Tungsten Oxide Modified V2O5-Sb2O3/TiO2 Monolithic Catalyst: NH3-SCR Activity and Sulfur Resistance
Abstract
:1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Activity Measurement
2.3. Characterizations
3. Results
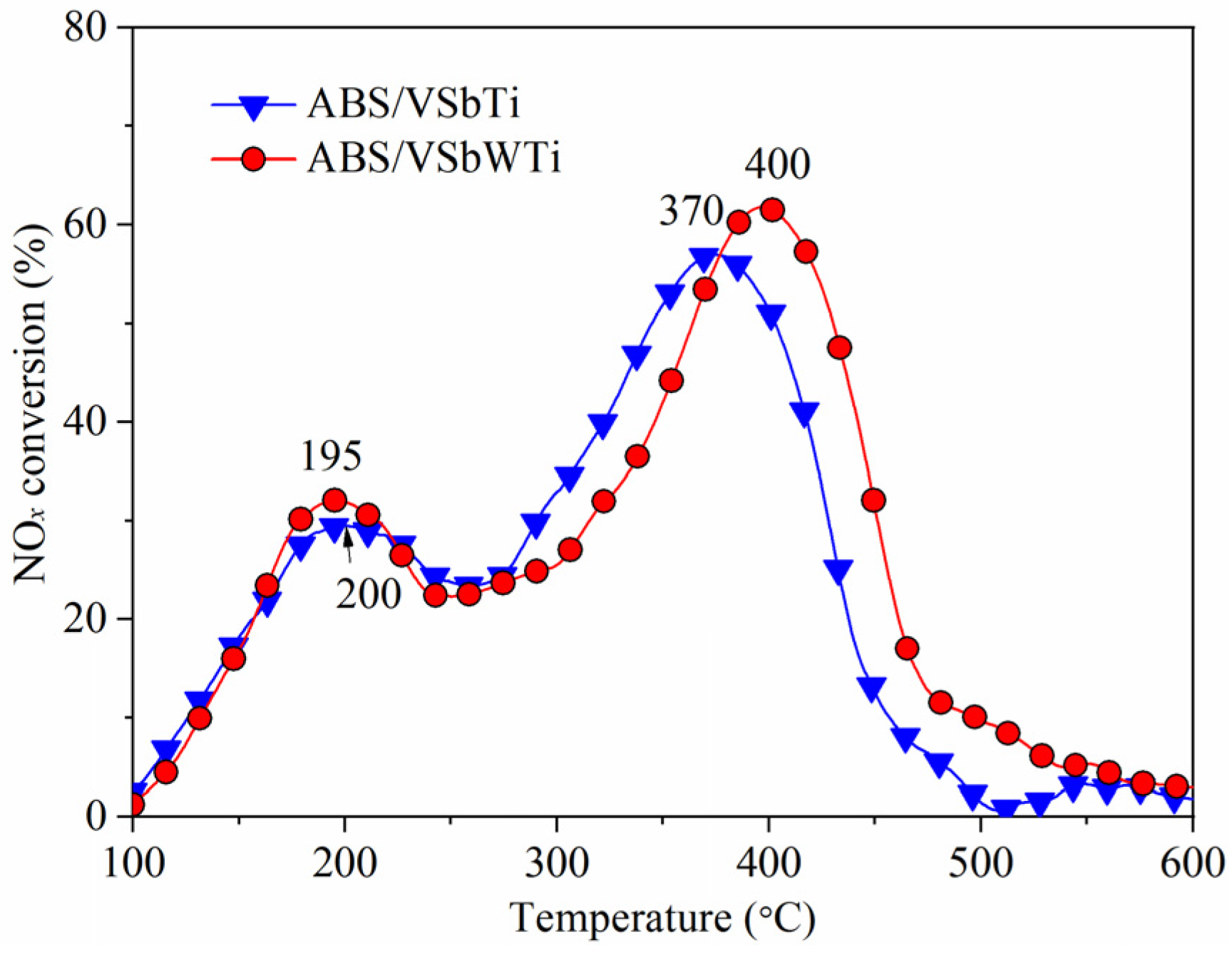
3.1. NH3-SCR Activity
3.2. Structural Properties
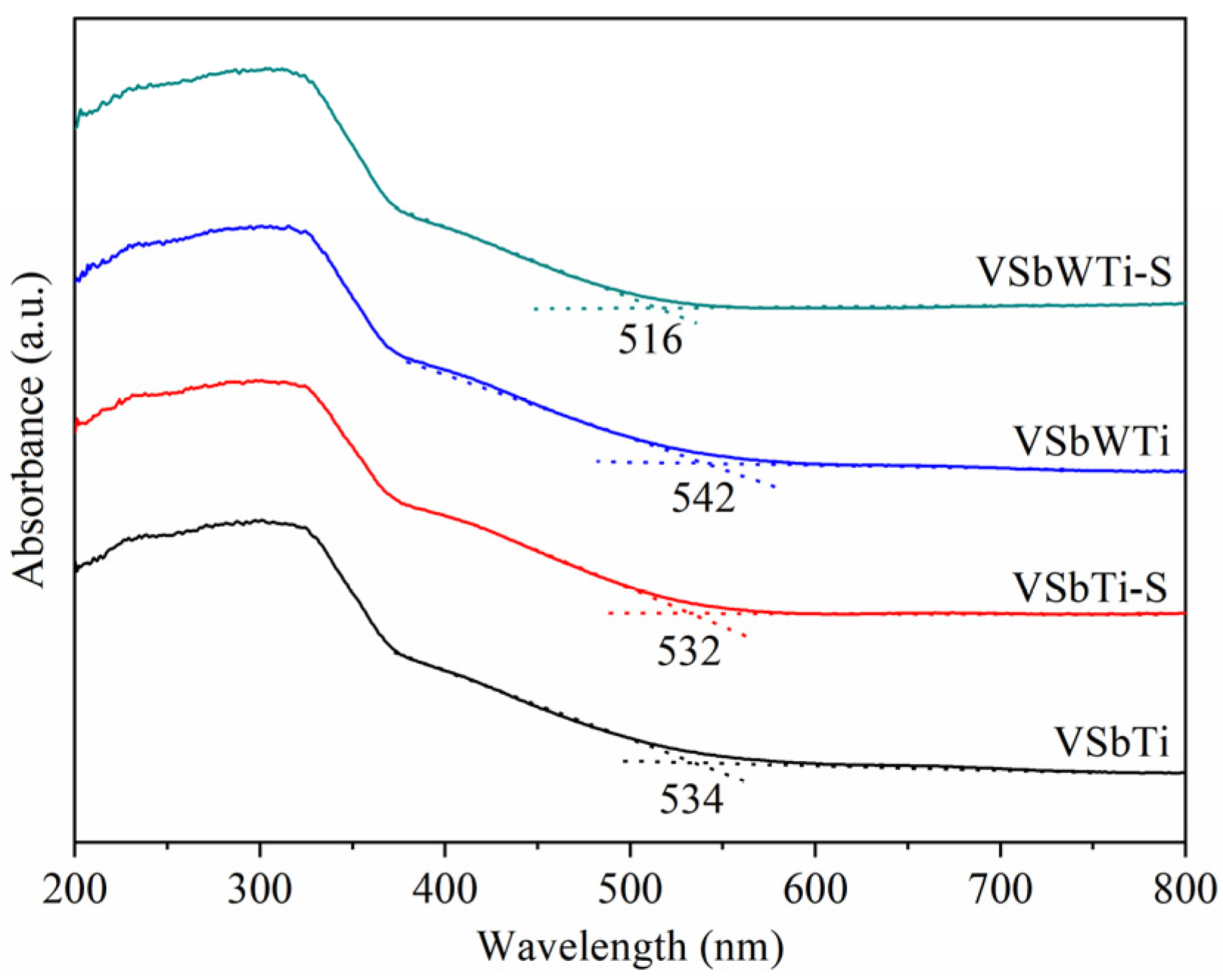
3.3. Surface Properties
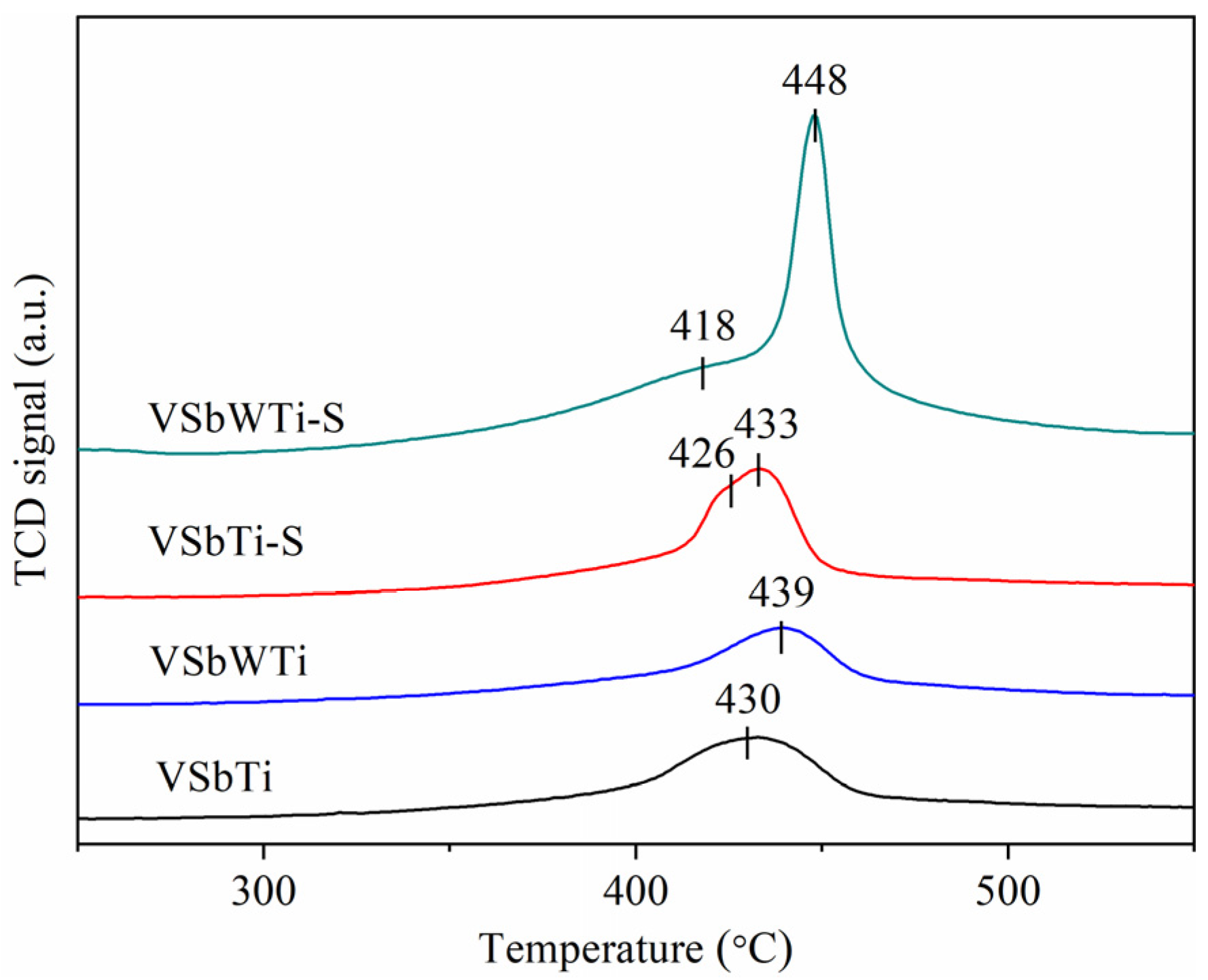
3.4. Reactivity of Ammonium Bisulfate with NO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, F.; Yu, Y.; He, H. Environmentally-benign catalysts for the selective catalytic reduction of NOx from diesel engines: Structure–activity relationship and reaction mechanism aspects. Chem. Commun. 2014, 50, 8445–8463. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Y.; Liu, S.; Chen, J.; Jia, W. Review on the latest developments in modified vanadium-titanium-based SCR catalysts. Chin. J. Catal. 2018, 39, 1347–1365. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, J. Numerical Investigation on the Intraphase and Interphase Mass Transfer Limitations for NH3-SCR over Cu-ZSM-5. Processes 2021, 9, 1966. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.-Y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective Catalytic Reduction of NOx with NH3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Lai, J.-K.; Wachs, I.E. A Perspective on the Selective Catalytic Reduction (SCR) of NO with NH3 by Supported V2O5-WO3/TiO2 Catalysts. ACS Catal. 2018, 8, 6537–6551. [Google Scholar] [CrossRef]
- Maitarad, P.; Meeprasert, J.; Shi, L.; Limtrakul, J.; Zhang, D.; Namuangruk, S. Mechanistic insight into the selective catalytic reduction of NO by NH3 over low-valent titanium-porphyrin: A DFT study. Catal. Sci. Technol. 2015, 6, 3878–3885. [Google Scholar] [CrossRef]
- Song, J.; Wang, Z.; Cheng, X.; Wang, X. State-of-Art Review of NO Reduction Technologies by CO, CH4 and H2. Processes 2021, 9, 563. [Google Scholar] [CrossRef]
- Mehdi, G.; Zhou, S.; Zhu, Y.; Shah, A.H.; Chand, K. Numerical Investigation of SCR Mixer Design Optimization for Improved Performance. Processes 2019, 7, 168. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Chen, G.; Guo, F.; Xie, J. Development of wide-temperature vanadium-based catalysts for selective catalytic reducing of NOx with ammonia: Review. Chem. Eng. J. 2018, 353, 507–518. [Google Scholar] [CrossRef]
