Abstract
An electrochemical method for the determination of the catalytic activity of L-asparaginase (ASNase) from Erwinia carotovora was proposed. Our approach is based on the electrooxidation of amino acids from L-asparaginase polypeptide backbones. The electrochemical behavior of ASNase on electrodes obtained by screen-printing modified with single-wall carbon nanotubes (SPE/SWCNTs) as sensing elements demonstrated a broad oxidation peak at 0.5–0.6 V centered at 0.531 ± 0.010 V. We have shown that in the presence of the substrate L-asparagine, the oxidation current of the enzyme was reduced in a concentration-dependent manner. The specificity of electrochemical analysis was confirmed in experiments with glycine, an amino acid with no substrate activity on ASNase and does not reduce the oxidation peak of L-asparaginase. The addition of glycine did not significantly influence the amplitude of the oxidation current. The innovative aspects of the proposed electrochemical sensor are the direct monitoring of ASNase catalytic activity and a reagentless approach, which does not require additional reagents or labels.
1. Introduction
L-asparaginase (ASNase, L-asparagine amidohydrolase (EC 3.5.1.1)) has been used in medicine as a pharmacological antitumor agent for acute lymphoblastic leukemia [1,2], non-Hodgkin’s lymphoma and other malignant tumors, against pathogenic bacterial infection [3], and in the food industry for preventing acrylamide formation in processed foods with high starch content [1,4,5]. ASNase is an amidohydrolase that catalyzes the conversion of L-asparagine (L-Asn) into L-aspartic acid and ammonia. Tumor cells lack L-asparagine synthetase and cannot synthesize L-Asn autonomously [6]. The deficiency of this amino acid can lead to abnormal protein synthesis in cancer cells and ultimately cause cell death [6]. Human cells cannot synthetize ASNase, and native L-ASNase from Escherichia coli (EcA) or Dickeya dadantii (formerly known as Erwinia chrysanthemi) (ErA) along with the pegylated form of E. coli asparaginase have been successfully used for the treatment of patients with acute lymphoblastic leukemia. The pharmacological dose of ASNase must be individually determined according to the patient’s clinical response and tolerance to ensure the maximum therapeutic effect and minimize side effects [6,7].
Estimation of ASNase catalytic activity is based on different approaches, such as the determination of L-Asn content or ammonia concentration measured by high-performance liquid chromatography (HPLC), direct amino acid quantification by circular dichroism (CD) [8], electrophoresis assays, and determination by a colorimetric assay from complexation with hydroxylamine [1]. The simultaneous production of L-aspartic acid (L-Asp) and NH4+ cations can also be used to quantify ASNase activity by conductometry, where the increase in conductivity corresponds to the rate of product formation [8,9]. The most commonly used Nessler reaction with potassium tetraiodine mercurate (II) K2HgI4 requires using highly toxic reagents with poor stability. In addition, the Nessler reaction has low reproducibility and complicated operations and is not suitable for detecting clinical samples [6]. From these viewpoints, sensitive, reliable, and robust methods of determination and catalytic activity monitoring of L-asparaginase are in great demand.
Physical, physico-chemical, and nanotechnological approaches have also contributed to the development of detection methods for the estimation of enzyme catalytic activity. An approach to measuring the activity of monomers or oligomers of the heme-containing enzyme CYP102A1 by atomic force microscopy (AFM) has been developed. Using AFM, it was found that the amplitude of fluctuations of the height of single CYP102A1 molecules performing the catalytic cycle is twice as great as the amplitude of fluctuations of the height of the same enzymes in the inactive state [10]. A new powerful and versatile approach for measuring enzymatic activities based on the detection of biochemical events using nanopores has been employed [11]. However, this sensitive and elegant approach for monitoring biocatalysis requires additional equipment for the registration of low currents (in the 10−12 A or 10−15 A range) and suitable biological or inorganic nanopores.
Electrochemistry, and especially bioelectrochemistry, has great potential in the field of enzymology and determination of such events as substrate binding, the oxidation state of the catalytic center, and structural fluctuations [12,13].
Enzyme electrodes are analytical devices based on the combination of the high specificity of biocatalytic reactions with the electrochemical transduction of the recognition event [14,15,16]. Analysis of enzyme electrode kinetics is important for designing a sensor or for optimization of parameters for enzyme-substrate interactions [17,18].
Based on the analysis of the electrochemical parameters of the bioelectrode, such as the dependence of the catalytic current generated by the enzyme during concentration-dependent substrate conversion, quantitative calculations of the Michaelis constant Km, substrate constant Ks, maximum reaction rate Vmax, catalytic rate constants and catalytic efficiency can be calculated [17].
