Fabrication and Characterization of Activated Carbon from Phyllostachys edulis Using Single-Step KOH Activation with Different Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Activated Carbon
- M: Activated carbon weight after activation
- M1: Activated carbon weight after wash
- M0: Initial weight of precursor
- m: Weight of KOH
2.3. Characterization of Activated Carbon
2.4. Statistical Analysis
3. Results and Discussion
3.1. Surface Morphology Characterization
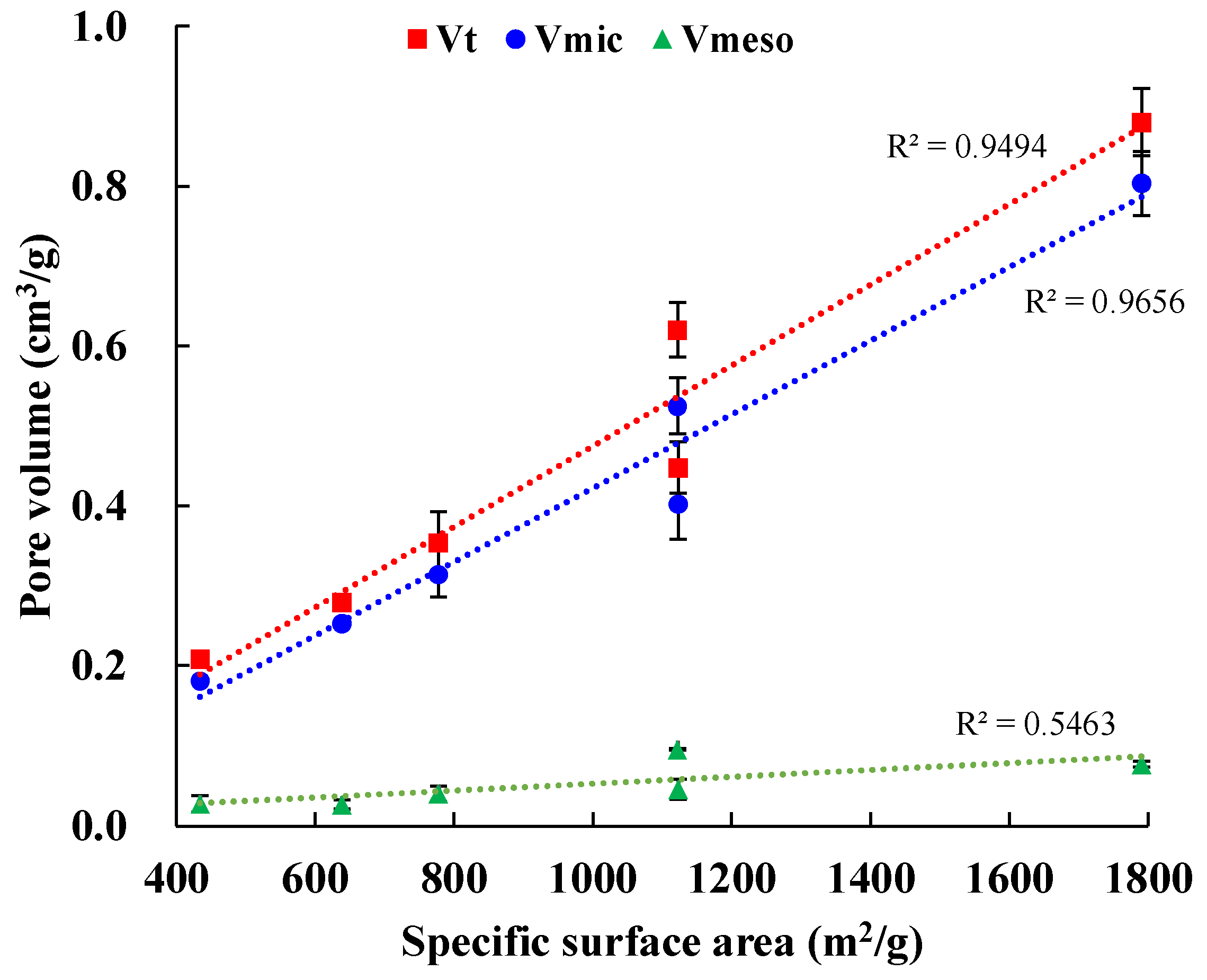
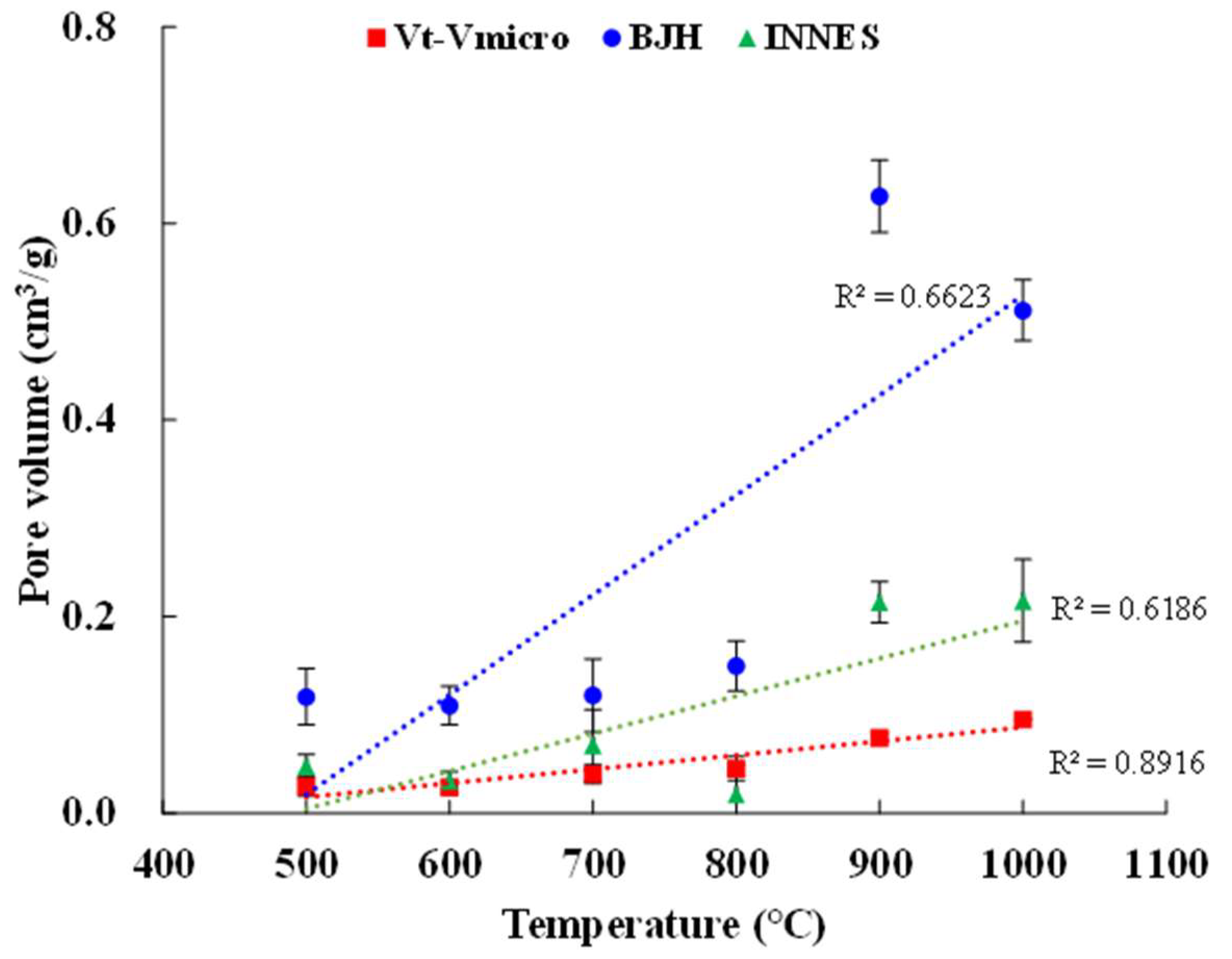
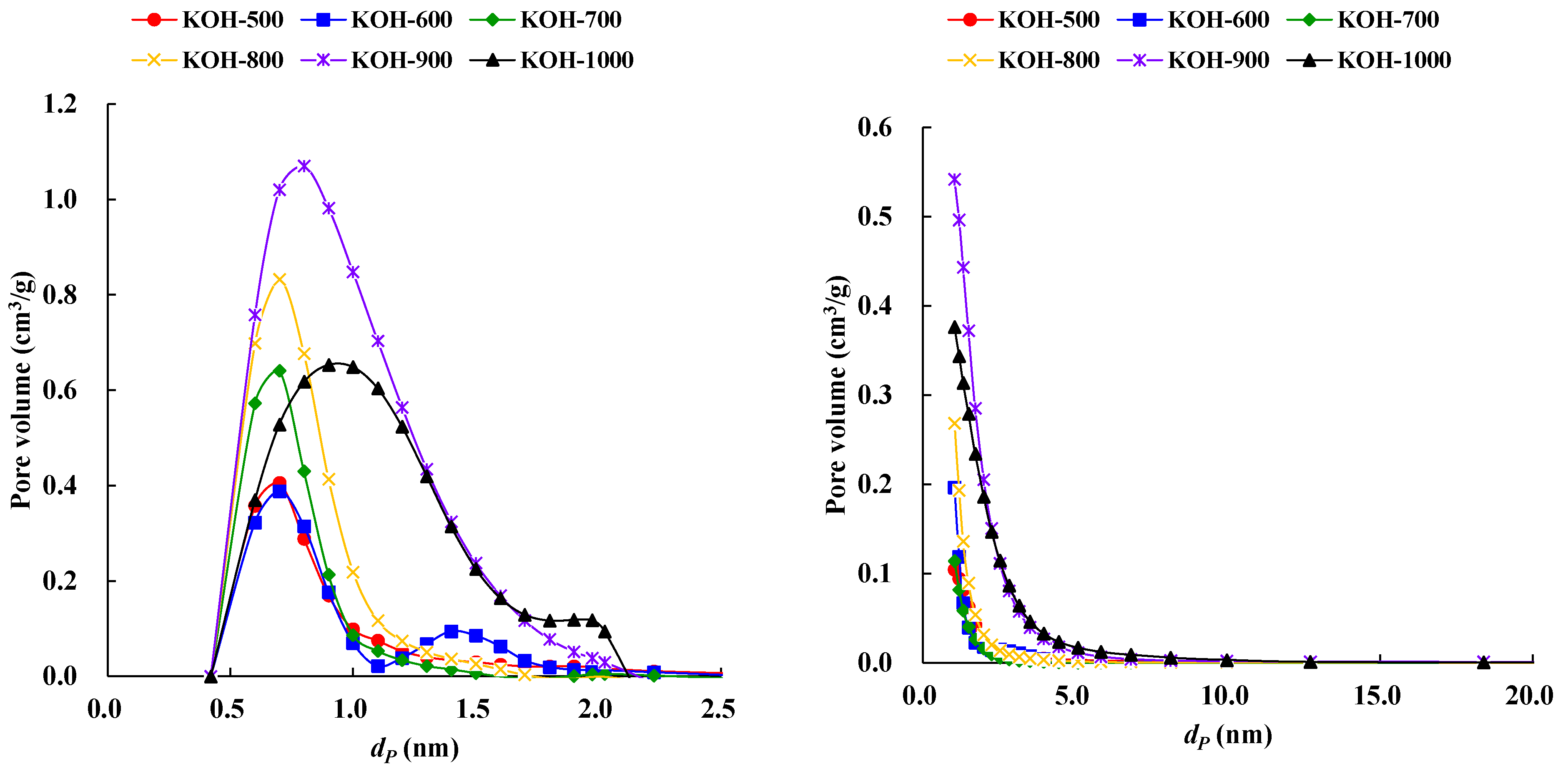
3.2. Surface Area and Porosity
