F-Doped Ni-Rich Layered Cathode Material with Improved Rate Performance for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coprecipitation Synthesis of LiNi0.8Co0.1Mn0.1O2−xFx (x = 0.0005, 0.001, 0.005)
2.2. Morphology and Structure Characterization
2.3. Electrochemical Measurements
3. Results
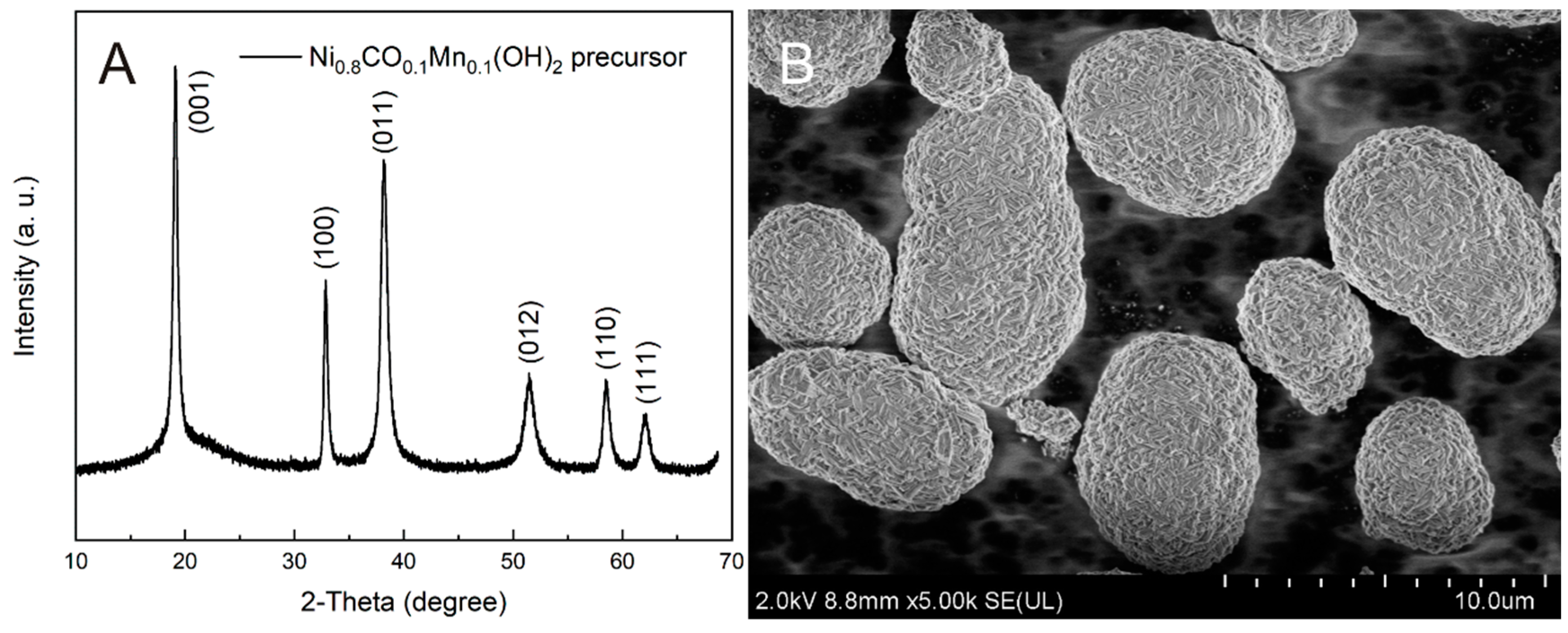
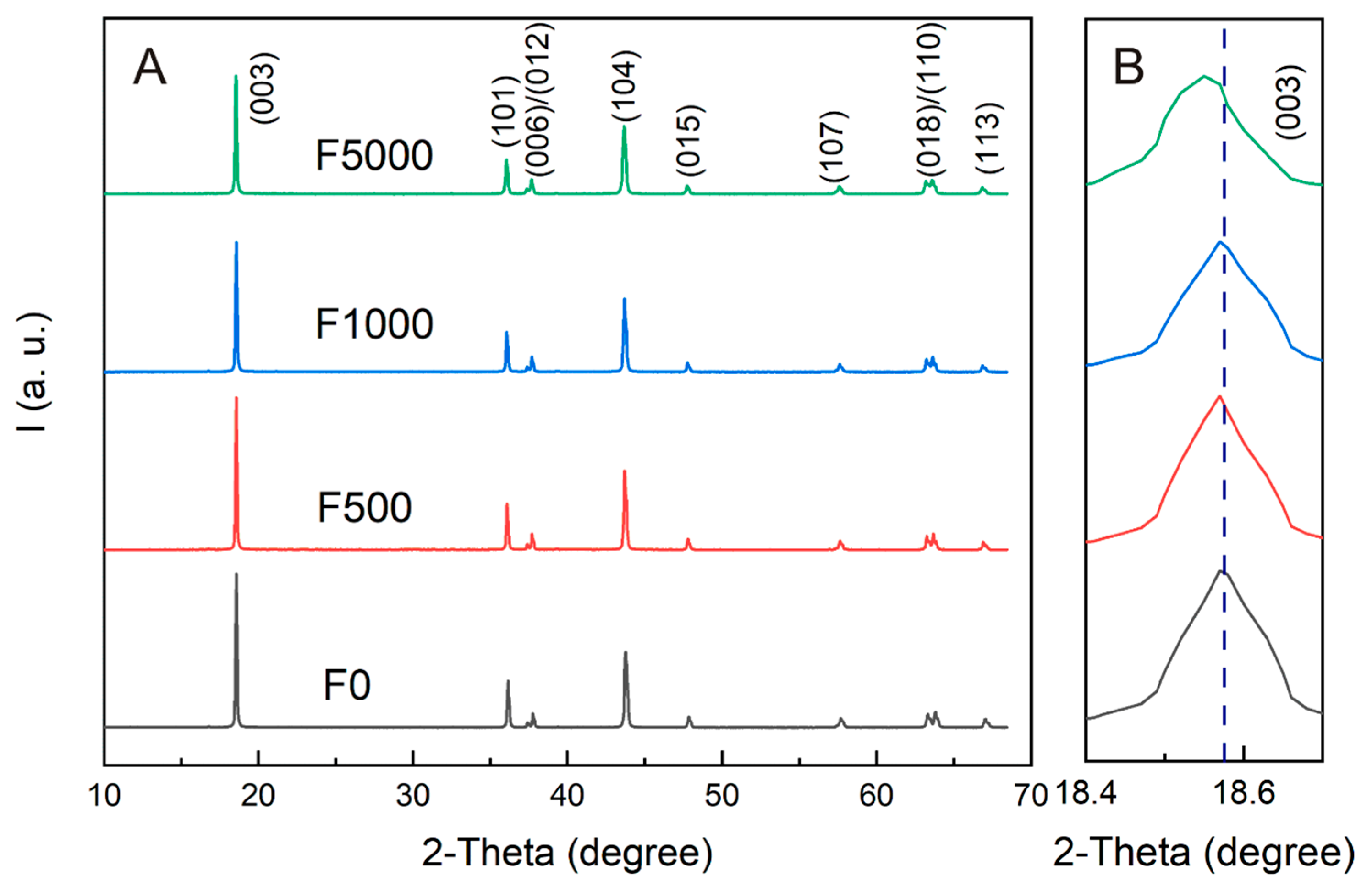
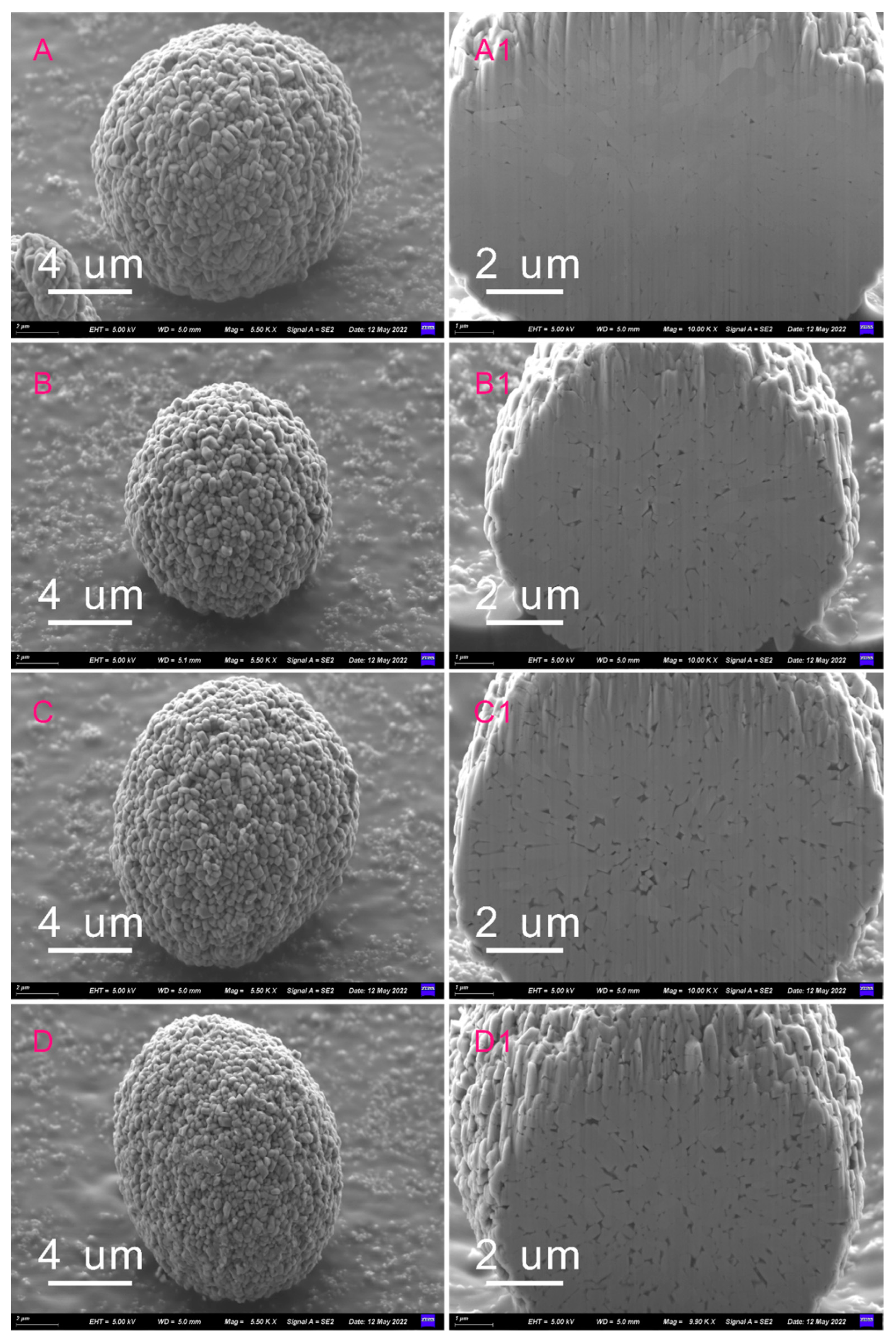
3.1. Structure and Morphology Analysis
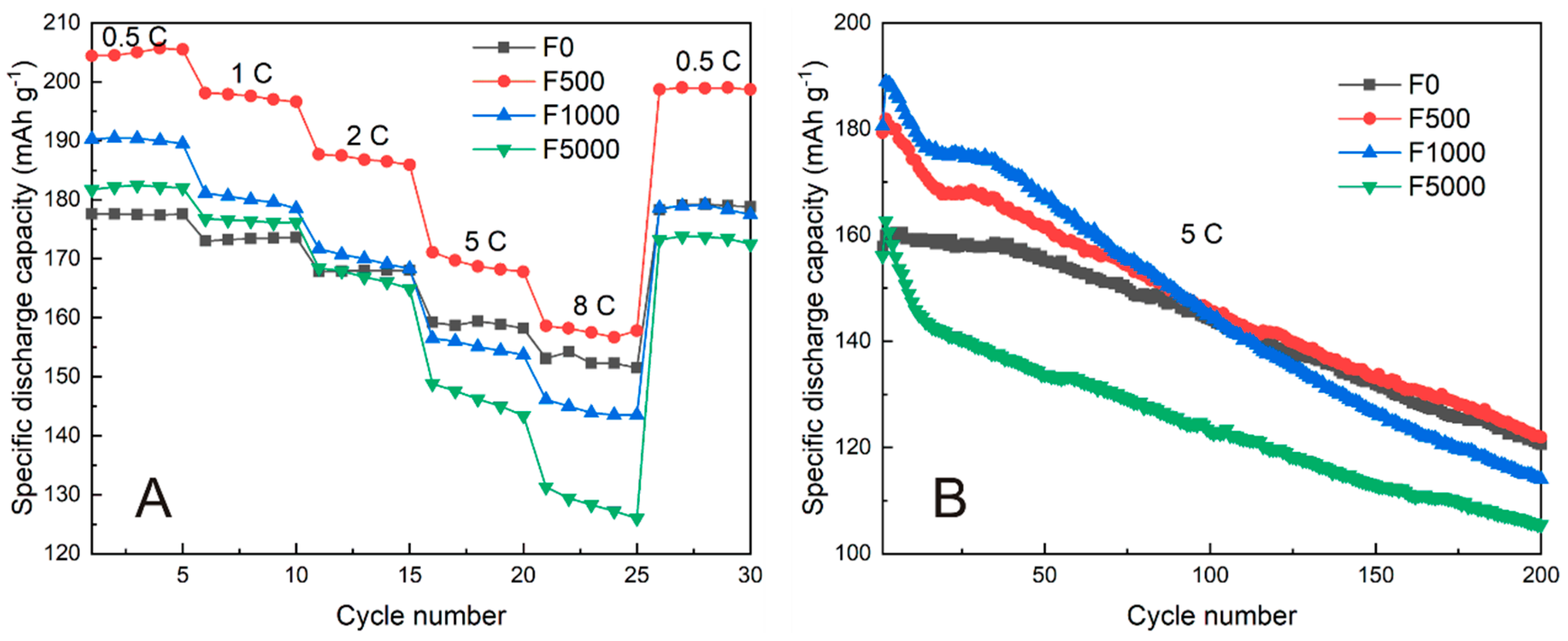
3.2. Electrochemical Performance
3.3. Density Functional Theory (DFT) Calculation
4. Discussion
4.1. EIS Analysis before and after Cycling
4.2. XPS Analysis before and after Cycling
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qian, R.; Liu, Y.; Cheng, T.; Li, P.; Chen, R.; Lyu, Y.; Guo, B. Enhanced Surface Chemical and Structural Stability of Ni-Rich Cathode Materials by Synchronous Lithium-Ion Conductor Coating for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 13813–13823. [Google Scholar] [CrossRef]
- Su, Y.; Chen, G.; Chen, L.; Li, W.; Zhang, Q.; Yang, Z.; Lu, Y.; Bao, L.; Tan, J.; Chen, R.; et al. Exposing the {010} Planes by Oriented Self-Assembly with Nanosheets To Improve the Electrochemical Performances of Ni-Rich Li[Ni0.8Co0.1Mn0.1]O2 Microspheres. ACS Appl. Mater. Interfaces 2018, 10, 6407–6414. [Google Scholar] [CrossRef]
- Su, Y.; Chen, G.; Chen, L.; Li, W.; Zhang, Q.; Lv, Z.; Li, C.; Li, L.; Liu, N.; Tan, G.; et al. High-Rate Struc-ture-Gradient Ni-Rich Cathode Material for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 36697–36704. [Google Scholar] [CrossRef]
- Liang, H.; Wang, Z.; Guo, H.; Wang, J.; Leng, J. Improvement in the electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode material by Li2ZrO3 coating. Appl. Surf. Sci. 2017, 423, 1045–1053. [Google Scholar] [CrossRef]
- Dong, S.; Zhou, Y.; Hai, C.; Zeng, J.; Sun, Y.; Shen, Y.; Li, X.; Ren, X.; Qi, G.; Zhang, X.; et al. Ultrathin CeO2 coating for improved cycling and rate performance of Ni-rich layered LiNi0.7Co0.2Mn0.1O2 cathode materials. Ceram. Int. 2019, 45, 144–152. [Google Scholar] [CrossRef]
