Optimization of Subcritical Fluid Extraction for Total Saponins from Hedera nepalensis Leaves Using Response Surface Methodology and Evaluation of Its Potential Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Accelerated Solvent Extraction (ASE) Procedure
2.3. HPLC Analysis
2.4. Experimental Design and Statistical Analytic
| Independent Variable | Factors | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Volume used (mL) | A | 80 | 100 | 120 |
| Extraction temperature (°C) | B | 140 | 170 | 200 |
| Extraction time (min) | C | 3 | 6 | 9 |
2.5. Antimicrobial Assay
2.5.1. Preparation of Test Organism Cultures
2.5.2. Inoculums Preparation
2.5.3. Qualitative Antibacterial Activity by Disc Diffusion Assay
2.5.4. Quantitative Antibacterial Activity by Minimum Inhibitory Concentration
3. Results and Discussion
3.1. Optimization of Extraction Using RSM
3.2. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Accelerated solvent extraction | ASE |
| American Type Culture Collection | ATCC |
| Analysis of variance | ANOVA |
| Blood agar plate | BAP |
| Box–Behnken design | BBD |
| Chocolate agar plate | CAP |
| Colony forming unit | CFU |
| Dimethyl sulfoxide | DMSO |
| Haemophilus influenza | H. influenza |
| High-performance liquid chromatography | HPLC |
| Klebsiella pneumoniae | K. pneumoniae |
| McFarland units | MFU |
| Minimum inhibitory concentration | MIC |
| Pseudomonas aeruginosa | P. aeruginosa |
| Response surface methodology | RSM |
| Standard error of mean | SEM |
| Staphylococcus aureus | S. aureus |
| Streptococcus pneumoniae | S. pneumoniae |
| Streptococcus pyogenes | S. pyogenes |
| Subcritical water extraction | SWE |
| Tryptic soy agar | TSA |
References
- Ahmad, B.; Munir, N.; Bashir, S.; Azam, S.; Khan, I.; Ayub, M. Biological screening of Hedera nepalensis. J. Med. Plants Res. 2012, 6, 5250–5257. [Google Scholar]
- Hiep, N.T.; Nhung, P.T.H.; Nguyen, N.H.; Duong, H.T.; Huyen, P.T.; Thom, V.T.; Khoi, N.M.; Long, D.D. Identification, preliminary genetic, and biochemical analyses the Hedera plants which naturally distribute in Vietnam. Pharmacogn. Res. 2020, 12, 450. [Google Scholar]
- Romman, M. Comparative Pharmacological and Biological Evaluation of the Stem and Leaves of Hedera Nepalensis from District Malakand Khyber Pakhtunkhwa, Pakistan; Islamia College Peshawar: Khyber Pakhtunkhwa, Pakistan, 2016. [Google Scholar]
- Bibi, Y.; Naeem, J.; Zahara, K.; Arshad, M.; Qayyum, A. In Vitro antimicrobial assessment of selected plant extracts from pakistan. Iran. J. Sci. Technol. Trans. A Sci. 2018, 42, 267–272. [Google Scholar] [CrossRef]
- Romman, M.; Jan, S.; Hamayun, M.; Ahmad, I.; Wali, S. In Vitro antioxidant activity of stem and leaves extracts of Hedera nepalensis K. Koch. Int. J. Biosci. IJB 2015, 7, 19–24. [Google Scholar]
- Lutsenko, Y.; Bylka, W.; Matlawska, I.; Darmohray, R. Hedera helix as a medicinal plant. Herba Pol. 2010, 56, 83–96. [Google Scholar]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated solvent extraction: A technique for sample preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Sun, H.; Ge, X.; Lv, Y.; Wang, A. Application of accelerated solvent extraction in the analysis of organic contaminants, bioactive and nutritional compounds in food and feed. J. Chromatogr. A 2012, 1237, 1–23. [Google Scholar] [CrossRef]
