Investigating the Molecular Mechanism of Qianghuo Shengshi Decoction in the Treatment of Ankylosing Spondylitis Based on Network Pharmacology and Molecular Docking Analysis
Abstract
1. Introduction
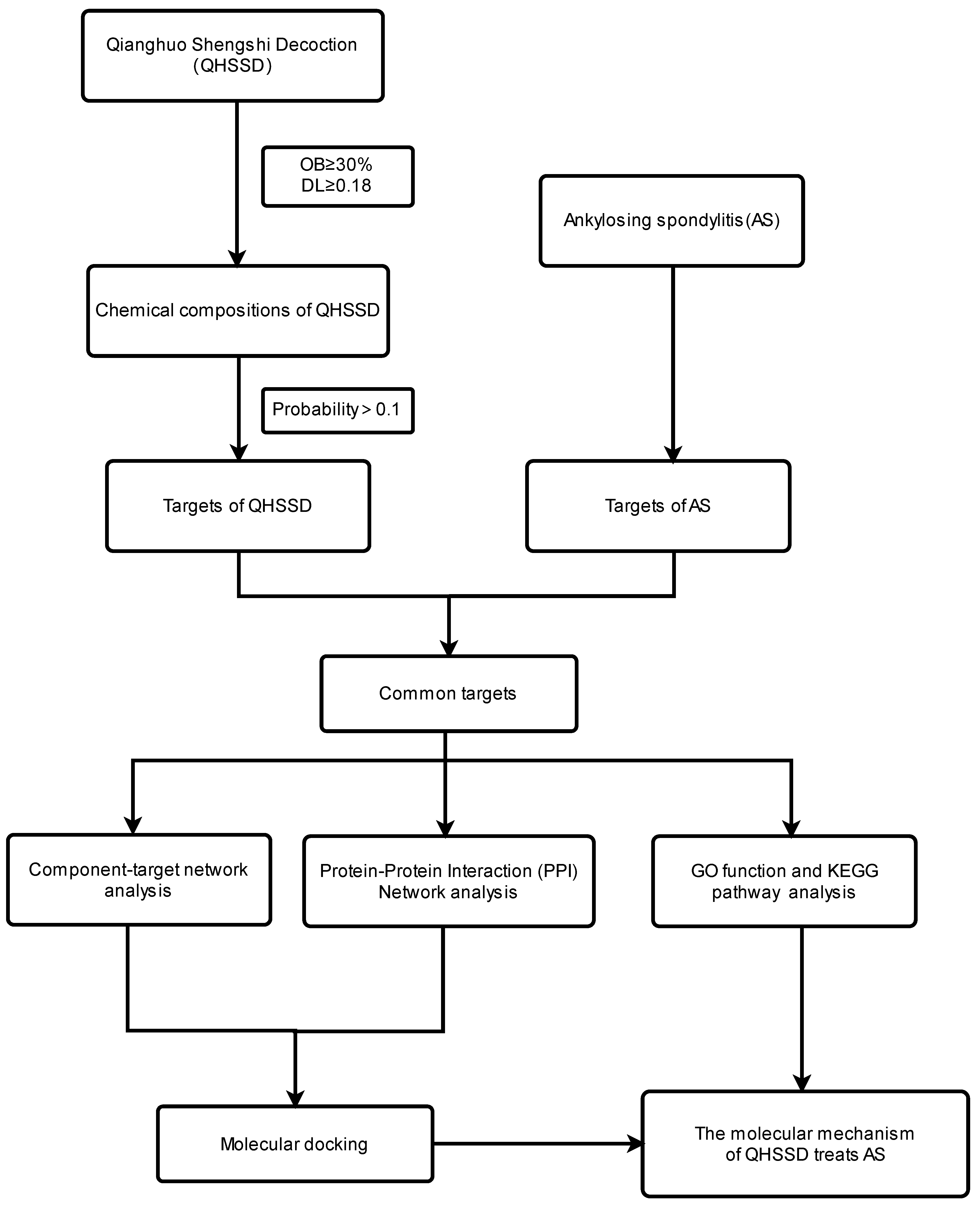
2. Methods
2.1. Obtaining the Chemical Components and Target of QHSSD
2.2. Obtaining the Potential Targets of AS
2.3. Obtaining Common Potential Targets of QHSSD and AS
2.4. Network Construction
2.4.1. Construction of Component–Target Interaction Network
2.4.2. Construction of Protein–Protein Interaction Network
2.5. Gene Ontology (GO) Function and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analysis
2.6. Molecular Docking
3. Results
3.1. The Chemical Components and Potential Targets of QHSSD
3.2. The Potential Targets of AS
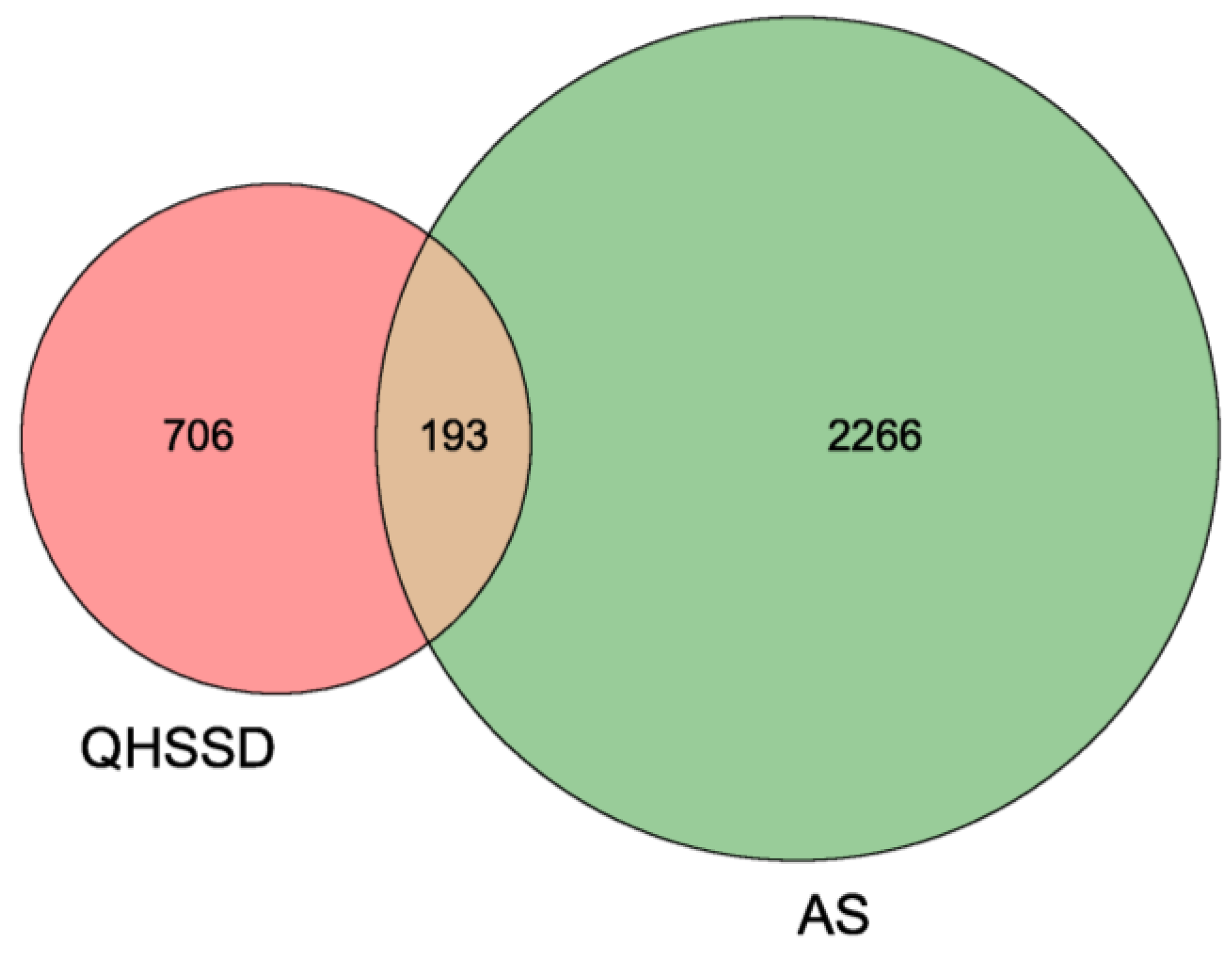
3.3. Common Potential Targets of QHSSD and AS
3.4. Network Construction
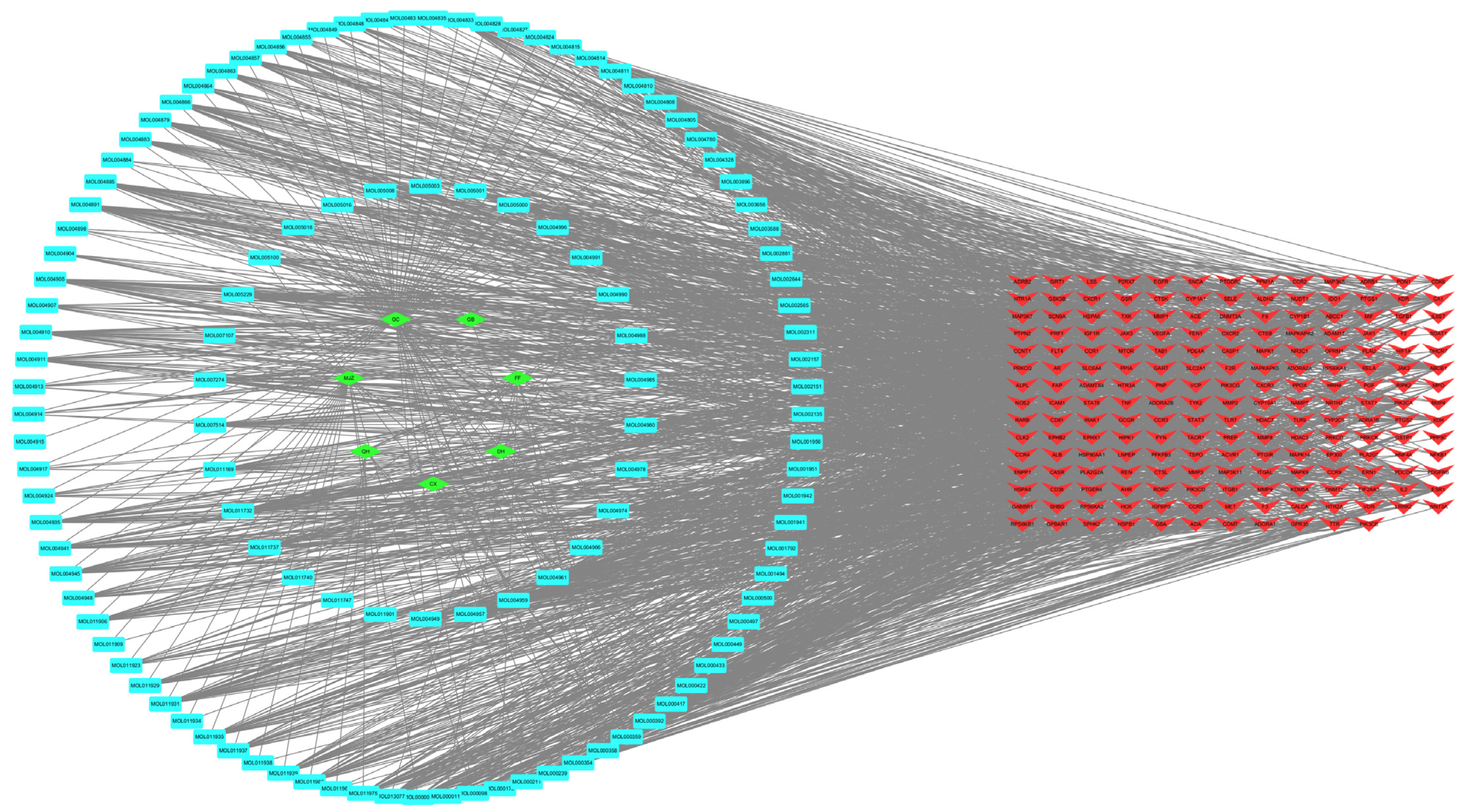
3.4.1. Component–Target Interaction Network
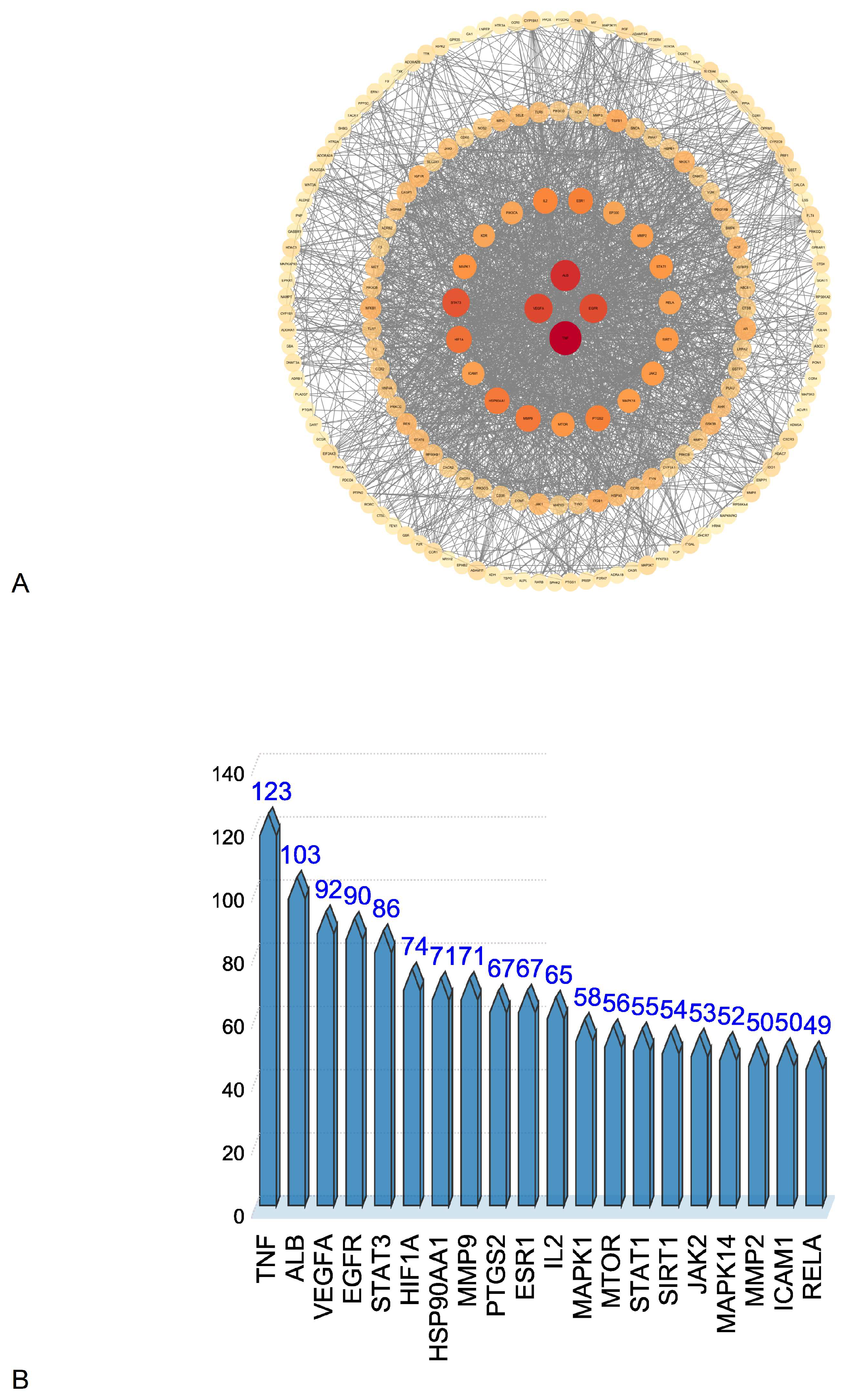
3.4.2. PPI Network
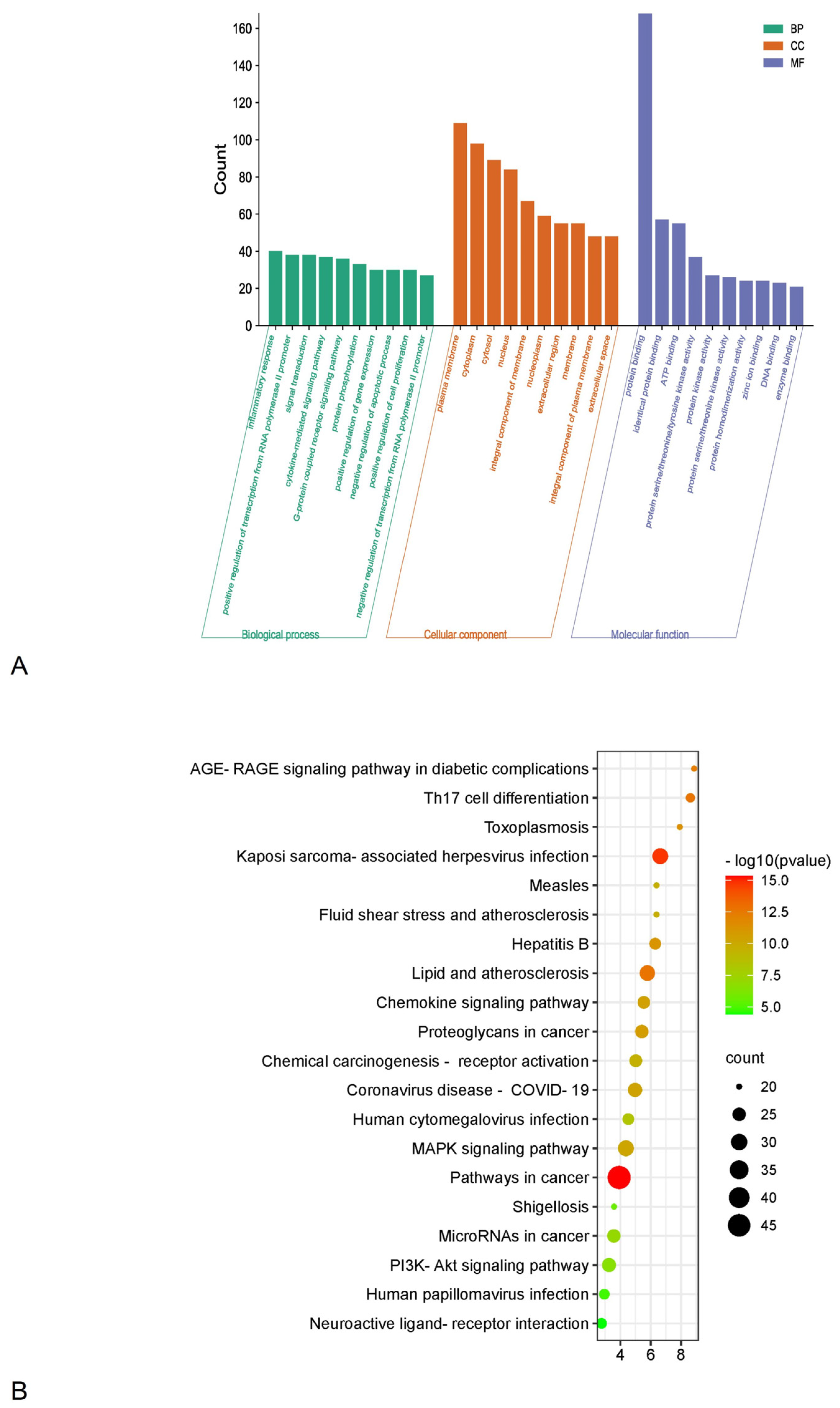
3.5. GO Function and KEGG Pathways Analysis
3.5.1. GO Function Analysis
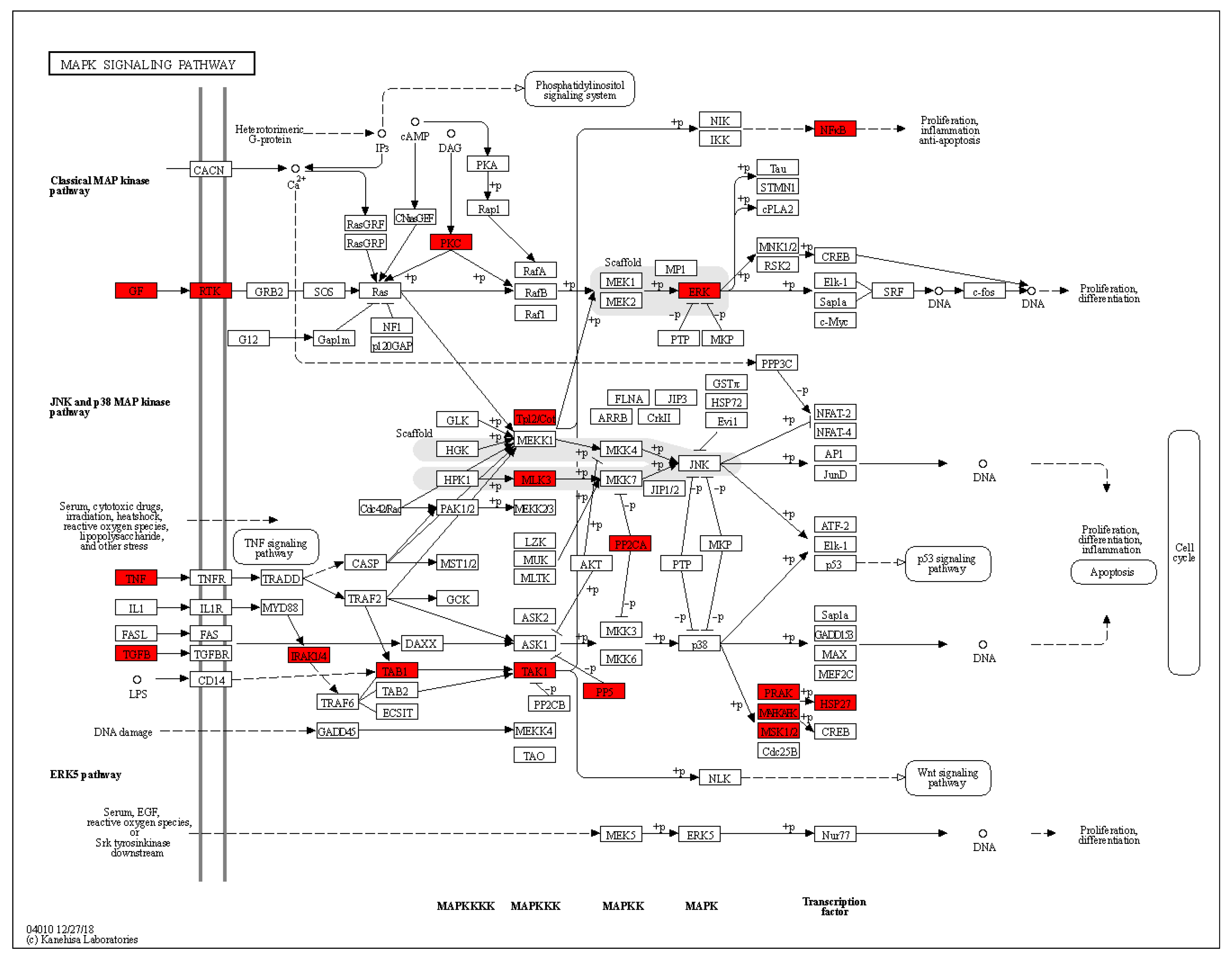
3.5.2. KEGG Pathways Analysis
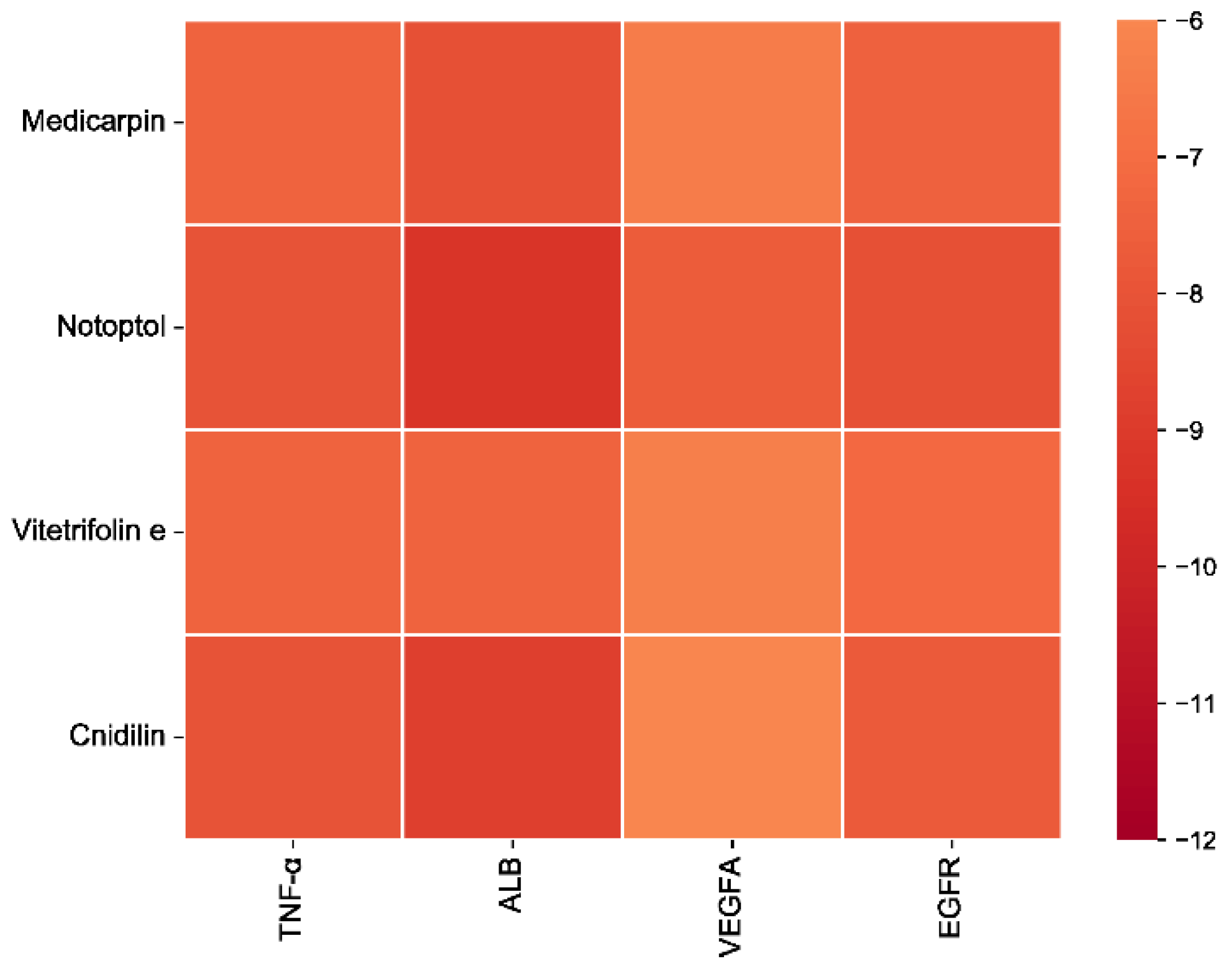
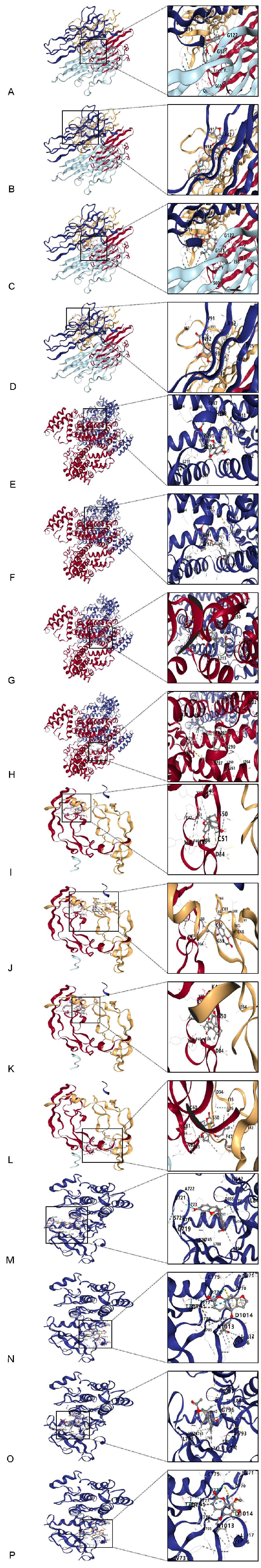
3.6. Molecular Docking
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Braun, J.; Sieper, J. Ankylosing spondylitis. Lancet 2007, 369, 1379–1390. [Google Scholar] [CrossRef]
- Zhu, W.; He, X.; Cheng, K.; Zhang, L.; Chen, D.; Wang, X.; Qiu, G.; Cao, X.; Weng, X. Ankylosing spondylitis: Etiology, pathogenesis, and treatments. Bone Res. 2019, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, H.; Wang, J.; Gao, X. Association between ERAP1 gene polymorphisms and ankylosing spondylitis susceptibility in Han population. Int. J. Clin. Exp. Pathol. 2015, 8, 11641–11646. [Google Scholar]
- Zochling, J.; van der Heijde, D.; Burgos-Vargas, R.; Collantes, E., Jr.; Davis, J.C.; Dijkmans, B.; Dougados, M.; Géher, P.; Inman, R.; Khan, M.A.; et al. ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann. Rheum. Dis. 2006, 65, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Salaffi, F.; Carotti, M.; Gasparini, S.; Intorcia, M.; Grassi, W. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: A comparison with a selected sample of healthy people. Health Qual. Life Outcomes 2009, 7, 25. [Google Scholar] [CrossRef]
