Google Scholar] [CrossRef]
- Kargar, S.; Paydar, M.M.; Safaei, A.S. A reverse supply chain for medical waste: A case study in Babol healthcare sector. Waste Manag. 2020, 113, 197–209. [Google Scholar] [CrossRef]
- Kocaoğlu, B.; Küçük, A. Evaluation of the performance of companies operating in the pharmaceutical sector for Reverse Logistics applications with TOPSIS and MOORA Methods. J. Transp. Logist. 2019, 4, 11–30. Available online: https://dergipark.org.tr/en/pub/jtl/issue/49457/632839 (accessed on 5 June 2022). [CrossRef]
- Shadkam, E. Cuckoo optimization algorithm in reverse logistics: A network design for COVID-19 waste management. Waste Manag. Res. J. Sustain. Circ. Econ. 2021, 40, 458–469. [Google Scholar] [CrossRef]
- Alfian, G.; Syafrudin, M.; Farooq, U.; Ma’Arif, M.R.; Syaekhoni, M.A.; Fitriyani, N.L.; Lee, J.; Rhee, J. Improving efficiency of RFID-based traceability system for perishable food by utilizing IoT sensors and machine learning model. Food Control 2020, 110, 107016. [Google Scholar] [CrossRef]
- Rayhana, R.; Xiao, G.; Liu, Z. RFID Sensing Technologies for Smart Agriculture. IEEE Instrum. Meas. Mag. 2021, 24, 50–60. [Google Scholar] [CrossRef]
- Ding, J.; Xu, H.; Li, P.; Zhu, F. Research on Food Safety Traceability Technology Based on RFID Security Authentication and 2-Dimensional Code. In International Conference on Innovative Mobile and Internet Services in Ubiquitous Computing; Springer: Cham, Switzerland, 2018; pp. 517–526. [Google Scholar] [CrossRef]
- Urbano, O.; Perles, A.; Pedraza, C.; Rubio-Arraez, S.; Castelló, M.L.; Ortola, M.D.; Mercado, R. Cost-Effective Implementation of a Temperature Traceability System Based on Smart RFID Tags and IoT Services. Sensors 2020, 20, 1163. [Google Scholar] [CrossRef] [PubMed]
- Peltier-Rivest, D.; Pacini, C. Detecting counterfeit pharmaceutical drugs: A multi-stakeholder forensic accounting strategy. J. Financ. Crime 2019, 26, 1027–1047. [Google Scholar] [CrossRef]
- Islam, I.; Islam, M.N. Digital intervention to reduce counterfeit and falsified medicines: A systematic review and future research agenda. J. King Saud Univ. Comput. Inf. Sci. 2022. [Google Scholar] [CrossRef]
- Wang, L.; Alexander, C.A. Cyber security during the COVID-19 pandemic. AIMS Electron. Electr. Eng. 2021, 5, 146–157. Available online: https://www.aimspress.com/aimspress-data/electreng/2021/2/PDF/electroneng-05-02-008.pdf (accessed on 5 June 2022). [CrossRef]
- Zoughalian, K.; Marchang, J.; Ghita, B. A Blockchain Secured Pharmaceutical Distribution System to Fight Counterfeiting. Int. J. Environ. Res. Public Health 2022, 19, 4091. [Google Scholar] [CrossRef]
- Abugabah, A.; Nizamuddin, N.; Abuqabbeh, A. A review of challenges and barriers implementing RFID technology in the Healthcare sector. Procedia Comput. Sci. 2020, 170, 1003–1010. [Google Scholar] [CrossRef]
- Nuuri, A. The Use of RFID Technology in the Patient Tracking System; Satakunta University of Applied Sciences: Satakunnankatu, Finland, 2022; Available online: https://urn.fi/URN:NBN:fi:amk-202204306603 (accessed on 5 June 2022).