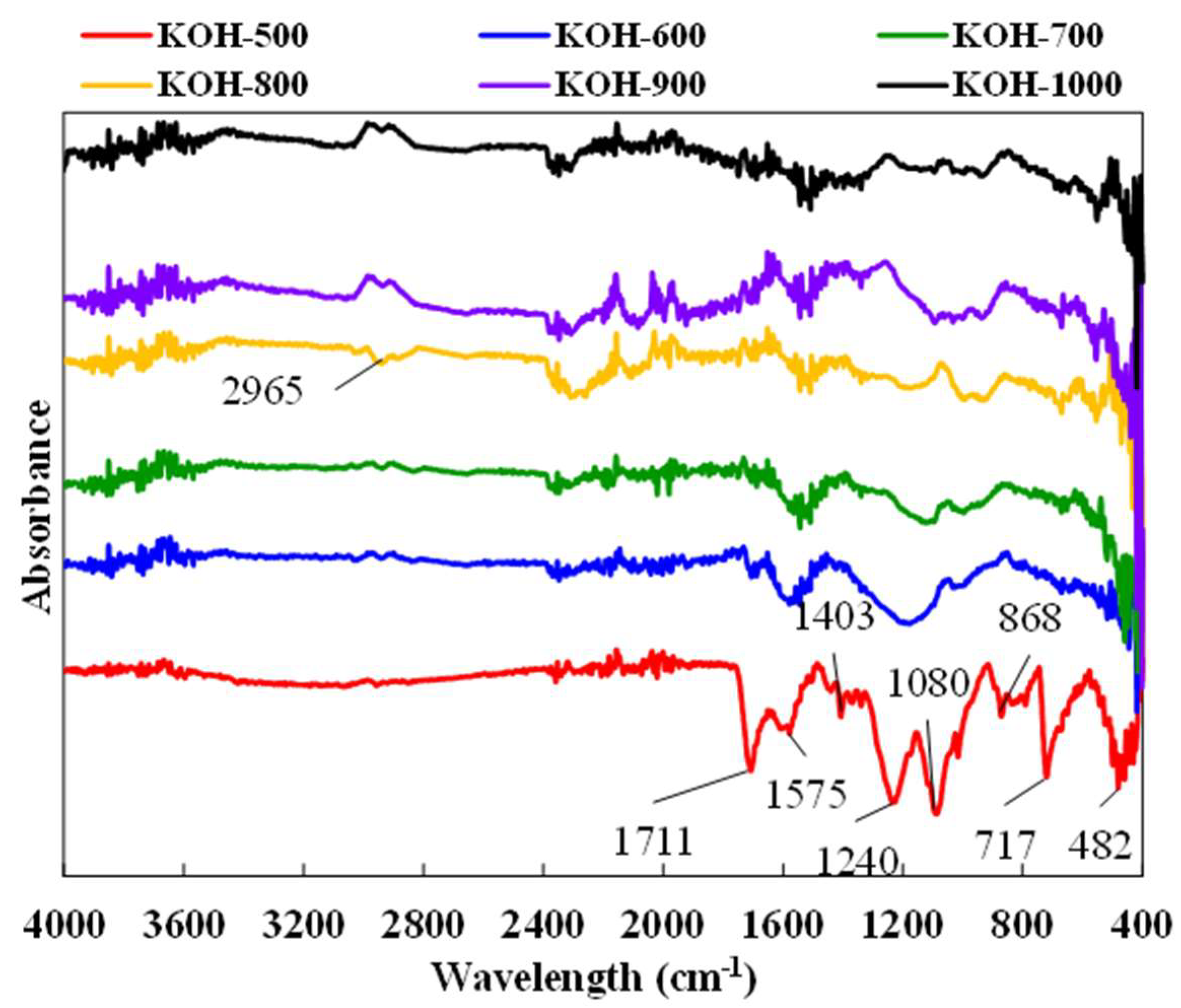
3.3. Surface Chemistry
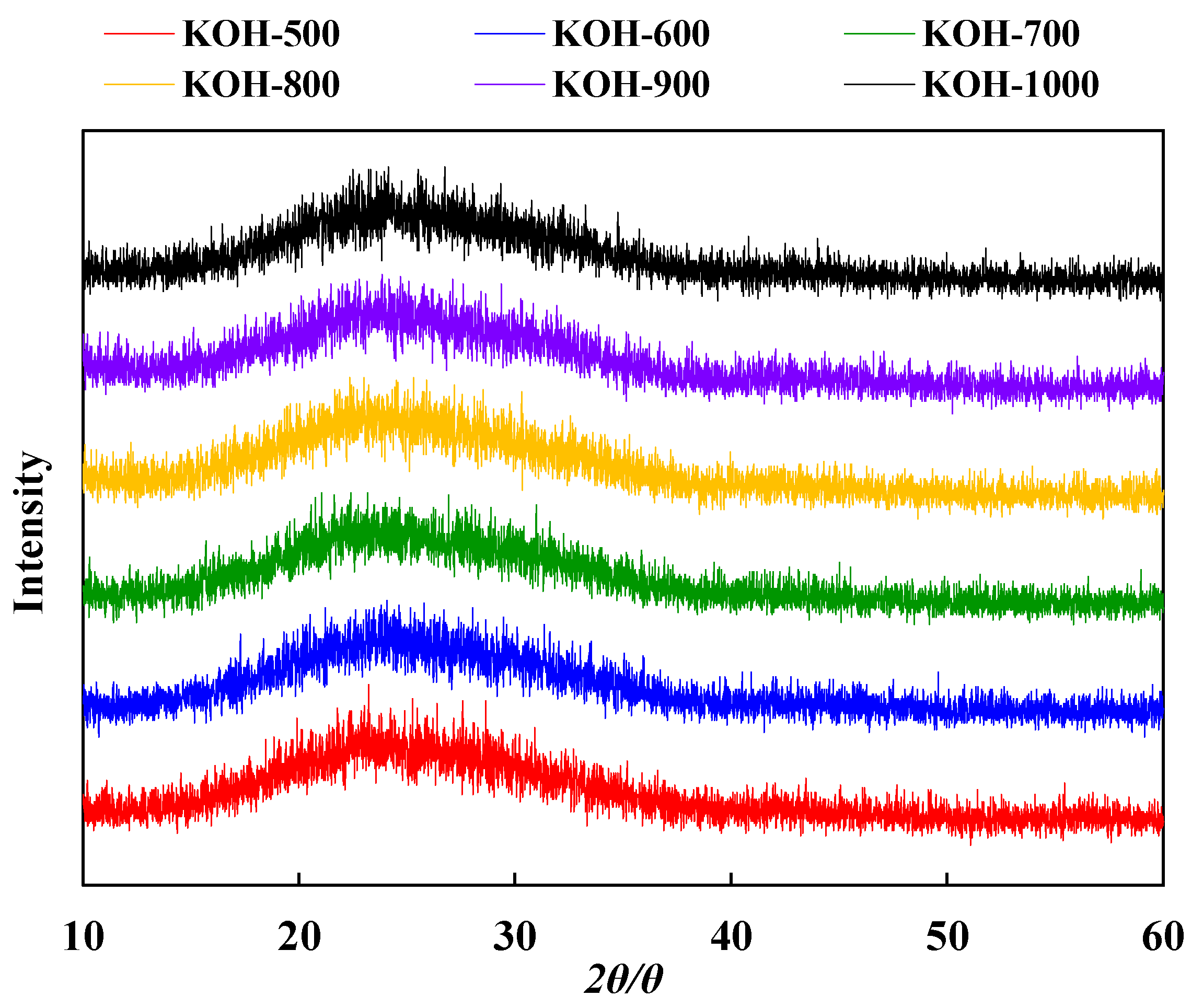
3.4. Structure Evolution of Activated Carbon
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNEP (United Nations Environment Programme). Converting Waste Agricultural Biomass into a Resource; United Nations Environment Programme Division of Technology, Industry and Economics International Environmental Technology Centre: Osaka/Shiga, Japan, 2015; Available online: www.unep.org/ietc/Portals/136/Publications/Waste%20Management/WasteAgriculturalBiomassEST_Compendium.pdf (accessed on 20 August 2022).
