Green Synthesis of 3-Hydroxybutyraldehyde from Acetaldehyde Catalyzed by La-Ca-Modified MgO/Al2O3
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Composite Oxide Catalysts
2.2. Characterization of Composite Oxide Catalysts
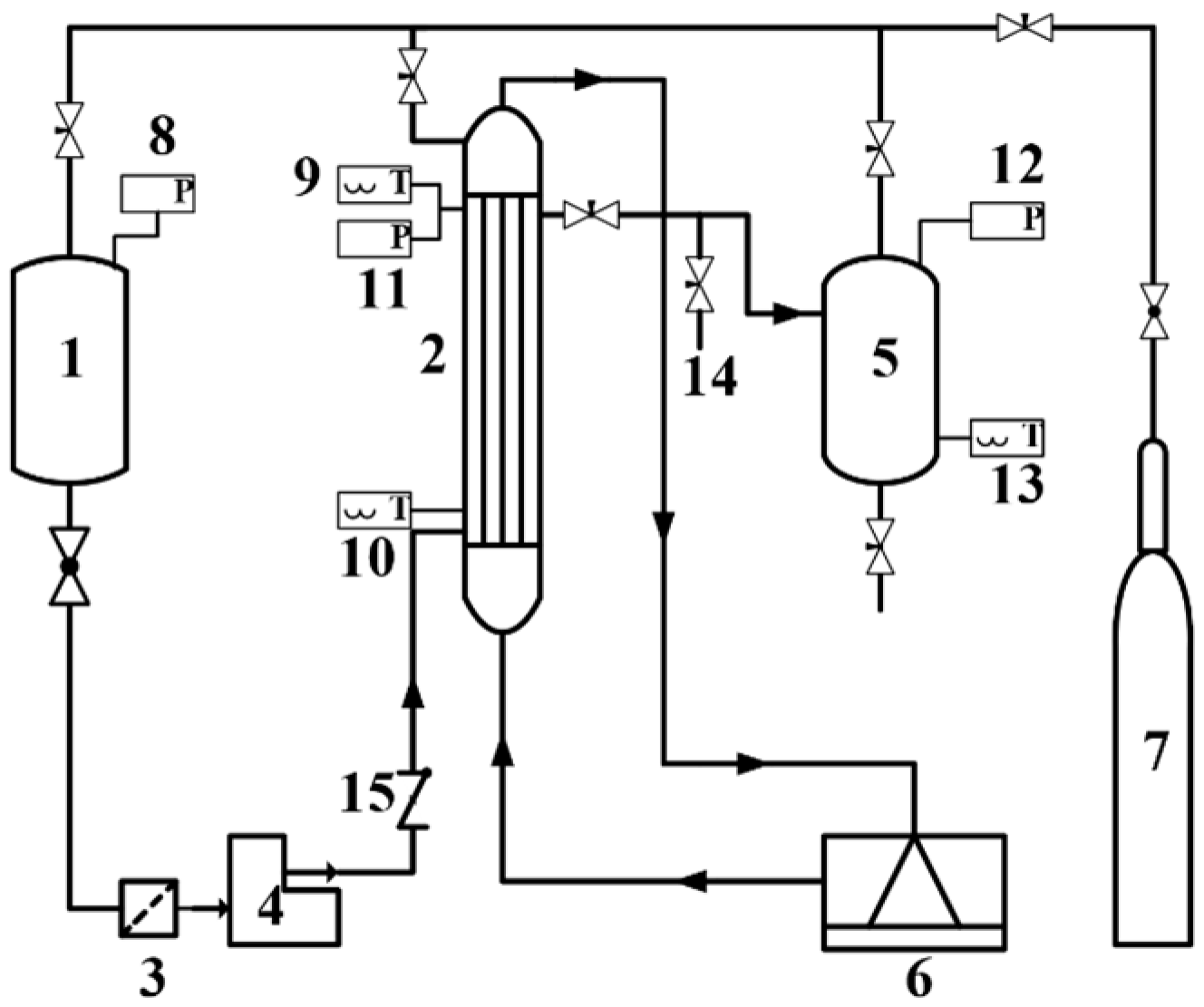
2.3. Evaluation of Catalyst Performances
3. Results and Discussion
3.1. Characterization of Catalyst Performances
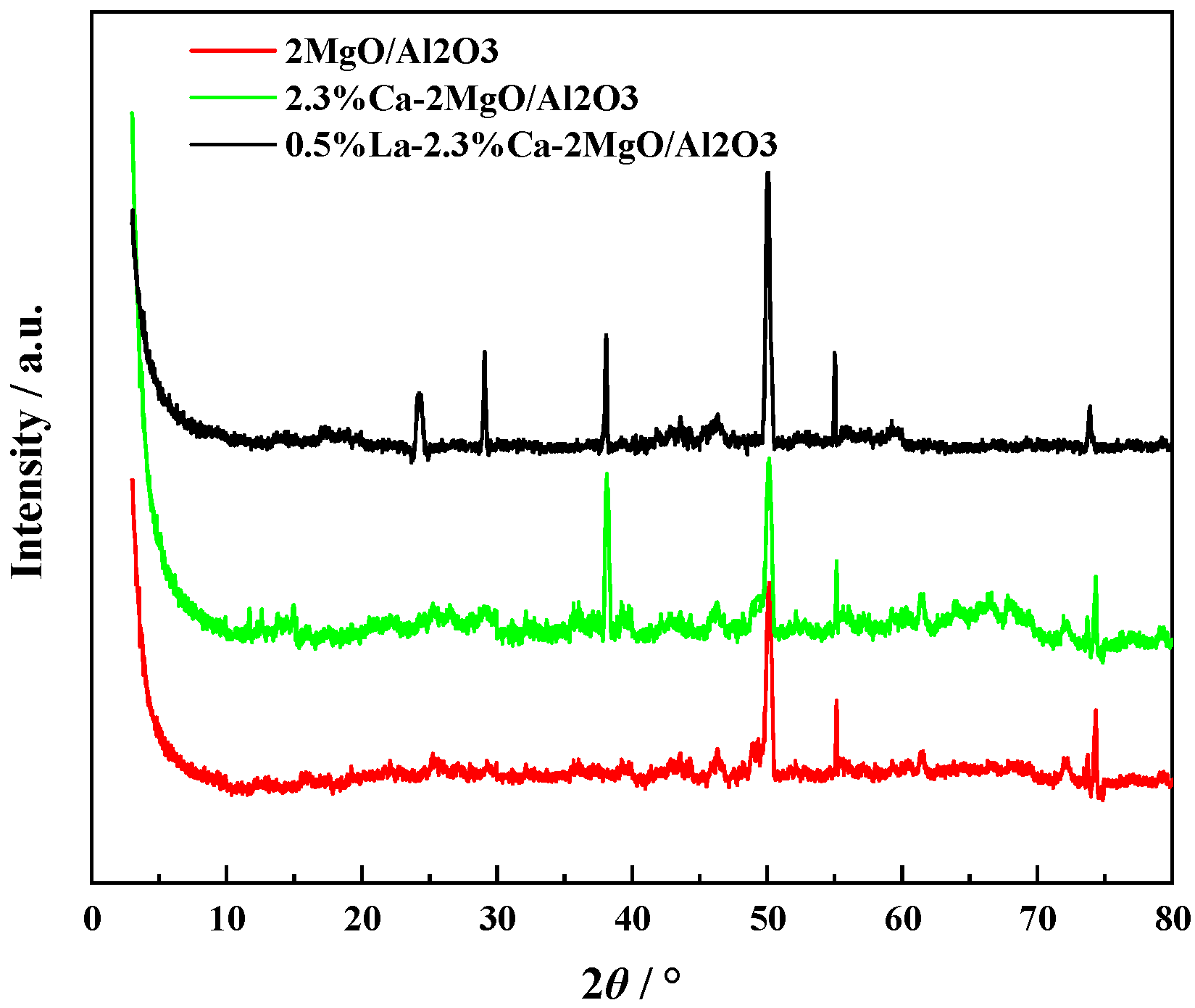
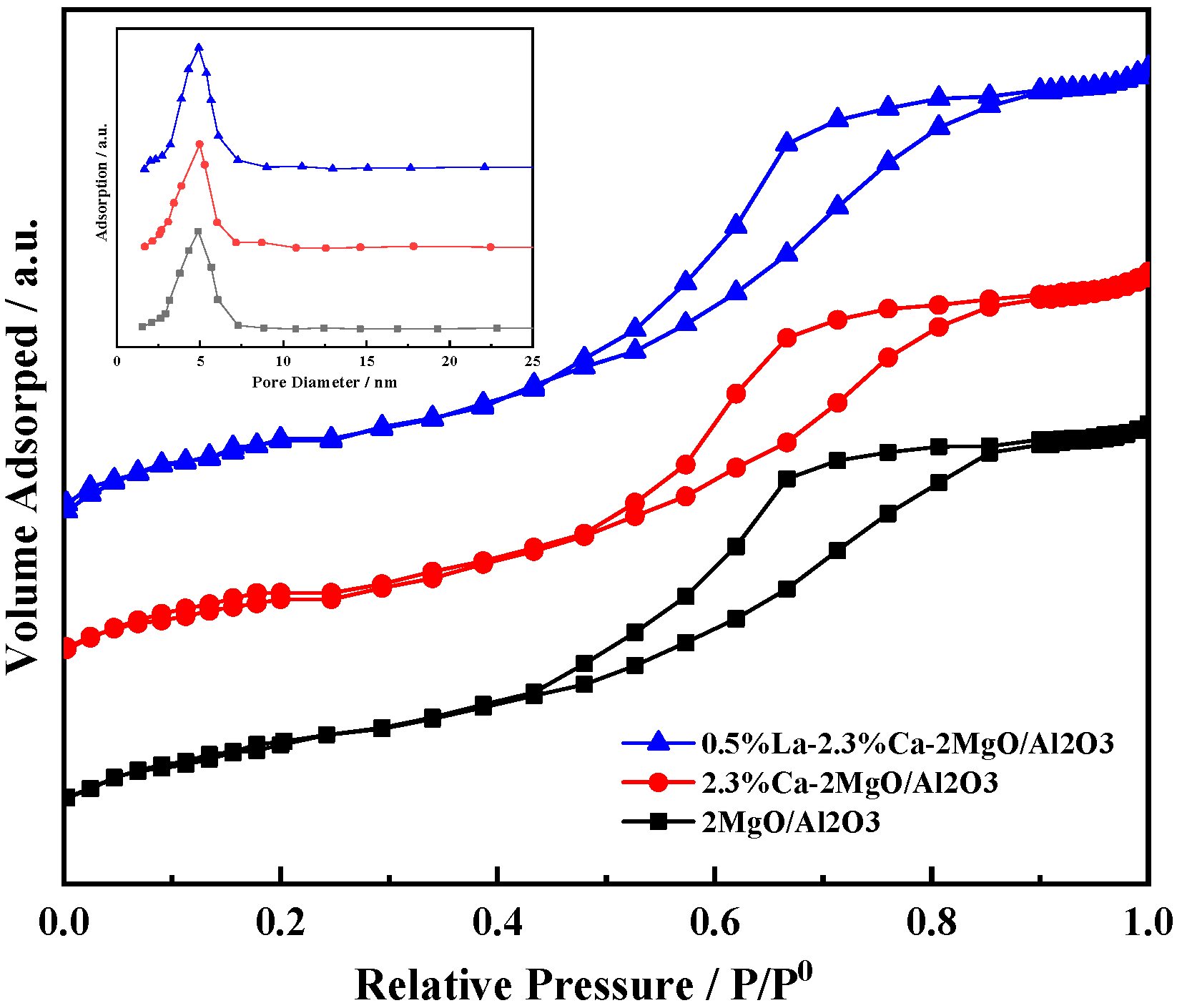
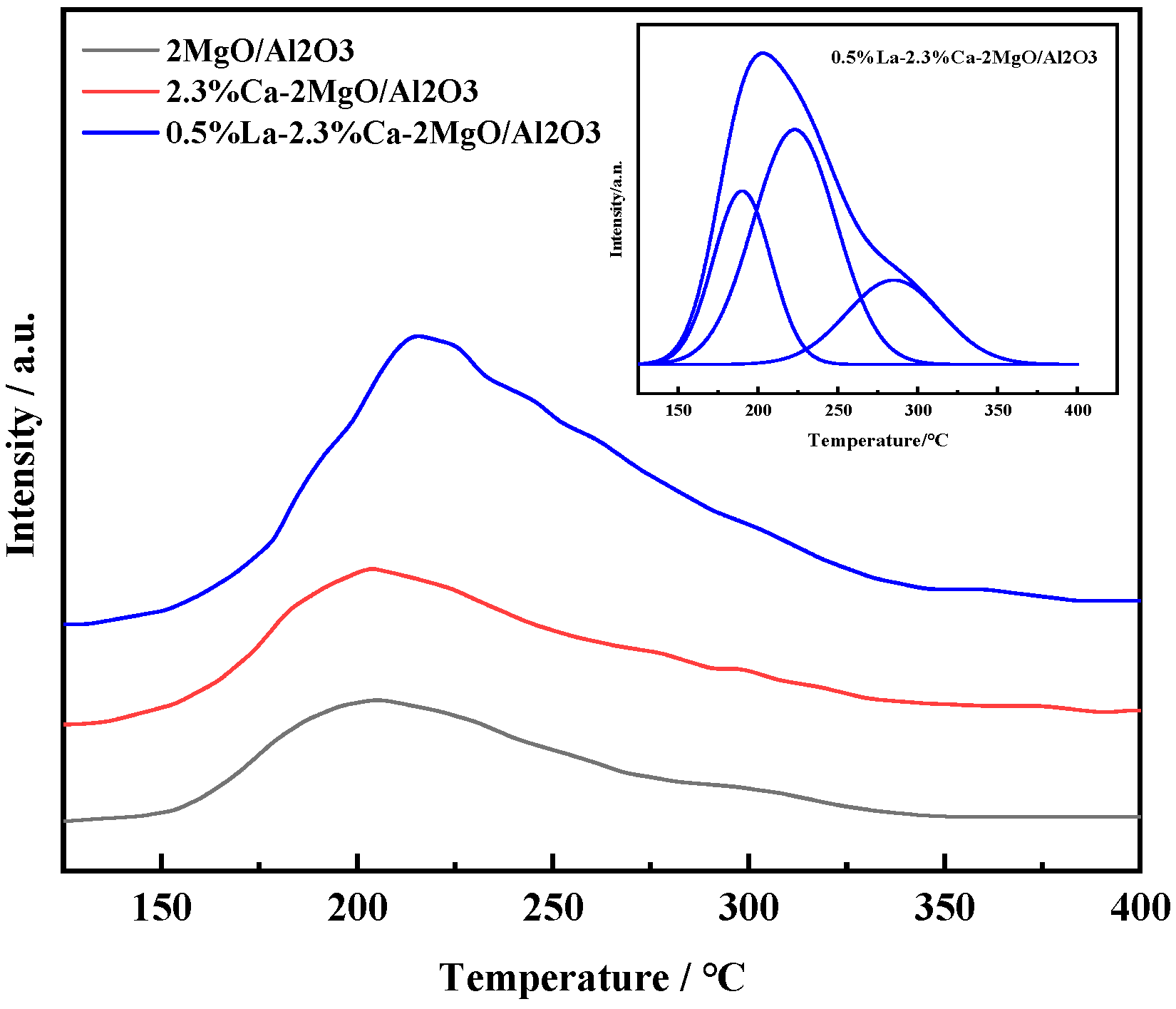
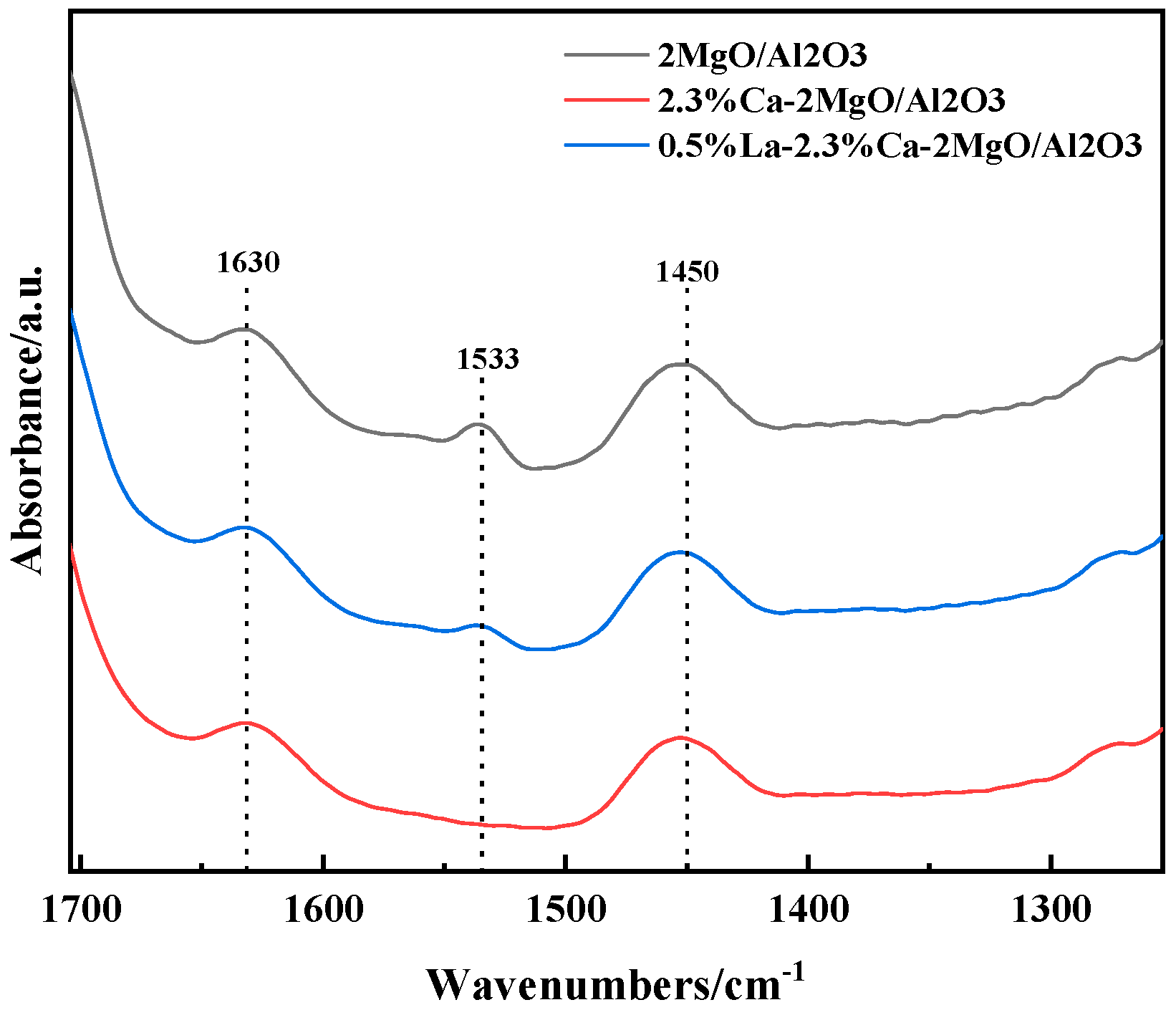
3.2. Characterization of Catalyst Structure
3.3. The Effect of Process Condition on Catalyst Performances
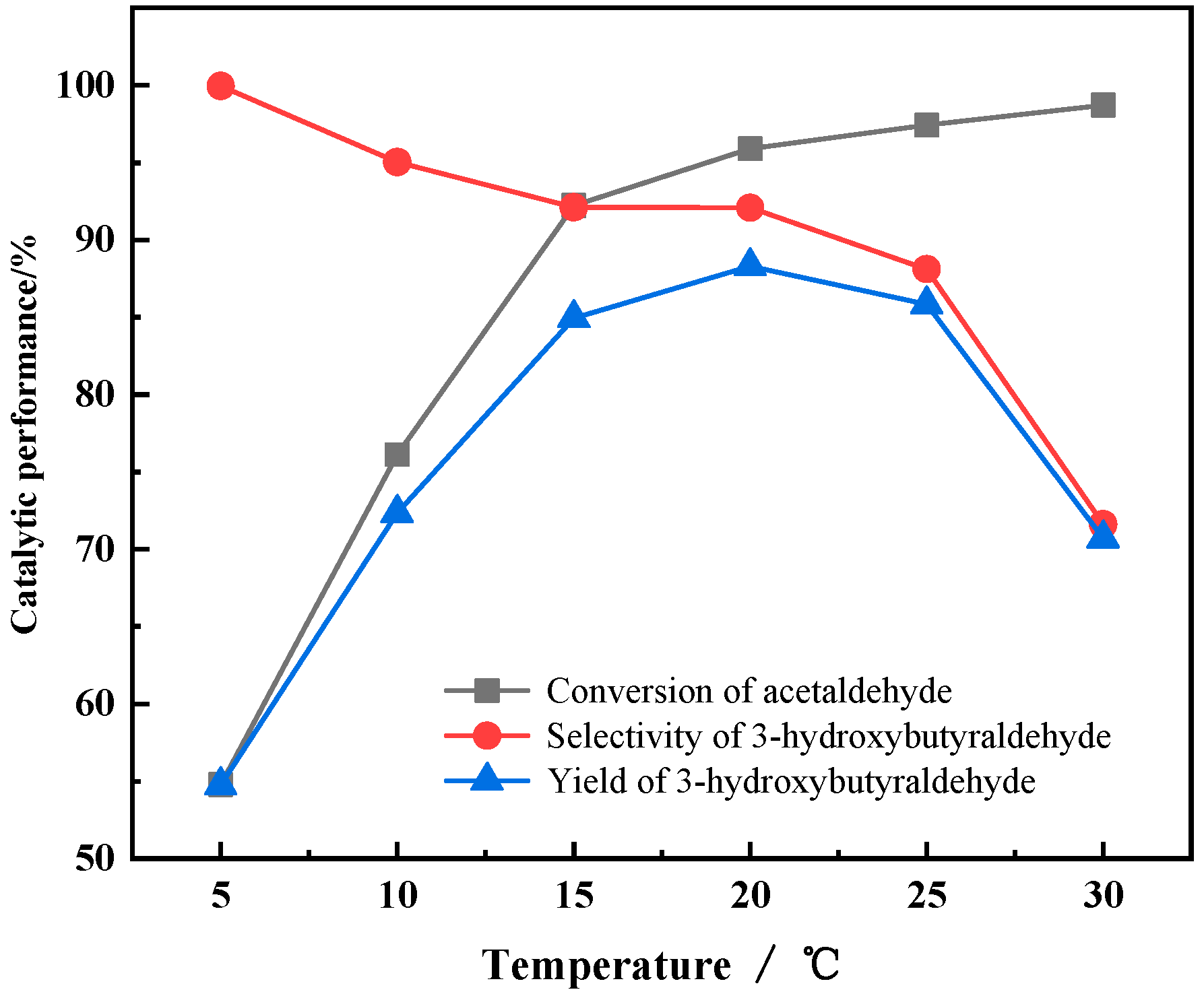
3.3.1. The Effect of Reaction Temperature on Catalyst Performances
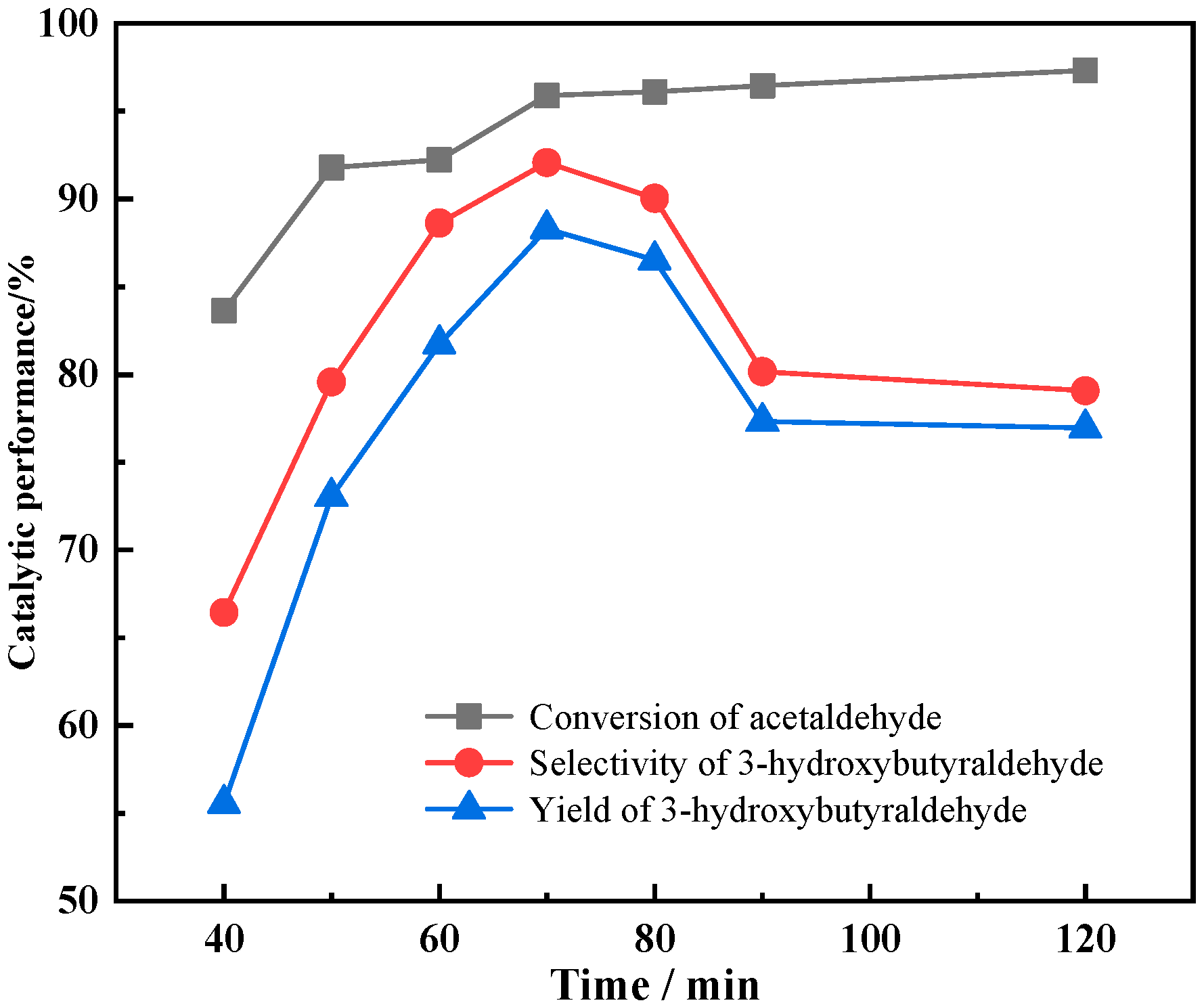
3.3.2. The Effect of Residence Time on Catalyst Performances
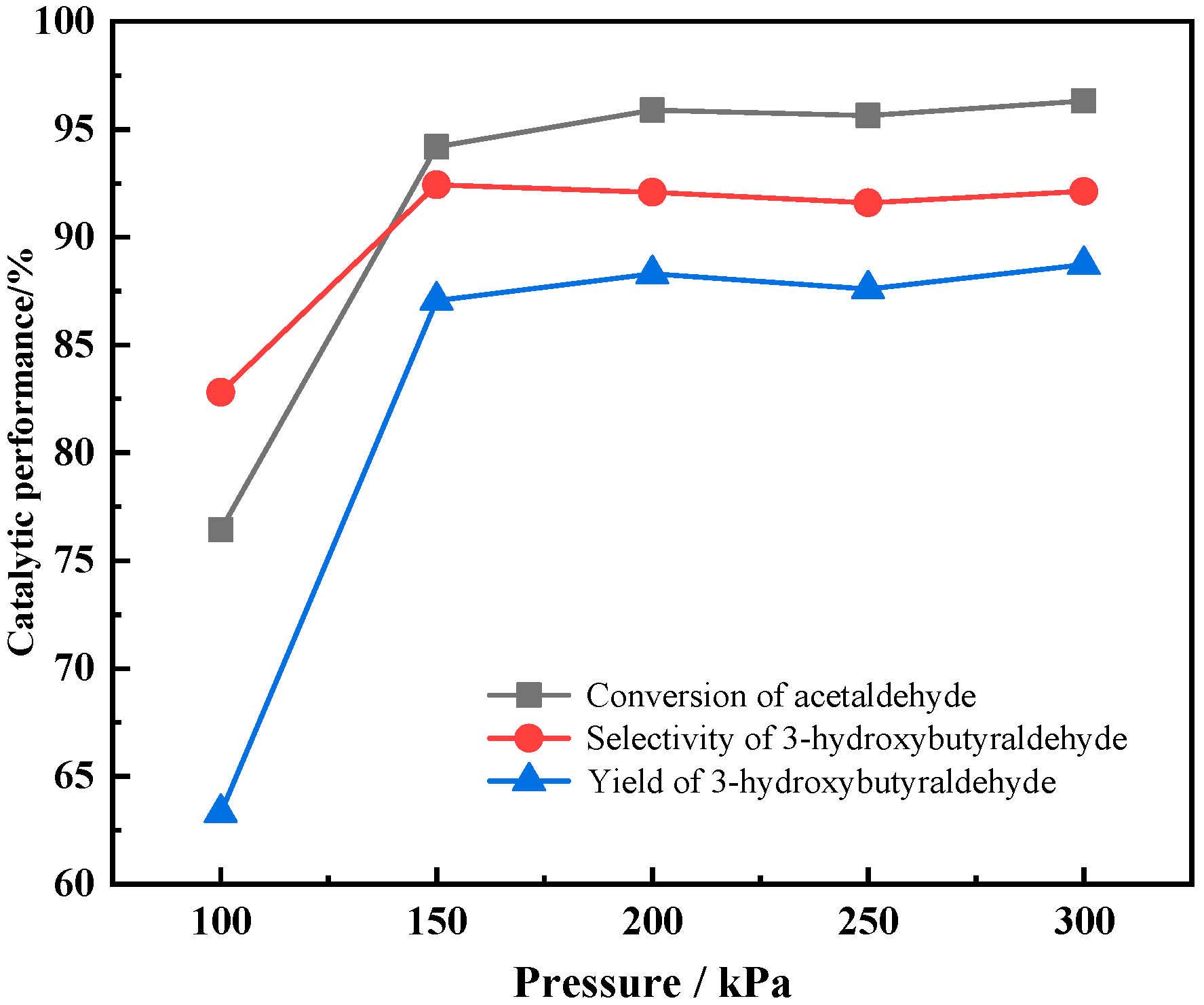
3.3.3. The Effect of Reaction Pressure on Catalyst Performances
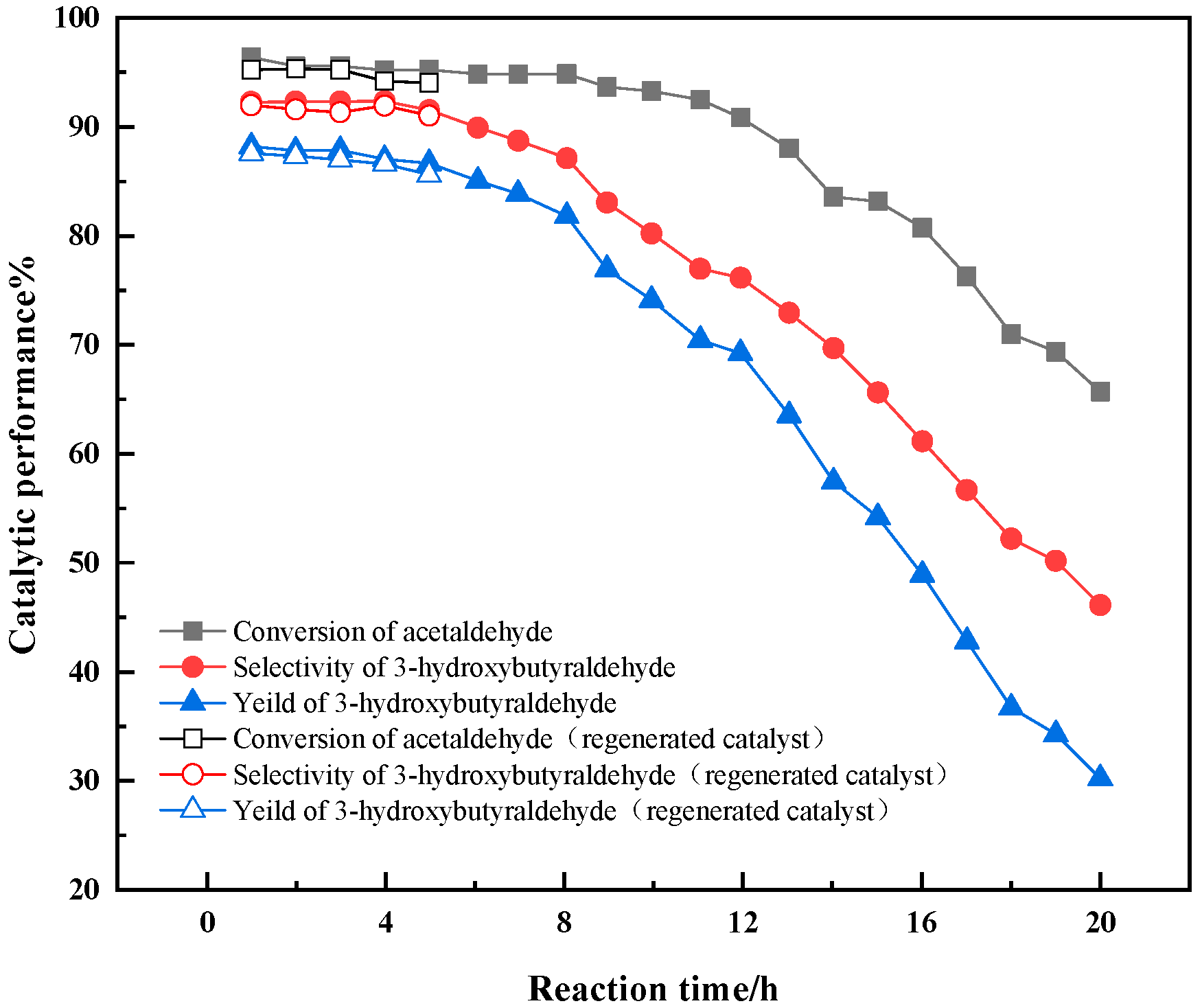
3.4. Catalyst Lifespan and Regeneration
4. Conclusions
- (1)
- A series of composite oxides 2MgO/Al2O3 are prepared by the co-precipitation method. Ca and La ions are introduced and applied to the catalytic condensation of AcH to synthesize 3-HBA. The catalytic activity of the composite materials is significantly improved. The catalyst performance of 0.5% La-2.3% Ca-2MgO/Al2O3 is better than those of other composite oxides used in the study. Under the conditions of an AcH content of 30%, reaction temperature of 23 °C, reaction time of 70 min, and reaction pressure of 200 kPa, the conversion of AcH reaches 95.89%, the selectivity of 3-HBA is 92.08%, and the yield is 88.30%.
- (2)