- Overman, C.L.; Kool, M.B.; Da Silva, J.; Geenen, R. The prevalence of severe fatigue in rheumatic diseases: An international study. Clin. Rheumatol. 2016, 35, 409–415. [Google Scholar] [CrossRef]
- Brophy, S.; Davies, H.; Dennis, M.S.; Cooksey, R.; Husain, M.J.; Irvine, E.; Siebert, S. Fatigue in ankylosing spondylitis: Treatment should focus on pain management. Semin. Arthritis Rheum. 2013, 42, 361–367. [Google Scholar] [CrossRef]
- Braun, J.; van den Berg, R.; Baraliakos, X.; Boehm, H.; Burgos-Vargas, R.; Estévez, E.C.; Dagfinrud, H.; Dijkmans, B.; Dougados, M.; Emery, P.; et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann. Rheum. Dis. 2011, 70, 896–904. [Google Scholar] [CrossRef]
- Bahadur, S.; Keshri, L.; Pathak, K. Adverse drug reactions and safety considerations of NSAIDs: Clinical analysis. Curr. Drug Saf. 2011, 6, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Xu, X.; Wang, X.; Li, B.; Wang, Y.; Li, Y.; Yang, L. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal _Radix Curcumae_ formula for application to cardiovascular disease. J. Ethnopharmacol. 2013, 145, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, W.; Huang, C.; Li, Y.; Yu, H.; Wang, Y.; Duan, J.; Ling, Y. A novel chemometric method for the prediction of human oral bioavailability. Int. J. Mol. Sci. 2012, 13, 6964–6982. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Li, Y.; Li, D.; Xu, L.; Hou, T. The application of _in silico_ drug-likeness predictions in pharmaceutical research. Adv. Drug Deliv. Rev. 2015, 86, 2–10. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Fishilevich, S.; Zimmerman, S.; Kohn, A.; Stein, T.I.; Olender, T.; Kolker, E.; Safran, M.; Lancet, R. Genic insights from integrated human proteomics in GeneCards. Database (Oxf.) 2016, 2016, 30. [Google Scholar] [CrossRef] [PubMed]