The electrochemical activity of protein molecules depends on two main structural characteristics, redox-active cofactors (heme or Flavin, for example) and electrochemically active amino acid residues [18,19,20,21,22,23,24,25]. Tyrosine, tryptophan, histidine, cysteine, cystine, and methionine yielded oxidation peaks at solid electrodes [19,20,21,22,23]. Amino acid oxidation is an irreversible electrochemical process. The changes in amino acid position and complex formation with metal ions or ligands influence the oxidation signal intensities. The electrochemical activity of amino acids is a valuable tool for the analysis and effective registration of protein posttranslational modifications or “modificomics,” protein-function analysis, and detection of conformational changes [24,25,26,27]. Electrochemical profiling of acetylcholinesterase wild-type AChE and mutant proteins was performed [28]. Amino acid substitutions introduced through site-directed mutagenesis of AChE were detected using square-wave voltammetry on a screen-printed carbon electrode. The authors underlined that complex conformational changes of the polypeptide chain have a more dominant influence on the oxidation profile of the mutants than the individual amino acid substitutions [28].
As shown earlier, Thr12, Tyr25, Thr89, Asp90, and Lys162 of ASNase participate in catalysis and substrate binding [29,30,31,32,33,34,35,36,37,38]. These amino acids are conserved for all bacterial asparaginases [29,34,35,36,37,38,39,40,41,42]. The mechanism of catalysis involves two triads, Thr12-Lys162-Asp90, which resembles the classical serine proteinase mechanism and participates in catalysis, and Thr12-Tyr25-Glu283, which participates in substrate binding and releases products of catalytic reactions [35,36,37].
In our investigation, the ability and susceptibility of the protein to conformational changes registered by electrochemical oxidation were used to estimate the catalytic activity of ASNase. We assumed that in the course of catalytic conversion of L-Asn as a substrate, structural fluctuations of ASNase occurred, which led to the rearrangement of the protein chain and changes in the intensities of electroactive amino acids as specific electrochemical labels [28]. These properties of a protein can be registered with voltammetric techniques such as differential pulse voltammetry.
2. Materials and Methods
2.1. Reagents and Materials
L-Asn and glycine (Gly) were purchased from Sigma–Aldrich (St. Louis, MO, USA). A water dispersion of 0.4% single-walled carbon nanotubes (SWCNTs, surface area 1000 m2/g) TUBALL™ BATT H2O stabilized by carboxymethylcellulose obtained from OCSIAL Ltd. (Luxembourg) https://tuball.com/ru/additives) (accessed on 16 June 2022) was used. Asparaginase from Erwinia carotovora was prepared as described previously [38].
Screen-printed electrodes (SPE) with graphite working (geometric area 0.0314 cm2), auxiliary electrodes, and silver/silver chloride reference electrodes (Ag/AgCl) were obtained from ColorElectronics, Moscow, Russia (http://www.colorel.ru) (accessed on 16 June 2022).
2.2. Equipment
Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) measurements were performed using an Autolab PGSTAT12 potentiostat/galvanostat (Metrohm Autolab, Utrecht, The Netherlands) equipped with GPES software (version 4.9.7). All electrochemical experiments were carried out at room temperature in 100 mM potassium phosphate with 50 mM NaCl at pH 7.4. CV experiments were carried out in a 1 mL electrochemical cell by potential sweeping from an initial potential of −300 mV to an end-point potential of +800 mV at different scan rates in a range of 10–100 mV/s. DPV experiments were carried out in a 60 µL drop applied onto the electrode to cover all three electrodes.
Electrochemical studies of L-asparaginase were performed in 0.1 M potassium-phosphate buffer containing 0.05 M NaCl, pH 7.4. For the preparation of the modified electrodes, 2 µL of SWCNT in carboxymethylcellulose diluted with water as 1:2 (OCSIAL, Luxembourg, https://tuball.com/ru/additives) (accessed on 16 June 2022) was dropped onto the working area of the SPE and incubated for 15 min. All potentials were referenced to the Ag/AgCl electrode. All experiments were performed in triplicate. The data are presented as average values ± standard deviations.
For further incorporation of the analyte, a 60-μL aliquot (ASNase, 3 μg/μL of electrolyte buffer) or ASNase with an appropriate concentration of amino acids L-Asn or Gly was dispensed onto the electrode surface. All electrochemical experiments were carried out at room temperature (23 ± 3 °C). A horizontal regimen of measurement was used for all experiments. The DPV method was used with the following parameters: pulse amplitude, 25 mV; potential step, 1 mV; pulse duration, 50 ms. Binding of amino acids with ASNase was performed for 5 min at room temperature.
Modified SPE/SWCNTs have been utilized for a single measurement to avoid surface fouling, cross-contamination, and blockage of the electrode surface by protein oxidation products. Each sample was tested with three independent measurements. The relative standard deviation in all cases did not exceed 10%. In the figures, data points represent the mean values of the detected peak currents (Ip) or potential maxima (Emax), with the error bars showing the confidential intervals. Each voltammogram was processed with Savitzky–Golay level 3 smoothing and baseline corrected to a peak width of 0.003 V using the GPES moving average baseline correction tool. The oxidation peak current and peak potential values were then recorded for each measurement.
SEM micrographs of carbon nanotubes were obtained using a Hitachi S-5500 scanning electron microscope (Hitachi, Tokyo, Japan) operating at 25 kV.