- Chang, H.; Li, J.; Su, W.; Shao, Y.; Hao, J. A novel mechanism for poisoning of metal oxide SCR catalysts: Base–acid explanation correlated with redox properties. Chem. Commun. 2014, 50, 10031–10034. [Google Scholar] [CrossRef]
- Guo, M.; Lis, B.M.; Ford, M.E.; Wachs, I.E. Effect of redox promoters (CeOx and CuOx) and surface sulfates on the selective catalytic reduction (SCR) of NO with NH3 by supported V2O5-WO3/TiO2 catalysts. Appl. Catal. B Environ. 2022, 306, 121108. [Google Scholar] [CrossRef]
- Zheng, Y.; Jensen, A.D.; Johnsson, J.E. Deactivation of V2O5-WO3-TiO2 SCR catalyst at a biomass-fired combined heat and power plant. Appl. Catal. B Environ. 2005, 60, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Xi, Y.; Ottinger, N.A.; Liu, Z.G. New insights into sulfur poisoning on a vanadia SCR catalyst under simulated diesel engine operating conditions. Appl. Catal. B Environ. 2014, 160–161, 1–9. [Google Scholar] [CrossRef]
- Magnusson, M.; Fridell, E.; Härelind, H. Improved low-temperature activity for marine selective catalytic reduction systems. Proc. Inst. Mech. Eng. Part M J. Eng. Marit. Environ. 2014, 230, 126–135. [Google Scholar] [CrossRef]
- Khatri, N.J.; Johnson, J.H.; Leddy, D.G. The Characterization of the Hydrocarbon and Sulfate Fractions of Diesel Particulate Matter; SAE Technical Paper 780111; SAE: Warrendale, PA, USA, 1978; pp. 469–492. [Google Scholar] [CrossRef]
- Wall, J.C.; Hoekman, S.K. Fuel Composition Effects on Heavy-Duty Diesel Particulate Emissions; SAE Technical Paper 841364; SAE: Warrendale, PA, USA, 1984; pp. 1–42. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, P.; Shen, Z.; Chen, S.; Wang, Q.; Cheng, D.; Zhang, D. Low-temperature NOx reduction over hydrothermally stable SCR catalysts by engineering low-coordinated Mn active sites. Chem. Eng. J. 2022, 442, 136182. [Google Scholar] [CrossRef]
- Xu, T.; Wang, C.; Wu, X.; Zhao, B.; Chen, Z.; Weng, D. Modification of MnCo2Ox catalysts by NbOx for low temperature selective catalytic reduction of NO with NH3. RSC Adv. 2016, 6, 97004–97011. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, R.-T.; Zhang, X.-F.; Liu, Y.-Z.; Duan, C.-P.; Wu, G.-L.; Pan, W.-G. Cerium Oxide-Based Catalysts for Low-Temperature Selective Catalytic Reduction of NOx with NH3: A Review. Energy Fuels 2021, 35, 2981–2998. [Google Scholar] [CrossRef]