- Amberger, J.S.; Hamosh, A. Searching Online Mendelian Inheritance in Man (OMIM): A Knowledgebase of Human Genes and Genetic Phenotypes. Curr. Protoc. Bioinform. 2017, 58, 1.2.1–1.2.12. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.; Saüch, J.; Sanz, F.; Furlong, L.I. The DisGeNET cytoscape app: Exploring and visualizing disease genomics data. Comput. Struct. Biotechnol. J. 2021, 19, 2960–2967. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612, Published Correction Appears in Nucleic Acids Res. 2021, 49, 10800. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Kir, J.; Liu, D.; Bryant, D.; Guo, Y.; Stephens, R.; Baseler, M.W.; Lane, H.C.; et al. DAVID Bioinformatics Resources: Expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007, 35, W169–W175. [Google Scholar] [CrossRef]
- Liu, Y.; Grimm, M.; Dai, W.T.; Hou, M.-C.; Xiao, Z.-X.; Cao, Y. CB-Dock: A web server for cavity detection-guided protein-ligand blind docking. Acta Pharm. Sin. 2020, 41, 138–144. [Google Scholar] [CrossRef]
- Tyagi, A.M.; Gautam, A.K.; Kumar, A.; Srivastava, K.; Bhargavan, B.; Trivedi, R.; Saravanan, S.; Yadav, D.K.; Singh, N.; Pollet, C.; et al. Medicarpin inhibits osteoclastogenesis and has nonestrogenic bone conserving effect in ovariectomized mice. Mol. Cell Endocrinol. 2010, 325, 101–109. [Google Scholar] [CrossRef]
- Mansoori, M.N.; Raghuvanshi, A.; Shukla, P.; Awasthi, P.; Trivedi, R.; Goel, A.; Singh, D. Medicarpin prevents arthritis in post-menopausal conditions by arresting the expansion of TH17 cells and pro-inflammatory cytokines. Int. Immunopharmacol. 2020, 82, 106299. [Google Scholar] [CrossRef]
- Azietaku, J.T.; Ma, H.; Yu, X.-A.; Li, J.; Oppong, M.B.; Cao, J.; An, M.; Chang, Y.-X. A review of the ethnopharmacology, phytochemistry and pharmacology of Notopterygium incisum. J. Ethnopharmacol. 2017, 202, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Hirata, N.; Kawaguchi, Y.; Yamazaki, M.; Naruto, S.; Shibano, M.; Taniguchi, M.; Baba, K.; Kubo, M. Melanogenesis stimulation in murine b16 melanoma cells by umberiferae plant extracts and their coumarin constituents. Biol. Pharm. Bull. 2005, 28, 1229–1233. [Google Scholar] [CrossRef]
- van der Heijde, D.; Kivitz, A.; Schiff, M.H.; Sieper, J.; Dijkmans, B.A.C.; Braun, J.; Dougados, M.; Reveille, J.D.; Wong, R.L.; Kupper, H.; et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: Results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006, 54, 2136–2146. [Google Scholar] [CrossRef]