3. Results and Discussion
Disposable screen-printed electrodes (SPEs) are widely used in electrochemical sensing of hemeproteins, drugs, DNA, drug-protein, and drug-DNA interactions [14,39,40,41]. They possess commercial availability, low cost, and the ability to modify the working surface with a broad choice of chemical materials with different structures. Carbon nanomaterials significantly improve the sensitivity of electrodes [34]. We used a drop-casting method to modify SPEs with a dispersion of SWCNTs in carboxymethylcellulose (SPE/SWCNT). Two-microliter drops of dispersion were applied to the working area of the SPE. As shown in Figure 1A,B, the images for SWCNTs demonstrated carbon nanomaterials as SWCNTs with an average diameter of 10 nm.
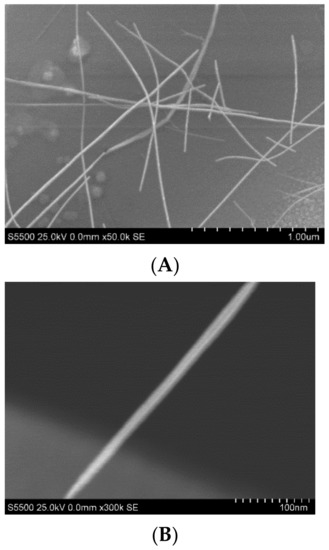
Figure 1.
SEM images of the water dispersion of single-wall carbon nanotubes (SWCNTs) stabilized by carboxymethylcellulose at higher (A) and lower (B) magnitudes.
Cyclic voltammetry (CV) measurements were used to assess the electroactive surface area of the modified SPE/SWCNTs. Figure 2A demonstrates the CV profiles for the ferri/ferro cyanide [Fe(CN)6]3−/4− redox probe with a couple of well-defined redox peaks for SPE/SWCNT. The linear dependence of the peak current versus the square root of the scan rate was found and confirmed as a diffusion-controlled process (Figure 2B) [42]. The anodic/cathodic peak currents for the SPE/SWCNT have much higher intensities than bare SPE with a lower ΔE peak-to-peak separation (Figure 2C). This indicates that a more reversible redox performance of [Fe(CN)6]3−/4− occurs when CNTs are used. SPE/SWCNTs possess a larger specific surface area according to the Randles–Sevcik equation [42]. The calculated values of the specific surface area corresponded to 0.0024 cm2 and 0.1258 cm2 for SPE and SPE/SWCNT, respectively. These results confirmed the good conductivity and electron transfer properties of carbon nanomaterials such as carbon nanotubes [14,39,40,41,42].
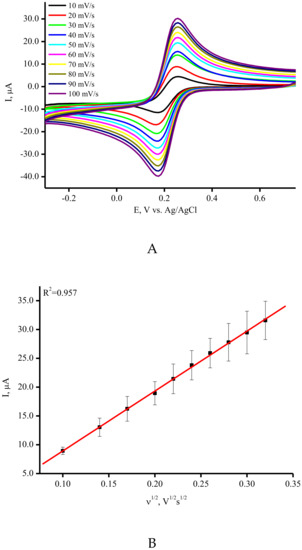
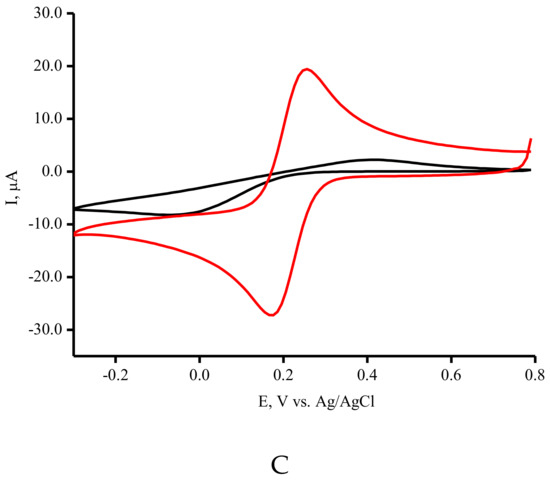
Figure 2.
(A) Typical CV curves for SPE/SWCNT. The measurements were carried out in 5 mM K3Fe(CN)6 at ambient temperature in a potential range from −400 mV to +800 mV (vs. Ag/AgCl) at scan rates in the range of 10–100 mV/s. (B) The dependence of peak current Ip on the square root of the scan rate in the range of 10–100 mV/s. (C) The typical CV curves for SPE (-) and SPE/SWCNT (-) in 5 mM K3Fe(CN)6 at 50 mV/s.