- Qin, C.; Cao, J.; Chen, J.; Dai, G.; Wu, T.; Chen, Y.; Tang, Y.; Li, A.; Chen, Y. Improvement of electrochemical performance of nickel rich LiNi0.6Co0.2Mn0.2O2 cathode active material by ultrathin TiO2 coating. Dalton Trans. 2016, 45, 9669–9675. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Y.; Fan, C.; Han, S. A highly stabilized single crystalline nickel-rich LiNi0.8Co0.1Mn0.1O2 cathode through a novel surface spinel-phase modification. Electrochim. Acta 2020, 341, 136075. [Google Scholar] [CrossRef]
- Li, X.; Kang, F.; Shen, W.; Bai, X. Improvement of structural stability and electrochemical activity of a cathode material LiNi0.7Co0.3O2 by chlorine doping. Electrochim. Acta 2007, 53, 1761–1765. [Google Scholar] [CrossRef]
- Wu, F.; Liu, N.; Chen, L.; Su, Y.; Tan, G.; Bao, L.; Zhang, Q.; Lu, Y.; Wang, J.; Chen, S.; et al. Improving the reversibility of the H2-H3 phase transitions for layered Ni-rich oxide cathode towards retarded structural transition and enhanced cycle stability. Nano Energy 2019, 59, 50–57. [Google Scholar] [CrossRef]
- Yue, P.; Wang, Z.; Wang, J.; Guo, H.; Xiong, X.; Li, X. Effect of fluorine on the electrochemical performance of spherical LiNi0.8Co0.1Mn0.1O2 cathode materials via a low temperature method. Powder Technol. 2013, 237, 623–626. [Google Scholar] [CrossRef]
- Jiang, Q.; Xu, L.; Li, X.; Zhang, H. Superior electrochemical properties of Li(Ni1/3Co1/3Mn1/3)O2/C synthesized by the precursor solid-phase method. Appl. Phys. A 2015, 121, 23–28. [Google Scholar] [CrossRef]
- He, Y.-S.; Ma, Z.-F.; Liao, X.-Z.; Jiang, Y. Synthesis and characterization of submicron-sized LiNi1/3Co1/3Mn1/3O2 by a simple self-propagating solid-state metathesis method. J. Power Sources 2007, 163, 1053–1058. [Google Scholar] [CrossRef]
- Lu, X.; Li, X.; Wang, Z.; Guo, H.; Yan, G.; Yin, X. A modified co-precipitation process to coat LiNi1/3Co1/3Mn1/3O2 onto LiNi0.8Co0.1Mn0.1O2 for improving the electrochemical performance. Appl. Surface Sci. 2014, 297, 182–187. [Google Scholar] [CrossRef]
- Li, L.-J.; Li, X.-H.; Wang, Z.-X.; Guo, H.-J.; Yue, P.; Chen, W.; Wu, L. Synthesis, structural and electrochemical properties of LiNi0.79Co0.1Mn0.1Cr0.01O2 via fast co-precipitation. J. Alloy Compd. 2010, 507, 172–177. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kim, H.; Kim, M.-S.; Youn, H.-C.; Kang, K.; Cho, B.-W.; Roh, K.C.; Kim, K.-B. Improved electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode material synthesized by citric acid assisted sol-gel method for lithium ion batteries. J. Power Sources 2016, 315, 261–268. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kim, M.-S.; Jeong, J.H.; Kim, D.-H.; Chung, K.Y.; Roh, K.C.; Kim, K.-B. Li3PO4 surface coating on Ni-rich LiNi0.6Co0.2Mn0.2O2 by a citric acid assisted sol-gel method: Improved thermal stability and high-voltage performance. J. Power Sources 2017, 360, 206–214. [Google Scholar] [CrossRef]
- Ju, S.H.; Kang, Y.C. The characteristics of Ni–Co–Mn–O precursor and Li(Ni1/3Co1/3Mn1/3)O2 cathode powders pre-pared by spray pyrolysis. Ceram. Int. 2009, 35, 1205–1210. [Google Scholar] [CrossRef]
- Park, S.H.; Yoon, C.S.; Kang, S.G.; Kim, H.S.; Moon, S.I.; Sun, Y.K. Synthesis and structural characterization of layered Li[Ni1/3Co1/3Mn1/3]O2 cathode materials by ultrasonic spray pyrolysis method. Electrochim. Acta 2004, 49, 557–563. [Google Scholar] [CrossRef]
- Li, T.; Li, X.; Wang, Z.; Guo, H.; Peng, W.; Zeng, K. Electrochemical properties of LiNi0.6Co0.2Mn0.2O2 as cathode material for Li-ion batteries prepared by ultrasonic spray pyrolysis. Mater. Lett. 2015, 159, 39–42. [Google Scholar] [CrossRef]
- Kwon, S.N.; Song, J.; Mumm, D.R. Effects of cathode fabrication conditions and cycling on the electrochemical performance of LiNiO2 synthesized by combustion and calcination. Ceram. Int. 2011, 37, 1543–1548. [Google Scholar] [CrossRef]
- Zhong, Z.; Ye, N.; Wang, H.; Ma, Z. Low temperature combustion synthesis and performance of spherical 0.5Li2MnO3–LiNi0.5Mn0.5O2 cathode material for Li-ion batteries. Chem. Eng. J. 2011, 175, 579–584. [Google Scholar] [CrossRef]
- Ahn, W.; Lim, S.N.; Jung, K.-N.; Yeon, S.-H.; Kim, K.-B.; Song, H.S.; Shin, K.-H. Combustion-synthesized LiNi0.6Mn0.2Co0.2O2 as cathode material for lithium ion batteries. J. Alloys Compd. 2014, 609, 143–149. [Google Scholar] [CrossRef]
- Zeng, J.; Hai, C.; Ren, X.; Li, X.; Shen, Y.; Dong, O.; Zhang, L.; Sun, Y.; Ma, L.; Zhang, X.; et al. Facile triethano-lamine-assisted combustion synthesized layered LiNi1/3Co1/3Mn1/3O2 cathode materials with enhanced electrochemical performance for lithium-ion batteries. J. Alloys Compd. 2018, 735, 1977–1985. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, W.; Ma, Y.; Jin, H.; Guo, L. Combustion synthesis of LiNi1/3Co1/3Mn1/3O2 powders with enhanced elec-trochemical performance in LIBs. J. Alloys Compd. 2015, 635, 207–212. [Google Scholar] [CrossRef]