- Ye, D.; Qu, R.; Song, H.; Gao, X.; Luo, Z.; Ni, M.; Cen, K. New insights into the various decomposition and reactivity behaviors of NH4HSO4 with NO on V2O5/TiO2 catalyst surfaces. Chem. Eng. J. 2016, 283, 846–854. [Google Scholar] [CrossRef]
- Arfaoui, J.; Ghorbel, A.; Petitto, C.; Delahay, G. Novel V2O5-CeO2-TiO2-SO42− nanostructured aerogel catalyst for the low temperature selective catalytic reduction of NO by NH3 in excess O2. Appl. Catal. B Environ. 2018, 224, 264–275. [Google Scholar] [CrossRef]
- Purbia, R.; Choi, S.Y.; Kim, H.J.; Ye, B.; Jeong, B.; Lee, D.H.; Park, H.; Kim, H.-D.; Baik, J.M. Cu- and Ce-promoted nano-heterostructures on vanadate catalysts for low-temperature NH3–SCR activity with improved SO2 and water resistance. Chem. Eng. J. 2022, 437, 135427. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Q. Optimization of Roasting Parameters for Recovery of Vanadium and Tungsten from Spent SCR Catalyst with Composite Roasting. Processes 2021, 9, 1923. [Google Scholar] [CrossRef]
- Cheng, K.; Liu, J.; Zhang, T.; Li, J.; Zhao, Z.; Wei, Y.; Jiang, G.; Duan, A. Effect of Ce doping of TiO2 support on NH3-SCR activity over V2O5-WO3/CeO2-TiO2 catalyst. J. Environ. Sci. 2014, 26, 2106–2113. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huo, Y.; Shi, X.; Liu, Z.; Shan, Y.; Yu, Y.; Shan, W.; He, H. Insight into the remarkable enhancement of NH3-SCR performance of Ce-Sn oxide catalyst by tungsten modification. Catal. Today 2022, in press. [Google Scholar] [CrossRef]
- Shi, Y.; Tan, S.; Li, S.; Zhao, J.; Xia, Y.; Lv, B.; Li, W. Inhibitory effect of SO2 on side reactions of NH3-SCR over olivine. Catal. Sci. Technol. 2015, 5, 3613–3623. [Google Scholar] [CrossRef]
- Chen, H.; Xia, Y.; Fang, R.; Huang, H.; Gan, Y.; Liang, C.; Zhang, J.; Zhang, W.; Liu, X. The effects of tungsten and hydrothermal aging in promoting NH3-SCR activity on V2O5/WO3-TiO2 catalysts. Appl. Surf. Sci. 2018, 459, 639–646. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, Q.; Zhao, W.; Li, Y. Surface characterization studies on F-doped V2O5/TiO2 catalyst for NO reduction with NH3 at low-temperature. Chem. Eng. J. 2014, 253, 207–216. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, X.; Feng, Y.; Si, Z.; Weng, D.; Shi, L. Low-temperature SCR activity and SO2 deactivation mechanism of Ce-modified V2O5-WO3/TiO2 catalyst. Prog. Nat. Sci. 2015, 25, 342–352. [Google Scholar] [CrossRef] [Green Version]