SPEs modified with SWCNTs (SPE/SWCNTs) were used for the electrochemical registration of ASNase. ASNase was deposited onto the modified active area of the working electrode. This step was accomplished by the direct adsorption method. As we have shown earlier, modification of the SPE with CNTs significantly enhances the ability of the electrode to detect amino acid and protein electrochemical oxidation [27]. To obtain the electrochemical response of ASNase on SPE/SWCNTs, we used a differential pulse voltammetry technique (DPV), which permits the registration of enzymes with good sensitivity. (Figure 3A). Electrochemical profiling of ASNase on SPE/SWCNT revealed a broad oxidation peak in the 0.5–0.6 V with a maximum amplitude of 0.593 ± 0.007 V. The oxidation peak was detected using DPV and corresponded to the irreversible oxidation of electroactive tyrosine (Tyr), tryptophan (Trp) and cysteine (Cys) residues [21,22,23,24,25,29,30,42]. No redox signals were observed for the SPE/SWCNTs in the absence of protein, confirming that the oxidation peak is attributed to ASNase. SPE/SWCNTs with two concentrations of ASNase (3 µg/µL and 5 µg/µL) also confirmed the nature of the electrochemical signal of the enzyme (Figure 3A). Sensitivity towards asparaginase was determined as 0.80 ± 0.07 µA/µg/µL of protein.
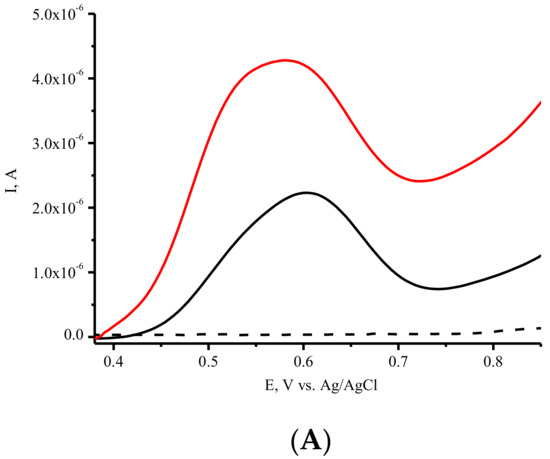
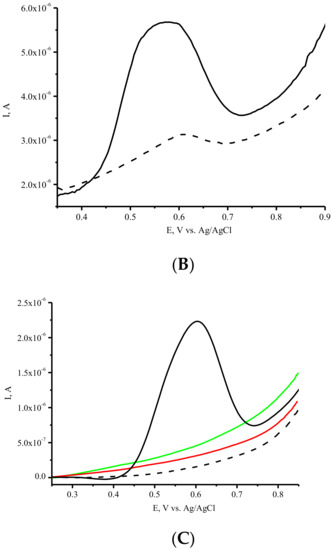
Figure 3.
(A). DPV electrochemical oxidation signals of SPE/SWCNT/ASNase, 3 µg/µL (-), and 5 µg/µL (-), SPE/SWCNT (---). (B). DPVs of the first (-) and the second (---) scan of SPE/SWCNT/ASNase (5 µg/µL). (C) DPVs of SPE/SWCNT/ASNase, 3 µg/µL (-), 33 mM L-Asn (-), and 33 mM Gly (-) on SPE/SWCNT, SPE/SWCNT (---). Supporting electrolyte: 0.1 M potassium-phosphate buffer containing 0.05 M NaCl, pH 7.4.
The second scan of DPV for ASNase on SPE/SWCNT revealed a lower peak current intensity, thus confirming the irreversible nature of amino acid electrooxidation (Figure 3B).
Structural rearrangements of proteins and especially hemoproteins during substrate binding can be registered using spectroscopy [43,44,45]. Upon substrate or inhibitor interaction with the iron porphyrin of cytochrome P450, spectral changes in the Soret band were observed [44]. The electrochemical response of heme proteins upon substrate binding may be registered as the appearance of catalytic current and shift of potential [17,18,46]. For enzymes without prosthetic groups, conformational changes in the polypeptide backbone registered using electrochemical oxidation of amino acids may be used as an effective tool for the investigation of enzyme-substrate interactions. The electrochemical signature of the enzyme based on the electrochemical oxidation of electroactive amino acids permits its use as an indicator for the assessment of the catalytic activity of ASNase in the presence of amino acids L-Asn and Gly. These amino acids have no significant electrooxidation properties on SPE/SWCNTs in the studied range of potentials (Figure 3C).
Dynamic conformational changes of enzymes during enzyme catalysis and interaction with the substrate are crucial to the catalytic activities and for the registration of these changes using the electrochemical analysis of such changes. Substrate binding was performed for 5 min at room temperature. Furthermore, DPV was employed to follow the direct electrooxidation of ASNase and its subsequent interaction with amino acids. The interplay of ASNase with L-Asn shows a significant decrease in the oxidation current (Figure 4A,B). A slight shift in reduction potential was registered for the interaction of ASNase with the substrate L-asparagine (Table 1). Significant differences in the registered oxidation behavior of ASNase were observed in the presence of L-asparagine, L-glutamine, and glycine, which has no substrate properties (Figure 4). Glycine at the same concentration did not produce significant conformational changes in the polypeptide chain, did not change the oxidation potential, and did not significantly influence the oxidation amino acid current, as confirmed by DPV (Figure 4B, Table 1). L-glutamine decreased the oxidation current of ASNase, by 43 percent in comparison with 23 percent in the presence of L-Asn (Figure 4B and Table 1), confirming the preference of the enzyme for L-Asn. The electrochemical approach permits to discriminate catalytic activity of enzyme ASNase towards amino acids with different roles in catalysis.