- Chun, A.M.S. Ground Rules for Humanitarian Design; Chun, A.M.S., Brisson, E., Eds.; Wiley Publishers: London, UK, 2015; ISBN 978-1-118-36159-7. [Google Scholar]
- Sha, T.; Liu, J.; Sun, M.; Li, L.; Bai, J.; Hu, Z.; Zhou, M. Green and low-cost synthesis of nitrogen-doped graphene-like mesoporous nanosheets from the biomass waste of okara for the amperometric detection of vitamin C in real samples. Talanta 2019, 200, 300–306. [Google Scholar] [CrossRef]
- Xu, C.; Nasrollahzadeh, M.; Selva, M.; Issaabadi, Z.; Luque, R. Waste-to-wealth: Biowaste valorization into valuable bio (nano) materials. Chem. Soc. Rev. 2019, 48, 4791–4822. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Hills, C.D.; Singh, R.S.; Atkinson, C.J. Biomass waste utilisation in low-carbon products: Harnessing a major potential resource. NPJ Clim. Atmos. Sci. 2019, 2, 35. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y. Recent progress in the conversion of biomass wastes into functional materials for value-added applications. Sci. Technol. Adv. Mater. 2020, 21, 787–804. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Bystrzejewski, M.; De Adhikari, A.; Huczko, A.; Wang, N. Methods for the conversion of biomass waste into value-added carbon nanomaterials: Recent progress and applications. Prog. Energy Combust. Sci. 2022, 92, 101023. [Google Scholar] [CrossRef]
- Hadoun, H.; Sadaoui, Z.; Souami, N.; Sahel, D.; Toumert, I. Characterization of mesoporous carbon prepared from date stems by H 3 PO 4 chemical activation. Appl. Surf. Sci. 2013, 280, 1–7. [Google Scholar] [CrossRef]
- Chandra, T.C.; Mirna, M.M.; Sunarso, J.; Sudaryanto, Y.; Ismadji, S. Activated carbon from durian shell: Preparation and characterization. J. Taiwan Inst. Chem. Eng. 2009, 40, 457–462. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Ouki, S.K.; Neufeld, R.D. Use of activated carbon for the recovery of chromium from industrial wastewaters. J. Chem. Technol. Biotechnol. 1997, 70, 3–8. [Google Scholar] [CrossRef]
- Valdés, H.; Sánchez-Polo, M.; Rivera-Utrilla, J.; Zaror, C.A. Effect of ozone treatment on surface properties of activated carbon. Langmuir 2002, 18, 2111–2116. [Google Scholar] [CrossRef]
- Cazetta, A.L.; Spessato, L.; Almeida, V.C. The use of chemometric tools for screening and optimization of variables in the preparation and application of carbon-based materials. J. Taiwan Inst. Chem. Eng. 2021, 121, 321–336. [Google Scholar] [CrossRef]
- Weber, F.J.; Hartmans, S. Use of activated carbon as a buffer in biofiltration of waste gases with fluctuating concentrations of toluene. Appl. Microbiol. Biotechnol. 1995, 43, 365–369. [Google Scholar] [CrossRef]
- Ukanwa, K.S.; Patchigolla, K.; Sakrabani, R.; Anthony, E.; Mandavgane, S. A Review of Chemicals to Produce Activated Carbon from Agricultural Waste Biomass. Sustainability 2019, 11, 6204. [Google Scholar] [CrossRef]
- Wang, Z.; Stapleton, C. Phyllostachys Edulis. Flora of China; Missouri Botanical Garden: St. Louis, MO, USA; Harvard University Herbaria: Cambridge, MA, USA, 2008. [Google Scholar]
- Nunes, L.J.R.; Matias, J.C.O.; Catalão, J.P.S. Biomass combustion systems: A review on the physical and chemical properties of the ashes. Renew. Sustain. Energy Rev. 2016, 53, 235–242. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Kim, K.H. Sampling, pretreatment, and analysis of particulate matter and trace metals emitted through charcoal combustion in cooking activities. TrAC-Trends Anal. Chem. 2016, 76, 52–59. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 1–9. [Google Scholar] [CrossRef]
- Chen, D.; Liu, D.; Zhang, H.; Chen, Y.; Li, Q. Bamboo pyrolysis using TG-FTIR and a lab-scale reactor: Analysis of pyrolysis behavior, product properties, and carbon and energy yields. Fuel 2015, 148, 79–86. [Google Scholar] [CrossRef]
- 20 Uses of Bamboo For Home, Office and Yourself (Infographic)-Bamboooz. Available online: http://www.bamboooz.com/20-uses-bamboo/ (accessed on 20 August 2022).