- The characterization of process conditions shows that reaction temperature has a significant effect on the selectivity of the product due to the fact that the aldol condensation of AcH is an exothermic process and the transformation of AcH is unfavorable at higher temperature.
- (3)
- The characterization results show that after the introduction of Ca and La ions into the composite oxide 2MgO/Al2O3, the acid and alkaline sites on the surface are significantly improved and optimized, but the surface area of the material decreases and the pore size increases. It is beneficial to the reaction from the perspective of the catalytic condensation of AcH to 3-HBA.
- (4)
- The investigation on the catalytic lifespan of 0.5% La-2.3% Ca-2MgO/Al2O3 shows that within 5 h, the conversion of AcH and the selectivity and yield of 3-HBA are high, and the catalytic activity recovers well after regeneration. Under the same process conditions, the catalytic performance of the composite oxide is equivalent to that of the traditional liquid alkaline catalyst, and it is environmentally friendly. Therefore, it is feasible and has broad prospects in industrial application to use this catalyst for the catalytic condensation of AcH to synthesize 3-HBA.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Abdel-Rahem, R.A. Influence of 1,3-Butanediol on the viscoelasticity of surfactant solutions. J. Surfact. Deterg. 2014, 17, 353–362. [Google Scholar] [CrossRef]
- Azarang, N.; Movagharnejad, K.; Pirdashti, M.; Ketabi, M. Densities, viscosities, and refractive indices of poly(ethylene glycol) 300 + 1,2-Ethanediol, 1,2-Propanediol, 1,3-Propanediol, 1,3-Butanediol, or 1,4-Butanediol binary liquid mixtures. J. Chem. Eng. Data 2020, 65, 3448–3462. [Google Scholar] [CrossRef]