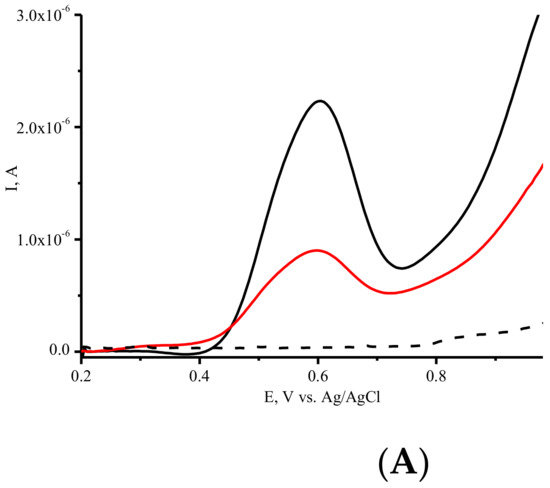
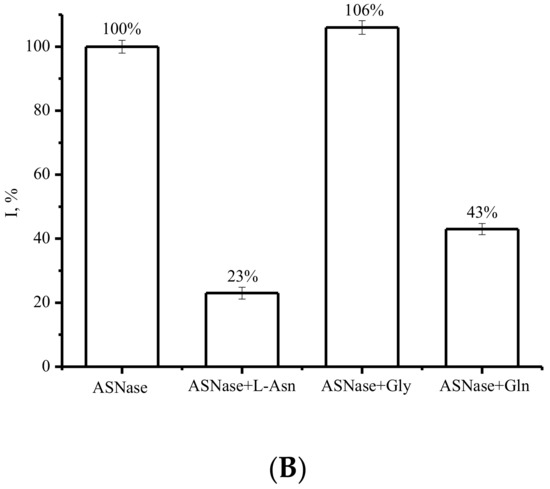
Figure 4.
(A) DPV electrochemical oxidation signals of SPE/SWCNT/ASNase (-), SPE/SWCNT/ASNase after interaction with 33 mM Asn (-), SPE/SWCNT (---). (B) Histograms corresponding to the average ASNase signals on SPE/SWCNT and ASNase on SPE/SWCNT after interaction with 33 mM L-Asn, after interaction with 33 mM Gly, after interaction with 33 mM L-Gln. Supporting electrolyte: 0.1 M potassium-phosphate buffer containing 0.05 M NaCl, pH 7.4.

Table 1.
Data from the differential pulse voltammetric (DPV) measurements of ASNase and ASNase after incubation with L-Asn, L-Gln, or Gly at SPE/SWCNTs (n = 3).
A concentration-dependent decrease in the oxidation current of ASNase was used to assess the Michaelis constant for substrate L-Asn. Electrochemical data were transformed into kinetic data using a nonlinear regression method using the OriginPro (version 8.5, Northampton, MA, USA) software package (R2 = 0.968). Curves were fitted to obtain the Km value [47,48].
Based on such dependencies, the electrochemical Michaelis constant for L-Asn was calculated and corresponded to 600 ± 70 µM (Figure 5). This parameter was calculated as 98 µM using the Nessler reagent [49]. The most commonly used Nessler reaction requires the use of highly toxic reagents with poor stability, and the Nessler reaction has low reproducibility and complicated operations and is not suitable for the detection of clinical samples [6]. The electrochemical approach depends on the type of electrode, electrode modifier, and accessibility of substrate to the active center of the enzyme. For these reasons, the difference between the Km value obtained using the Nessler reaction and the electrochemical approach may be registered. The fluorescence analysis method requires the synthesis of a fluorogenic probe for the assessment of the catalytic activity of the enzyme. From this viewpoint, the electrochemical approach is the most robust and effective.
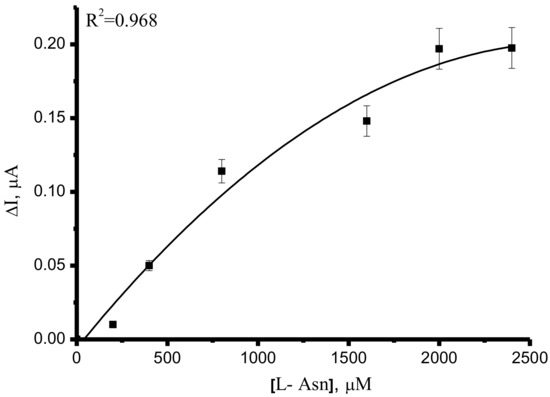
Figure 5.
The dependence of the difference in the asparaginase oxidation current ΔI = Ienz − I (enz + Asn) vs. L-Asn concentration as an electrochemical equivalent of the Michaelis–Menten plot. The values are the means from at least 3 experiments ± S.D.
We assumed that amino acids with the ability for electrochemical oxidation, such as Tyr, Trp, His, Cys, Cys-Cys, and Met [19,20,21,22,23,24,25,26,27], are responsible for the response to substrate interactions. Tyr29 is a crucial amino acid in the active site of bacterial L-asparaginases and Tyr29 is the most important candidate for the main role in electrochemical registration of enzyme-substrate interactions in ASNase from Erwinia carotovora [31,47]. Tyr29 of L-asparaginase from Erwinia carotovora is involved in substrate binding and release of reaction product [31].