- Arfaoui, J.; Ghorbel, A.; Petitto, C.; Delahay, G. New CeO2-TiO2, WO3-TiO2 and WO3-CeO2-TiO2 mesoporous aerogel catalysts for the low temperature selective catalytic reduction of NO by NH3. J. Porous Mater. 2021, 28, 1535–1543. [Google Scholar] [CrossRef]
- Lietti, L.; Alemany, J.; Forzatti, P.; Busca, G.; Ramis, G.; Giamello, E.; Bregani, F. Reactivity of V2O5-WO3/TiO2 catalysts in the selective catalytic reduction of nitric oxide by ammonia. Catal. Today 1996, 29, 143–148. [Google Scholar] [CrossRef]
- Paganini, M.C.; Dall’Acqua, L.; Giamello, E.; Lietti, L.; Forzatti, P.; Busca, G. An EPR Study of the Surface Chemistry of the V2O5-WO3/TiO2 Catalyst: Redox Behaviour and State of V(IV). J. Catal. 1997, 166, 195–205. [Google Scholar] [CrossRef]
- Jaegers, N.R.; Lai, J.; He, Y.; Walter, E.; Dixon, D.A.; Vasiliu, M.; Chen, Y.; Wang, C.; Hu, M.Y.; Mueller, K.T.; et al. Mechanism by which Tungsten Oxide Promotes the Activity of Supported V2O5/TiO2 Catalysts for NOX Abatement: Structural Effects Revealed by 51 V MAS NMR Spectroscopy. Angew. Chem. Int. Ed. 2019, 58, 12609–12616. [Google Scholar] [CrossRef]
- Sun, C.; Dong, L.; Yu, W.; Liu, L.; Li, H.; Gao, F.; Dong, L.; Chen, Y. Promotion effect of tungsten oxide on SCR of NO with NH3 for the V2O5-WO3/Ti0.5Sn0.5O2 catalyst: Experiments combined with DFT calculations. J. Mol. Catal. A Chem. 2011, 346, 29–38. [Google Scholar] [CrossRef]
- Nuguid, R.J.G.; Ferri, D.; Marberger, A.; Nachtegaal, M.; Kröcher, O. Modulated Excitation Raman Spectroscopy of V2O5/TiO2: Mechanistic Insights into the Selective Catalytic Reduction of NO with NH3. ACS Catal. 2019, 9, 6814–6820. [Google Scholar] [CrossRef]
- Kang, T.H.; Youn, S.; Kim, D.H. Improved catalytic performance and resistance to SO2 over V2O5-WO3/TiO2 catalyst physically mixed with Fe2O3 for low-temperature NH3-SCR. Catal. Today 2020, 376, 95–103. [Google Scholar] [CrossRef]