- Zhu, Y.; Cheng, X.; Pang, X.; Yu, L. Heterotactic enthalpic interactions of L-Arginine and L-Proline with 1,3-Butanediol and 2,3-Butanediol in aqueous solutions. J. Chem. Eng. Data 2010, 55, 3813–3816. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Lu, J.; Zhang, C.; Wang, M.; Li, M.; Liu, X.; Wang, F. Conversion of isobutene and formaldehyde to diol using praseodymium-doped CeO2 catalyst. ACS Catal. 2016, 6, 8248–8254. [Google Scholar] [CrossRef]
- Song, H.; Tang, Z.; Chen, J. Prins condensation of formaldehyde with alkene using functional acid ionic liquid as catalyst. J. Mol. Catal. 2008, 22, 403–407. [Google Scholar]
- Windhorst, K.A.; Cortez, O. 1,3-Butylene Glycl with Reduced Odor. U.S. Patent US8445733B1, 21 May 2013. [Google Scholar]
- Tsuji, Y.; Tagawa, K. 1,3-Butylene Glygol of High Purity and Method for Producing the Same. U.S. Patent US6376725B1, 23 April 2002. [Google Scholar]
- Matsuyama, A.; Yamamoto, H.; Kawada, N.; Kobayashi, Y. Industrial Production of (R)-1,3-Butanediol by new biocatalysts. J. Mol. Catal. B Enzym. 2001, 11, 513–521. [Google Scholar] [CrossRef]
- Iwata, M.; Yazaki, R.; Suzuki, Y.; Kumagai, N.; Shibasaki, M. Direct catalytic asymmetric aldol reactions of thioamides: Toward a stereo controlled synthesis of 1,3-Polyols. J. Am. Chem. Soc. 2009, 131, 18244–18245. [Google Scholar] [CrossRef]
- Young, D.Z.; Hanspal, S.; Davis, J.R. Aldol condensation of acetaldehyde over titania, hydroxyapatite, and magnesia. ACS Catal. 2016, 6, 3193–3202. [Google Scholar] [CrossRef]
- Rekoske, J.E.; Barteau, M.A. Kinetics, selectivity, and deactivation in the aldol condensation of acetaldehyde on anatase titanium dioxide. Ind. Eng. Chem. Res. 2011, 50, 41–51. [Google Scholar] [CrossRef]
- Yan, T.; Yao, S.; Dai, W.; Wu, G.; Guan, N.; Li, L. Self-aldol condensation of aldehydes over Lewis acidic rare-earth cations stabilized by zeolites. Chin. J. Catal. 2021, 42, 595–605. [Google Scholar] [CrossRef]