- Lan, F.L.; Lay, H.L.; Teng, W.F. Study on the moso bamboo charcoal property. Crop Environ. Bioinform. 2008, 5, 180–186. [Google Scholar]
- Wang, S.; Tsai, M.; Lo, S.; Tsai, M. Effects of manufacturing conditions on the adsorption capacity of heavy metal ions by Makino bamboo charcoal. Bioresour. Technol. 2008, 99, 7027–7033. [Google Scholar] [CrossRef]
- Jiang, S. Training Manual of Bamboo Charcoal for Producers and Consumers; Bamboo Engineering Research Center, Nanjing Forestry University: Nanjing, China, 2004; Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.terrapreta.bioenergylists.org/files/Training%20Manual.pdf (accessed on 20 August 2022).
- Horikawa, T.; Kitakaze, Y.; Sekida, T.; Hayashi, J.; Katoh, M. Characteristics and humidity control capacity of activated carbon from bamboo. Bioresour. Technol. 2010, 101, 3964–3969. [Google Scholar] [CrossRef]
- Huang, P.H.; Jhan, J.W.; Cheng, Y.M.; Cheng, H.H. Effects of carbonization parameters of Moso-bamboo-based porous charcoal on capturing carbon dioxide. Sci. World J. 2014, 2014, 937867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariana, M.; Mistar, E.M.; Alfatah, T.; Supardan, M.D. High-porous activated carbon derived from Myristica fragrans shell using one-step KOH activation for methylene blue adsorption. Bioresour. Technol. Rep. 2021, 16, 100845. [Google Scholar] [CrossRef]
- Angin, D. Production and characterization of activated carbon from sour cherry stones by zinc chloride. Fuel 2014, 115, 804–811. [Google Scholar] [CrossRef]
- Lee, J.H.; Heo, Y.J.; Park, S.J. Effect of silica removal and steam activation on extra-porous activated carbons from rice husks for methane storage. Int. J. Hydrogen Energy 2018, 43, 22377–22384. [Google Scholar] [CrossRef]
- Alfatah, T.; Mistar, E.M.; Supardan, M.D. Porous structure and adsorptive properties of activated carbon derived from Bambusa vulgaris striata by two-stage KOH/NaOH mixture activation for Hg2+ removal. J. Water Process Eng. 2021, 43, 102294. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B.; Gupta, V.; Sharma, P. Chemically activated carbon from lignocellulosic wastes for heavy metal wastewater remediation: Effect of activation conditions. J. Colloid Interface Sci. 2017, 493, 228–240. [Google Scholar] [CrossRef]
- Hu, Z.; Li, X.; Tu, Z.; Wang, Y.; Dacres, O.D.; Sun, Y.; Yao, H. ‘Thermal dissolution carbon enrichment’ treatment of biomass wastes: Supercapacitor electrode preparation using the residue. Fuel Process. Technol. 2019, 205, 106430. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Comparisons of porous and adsorption properties of carbons activated by steam and KOH. J. Colloid Interface Sci. 2005, 283, 49–56. [Google Scholar] [CrossRef]
- Nagaraju, G.; Lim, J.H.; Cha, S.M.; Yu, J.S. Three-dimensional activated porous carbon with meso/macropore structures derived from fallen pine cone flowers: A low-cost counter electrode material in dye-sensitized solar cells. J. Alloys Compd. 2017, 693, 1297–1304. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Sun, J.; Li, W.; Zhang, J.; Zhao, C. Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption. Chem. Eng. J. 2020, 381, 122736. [Google Scholar] [CrossRef]
- Li, H.; Shi, F.; An, Q.; Zhai, S.; Wang, K.; Tong, Y. Three-dimensional hierarchical porous carbon derived from lignin for supercapacitors: Insight into the hydrothermal carbonization and activation. Int. J. Biol. Macromol. 2021, 166, 923–933. [Google Scholar] [CrossRef]