4. Conclusions
The catalytic activity of ASNase from Erwinia carotovora was registered electrochemically using the DPV technique. The electrooxidation of amino acids from L-asparaginase backbones was used as a measuring tool. ASNase was immobilized on SPE modified by carbon nanotubes. The substrate L-asparagine reduced the oxidation current of the enzyme in a concentration-dependent manner. The electrochemical L-asparagine Michaelis constant corresponded to 600 ± 70 µM and was the same order of magnitude as the biochemical value, calculated with analysis of product formation measured with Nessler reagent. The addition of glycine did not significantly influence the amplitude of the oxidation current. The proposed electrochemical method permits the registration of direct ASNase electrochemical oxidation for monitoring substrate binding and does not require specific labels of protein or substrate. However, the electrochemical approach depends on the type of electrode, electrode modifier, and accessibility of substrate to the active center of the enzyme. The future perspective of this method is the construction of new nanocomposite materials for the modification of working electrodes with enhanced sensitivity to the electrochemical oxidation of amino acids, investigation of mutant forms of asparaginase from different sources, a range of amino acids, and inhibitors of this enzyme.
Author Contributions
Conceptualization, V.S. and D.Z.; methodology, V.S. and T.B. designed the experiments and analyzed the obtained data. Formal analysis and investigation: T.B., V.P. and V.S. performed the electrochemical experiments, S.K. performed the SEM experiments. Writing—original draft preparation: V.S. prepared an original draft. Writing—review and editing, D.Z. carried out a review and editing. M.P. and S.A. cloned and purified L-asparaginase. Supervision, V.S. and D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The work was performed within the framework of the Program for Basic Research in the Russian Federation for a long-term period (2021–2030) (No. 122030100168-2).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Magri, A.; Soler, M.F.; Lopes, A.M.; Cilli, E.M.; Barber, P.S.; Pessoa, A.; Pereira, J.F. A critical analysis of L-asparaginase activity quantification methods-colorimetric methods versus high-performance liquid Chromatography. Anal. Bioanal. Chem. 2018, 410, 6985–6990. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.M.; Roberts, J.; Loeb, E.; Khan, A.; MacLellan, A.; Hill, R.W. L-asparaginase Therapy for leukemia and other malignant neoplasms. JAMA 1967, 202, 882–888. [Google Scholar] [CrossRef]
- Vimal, A.; Kumar, A. Biotechnological production and practical application of L-asparaginase enzyme. Biotechnol. Genet. Eng. Rev. 2017, 8725, 1–22. [Google Scholar] [CrossRef]
- Aiswarya, R.; Baskar, G. Enzymatic mitigation of acrylamide in fried potato chips using asparaginase from Aspergillus terreus. Int. J. Food Sci. Technol. 2018, 53, 491–498. [Google Scholar] [CrossRef]
- Bleyer, A.; Asselin, B.L.; Koontz, S.E.; Hunger, S.P. Clinical application of asparaginase activity levels following treatment with Pegaspargase. Pediatr. Blood Cancer 2002, 62, 1102–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Qin, W.; Chen, D.; Wang, N.; Zhang, C.; Fang, Z.; Fang, B.; Du, W.; Yang, N.; Wu, Q.; et al. Design, synthesis and application of fluorogenic probe for detecting L-asparaginase in serum samples. Res. Chem. 2021, 3, 100103. [Google Scholar] [CrossRef]
- Karpel-Massler, G.; Ramani, D.; Shu, C.; Halatsch, M.E.; Westhoff, M.A.; Bruce, J.N.; Canoll, P.; Siegeli, M.D. Metabolic reprogramming of glioblastoma cells by L-asparaginase sensitizes for apoptosis in vitro and in vivo. Oncotarget 2016, 7, 33512–33528. [Google Scholar] [CrossRef] [Green Version]
- Kudryashova, E.V.; Sukhoverkov, K.V. Reagent-free L-asparaginase activity assay based on CD spectroscopy and conductometry. Anal. Bioanal. Chem. 2016, 408, 1183–1189. [Google Scholar] [CrossRef]