- Barrett, C.J.; Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Single-reactor process for sequential aldol-condensation and hydrogenation of biomass-derived compounds in water. Appl. Catal. B 2006, 66, 111–118. [Google Scholar] [CrossRef]
- Valihura, K.V.; Larina, O.V.; Kyriienko, P.I.; Balakin, D.Y.; Soloviev, S.O. Influence of modifying additives of Lanthanum and Cerium oxides on Acid-Base characteristics and catalytic properties of MgO-Al2O3 systems in the processes of gas-phase conversion of ethanol to 1-butanol. Theor. Exp. Chem. 2021, 56, 404–411. [Google Scholar] [CrossRef]
- Singh, M.; Zhou, N.; Dilip, K.P.; Klabunde, K.J. IR Spectral evidence of aldol condensation: Acetaldehyde adsorption over TiO2 surface. J. Catal. 2008, 260, 371–379. [Google Scholar] [CrossRef]
- Kagunya, W.; Jones, W. Aldol condensation of acetaldehyde using calcined layered double hydroxides. Appl. Clay Sci. 1995, 10, 95–102. [Google Scholar] [CrossRef]
- Korolova, V.; Kikhtyanin, O.; Veselý, M.; Vrtiška, D.; Paterová, I.; Fíla, V.; Čapek, L.; Kubička, D. On the Effect of the M3+ Origin on the Properties and Aldol Condensation Performance of MgM3+ Hydrotalcites and Mixed Oxides. Catalysts 2021, 11, 992–1008. [Google Scholar] [CrossRef]
- McNeice, P.; Marr, P.C.; Marr, A.C. Basic ionic liquids for catalysis: The road to greater stability. Catal. Sci. Technol. 2021, 11, 726–741. [Google Scholar] [CrossRef]
- Wu, Y.H.; Ren, W.H.; Liang, Z.L.; Liu, Z.N.; Wu, G.S. Liquid-phase aldol condensation of AcH and its kinetics. Chem. React. Eng. Technol. 2013, 29, 75–80. [Google Scholar]
- Evangelista, J.P.D.C.; Gondimm, A.D.; Di Souza, L.; Araujo, A.S. Alumina-supported Potassium compounds as heterogeneous catalysts for biodiesel production: A Review. Renew. Sustain. Energy Rev. 2016, 59, 887–894. [Google Scholar] [CrossRef]