- Braun, J.; Bollow, M.; Neure, L.; Seipelt, E.; Seyrekbasan, F.; Herbst, H.; Eggens, U.; Distler, A.; Sieper, J. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 1995, 38, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Sveaas, S.H.; Berg, I.J.; Provan, S.; Semb, A.; Olsen, I.C.; Ueland, T.; Aukrust, P.; Vøllestad, N.; Hagen, K.; Kvien, T.; et al. Circulating levels of inflammatory cytokines and cytokine receptors in patients with ankylosing spondylitis: A cross-sectional comparative study. Scand. J. Rheumatol. 2015, 44, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Zaragoza, J.F.; Gutiérrez-Franco, J.; Durán-Avelar, M.J.; Vibanco-Pérez, N.; Ortiz-Martínez, L.; Ayón-Pérez, M.F.; Vázquez-Reyes, A.; Agraz-Cibrián, J.M. Neutrophil extracellular traps and inflammatory response: Implications for the immunopathogenesis of ankylosing spondylitis. Int. J. Rheum. Dis. 2021, 24, 426–433. [Google Scholar] [CrossRef]
- Li, S.; Yin, Y.; Yao, L.; Lin, Z.; Sun, S.; Zhang, J.; Li, X. TNF-α treatment increases DKK1 protein levels in primary osteoblasts via upregulation of DKK1 mRNA levels and downregulation of miR-335-5p. Mol. Med. Rep. 2020, 22, 1017–1025. [Google Scholar] [CrossRef]
- Wu, Q.; Inman, R.D.; Davis, K.D. Tumor necrosis factor inhibitor therapy in ankylosing spondylitis: Differential effects on pain and fatigue and brain correlates. Pain 2015, 156, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Qian, H.; Yuan, X.; Chen, S.; Liu, Y.; Wang, B.; Shi, G. Immunological Changes in Peripheral Blood of Ankylosing Spondylitis Patients during Anti-TNF-α Therapy and Their Correlations with Treatment Outcomes. J. Immunol. Res. 2021, 2021, 1017938. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, L.J.; Zochling, J.; Boonen, A.; Chen, S.; Liu, Y.; Wang, B.; Shi, G. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst. Rev. 2015, 4, CD005468. [Google Scholar] [CrossRef]
- Lata, M.; Hettinghouse, A.S.; Liu, C.J. Targeting tumor necrosis factor receptors in ankylosing spondylitis. Ann. N. Y. Acad. Sci. 2019, 1442, 5–16. [Google Scholar] [CrossRef]