- Jian, X.; Wenren, H.; Huang, S.; Shi, S.; Wang, X.; Gu, C.; Tu, J. Oxalic acid-assisted combustion synthesized LiVO3 cathode material for lithium ion batteries. J. Power Sources 2014, 246, 417–422. [Google Scholar] [CrossRef]
- Manikandan, P.; Ananth, M.; Kumar, T.P.; Raju, M.; Periasamy, P.; Manimaran, K. Solution combustion synthesis of layered LiNi0.5Mn0.5O2 and its characterization as cathode material for lithium-ion cells. J. Power Sources 2011, 196, 10148–10155. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, N.; Li, G.-D.; Guo, F.-F.; Zhao, J.; Zhao, Y.; Jia, T.; Fu, F.; Li, J. High rate performance of LiNi1/3Co1/3Mn1/3O2 cathode material synthesized by a carbon gel–combustion process for lithium ion batteries. Mater. Res. Bull. 2016, 73, 192–196. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef]
- Wu, Z.; Ji, S.; Liu, T.; Duan, Y.; Xiao, S.; Lin, Y.; Xu, K.; Pan, F. Aligned Li+ tunnels in core–shell Li(NixMnyCoz)O2@LiFePO4 enhances its high voltage cycling stability as Li-ion battery cathode. Nano Lett. 2016, 16, 6357–6363. [Google Scholar] [CrossRef]
- Jung, S.K.; Gwon, H.; Hong, J.; Park, K.Y.; Seo, D.H.; Kim, H.; Hyun, J.; Yang, W.; Kang, K. Understanding the degradation mechanisms of LiNi0.5Co0.2Mn0.3O2 cathode material in lithium ion batteries. Adv. Energy Mater. 2014, 4, 1300787. [Google Scholar] [CrossRef]
- Börner, M.; Horsthemke, F.; Kollmer, F.; Haseloff, S.; Friesen, A.; Niehoff, P.; Nowak, S.; Winter, M.; Schappacher, F. Degradation effects on the surface of commercial LiNi0.5Co0.2Mn0.3O2 electrodes. J. Power Sources 2016, 335, 45–55. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Q.; Dai, S.; Li, W.; Liu, X.; Ding, F.; Zhang, J. Synergistic Effect of F(-) Doping and LiF Coating on Improving the High-Voltage Cycling Stability and Rate Capacity of LiNi0.5Co0.2Mn0.3O2 Cathode Materials for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 34153–34162. [Google Scholar] [CrossRef] [PubMed]
- Schipper, F.; Bouzaglo, H.; Dixit, M.; Erickson, E.M.; Weigel, T.; Talianker, M.; Grinblat, J.; Burstein, L.; Schmidt, M.; Lampert, J.; et al. From Surface ZrO2 Coating to Bulk Zr Doping by High Temperature Annealing of Nickel-Rich Lithiated Oxides and Their Enhanced Electrochemical Performance in Lithium Ion Batteries. Adv. Energy Mater. 2018, 8, 1701682. [Google Scholar] [CrossRef]