- Drainas, D.; Drainas, C. A conductimetric method for assaying asparaginase activity in Aspergillus nidulans. Eur. J. Biochem. 1985, 593, 591–593. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Bukharina, N.S.; Pleshakova, T.O.; Frantsuzov, P.A.; Krokhin, N.V.; Ziborov, V.S.; Archakov, A.I. Atomic Force Microscopy Visualization and Measurement of the Activity and Physicochemical Properties of Single Monomeric and Oligomeric Enzymes. Biophysics 2011, 56, 892–896. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhang, S.; Liu, L.; Wu, H.C. Measuring Enzymatic Activities with Nanopores. Chem. Bio. Chem. 2020, 21, 2089–2097. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Zhang, J.; Li, G. Electrochemical Sensors for Clinic Analysis. Sensors 2008, 8, 2043–2081. [Google Scholar] [CrossRef] [Green Version]
- Davis, C.; Wang, S.X.; Sepunaru, L. What can electrochemistry tell us about individual enzymes? Curr. Opin. Electrochem. 2021, 25, 100643. [Google Scholar] [CrossRef]
- Ferrari, A.G.M.; Rowley-Neale, S.J. Banks CE. Screen-printed electrodes: Transitioning the laboratory in-to-the field. Talanta Open 2021, 3, 100032. [Google Scholar] [CrossRef]
- Ghosh, T.; Sarkar, P.; Turner, A.P.F. A novel third generation uric acid biosensor using uricase electroactivated with ferrocene on a Nafion coated glassy carbon electrode. Bioelectrochemistry 2015, 102, 1–9. [Google Scholar] [CrossRef]
- Ambaye, A.D.; Kefeni, K.K.; Mishra, S.B.; Nxumalo, E.N.; Ntsendwana, B. Recent developments in nanotechnology-based printing electrode systems for electrochemical sensors. Talanta 2021, 225, 121951. [Google Scholar] [CrossRef]
- Shumyantseva, V.; Masamrekh, R.; Bulko, T.; Archakov, A. From electrochemistry to enzyme kinetics of cytochrome P450. Biosens. Bioelectron. 2018, 121, 192–204. [Google Scholar] [CrossRef]
- Shumyantseva, V.; Bulko, T.; Kuzikov, A.; Masamrekh, R.; Konyakhina, A.; Romanenko, I.; Max, J.B.; Köhler, M.; Gilep, A.; Usanov, S.; et al. All-electrochemical nanocomposite two-electrode setup for quantification of drugs and study of their electrocatalytical conversion by cytochromes P450. Electrochim. Acta 2020, 336, 135579. [Google Scholar] [CrossRef]
- Brabec, V.; Mornstein, V. Electrochemical behavior of proteins at graphite electrodes. I. Electrooxidation of proteins as a new probe of protein structure and reactions. Biochim. Biophys. Acta 1980, 625, 43–50. [Google Scholar] [CrossRef]
- Brabec, V.; Mornstein, V. Electrochemical behavior of proteins at graphite electrodes. II. Electrooxidation of amino acids. Biophys. Chem. 1980, 12, 159–165. [Google Scholar] [CrossRef]
- Malfoy, B.; Reynaud, J.A. Electrochemical investigations of amino acids at solid electrodes: Part II. Amino acids containing no sulfur atoms: Tryptophan, tyrosine, histidine and derivatives. J. Electroanal. Chem. 1980, 114, 213–223. [Google Scholar] [CrossRef]
- Reynaud, J.A.; Malfoy, B.; Bere, A. The electrochemical oxidation of three proteins: RNAase A, bovine serum albumin and concanavalin A at solid electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1980, 116, 595–606. [Google Scholar] [CrossRef]
- Reynaud, J.A.; Malfoy, B.; Canesson, P. Electrochemical investigations of amino acids at solid electrodes: Part I. Sulfur components: Cystine, cysteine, methionine. J. Electroanal. Chem. 1980, 114, 195–211. [Google Scholar] [CrossRef]
- Shumyantseva, V.; Suprun, E.; Bulko, T.; Archakov, A. Electrochemical methods for detection of posttranslational modifications of proteins. Biosens. Bioelectron. 2014, 61, 131–139. [Google Scholar] [CrossRef]
- Reinders, J.; Sickmann, A. Modificomics: Posttranslational modifications beyond protein phosphorylation and glycosylation. Biomol. Eng. 2007, 24, 169–177. [Google Scholar] [CrossRef]
- Wei, M.Y.; Famouri, P.; Guo, L.H. Electrocatalytic oxidation of tyrosines shows signal enhancement in label-free protein biosensors. Trends Anal. Chem. 2012, 39, 130–148. [Google Scholar] [CrossRef]
- Shumyantseva, V.; Bulko, T.; Kuzikov, A.; Masamrekh, R.; Archakov, A. Analysis of l-tyrosine based on electrocatalytic oxidative reactions via screen-printed electrodes modified with multi-walled carbon nanotubes and nanosized titanium oxide (TiO2). Amino Acids 2018, 50, 823–829. [Google Scholar] [CrossRef]
- Somji, M.; Dounin, V.; Muench, S.B.; Schulze, H.; Bachmann, T.T.; Kerman, K. Electroanalysis of amino acid substitutions in bioengineered acetylcholinesterase. Bioelectrochemistry 2012, 88, 110–113. [Google Scholar] [CrossRef]
- Borek, D.; Jaskólski, M. Sequence analysis of enzymes with asparaginase activity. Acta Biochim. Pol. 2001, 48, 893–902. [Google Scholar] [CrossRef]