- Tahir, H. Therapies in ankylosing spondylitis-from clinical trials to clinical practice. Rheumatology (Oxford) 2018, 57 (Suppl. S6), vi23–vi28. [Google Scholar] [CrossRef]
- Hou, L.Q.; Jiang, G.X.; Chen, Y.F.; Yang, X.-M.; Meng, L.; Xue, M.; Liu, X.-G.; Chen, X.-C.; Li, X. The Comparative Safety of TNF Inhibitors in Ankylosing Spondylitis-a Meta-Analysis Update of 14 Randomized Controlled Trials. Clin. Rev. Allergy Immunol. 2018, 54, 234–243. [Google Scholar] [CrossRef]
- Ruot, B.; Béchereau, F.; Bayle, G.; Breuillé, D.; Obled, C. The response of liver albumin synthesis to infection in rats varies with the phase of the inflammatory process. Clin. Sci. 2002, 102, 107–114. [Google Scholar] [CrossRef]
- Zhong, Z.; Huang, Y.; Liu, Y.; Chen, J.; Liu, M.; Huang, Q.; Zheng, S.; Guo, X.; Deng, W.; Li, T. Correlation between C-Reactive Protein to Albumin Ratio and Disease Activity in Patients with Axial Spondyloarthritis. Dis. Markers 2021, 2021, 6642486. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454, Published Correction Appears in N. Engl. J. Med. 1999, 340, 1376. [Google Scholar] [CrossRef]
- Liu, M.; Huang, Y.; Huang, Z.; Zhong, Z.; Deng, W.; Huang, Z.; Huang, Q.; Li, T. The role of fibrinogen to albumin ratio in ankylosing spondylitis: Correlation with disease activity. Clin. Chim. Acta 2020, 505, 136–140. [Google Scholar] [CrossRef]
- Yang, W.M.; Zhang, W.H.; Ying, H.Q.; Xu, Y.-M.; Zhang, J.; Min, Q.-H.; Huang, B.; Lin, J.; Chen, J.-J.; Wang, X.-Z. Two new inflammatory markers associated with disease activity score-28 in patients with rheumatoid arthritis: Albumin to fibrinogen ratio and C-reactive protein to albumin ratio. Int. Immunopharmacol. 2018, 62, 293–298. [Google Scholar] [CrossRef]
- Kinoshita, A.; Onoda, H.; Imai, N.; Iwaku, A.; Oishi, M.; Tanaka, K.; Fushiya, N.; Koike, K.; Nishino, H.; Matsushima, M. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann. Surg. Oncol. 2015, 22, 803–810. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, G.; Wang, N.; Xue, Q. Risk Factors of Renal Involvement Based on Different Manifestations in Patients with Ankylosing Spondylitis. Kidney Blood Press. Res. 2018, 43, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Kasama, T.; Kobayashi, K.; Yoda, Y.; Shiozawa, F.; Hanyuda, M.; Negishi, M.; Ide, H.; Adachi, M. Vascular endothelial growth factor expression and regulation of murine collagen-induced arthritis. J. Immunol. 2000, 164, 5922–5927. [Google Scholar] [CrossRef] [PubMed]