- Makhonina, E.; Maslennikova, L.; Volkov, V.; Medvedeva, A.; Rumyantsev, A.; Koshtyal, Y.; Maximov, M.Y.; Pervov, V.; Eremenko, I. Li-rich and Ni-rich transition metal oxides: Coating and core-shell structures. Appl. Surf. Sci. 2018, 474, 25–33. [Google Scholar] [CrossRef]
- Peng, Z.; Yang, G.; Li, F.; Zhu, Z.; Liu, Z. Improving the cathode properties of Ni-rich LiNi0.6Co0.2Mn0.2O2 at high voltages under 5 C by Li2SiO3 coating and Si4+ doping. J. Alloys Compd. 2018, 762, 827–834. [Google Scholar] [CrossRef]
- Li, Y.-C.; Xiang, W.; Xiao, Y.; Wu, Z.-G.; Xu, C.-L.; Xu, W.; Xu, Y.-D.; Wu, C.; Yang, Z.-G.; Guo, X.-D. Synergy of doping and coating induced heterogeneous structure and concentration gradient in Ni-rich cathode for enhanced electrochemical performance. J. Power Sources 2019, 423, 144–151. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Xiong, D.; Hao, Y.; Li, J.; Kou, H.; Yan, B.; Li, D.; Lu, S.; Koo, A.; et al. Significantly improving cycling performance of cathodes in lithium ion batteries: The effect of Al2O3 and LiAlO2 coatings on LiNi0.6Co0.2Mn0.2O. Nano Energy 2018, 44, 111–120. [Google Scholar] [CrossRef]
- Liang, L.; Du, K.; Lu, W.; Peng, Z.; Cao, Y.; Hu, G. Synthesis and characterization of concentration–gradient LiNi0.6Co0.2Mn0.2O2 cathode material for lithium ion batteries. J. Alloys Compd. 2014, 613, 296–305. [Google Scholar] [CrossRef]
- Ding, Z.; Feng, Y.; Ji, R.; Zhang, D.; Chen, L.; Wang, S.; Ivey, D.G.; Wei, W. Improving the electrochemical cyclability of lithium manganese orthosilicate through the pillaring effects of gradient Na substitution. J. Power Sources 2017, 349, 18–26. [Google Scholar] [CrossRef]
- Liu, H.; Bugnet, M.; Tessaro, M.Z.; Harris, K.J.; Dunham, M.J.R.; Jiang, M.; Goward, G.R.; Botton, G.A. Spatially resolved surface valence gradient and structural transformation of lithium transition metal oxides in lithium-ion batteries. Phys. Chem. Chem. Phys. 2016, 18, 29064–29075. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Xu, G.; Miao, C.; Wen, M.; Xu, M.; Wang, C.; Xiao, W. Strengthened the structural stability of in-situ F− doping Ni-rich LiNi0.8Co0.15Al0.05O2 cathode materials for lithium-ion batteries. Chem. Eng. J. 2022, 438, 135537. [Google Scholar] [CrossRef]