- Xu, C.; Liu, J.; Zhao, Z.; Yu, F.; Cheng, K.; Wei, Y.; Duan, A.; Jiang, G. NH3-SCR denitration catalyst performance over vanadium–titanium with the addition of Ce and Sb. J. Environ. Sci. 2015, 31, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Haheon, P.; Chungsoon, H.; Ohyoung, J. Selective catalytic NOx reduction on Antimony promoted V2O5-Sb/TiO2 catalyst. Rare Met. 2006, 25, 84–88. [Google Scholar] [CrossRef]
- Phil, H.H.; Reddy, M.P.; Kumar, P.A.; Ju, L.K.; Hyo, J.S. SO2 resistant antimony promoted V2O5/TiO2 catalyst for NH3-SCR of NOx at low temperatures. Appl. Catal. B Environ. 2008, 78, 301–308. [Google Scholar] [CrossRef]
- Xu, T.; Wu, X.; Gao, Y.; Lin, Q.; Hu, J.; Weng, D. Comparative study on sulfur poisoning of V2O5-Sb2O3/TiO2 and V2O5-WO3/TiO2 monolithic catalysts for low-temperature NH3-SCR. Catal. Commun. 2017, 93, 33–36. [Google Scholar] [CrossRef]
- Kumar, P.A.; Jeong, Y.E.; Gautam, S.; Ha, H.P.; Lee, K.J.; Chae, K.H. XANES and DRIFTS study of sulfated Sb/V/Ce/TiO2 catalysts for NH3-SCR. Chem. Eng. J. 2015, 275, 142–151. [Google Scholar] [CrossRef]
- Shen, M.; Li, C.; Wang, J.; Xu, L.; Wang, W.; Wang, J. New insight into the promotion effect of Cu doped V2O5/WO3-TiO2 for low temperature NH3-SCR performance. RSC Adv. 2015, 5, 35155–35165. [Google Scholar] [CrossRef]
- Albonetti, S.; Blasioli, S.; Bonelli, R.; Mengou, J.E.; Scirè, S.; Trifirò, F. The role of acidity in the decomposition of 1,2-dichlorobenzene over TiO2-based V2O5/WO3 catalysts. Appl. Catal. A Gen. 2008, 341, 18–25. [Google Scholar] [CrossRef]
- Kubacka, A.; Iglesias-Juez, A.; di Michiel, M.; Becerro, A.I.; Fernández-García, M. Morphological and structural behavior of TiO2 nanoparticles in the presence of WO3: Crystallization of the oxide composite system. Phys. Chem. Chem. Phys. 2014, 16, 19540–19549. [Google Scholar] [CrossRef] [Green Version]
- Maqbool, M.S.; Pullur, A.K.; Ha, H.P. Novel sulfation effect on low-temperature activity enhancement of CeO2-added Sb-V2O5/TiO2 catalyst for NH3-SCR. Appl. Catal. B Environ. 2014, 152–153, 28–37. [Google Scholar] [CrossRef]
- Kwon, D.W.; Park, K.H.; Hong, S.C. Enhancement of SCR activity and SO2 resistance on VOx/TiO2 catalyst by addition of molybdenum. Chem. Eng. J. 2016, 284, 315–324. [Google Scholar] [CrossRef]