- Ben-Daya, M.; Hassini, E.; Bahroun, Z.; Banimfreg, B.H. The role of internet of things in food supply chain quality management: A review. Qual. Manag. J. 2020, 28, 17–40. [Google Scholar] [CrossRef]
- Thibaud, M.; Chi, H.; Zhou, W.; Piramuthu, S. Internet of Things (IoT) in high-risk Environment, Health and Safety (EHS) industries: A comprehensive review. Decis. Support Syst. 2018, 108, 79–95. [Google Scholar] [CrossRef]
- Shi, X.; An, X.; Zhao, Q.; Liu, H.; Xia, L.; Sun, X.; Guo, Y. State-of-the-Art Internet of Things in Protected Agriculture. Sensors 2019, 19, 1833. [Google Scholar] [CrossRef]
- Ayaz, M.; Ammad-Uddin, M.; Sharif, Z.; Mansour, A.; Aggoune, E.-H.M. Internet-of-Things (IoT)-Based Smart Agriculture: Toward Making the Fields Talk. IEEE Access 2019, 7, 129551–129583. [Google Scholar] [CrossRef]
- Sheth, A.; Jaimini, U.; Yip, H.Y. How Will the Internet of Things Enable Augmented Personalized Health? IEEE Intell. Syst. 2018, 33, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, K.T.; Alsahlany, A.M.; Wadi, S.M.; Kadhum, H.T. An Overview of Patient’s Health Status Monitoring System Based on Internet of Things (IoT). Wirel. Pers. Commun. 2020, 114, 2235–2262. [Google Scholar] [CrossRef]
- Atlam, H.F.; Walters, R.J.; Wills, G.B. Internet of things: State-of-the-art, challenges, applications, and open issues. Int. J. Intell. Comput. Res. (IJICR) 2018, 9, 928–938. [Google Scholar] [CrossRef]
- Usak, M.; Kubiatko, M.; Shabbir, M.S.; Dudnik, O.V.; Jermsittiparsert, K.; Rajabion, L. Health care service delivery based on the Internet of things: A systematic and comprehensive study. Int. J. Commun. Syst. 2020, 33, e4179. [Google Scholar] [CrossRef]
- Evans, J.D. Improving the Transparency of the Pharmaceutical Supply Chain through the Adoption of Quick Response (QR) Code, Internet of Things (IoT), and Blockchain Technology: One Result: Ending the Opioid Crisis. Pittsburgh J. Technol. Law Policy 2019, 19, 35. [Google Scholar] [CrossRef]
- Singh, R.; Dwivedi, A.D.; Srivastava, G. Internet of Things Based Blockchain for Temperature Monitoring and Counterfeit Pharmaceutical Prevention. Sensors 2020, 20, 3951. [Google Scholar] [CrossRef]
- Chen, S.; Liu, X.; Yan, J.; Hu, G.; Shi, Y. Processes, benefits, and challenges for adoption of blockchain technologies in food supply chains: A thematic analysis. Inf. Syst. e-Bus. Manag. 2021, 19, 909–935. [Google Scholar] [CrossRef]
- Labaran, M.J.; Hamma-Adama, M. The Nigerian Pharmaceutical Supply Chain: Blockchain Adoption, Counterfeit Drugs and Successful Deployment of COVID-19 Vaccine in Nigeria. J. Sci. Res. Rep. 2021, 27, 20–36. [Google Scholar] [CrossRef]
- Menon, S.; Jain, K. Blockchain technology for transparency in agri-food supply chain: Use cases, imitations, and future directions. IEEE Trans. Eng. Manag. 2021, 1–15. [Google Scholar] [CrossRef]
- Galvez, J.F.; Mejuto, J.C.; Simal-Gandara, J. Future challenges on the use of blockchain for food traceability analysis. TrAC Trends Anal. Chem. 2018, 107, 222–232. [Google Scholar] [CrossRef]
- Majdalawieh, M.; Nizamuddin, N.; Alaraj, M.; Khan, S.; Bani-Hani, A. Blockchain-based solution for Secure and Transparent Food Supply Chain Network. Peer-to-Peer Netw. Appl. 2021, 14, 3831–3850. [Google Scholar] [CrossRef]
- Tsoukas, V.; Gkogkidis, A.; Kampa, A.; Spathoulas, G.; Kakarountas, A. Blockchain technology in food supply chain: A state of the art. In Proceedings of the 2021 6th South-East Europe Design Automation, Computer Engineering, Computer Networks and Social Media Conference (SEEDA-CECNSM), Preveza, Greece, 24–26 September 2021; pp. 1–8. [Google Scholar] [CrossRef]
- Bapatla, A.K.; Mohanty, S.P.; Kougianos, E. PharmaChain: A Blockchain to Ensure Counterfeit Free Pharmaceutical Supply Chain. arXiv 2022, arXiv:2202.02592. Available online: https://arxiv.org/abs/2202.02592 (accessed on 5 June 2022).