- Wang, J.; Nie, Y.; Miao, C.; Tan, Y.; Wen, M.; Xiao, W. Enhanced electrochemical properties of Ni-rich layered cathode materials via Mg(2+) and Ti(4+)co-doping for lithium-ion batteries. J. Colloid. Interface Sci. 2021, 601, 853–862. [Google Scholar] [CrossRef]
- Xiao, W.; Nie, Y.; Miao, C.; Wang, J.; Tan, Y.; Wen, M. Structural design of high-performance Ni-rich LiNi0.83Co0.11Mn0.06O2 cathode materials enhanced by Mg(2+) doping and Li3PO4 coating for lithium ion battery. J. Colloid. Interface Sci. 2022, 607, 1071–1082. [Google Scholar] [CrossRef]
- Ding, X.; Li, Y.-X.; Deng, M.-M.; Wang, S.; Aqsa, Y.; Hu, Q.; Chen, C.-H. Cesium doping to improve the electrochemical per-formance of layered Li1.2Ni0.13Co0.13Mn0.54O2 cathode material. J. Alloys Compd. 2019, 791, 100–108. [Google Scholar] [CrossRef]
- Shang, G.; Tang, Y.; Lai, Y.; Wu, J.; Yang, X.; Li, H.; Peng, C.; Zheng, J.; Zhang, Z. Enhancing structural stability unto 4.5 V of Ni-rich cathodes by tungsten-doping for lithium storage. J. Power Sources 2019, 423, 246–254. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, S.; Han, B.; Lin, G.; Huang, Q.; Ivey, D.G.; Yang, C.; Wang, P.; Wei, W. Towards rational design of high per-formance Ni-rich layered oxide cathodes: The interplay of borate-doping and excess lithium. J. Power Sources 2019, 431, 40–47. [Google Scholar] [CrossRef]
- Kim, S.-B.; Kim, H.; Park, D.-H.; Kim, J.-H.; Shin, J.-H.; Jang, J.-S.; Moon, S.-H.; Choi, J.-H.; Park, K.-W. Li-ion diffusivity and electrochemical performance of Ni-rich cathode material doped with fluoride ions. J. Power Sources 2021, 506, 230219. [Google Scholar] [CrossRef]
- Dai, K.-H.; Xie, Y.-T.; Wang, Y.-J.; Song, Z.-S.; Qilu. Effect of fluorine in the preparation of Li(Ni1/3Co1/3Mn1/3)O2 via hydroxide co-precipitation. Electrochim. Acta 2008, 53, 3257–3261. [Google Scholar] [CrossRef]
- Lee, M.H.; Kang, Y.J.; Myung, S.T.; Sun, Y.K. Synthetic optimization of Li[Ni1/3Co1/3Mn1/3]O2 via co-precipitation. Electrochim. Acta 2004, 50, 939–948. [Google Scholar] [CrossRef]
- Su, Y.; Yang, Y.; Chen, L.; Lu, Y.; Bao, L.; Chen, G.; Yang, Z.; Zhang, Q.; Wang, J.; Chen, R.; et al. Improving the cycling stability of Ni-rich cathode materials by fabricating surface rock salt phase. Electrochim. Acta 2018, 292, 217–226. [Google Scholar] [CrossRef]