- Tang, F.; Xu, B.; Shi, H.; Qiu, J.; Fan, Y. The poisoning effect of Na+ and Ca2+ ions doped on the V2O5/TiO2 catalysts for selective catalytic reduction of NO by NH3. Appl. Catal. B Environ. 2010, 94, 71–76. [Google Scholar] [CrossRef]
- Youn, S.; Jeong, S.; Kim, D.H. Effect of oxidation states of vanadium precursor solution in V2O5/TiO2 catalysts for low temperature NH3 selective catalytic reduction. Catal. Today 2014, 232, 185–191. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, X.; Lin, Q.; Hu, J.; Ran, R.; Weng, D. SO2 promoted V2O5-MoO3/TiO2 catalyst for NH3-SCR of NO at low temperatures. Appl. Catal. A Gen. 2018, 570, 42–50. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, X.; Cao, L.; Ma, Y.; Ran, R.; Si, Z.; Weng, D.; Ma, Z.; Wang, B. Crystal orientation-dependent activity of tungsten-based catalysts for selective catalytic reduction of NO with NH3. J. Catal. 2019, 375, 294–303. [Google Scholar] [CrossRef]
- Yanyan, L.; Kurniawan, T.A.; Ying, Z.; Albadarin, A.B.; Walker, G. Enhanced photocatalytic degradation of acetaminophen from wastewater using WO3/TiO2/SiO2 composite under UV–VIS irradiation. J. Mol. Liq. 2017, 243, 761–770. [Google Scholar] [CrossRef] [Green Version]
- Srinivas, D.; Hölderich, W.; Kujath, S.; Valkenberg, M.; Raja, T.; Saikia, L.; Hinze, R.; Ramaswamy, V. Active sites in vanadia/titania catalysts for selective aerial oxidation of β-picoline to nicotinic acid. J. Catal. 2008, 259, 165–173. [Google Scholar] [CrossRef]
- Song, L.; Chao, J.; Fang, Y.; He, H.; Li, J.; Qiu, W.; Zhang, G. Promotion of ceria for decomposition of ammonia bisulfate over V2O5-MoO3/TiO2 catalyst for selective catalytic reduction. Chem. Eng. J. 2016, 303, 275–281. [Google Scholar] [CrossRef]
- Shi, Y.-J.; Shu, H.; Zhang, Y.-H.; Fan, H.-M.; Zhang, Y.-P.; Yang, L.-J. Formation and decomposition of NH4HSO4 during selective catalytic reduction of NO with NH3 over V2O5-WO3/TiO2 catalysts. Fuel Process. Technol. 2016, 150, 141–147. [Google Scholar] [CrossRef]
- Ye, D.; Qu, R.; Song, H.; Zheng, C.; Gao, X.; Luo, Z.; Ni, M.; Cen, K. Investigation of the promotion effect of WO3 on the decomposition and reactivity of NH4HSO4 with NO on V2O5-WO3/TiO2 SCR catalysts. RSC Adv. 2016, 6, 55584–55592. [Google Scholar] [CrossRef]