- Dobrucka, R.; Cierpiszewski, R. Active and intelligent packaging food-Research and development-A Review. Pol. J. Food Nutr. Sci. 2014, 64, 1. Available online: https://yadda.icm.edu.pl/yadda/element/bwmeta1.element.agro-840f6a8c-9921-49f7-a0f3-e0406a18c77c (accessed on 5 June 2022). [CrossRef]
- Realini, C.E.; Marcos, B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef] [PubMed]
- BArskA, A.; WyrWA, J. Innovations in the food packaging market–intelligent packaging–A review. Czech J. Food Sci. 2017, 35, 1–6. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, H.; McClements, D.J.; Chen, L.; Jiao, A.; Tian, Y.; Miao, M.; Jin, Z. Recent advances in intelligent food packaging materials: Principles, preparation and applications. Food Chem. 2022, 375, 131738. [Google Scholar] [CrossRef]
- Fernandez, C.M.; Alves, J.; Gaspar, P.D.; Lima, T.M.; Silva, P.D. Innovative processes in smart packaging. A systematic review. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef]
- Fathima, A.; Chiome, T.J.; Catherine, A.A.; Egbuna, C.; Achar, R.R.; Srinivasan, A. Smart Use of Nanomaterials as Sensors for Detection and Monitoring of Food Spoilage. In Application of Nanotechnology in Food Science, Processing and Packaging; Springer: Cham, Switzerland, 2022; pp. 169–188. [Google Scholar] [CrossRef]
- Rostamzad, H. Active and intelligent biodegradable films and polymers. In Biodegradable Polymers, Blends and Composites; Woodhead Publishing: Sawston, UK, 2022; pp. 415–430. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Ko, S. Packaging technology for home meal replacement: Innovations and future prospective. Food Control 2022, 132, 108470. [Google Scholar] [CrossRef]
- Chausali, N.; Saxena, J.; Prasad, R. Recent trends in nanotechnology applications of bio-based packaging. J. Agric. Food Res. 2022, 7, 100257. [Google Scholar] [CrossRef]
- Hui, T.K.L.; Mohammed, B.; Donyai, P.; McCrindle, R.; Sherratt, R.S. Enhancing Pharmaceutical Packaging through a Technology Ecosystem to Facilitate the Reuse of Medicines and Reduce Medicinal Waste. Pharmacy 2020, 8, 58. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Hua, D.; Huang, C.; Samal, S.K.; Xiong, R.; Sauvage, F.; Braeckmans, K.; Remaut, K.; De Smedt, S.C. Materials and Technologies to Combat Counterfeiting of Pharmaceuticals: Current and Future Problem Tackling. Adv. Mater. 2020, 32, 1905486. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, D.; Cheung, W.M. Smart Packaging: Opportunities and Challenges. Procedia CIRP 2018, 72, 1022–1027. [Google Scholar] [CrossRef]
- D’Uva, N.; Camera, F.; Amendola, S.; Nappi, S.; Miozzi, C.; Occhiuzzi, C.; Marrocco, G. Batteryless wireless temperature/humidity sensor for item-level smart pharma packaging. In Proceedings of the 2021 IEEE International Workshop on Metrology for Industry 4.0 & IoT (MetroInd4. 0 & IoT), Rome, Italy, 7–9 June 2021; pp. 145–149. [Google Scholar] [CrossRef]
- Bodena, D.; Teklemariam, Z.; Balakrishnan, S.; Tesfa, T. Bacterial contamination of mobile phones of health professionals in Eastern Ethiopia: Antimicrobial susceptibility and associated factors. Trop. Med. Health 2019, 47, 1–10. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Lima, C.M.; Fujishima, M.A.T.; de Paula Lima, B.; Mastroianni, P.C.; de Sousa, F.F.O.; da Silva, J.O. Microbial contamination in herbal medicines: A serious health hazard to elderly consumers. BMC Complement. Med. Ther. 2020, 20, 17. [Google Scholar] [CrossRef] [PubMed]