- Gao, H.; Jiao, L.; Peng, W.; Liu, G.; Yang, J.; Zhao, Q.; Qi, Z.; Si, Y.; Wang, Y.; Yuan, H. Enhanced electrochemical performance of LiFePO4/C via Mo-doping at Fe site. Electrochim. Acta 2011, 56, 9961–9967. [Google Scholar] [CrossRef]
- Liu, X.; He, P.; Li, H.; Ishida, M.; Zhou, H. Improvement of electrochemical properties of LiNi1/3Co1/3Mn1/3O2 by coating with V2O5 layer. J. Alloys Compd. 2013, 552, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First Principles Methods Using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Zheng, J.; Cui, S.; Song, X.; Su, Y.; Deng, W.; Wu, Z.; Wang, X.; Wang, W.; Rao, M.; et al. Kinetics Tuning of Li-Ion Diffusion in Layered Li(NixMnyCoz)O2. J. Am. Chem. Soc. 2015, 137, 8364–8367. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yin, Z.; Wang, Z.; Li, X.; Guo, H. Effect of heat-treatment on electrochemical performance of Li3VO4-coated LiNi1/3Co1/3Mn1/3O2 cathode materials. Mater. Lett. 2015, 160, 298–301. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, J.; Huang, J.; Zeng, R.; Zhang, J.; Chen, H.; Shi, G. Improving the Ni-rich LiNi0.5Co0.2Mn0.3O2 cathode properties at high operating voltage by double coating layer of Al2O3 and AlPO4. J. Alloys Compd. 2017, 724, 1109–1116. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Luo, L.; Ming, X.; Li, J.; Lei, T.; Deng, S.; Guo, J.; Zhu, J.; Chang, S. Surface in-situ reconstruction of LiNi0.8Co0.1Mn0.1O2 cathode materials interacting with antimony compounds and the electrochemical performances. J. Electroanal. Chem. 2019, 854, 113582. [Google Scholar] [CrossRef]
- Liu, S.; Dang, Z.; Liu, D.; Zhang, C.; Huang, T.; Yu, A. Comparative studies of zirconium doping and coating on LiNi0.6Co0.2Mn0.2O2 cathode material at elevated temperatures. J. Power Sources 2018, 396, 288–296. [Google Scholar] [CrossRef]
- Li, Y.-C.; Xiang, W.; Wu, Z.-G.; Xu, C.-L.; Xu, Y.-D.; Xiao, Y.; Yang, Z.-G.; Wu, C.-J.; Lv, G.-P.; Guo, X.-D. Construction of homogeneously Al3+ doped Ni rich Ni-Co-Mn cathode with high stable cycling performance and storage stability via scalable continuous precipitation. Electrochim. Acta 2018, 291, 84–94. [Google Scholar] [CrossRef]
- Wang, H.; Chu, Y.; Pan, Q.; Yang, G.; Lai, A.; Liu, Z.; Zheng, F.; Hu, S.; Huang, Y.; Li, Q. Enhanced interfacial reaction interface stability of Ni-rich cathode materials by fabricating dual-modified layer coating for lithium-ion batteries. Electrochim. Acta 2021, 366, 137476. [Google Scholar] [CrossRef]