- Xie, W.; Chen, J. Heterogeneous interesterification of triacylglycerols catalyzed by using Potassium-doped Alumina as a solid catalyst. J. Agric. Food Chem. 2014, 62, 10414–10421. [Google Scholar] [CrossRef]
- Fang, P.; He, M.; Xie, Y.; Luo, M.F. XRD and Raman Spectroscopic comparative study on phase transformation of γ-Al2O3 at high temperature. Spectrosc. Spectr. Anal. 2006, 11, 2039–2042. [Google Scholar]
- Khemthong, P.; Luadthong, C.; Nualpaeng, W.; Changsuwan, P.; Tongprem, P.; Viriya-Empikul, N.; Faungnawakij, K. Industrial eggshell wastes as the heterogeneous catalysts for microwave-assisted biodiesel production. Catal. Today 2012, 190, 112–116. [Google Scholar] [CrossRef]
- Kumar, V.S.; Lee, Z.H.; Sim, J.H.; Law, S.C.; Mohamed, A.R. Improved CO2 Sorption Performance of Calcium Oxide (CaO) Sorbent with Nickel Oxide. Earth Environ. Sci. 2019, 268, 012026. [Google Scholar]
- Maheshwaran, G.; Muneeswari, R.S.; Bharathi, A.N.; Kumar, M.K.; Sudhahar, S. Eco-friendly synthesis of lanthanum oxide nanoparticles by Eucalyptus globulus leaf extracts for effective biomedical applications. Mater. Lett. 2021, 283, 128799. [Google Scholar] [CrossRef]
- Bang, Y.; Park, S.; Han, S.J.; Yoo, J.; Song, J.H.; Choi, J.H.; Kang, K.H.; Song, I.K. Hydrogen Production by Steam Reforming of liquefied Natural Gas (LNG) over mesoporous Ni/Al2O3 catalyst prepared by an EDTA-assisted impregnation method. Appl. Catal. B 2016, 180, 179–188. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Čapek, L.; Smoláková, L.; Tišler, Z.; Kadlec, D.; Lhotka, M.; Diblíková, P.; Kubička, D. Influence of Mg-Al mixed oxide compositions on their properties and performance in aldol Condensation. Ind. Eng. Chem. Res. 2017, 56, 13411–13422. [Google Scholar] [CrossRef]