- Borek, D.; Kozak, M.; Pei, J.; Jaskolski, M. Crystal structure of active site mutant of antileukemic L-asparaginase reveals conserved zinc-binding site. FEBS J. 2014, 281, 111. [Google Scholar] [CrossRef]
- Pokrovskaya, M.V.; Pokrovsky, V.S.; Aleksandrova, S.S.; Sokolov, N.N.; Zhdanov, D.D. Molecular Analysis of L-Asparaginases for Clarification of the Mechanism of Action and Optimization of Pharmacological Functions. Pharmaceutics 2022, 14, 599. [Google Scholar] [CrossRef] [PubMed]
- Derst, C.; Wehner, A.; Specht, V.; Rohm, K.H. States and functions of tyrosine residues in Escherichia coli asparaginase II. Eur. J. Biochem. 1994, 224, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Aung, H.P.; Bocola, M.; Schleper, S.; Röhm, K.H. Dynamics of a mobile loop at the active site of Escherichia coli asparaginase. Biochim. Biophys. Acta 2000, 1481, 349–359. [Google Scholar] [CrossRef]
- Lubkowski, J.; Vanegas, J.M.; Chan, W.K.; Lorenzi, P.L.; Weinstein, J.N.; Sukharev, S.; Fushman, D.; Rempe, S.; Anishkin, A.; Wlodawer, A. Mechanism of Catalysis by L-asparaginase. Biochemistry 2020, 59, 1927–1945. [Google Scholar] [CrossRef]
- Lubkowski, J.; Wlodawer, A. Geometric considerations support the double-displacement catalytic mechanism of L -asparaginase. Protein Sci. 2019, 28, 1850–1864. [Google Scholar] [CrossRef]
- Lubkowski, J.; Wlodawer, A. Structural and biochemical properties of L-asparaginase. FEBS J. 2021, 288, 4183–4209. [Google Scholar] [CrossRef]
- Agrawal, S.; Jana, U.K.; Kango, N. Heterologous expression and molecular modeling of L-asparaginase from Bacillus subtilis ETMC-2. Int. J. Biol. Macromol. 2021, 192, 28–37. [Google Scholar] [CrossRef]
- Papageorgiou, A.C.; Posypanova, G.A.; Andersson, C.S.; Sokolov, N.N.; Krasotkina, J. Structural and functional insights into Erwinia carotovora L-asparaginase. FEBS J. 2008, 275, 4306–4316. [Google Scholar] [CrossRef]
- Sigolaeva, L.V.; Bulko, T.V.; Kozin, M.S.; Zhang, W.; Köhler, M.; Romanenko, I.; Yuan, J.; Schacher, F.H.; Pergushov, D.V.; Shumyantseva, V.V. Long-term stable poly(ionic liquid)/MWCNTs inks enable enhanced surface modification for electrooxidative detection and quantification of dsDNA. Polymer 2019, 168, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Manna, S.; Sharma, A.; Satpati, A.K. Electrochemical methods in understanding the redox processes of drugs and biomolecules and their sensing. Curr. Opin. Electrochem. 2022, 32, 100886. [Google Scholar] [CrossRef]
- Carrara, S.; Baj-Rossi, C.; Boero, C.; De Micheli, G. Do Carbon Nanotubes contribute to Electrochemical Biosensing? Electrochim. Acta 2014, 128, 102–112. [Google Scholar] [CrossRef] [Green Version]
- Wang, J. Analytical Electrochemistry, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; p. 32. [Google Scholar]
- Schenkman, J.B.; Remmer, H.; Estabrook, R.W. Spectral studies of drug interaction with hepatic microsomal cytochrome P-450. Mol. Pharmacol. 1967, 3, 113–123. [Google Scholar]
- Guengerich, F.P.; McCarty, K.D.; Chapman, J.G.; Tateishi, Y. Stepwise binding of inhibitors to human cytochrome P450 17A1 and rapid kinetics of inhibition of androgen biosynthesis. J. Biol. Chem. 2021, 297, 100969. [Google Scholar] [CrossRef]
- Guengerich, F.P.; McCarty, K.D.; Chapman, J.G. Kinetics of cytochrome P450 3A4 inhibition by heterocyclic drugs defines a general sequential multistep binding process. J. Biol. Chem. 2020, 296, 100223. [Google Scholar] [CrossRef]
- Schneider, E.; Clark, D.S. Cytochrome P450 (CYP) enzymes and the development of CYP biosensors. Biosens. Bioelectron. 2013, 39, 1–13. [Google Scholar] [CrossRef]
- Rusling, J.F.; Wang, B.; Yun, S. Electrochemistry of redox enzymes. In Bioelectrochemistry: Fundametals, Experimental Techniques and Applications; Bartlett, P.N., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 39–85. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Bulko, T.V.; Koroleva, P.I.; Shikh, E.V.; Makhova, A.A.; Kisel, M.S.; Haidukevich, I.V.; Gilep, A.A. Human Cytochrome P450 2C9 and its polymorphic modifications: Electroanalysis, catalytic properties, and approaches to the regulation of enzymatic activity. Processes 2022, 10, 383. [Google Scholar] [CrossRef]
- Krasotkina, J.; Borisova, A.A.; Gervaziev, Y.V.; Sokolov, N.N. One-step purification and kinetic properties of the recombinant L-asparaginase from Erwinia carotovora. Biotechnol. Appl. Biochem. 2004, 39, 215–221. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).