| Keywords | Search Strings | Databases |
|---|---|---|
| 1. Identify factors that affect food quality and proposed solutions to achieve public health safety. | Factors (OR issues) affect food quality AND Solutions for food quality AND Food supply chain | Web of Science Scopus ScienceDirect Emerald insight Wiley IEEE Research gate Google Scholar |
| 2. Identify factors that affect drug safety and proposed solutions to achieve public health safety. | Factors (OR issues) affect food quality AND Solutions for food quality AND Pharmaceutical supply chain | |
| 3. Identify similar solutions to overcome public health issues in the food and drug industries and achieve an integrated supply chain. | Effective solutions (OR tools) AND Emerging technologies AND Integrated supply chain AND Food quality (OR Drug safety) AND Similar issues |
| Exclusions | Description |
|---|---|
| 1. Initial search (Filter 1) | The search engines were used, and the abstract, introduction, and conclusion of articles in selected journals were skimmed. This search generated 298 papers. |
| 2. First exclusion (Filter 2) | The filter generated 245 papers. Duplicate papers were removed, and papers irrelevant to food or drug issues in their prospective supply chains or those that could be classified as similar for both were also removed. |
| 3. Second exclusion (Filter 3) | The filter was applied to refine the final selection and generated 205 articles. Only those with relevant information for each objective of this study, such as basic information for the introduction, factors that affect food quality and drug safety, and solutions to achieve an integrated supply chain and ensure public health safety, were included. |
| Food | Drugs | |
|---|---|---|
| Number of articles | 112 | 56 |
| Original research | 84 | 48 |
| Literature review | 28 | 8 |
| Region | ||
| Africa | 7 | 7 |
| Asia | 12 | 9 |
| Europe | 10 | 4 |
| North America | 7 | 4 |
| Oceania | 2 | - |
| South America | 6 | 4 |
| Australia and Pacific | 3 | - |
| Worldwide | 55 | 36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haji, M.; Kerbache, L.; Al-Ansari, T. Food Quality, Drug Safety, and Increasing Public Health Measures in Supply Chain Management. Processes 2022, 10, 1715. https://doi.org/10.3390/pr10091715
Haji M, Kerbache L, Al-Ansari T. Food Quality, Drug Safety, and Increasing Public Health Measures in Supply Chain Management. Processes. 2022; 10(9):1715. https://doi.org/10.3390/pr10091715
Chicago/Turabian StyleHaji, Mona, Laoucine Kerbache, and Tareq Al-Ansari. 2022. "Food Quality, Drug Safety, and Increasing Public Health Measures in Supply Chain Management" Processes 10, no. 9: 1715. https://doi.org/10.3390/pr10091715
APA StyleHaji, M., Kerbache, L., & Al-Ansari, T. (2022). Food Quality, Drug Safety, and Increasing Public Health Measures in Supply Chain Management. Processes, 10(9), 1715. https://doi.org/10.3390/pr10091715