- Yang, L.; Feng, Y.; Cao, M.; Yao, J. Two-step preparation of hierarchical porous carbon from KOH-activated wood sawdust for supercapacitor. Mater. Chem. Phys. 2019, 238, 121956. [Google Scholar] [CrossRef]
- Wan, L.; Song, P.; Liu, J.; Chen, D.; Xiao, R.; Zhang, Y.; Chen, J.; Xie, M.; Du, C. Facile synthesis of nitrogen self-doped hierarchical porous carbon derived from pine pollen via MgCO3 activation for high-performance supercapacitors. J. Power Sources 2019, 438, 227013. [Google Scholar] [CrossRef]
- Mistar, E.M.; Alfatah, T.; Supardan, M.D. Synthesis and characterization of activated carbon from Bambusa vulgaris striata using two-step KOH activation. J. Mater. Res. Technol. 2020, 9, 6278–6286. [Google Scholar] [CrossRef]
- Herde, Z.D.; Dharmasena, R.; Sumanasekera, G.; Tumuluru, J.S.; Satyavolu, J. Impact of hydrolysis on surface area and energy storage applications of activated carbons produced from corn fiber and soy hulls. Carbon Resour. Convers. 2020, 3, 19–28. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, J.; Jin, C.; Wang, Z.; Wang, T.; Cheng, X.; Ma, C. Effects of temperature, oxygen and steam on pore structure characteristics of coconut husk activated carbon powders prepared by one-step rapid pyrolysis activation process. Bioresour. Technol. 2020, 310, 123413. [Google Scholar] [CrossRef]
- Fu, P.; Hu, S.; Xiang, J.; Yi, W.; Bai, X.; Sun, L.; Su, S. Evolution of char structure during steam gasification of the chars produced from rapid pyrolysis of rice husk. Bioresour. Technol. 2012, 114, 691–697. [Google Scholar] [CrossRef]
- Norouzi, S.; Heidari, M.; Alipour, V.; Rahmanian, O.; Fazlzadeh, M.; Mohammadi-Moghadam, F.; Nourmoradi, H.; Goudarzi, B.; Dindarloo, K. Preparation, characterization and Cr(VI) adsorption evaluation of NaOH-activated carbon produced from Date Press Cake; an agro-industrial waste. Bioresour. Technol. 2018, 258, 48–56. [Google Scholar] [CrossRef]
- Ikuo, A. Production Methods of Activated Carbon. No. 225. 2006, pp. 378–381. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.jstage.jst.go.jp/article/tanso1949/2006/225/2006_225_373/_pdf (accessed on 28 February 2022).
- Hayashi, J.; Hasegawa, I.; Hagihara, T.; Takara, T. Production of activated carbon with a large specific surface area from banana peel and its methane adsorption ability. Carbon 2021, 176, 656. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Zhang, Y.; He, J.; Wang, Y.; Wang, K.; Xu, Y.; Li, H.; Wang, Y. Biomass-derived microporous carbon with large micropore size for high-performance supercapacitors. J. Power Sources 2020, 448, 227396. [Google Scholar] [CrossRef]
- Wang, Q.; Tuohedi, N. Polyurethane foams and bio-polyols from liquefied cotton stalk agricultural waste. Sustainability 2020, 12, 4214. [Google Scholar] [CrossRef]
- Chen, S.; Xia, Y.; Zhang, B.; Chen, H.; Chen, G.; Tang, S. Disassembly of lignocellulose into cellulose, hemicellulose, and lignin for preparation of porous carbon materials with enhanced performances. J. Hazard. Mater. 2020, 408, 124956. [Google Scholar] [CrossRef] [PubMed]
- Al Bahri, M.; Calvo, L.; Gilarranz, M.A.; Rodriguez, J.J. Activated carbon from grape seeds upon chemical activation with phosphoric acid: Application to the adsorption of diuron from water. Chem. Eng. J. 2012, 203, 348–356. [Google Scholar] [CrossRef]
- Qin, C.; Wang, S.; Wang, Z.; Ji, K.; Wang, S.; Zeng, X.; Liu, G. Hierarchical porous carbon derived from Gardenia jasminoides Ellis flowers for high performance supercapacitor. J. Energy Storage 2021, 33, 102061. [Google Scholar] [CrossRef]