- Garbarino, G.; Wang, C.; Valsamakis, I.; Chitsazan, S.; Riani, P.; Finocchio, E.; Flytzani-Stephanopoulos, M.; Busca, G. Acido-basicity of lanthana/alumina catalysts and their activity in ethanol conversion. Appl. Catal. B Environ. 2017, 200, 458–468. [Google Scholar] [CrossRef] [Green Version]
- Bao, Q.; Zhu, W.; Yan, J.; Zhang, C.; Ning, C.; Zhang, Y.; Hao, M.; Wang, Z. Vapor phase aldol condensation of methyl acetate with formaldehyde over a Ba–La/Al2O3 catalyst: The stabilizing role of La and effect of acid–base properties. RSC Adv. 2017, 7, 52304. [Google Scholar] [CrossRef] [Green Version]
- Bao, Q.; Bu, T.; Yan, J.; Zhang, C.; Ning, C.; Zhang, Y.; Hao, M.; Zhang, W.; Wang, Z. Synthesis of Methyl Acrylate by Aldol Condensation of Methyl Acetate with Formaldehyde Over Al2O3-Supported Barium Catalyst. Catal. Lett. 2017, 147, 1540–1550. [Google Scholar] [CrossRef]
- Bai, S.; Dai, Q.; Chu, X.; Wang, X. Dehydrochlorination of 1,2-dichloroethane over Ba-modified Al2O3 catalysts. RSC Adv. 2016, 6, 52564–52574. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.X.; Li, X.G.; Xiao, W.D.; Chen, D. Hydrogenation of CO to olefins over a supported iron catalyst on MgAl2O4 spinel: Effects of the spinel synthesis method. RSC Adv. 2020, 10, 40815–40829. [Google Scholar] [CrossRef]
- Wang, K.; Miao, C.; Liu, Y.; Cai, L.; Jones, W.; Fan, J.; Li, D.; Feng, J. Vacancy enriched ultrathin TiMgAl-layered double hydroxide/graphene oxides composites as highly efficient visible-light catalysts for CO2 reduction. Appl. Catal. B Environ. 2020, 270, 118878. [Google Scholar] [CrossRef]
- Chen, L.F.; Noreña, L.E.; Navarrete, J.; Wang, J.A. Improvement of surface acidity and structural regularity of Zr-modified mesoporous MCM-41. Mater. Chem. Phys. 2006, 97, 236–242. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Wang, S.J.; Wang, J.W.; Lou, L.L.; Zhang, C.; Liu, S. Synthesis and characterization of Zr-SBA-15 supported tungsten oxide as a new mesoporous solid acid. Solid State Sci. 2009, 11, 1412–1418. [Google Scholar] [CrossRef]