- Jangjou, Y.; Do, Q.; Gu, Y.; Lim, L.-G.; Sun, H.; Wang, D.; Kumar, A.; Li, J.; Grabow, L.C.; Epling, W.S. Nature of Cu Active Centers in Cu-SSZ-13 and Their Responses to SO2 Exposure. ACS Catal. 2018, 8, 1325–1337. [Google Scholar] [CrossRef]
- Liang, Q.; Li, J.; He, H.; Yue, T.; Tong, L. Effects of SO2 and H2O on low-temperature NO conversion over F-V2O5-WO3/TiO2 catalysts. J. Environ. Sci. 2019, 90, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, H. Thermal decomposition temperatures of metal sulfates. Thermochim. Acta 1984, 80, 23–33. [Google Scholar] [CrossRef]
- Han, L.; Gao, M.; Hasegawa, J.-Y.; Li, S.; Shen, Y.; Li, H.; Shi, L.; Zhang, D. SO2-Tolerant Selective Catalytic Reduction of NOx over Meso-TiO2@Fe2O3@Al2O3 Metal-Based Monolith Catalysts. Environ. Sci. Technol. 2019, 53, 6462–6473. [Google Scholar] [CrossRef]
- Ye, D.; Qu, R.; Zheng, C.; Cen, K.; Gao, X. Mechanistic investigation of enhanced reactivity of NH4HSO4 and NO on Nb- and Sb-doped VW/Ti SCR catalysts. Appl. Catal. A Gen. 2018, 549, 310–319. [Google Scholar] [CrossRef]
- Zhu, Z.; Niu, H.; Liu, Z.; Liu, S. Decomposition and Reactivity of NH4HSO4 on V2O5/AC Catalysts Used for NO Reduction with Ammonia. J. Catal. 2000, 195, 268–278. [Google Scholar] [CrossRef]
- Xu, T.; Wu, X.; Liu, X.; Cao, L.; Lin, Q.; Weng, D. Effect of barium sulfate modification on the SO2 tolerance of V2O5/TiO2 catalyst for NH3-SCR reaction. J. Environ. Sci. 2017, 57, 110–117. [Google Scholar] [CrossRef]
- Dong, G.-J.; Zhang, Y.-F.; Zhao, Y.; Bai, Y. Effect of the pH value of precursor solution on the catalytic performance of V2O5-WO3/TiO2 in the low temperature NH3-SCR of NOx. J. Fuel Chem. Technol. 2014, 42, 1455–1463. [Google Scholar] [CrossRef]
- Song, I.; Youn, S.; Lee, H.; Lee, S.G.; Cho, S.J.; Kim, D.H. Effects of microporous TiO2 support on the catalytic and structural properties of V2O5/microporous TiO2 for the selective catalytic reduction of NO by NH3. Appl. Catal. B Environ. 2017, 210, 421–431. [Google Scholar] [CrossRef]
- Liu, L.; Wu, X.; Ma, Y.; Zhang, X.; Ran, R.; Si, Z.; Weng, D. Potassium deactivation of Cu-SSZ-13 catalyst for NH3-SCR: Evolution of salts, zeolite and copper species. Chem. Eng. J. 2019, 383, 123080. [Google Scholar] [CrossRef]