- Byamba-Ochir, N.; Shim, W.G.; Balathanigaimani, M.S.; Moon, H. Highly porous activated carbons prepared from carbon rich Mongolian anthracite by direct NaOH activation. Appl. Surf. Sci. 2016, 379, 331–337. [Google Scholar] [CrossRef]
- Shi, M.; Xin, Y.; Chen, X.; Zou, K.; Jing, W.; Sun, J.; Liu, Y. Coal-derived porous activated carbon with ultrahigh specific surface area and excellent electrochemical performance for supercapacitors. J. Alloys Compd. 2021, 859, 157856. [Google Scholar] [CrossRef]
- Lu, W.; Cao, X.; Hao, L.; Zhou, Y.; Wang, Y. Activated carbon derived from pitaya peel for supercapacitor applications with high capacitance performance. Mater. Lett. 2020, 264, 127339. [Google Scholar] [CrossRef]
- Wan, L.; Chen, D.; Liu, J.; Zhang, Y.; Chen, J.; Du, C.; Xie, M. Facile preparation of porous carbons derived from orange peel via basic copper carbonate activation for supercapacitors. J. Alloys Compd. 2020, 823, 153747. [Google Scholar] [CrossRef]


| Parameter | Value | |
|---|---|---|
| Proximate analysis | Moisture | 7.0 |
| Volatile Matter | 81.0 | |
| Ash | 0.2 | |
| Fixed Carbon | 11.8 | |
| Ultimate analysis | C | 49.0 |
| H | 6.0 | |
| N | 0.3 | |
| O | 44.5 | |
| S | 0.0 | |
| Lignocellulosic analysis | Holocellulose | 72.4 |
| Lignin | 27.6 |
| SBET (m2/g) | Vt (cm3/g) | Vmic (cm3/g) | Vmeso (cm3/g) | Vmic/Vt (%) | dave (nm) | |
|---|---|---|---|---|---|---|
| KOH-1000 | 1122 | 0.6203 | 0.525 | 0.0954 | 84.63 | 2.16 |
| KOH-900 | 1791 | 0.8801 | 0.8034 | 0.0767 | 91.29 | 1.87 |
| KOH-800 | 1123 | 0.4479 | 0.4025 | 0.0454 | 89.86 | 1.51 |
| KOH-700 | 778.1 | 0.3542 | 0.3144 | 0.0398 | 88.76 | 1.67 |
| KOH-600 | 639.3 | 0.2795 | 0.2533 | 0.0262 | 90.63 | 1.78 |
| KOH-500 | 434.6 | 0.2089 | 0.1814 | 0.0275 | 86.83 | 2.02 |
| Raw Material | Activator | Impregnation Ratio | Activation Temperature (°C) | Holding Time (h) | SBET | Vt (cm3/g) | References |
|---|---|---|---|---|---|---|---|
| (m2/g) | |||||||
| Lignin | KOH | 1:1 | 800 | 3 | 1504 | 0.757 | [36] |
| Pistachio shells | KOH | 1:1 | 780 | 1 | 1096 | 0.61 | [33] |
| Pinecone | KOH | 1:1 | 900 | 2 | 1589 | - | [34] |
| Wood sawdust | KOH | 5 g 100 mL | 800 | 1.5 | 1185 | 0.562 | [37] |
| 5 wt.% | |||||||
| Pine pollen | MgCO3 | 6:1 | 850 | 2 | 1311.2 | 0.69 | [38] |
| Sugarcane bagasse | NaOH | 30 wt.% | 850 | 1.5 | 1149 | 1.73 | [35] |
| Bambusa vulgaris striata | KOH | 3:1 | 800 | 1 | 980 | 0.559 | [39] |
| Moso bamboo | KOH | 1:1 | 900 | 1 | 1790.8 | 0.88 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Wang, Q. Fabrication and Characterization of Activated Carbon from Phyllostachys edulis Using Single-Step KOH Activation with Different Temperatures. Processes 2022, 10, 1712. https://doi.org/10.3390/pr10091712
Guo Y, Wang Q. Fabrication and Characterization of Activated Carbon from Phyllostachys edulis Using Single-Step KOH Activation with Different Temperatures. Processes. 2022; 10(9):1712. https://doi.org/10.3390/pr10091712
Chicago/Turabian StyleGuo, Yue, and Qingyue Wang. 2022. "Fabrication and Characterization of Activated Carbon from Phyllostachys edulis Using Single-Step KOH Activation with Different Temperatures" Processes 10, no. 9: 1712. https://doi.org/10.3390/pr10091712
APA StyleGuo, Y., & Wang, Q. (2022). Fabrication and Characterization of Activated Carbon from Phyllostachys edulis Using Single-Step KOH Activation with Different Temperatures. Processes, 10(9), 1712. https://doi.org/10.3390/pr10091712