- Drouart, M.; Saas, P.; Billot, M.; Cedoz, J.; Tiberghien, P.; Wendling, D.; Toussirot, E. High serum vascular endothelial growth factor correlates with disease activity of spondylarthropathies. Clin. Exp. Immunol. 2003, 132, 158–162. [Google Scholar] [CrossRef]
- Lin, T.T.; Lu, J.; Qi, C.Y.; Yuan, L.; Li, X.-L.; Xia, L.-P.; Shen, H. Elevated serum level of IL-27 and VEGF in patients with ankylosing spondylitis and associate with disease activity. Clin. Exp. Med. 2015, 15, 227–231. [Google Scholar] [CrossRef]
- Carvalho, J.F.; Blank, M.; Shoenfeld, Y. Vascular endothelial growth factor (VEGF) in autoimmune diseases. J. Clin. Immunol. 2007, 27, 246–256. [Google Scholar] [CrossRef]
- Distler, J.H.; Hirth, A.; Kurowska-Stolarska, M.; Gay, R.E.; Gay, S.; Distler, O. Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q. J. Nucl. Med. 2003, 47, 149–161. [Google Scholar]
- Yamamoto, T. Angiogenic and inflammatory properties of psoriatic arthritis. ISRN Dermatol. 2013, 2013, 630620. [Google Scholar] [CrossRef][Green Version]
- Fearon, U.; Griosios, K.; Fraser, A.; Reece, R.; Emery, P.; Jones, P.F.; Veale, D. Angiopoietins, growth factors, and vascular morphology in early arthritis. J. Rheumatol. 2003, 30, 260–268. [Google Scholar]
- Liu, K.; He, Q.; Tan, J.; Liao, G. Expression of TNF-α, VEGF, and MMP-3 mRNAs in synovial tissues and their roles in fibroblast-mediated osteogenesis in ankylosing spondylitis. Genet. Mol. Res. 2015, 14, 6852–6858. [Google Scholar] [CrossRef]
- Harrison, P.T.; Vyse, S.; Huang, P.H. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin. Cancer Biol. 2020, 61, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-M.; Chen, H.-H.; Chen, D.-C.; Huang, Y.-C.; Liu, S.-P.; Lin, Y.-J.; Chang, Y.-Y.; Lin, H.-W.; Chen, S.-Y.; Tsai, F.-J. Rheumatoid arthritis is associated with rs17337023 polymorphism and increased serum level of the EGFR protein. PLoS ONE 2017, 12, e0180604. [Google Scholar] [CrossRef] [PubMed]