- Jia, A.; Li, J.; Zhang, Y.; Song, Y.; Liu, S. Synthesis and characterization of nanosized micromesoporous Zr–SiO2 via ionic liquid templating. Mater. Sci. Eng. 2009, 28, 1217–1226. [Google Scholar] [CrossRef]
- Hernández-Giménez, A.M.; Ruiz-Martínez, J.; Puértolas, B.; Pérez-Ramírez, J.; Bruijnincx, P.C.; Weckhuysen, B.M. Operando Spectroscopy of the Gas-Phase Aldol Condensation of Propanal over Solid Base Catalysts. Top. Catal. 2017, 60, 1522–1536. [Google Scholar] [CrossRef]
- Parry, E. An infrared study of pyridine adsorbed on acidic solids characterization of surface acidity. J. Catal. 1963, 2, 371. [Google Scholar] [CrossRef]
- Tišler, Z.; Vondrová, P.; Hrachovcová, K.; Štěpánek, K.; Velvarská, R.; Kocík, J.; Svobodová, E. Aldol Condensation of Cyclohexanone and Furfural in Fixed-Bed Reactor. Catalysts 2019, 9, 1068. [Google Scholar] [CrossRef] [Green Version]
- Vrbková, E.; Tišler, Z.; Vyskočilová, E.; Kadlec, D.; Červený, L. Aldol Condensation of Benzaldehyde and Heptanal: A Comparative Study of Laboratory and Industrially Prepared Mg-Al Mixed Oxides. Chem. Technol. Biotechnol. 2017, 93, 166–173. [Google Scholar] [CrossRef]

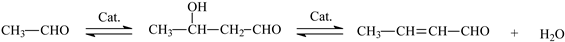 | ||||
|---|---|---|---|---|
| NO. | Catalyst | Conversion of AcH/% | Selectivity of 3-HBA/% | Yield of 3-HBA/% |
| 1 | 20%wt NaOH | 95.39 | 93.45 | 89.14 |
| 2 | MgO/Al2O3 | 64.13 | 86.42 | 55.42 |
| 3 | 2MgO/Al2O3 | 82.61 | 97.39 | 80.45 |
| 4 | 3MgO/Al2O3 | 88.23 | 85.38 | 75.33 |
| 5 | 4MgO/Al2O3 | 92.45 | 81.25 | 75.12 |
| 6 | 2.0%Ca-2MgO/Al2O3 | 84.83 | 77.43 | 65.68 |
| 7 | 2.3%Ca-2MgO/Al2O3 | 92.45 | 90.14 | 83.33 |
| 8 | 2.7%Ca-2MgO/Al2O3 | 88.50 | 82.92 | 73.38 |
| 9 | 3.0%Ca-2MgO/Al2O3 | 89.75 | 87.35 | 78.40 |