- Kong, M.; Liu, Q.; Zhou, J.; Jiang, L.; Tian, Y.; Yang, J.; Ren, S.; Li, J. Effect of different potassium species on the deactivation of V2O5-WO3/TiO2 SCR catalyst: Comparison of K2SO4, KCl and K2O. Chem. Eng. J. 2018, 348, 637–643. [Google Scholar] [CrossRef]
- Orsenigo, C.; Lietti, L.; Tronconi, E.; Forzatti, P.; Bregani, F. Dynamic Investigation of the Role of the Surface Sulfates in NOx Reduction and SO2 Oxidation over V2O5−WO3/TiO2 Catalysts. Ind. Eng. Chem. Res. 1998, 37, 2350–2359. [Google Scholar] [CrossRef]
- Liu, F.; Asakura, K.; He, H.; Shan, W.; Shi, X.; Zhang, C. Influence of sulfation on iron titanate catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 2011, 103, 369–377. [Google Scholar] [CrossRef]
- Chen, C.; Wu, X.; Yu, W.; Gao, Y.; Weng, D.; Shi, L.; Geng, C. Potassium poisoning of titania supported deNOx catalysts: Preservation of vanadia and sacrifice of tungsten oxide. Chin. J. Catal. 2015, 36, 1287–1294. [Google Scholar] [CrossRef]
- Dunn, J.P.; Koppula, P.R.; Stenger, H.G.; Wachs, I.E. Oxidation of sulfur dioxide to sulfur trioxide over supported vanadia catalysts. Appl. Catal. B Environ. 1998, 19, 103–117. [Google Scholar] [CrossRef]


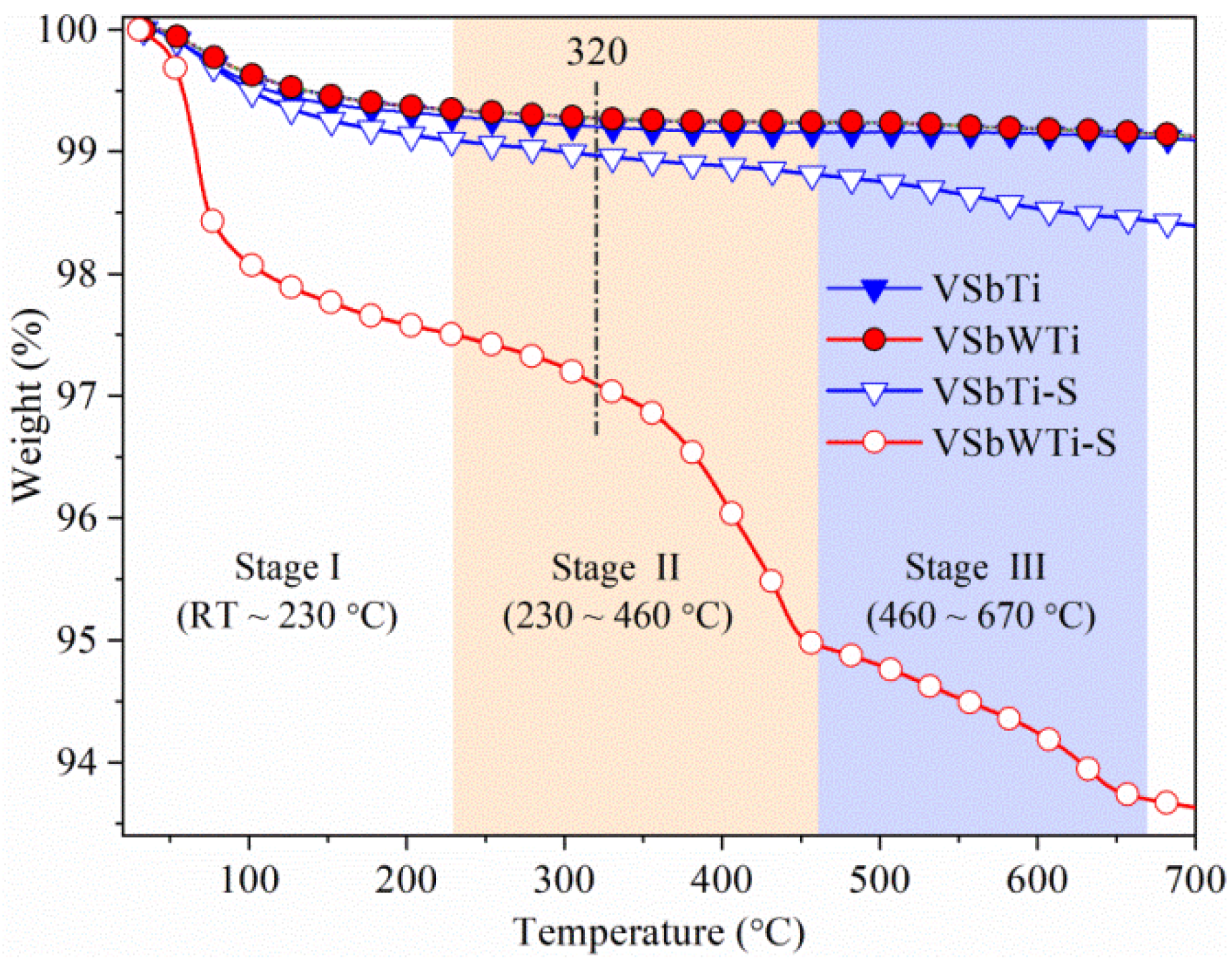

| Catalyst | Weight Loss (%) | |
|---|---|---|
| Stage II (230–460 °C) | Stage III (460–670 °C) | |
| VSbTi-S | 0.28 | 0.38 |
| VSbWTi-S | 2.54 | 1.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Wu, X.; Ma, Y.; Wang, J.; Ran, R.; Si, Z.; Weng, D. Tungsten Oxide Modified V2O5-Sb2O3/TiO2 Monolithic Catalyst: NH3-SCR Activity and Sulfur Resistance. Processes 2022, 10, 1333. https://doi.org/10.3390/pr10071333
Liu L, Wu X, Ma Y, Wang J, Ran R, Si Z, Weng D. Tungsten Oxide Modified V2O5-Sb2O3/TiO2 Monolithic Catalyst: NH3-SCR Activity and Sulfur Resistance. Processes. 2022; 10(7):1333. https://doi.org/10.3390/pr10071333
Chicago/Turabian StyleLiu, Liping, Xiaodong Wu, Yue Ma, Jinyi Wang, Rui Ran, Zhichun Si, and Duan Weng. 2022. "Tungsten Oxide Modified V2O5-Sb2O3/TiO2 Monolithic Catalyst: NH3-SCR Activity and Sulfur Resistance" Processes 10, no. 7: 1333. https://doi.org/10.3390/pr10071333
APA StyleLiu, L., Wu, X., Ma, Y., Wang, J., Ran, R., Si, Z., & Weng, D. (2022). Tungsten Oxide Modified V2O5-Sb2O3/TiO2 Monolithic Catalyst: NH3-SCR Activity and Sulfur Resistance. Processes, 10(7), 1333. https://doi.org/10.3390/pr10071333