- Killock, D. Experimental arthritis: Targeting EGFR to fight synovitis. Nat. Rev. Rheumatol. 2012, 8, 247. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, X.; Xu, K.; Hu, P.; Bao, J.; Li, J.; Zhu, J.; Wu, L. miR-7/EGFR/MEGF9 axis regulates cartilage degradation in osteoarthritis via PI3K/AKT/mTOR signaling pathway. Bioengineered 2021, 12, 8622–8634. [Google Scholar] [CrossRef]
- Colomb, F.; Vidal, O.; Bobowski, M.; Krzewinski-Recchi, M.-A.; Harduin-Lepers, A.; Mensier, E.; Jaillard, S.; Lafitte, J.-J.; Delannoy, P.; Groux-Degroote, S. TNF induces the expression of the sialyltransferase ST3Gal IV in human bronchial mucosa via MSK1/2 protein kinases and increases FliD/sialyl-Lewis(x)-mediated adhesion of Pseudomonas aeruginosa. Biochem. J. 2014, 457, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Wang, C.; Wang, B.; Wang, L.; Huang, J.; Wang, J.; Li, C. Qianghuo Shengshi decoction exerts anti-inflammatory and analgesic via MAPKs/CREB signaling pathway. J. Ethnopharmacol. 2022, 284, 114776. [Google Scholar] [CrossRef]
- Wu, Y.; Xiao, H.; Pi, J.; Zhang, H.; Pan, A.; Pu, Y.; Liang, Z.; Shen, J.; Du, J. EGFR promotes the proliferation of quail follicular granulosa cells through the MAPK/extracellular signal-regulated kinase (ERK) signaling pathway. Cell Cycle 2019, 18, 2742–2756. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Hu, J.; Jiang, J.; Xiao, G.; Yang, R.; Li, S.; Li, Y.; Huang, H.; Zhong, H.; Bi, X. Network Pharmacology and Molecular Docking-Based Prediction of the Mechanism of Qianghuo Shengshi Decoction against Rheumatoid Arthritis. Biomed. Res. Int. 2021, 2021, 6623912. [Google Scholar] [CrossRef]
- Scott, D.L.; Wolfe, F.; Huizinga, T.W.J. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
| Molecule ID | Molecule Name | Target Name | Vina Score | Cavity Size | Center | Size | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||||
| MOL002565 | Medicarpin | TNF-α | −7.4 | 233 | −12 | 69 | 18 | 20 | 20 | 20 |
| MOL011975 | Notoptol | TNF-α | −8 | 1253 | −5 | 82 | 28 | 23 | 23 | 23 |
| MOL011931 | Vitetrifolin E | TNF-α | −7.4 | 233 | −12 | 69 | 18 | 20 | 20 | 20 |
| MOL001956 | Cnidilin | TNF-α | −8 | 1253 | −5 | 82 | 28 | 21 | 21 | 21 |