- Mottaleb, M.A.; Sarker, S.D. Accelerated solvent extraction for natural products isolation. In Natural Products Isolation; Springer: Berlin/Heidelberg, Germany, 2012; pp. 75–87. [Google Scholar]
- Brachet, A.; Rudaz, S.; Mateus, L.; Christen, P.; Veuthey, J.L. Optimisation of accelerated solvent extraction of cocaine and benzoylecgonine from coca leaves. J. Sep. Sci. 2001, 24, 865–873. [Google Scholar] [CrossRef]
- Hiep, N.T.; Duong, H.T.; Anh, D.T.; Hoai Nguyen, N.; Thai, D.Q.; Linh, D.T.T.; Anh, V.T.H.; Khoi, N.M. Subcritical Water Extraction of Epigallocatechin Gallate from Camellia sinensis and Optimization Study Using Response Surface Methodology. Processes 2020, 8, 1028. [Google Scholar] [CrossRef]
- Lee, H.K.; Koh, H.L.; Ong, E.S.; Woo, S.O. Determination of ginsenosides in medicinal plants and health supplements by pressurized liquid extraction (PLE) with reversed phase high performance liquid chromatography. J. Sep. Sci. 2002, 25, 160–166. [Google Scholar] [CrossRef]
- Ong, E.S.; Len, S.M. Pressurized hot water extraction of berberine, baicalein and glycyrrhizin in medicinal plants. Anal. Chim. Acta 2003, 482, 81–89. [Google Scholar] [CrossRef]
- Güçlü-Üstündağ, Ö.; Balsevich, J.; Mazza, G. Pressurized low polarity water extraction of saponins from cow cockle seed. J. Food Eng. 2007, 80, 619–630. [Google Scholar] [CrossRef]
- Gil-Ramirez, A.; Salas-Veizaga, D.M.; Grey, C.; Karlsson, E.N.; Rodriguez-Meizoso, I.; Linares-Pastén, J.A. Integrated process for sequential extraction of saponins, xylan and cellulose from quinoa stalks (Chenopodium quinoa Willd.). Ind. Crops Prod. 2018, 121, 54–65. [Google Scholar] [CrossRef]
- Elisha, I.L.; Botha, F.S.; McGaw, L.J.; Eloff, J.N. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complement. Altern. Med. 2017, 17, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, D.I.; Hughes, D. Persistence of antibiotic resistance in bacterial populations. FEMS Microbiol. Rev. 2011, 35, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Riswanto, F.D.O.; Rohman, A.; Pramono, S.; Martono, S. Application of response surface methodology as mathematical and statistical tools in natural product research. J. Appl. Pharm. Sci 2019, 9, 125–133. [Google Scholar]
- Do Thi, T.L.; Hoang, T.D.; Nguyen, T.H.; Pham, T.H.; Nguyen, M.K.; Dinh, D.L. Simultaneous Quantification of Hederacoside C and α-hederin in Hedera Nepalensis K. Koch Using HPLC-UV. VNU J. Sci. Med. Pharm. Sci. 2020, 36, 17–23. [Google Scholar]
- Venter, G. Non-Dimensional Response Surfaces for Structural Optimization with Uncertainty; University of Florida: Gainesville, FL, USA, 1998. [Google Scholar]
- Kumar, A.; Prasad, B.; Mishra, I. Process parametric study for ethene carboxylic acid removal onto powder activated carbon using Box-Behnken design. Chem. Eng. Technol. Ind. Chem. Plant Equip. Process Eng. Biotechnol. 2007, 30, 932–937. [Google Scholar] [CrossRef]
- Mokhtar, W.N.A.W.; Bakar, W.A.W.A.; Ali, R.; Kadir, A.A.A. Deep desulfurization of model diesel by extraction with N, N-dimethylformamide: Optimization by Box–Behnken design. J. Taiwan Inst. Chem. Eng. 2014, 45, 1542–1548. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.-Y.; Gong, X.-F. Microwave-assisted extraction used for the isolation of total triterpenoid saponins from Ganoderma atrum. J. Food Eng. 2007, 81, 162–170. [Google Scholar] [CrossRef]