| 10 | 4.0%Ca-2MgO/Al2O3 | 90.17 | 85.42 | 77.02 |
| 11 | 0.1%La-2.3%Ca-2MgO/Al2O3 | 92.23 | 89.89 | 82.91 |
| 12 | 0.3%La-2.3%Ca-2MgO/Al2O3 | 94.19 | 90.43 | 85.18 |
| 13 | 0.5%La-2.3%Ca-2MgO/Al2O3 | 95.89 | 92.08 | 88.30 |
| 14 | 0.8%La-2.3%Ca-2MgO/Al2O3 | 92.53 | 89.10 | 82.44 |
| 15 | 1.0%La-2.3%Ca-2MgO/Al2O3 | 92.40 | 84.10 | 77.71 |
| Composite Oxides | MgO:Al2O3(ICP) | CaO/wt%(ICP) | La2O3/wt%(ICP) | Specific Surface Area/m2/g | Pore Volume/cm3/g | Pore Size/nm |
|---|---|---|---|---|---|---|
| 2MgO/Al2O3 | 2.11:1 | 0 | 0 | 322.8 | 0.46 | 4.9 |
| 2.3%Ca-2MgO/Al2O3 | 2.17:1 | 2.42 | 0 | 285.3 | 0.45 | 5.2 |
| 0.5%La-2.3%Ca-2MgO/Al2O3 | 2.09:1 | 2.39 | 0.44 | 280.9 | 0.44 | 5.3 |
| Composite Oxides | 2MgO/Al2O3 | 2.3%Ca-2MgO/Al2O3 | 0.5%La-2.3%Ca-2MgO/Al2O3 |
|---|---|---|---|
| total alkali content/mmol/g | 0.135 | 0.157 | 0.916 |
| total acid content/mmol/g | 0.574 | 0.391 | 0.413 |
| T1(150~250 °C) | 0.052 | 0.038 | 0.209 |
| T2(250~320 °C) | 0.357 | 0.287 | 0.031 |
| T3(320~500 °C) | 0.165 | 0.066 | 0.173 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.; Li, W. Green Synthesis of 3-Hydroxybutyraldehyde from Acetaldehyde Catalyzed by La-Ca-Modified MgO/Al2O3. Processes 2022, 10, 1302. https://doi.org/10.3390/pr10071302
Ren H, Li W. Green Synthesis of 3-Hydroxybutyraldehyde from Acetaldehyde Catalyzed by La-Ca-Modified MgO/Al2O3. Processes. 2022; 10(7):1302. https://doi.org/10.3390/pr10071302
Chicago/Turabian StyleRen, Hailun, and Weihong Li. 2022. "Green Synthesis of 3-Hydroxybutyraldehyde from Acetaldehyde Catalyzed by La-Ca-Modified MgO/Al2O3" Processes 10, no. 7: 1302. https://doi.org/10.3390/pr10071302
APA StyleRen, H., & Li, W. (2022). Green Synthesis of 3-Hydroxybutyraldehyde from Acetaldehyde Catalyzed by La-Ca-Modified MgO/Al2O3. Processes, 10(7), 1302. https://doi.org/10.3390/pr10071302