| MOL002565 | Medicarpin | ALB | −8.1 | 2341 | −28 | 15 | 40 | 20 | 20 | 20 |
| MOL011975 | Notoptol | ALB | −9.3 | 2341 | −28 | 15 | 40 | 23 | 23 | 23 |
| MOL011931 | Vitetrifolin E | ALB | −7.4 | 1260 | −35 | −8 | 10 | 21 | 21 | 21 |
| MOL001956 | Cnidilin | ALB | −8.8 | 7244 | −19 | −12 | −4 | 35 | 30 | 21 |
| MOL002565 | Medicarpin | VEGFA | −6.4 | 537 | 27 | −23 | 15 | 20 | 20 | 20 |
| MOL011975 | Notoptol | VEGFA | −7.7 | 537 | 27 | −23 | 15 | 23 | 23 | 23 |
| MOL011931 | Vitetrifolin E | VEGFA | −6.3 | 537 | 27 | −23 | 15 | 21 | 21 | 21 |
| MOL001956 | Cnidilin | VEGFA | −6 | 548 | 19 | −55 | 8 | 21 | 21 | 21 |
| MOL002565 | Medicarpin | EGFR | −7.5 | 555 | −52 | 2 | 24 | 20 | 20 | 20 |
| MOL011975 | Notoptol | EGFR | −8.2 | 643 | −64 | −7 | 31 | 23 | 23 | 23 |
| MOL011931 | Vitetrifolin E | EGFR | −7.2 | 555 | −52 | 2 | 24 | 21 | 21 | 21 |
| MOL001956 | Cnidilin | EGFR | −7.8 | 643 | −64 | −7 | 31 | 21 | 21 | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, S.; Xiao, X.; Luo, W.; Zhang, X.; Zhang, J.; Tang, S. Investigating the Molecular Mechanism of Qianghuo Shengshi Decoction in the Treatment of Ankylosing Spondylitis Based on Network Pharmacology and Molecular Docking Analysis. Processes 2022, 10, 1487. https://doi.org/10.3390/pr10081487
Luo S, Xiao X, Luo W, Zhang X, Zhang J, Tang S. Investigating the Molecular Mechanism of Qianghuo Shengshi Decoction in the Treatment of Ankylosing Spondylitis Based on Network Pharmacology and Molecular Docking Analysis. Processes. 2022; 10(8):1487. https://doi.org/10.3390/pr10081487
Chicago/Turabian StyleLuo, Simin, Xiang Xiao, Wenting Luo, Xuan Zhang, Jian Zhang, and Songqi Tang. 2022. "Investigating the Molecular Mechanism of Qianghuo Shengshi Decoction in the Treatment of Ankylosing Spondylitis Based on Network Pharmacology and Molecular Docking Analysis" Processes 10, no. 8: 1487. https://doi.org/10.3390/pr10081487
APA StyleLuo, S., Xiao, X., Luo, W., Zhang, X., Zhang, J., & Tang, S. (2022). Investigating the Molecular Mechanism of Qianghuo Shengshi Decoction in the Treatment of Ankylosing Spondylitis Based on Network Pharmacology and Molecular Docking Analysis. Processes, 10(8), 1487. https://doi.org/10.3390/pr10081487