| Sample | I(003) (Counts) | I(003)/I(104) | Molar Ration of Li:Ni:Co:Mn | Molar Percent of F |
|---|---|---|---|---|
| F0 | 22057 | 2.0661 | 1.01:0.79:0.099:0.098 | 0 |
| F500 | 21427 | 1.9120 | 1.01:0.80:0.098:0.099 | 0.049% |
| F1000 | 18490 | 1.7762 | 1.01:0.80:0.099:0.098 | 0.10% |
| F5000 | 16675 | 1.7271 | 1.01:0.80:0.099:0.099 | 0.51% |
| Sample | a (Å) | c (Å) | c/a |
|---|---|---|---|
| F0 | 2.8679 | 14.1701 | 4.9409 |
| F500 | 2.8741 | 14.2023 | 4.9415 |
| F1000 | 2.8773 | 14.2418 | 4.9497 |
| F5000 | 2.8783 | 14.2433 | 4.9485 |
| Sample | RE (ohms) | RSEI (ohms) | RCT (ohms) | D (cm2 s−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| F0 | 1.76 | 2.08 | 19.84 | 23.12 | 4.26 | 87.89 | 3.81 × 10−12 | 1.60 × 10−12 |
| F500 | 2.73 | 2.17 | 38.97 | 30.63 | 19.16 | 141.30 | 1.04 × 10−12 | 1.00 × 10−12 |
| F1000 | 2.72 | 1.94 | 64.84 | 23.51 | 45.90 | 171.30 | 3.67 × 10−12 | 1.28 × 10−12 |
| F5000 | 2.34 | 2.32 | 47.92 | 30.88 | 38.15 | 174.20 | 1.85 × 10−12 | 1.25 × 10−12 |
| Sample | M-F | Lattice O | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| F0 | 0 | 63.21% | 56.53% | 12.66% |
| F500 | 2.27% | 35.90% | 65.20% | 13.98% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, J.; Shen, Y.; Ren, X.; Li, X.; Sun, Y.; Zhang, G.; Wu, Z.; Zhu, S.; Hai, C.; Zhou, Y. F-Doped Ni-Rich Layered Cathode Material with Improved Rate Performance for Lithium-Ion Batteries. Processes 2022, 10, 1573. https://doi.org/10.3390/pr10081573
Zeng J, Shen Y, Ren X, Li X, Sun Y, Zhang G, Wu Z, Zhu S, Hai C, Zhou Y. F-Doped Ni-Rich Layered Cathode Material with Improved Rate Performance for Lithium-Ion Batteries. Processes. 2022; 10(8):1573. https://doi.org/10.3390/pr10081573
Chicago/Turabian StyleZeng, Jinbo, Yue Shen, Xiufeng Ren, Xiang Li, Yanxia Sun, Guotai Zhang, Zhaowei Wu, Shenglong Zhu, Chunxi Hai, and Yuan Zhou. 2022. "F-Doped Ni-Rich Layered Cathode Material with Improved Rate Performance for Lithium-Ion Batteries" Processes 10, no. 8: 1573. https://doi.org/10.3390/pr10081573
APA StyleZeng, J., Shen, Y., Ren, X., Li, X., Sun, Y., Zhang, G., Wu, Z., Zhu, S., Hai, C., & Zhou, Y. (2022). F-Doped Ni-Rich Layered Cathode Material with Improved Rate Performance for Lithium-Ion Batteries. Processes, 10(8), 1573. https://doi.org/10.3390/pr10081573