- Shi, J.; Arunasalam, K.; Yeung, D.; Kakuda, Y.; Mittal, G.; Jiang, Y. Saponins from edible legumes: Chemistry, processing, and health benefits. J. Med. Food 2004, 7, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Tan, C.; Ho, C. Effects of extraction solvent system, time and temperature on total phenolic content of henna (Lawsonia inermis) stems. Int. Food Res. J. 2013, 20, 3117. [Google Scholar]
- He, J.; Wu, Z.Y.; Zhang, S.; Zhou, Y.; Zhao, F.; Peng, Z.Q.; Hu, Z.W. Optimization of microwave-assisted extraction of tea saponin and its application on cleaning of historic silks. J. Surfactants Deterg. 2014, 17, 919–928. [Google Scholar] [CrossRef]
- Ullah, S.; Abbasi, M.; Raza, M.; Khan, S.; Muhammad, B.; Rehman, A.; Mughal, M. Antibacterial activity of some selected plants of Swat valley. Biosci. Res. 2011, 8, 15–18. [Google Scholar]
- Li, W.; Zhao, L.-C.; Sun, Y.-S.; Lei, F.-J.; Wang, Z.; Gui, X.-B.; Wang, H. Optimization of pressurized liquid extraction of three major acetophenones from Cynanchum bungei using a box-behnken design. Int. J. Mol. Sci. 2012, 13, 14533–14544. [Google Scholar] [CrossRef]
- Choi, M.P.; Chan, K.K.; Leung, H.W.; Huie, C.W. Pressurized liquid extraction of active ingredients (ginsenosides) from medicinal plants using non-ionic surfactant solutions. J. Chromatogr. A 2003, 983, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Kim, H.K.; Yang, E.S.; Lee, K.Y.; Du Kim, S.; Kim, Y.C.; Sung, S.H. Optimization of pressurized liquid extraction for spicatoside A in Liriope platyphylla. Sep. Purif. Technol. 2010, 71, 168–172. [Google Scholar] [CrossRef]
- Alzorqi, I.; Singh, A.; Manickam, S.; Al-Qrimli, H.F. Optimization of ultrasound assisted extraction (UAE) of β-D-glucan polysaccharides from Ganoderma lucidum for prospective scale-up. Resour. Effic. Technol. 2017, 3, 46–54. [Google Scholar] [CrossRef]
- Chan, C.-H.; See, T.-Y.; Yusoff, R.; Ngoh, G.-C.; Kow, K.-W. Extraction of bioactives from Orthosiphon stamineus using microwave and ultrasound-assisted techniques: Process optimization and scale up. Food Chem. 2017, 221, 1382–1387. [Google Scholar] [CrossRef]
- Green, D.W. The bacterial cell wall as a source of antibacterial targets. Expert Opin. Ther. Targets 2002, 6, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Zia, N.; Khalil, A.T.; Ayaz, M.; Khalil, A.; Ahmad, I. Nanoantibiotics: Recent developments and future prospects. Front. Clin. Drug Res. Anti. Infect. 2019, 5, 158–174. [Google Scholar]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In Vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J. Trop. Med. 2019, 5, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, G.; Khan, A.A.; Alamzeb, M.; Ali, S.; Alam, M.; Rauf, A.; Ullah, W. Biological screening of ethyl acetate extract of Hedera nepalensis stem. Afr. J. Pharm. Pharmacol. 2012, 6, 2934–2937. [Google Scholar] [CrossRef] [Green Version]
- Kavya, N.; Adil, L.; Senthilkumar, P. A Review on Saponin Biosynthesis and its Transcriptomic Resources in Medicinal Plants. Plant Mol. Biol. Report. 2021, 39, 833–840. [Google Scholar] [CrossRef]
- Fialová, S.B.; Rendeková, K.; Mučaji, P.; Nagy, M.; Slobodníková, L. Antibacterial Activity of Medicinal Plants and Their Constituents in the Context of Skin and Wound Infections, Considering European Legislation and Folk Medicine—A Review. Int. J. Mol. Sci. 2021, 22, 10746. [Google Scholar] [CrossRef]
- Akhtar, M.; Shaukat, A.; Zahoor, A.; Chen, Y.; Wang, Y.; Yang, M.; Guo, M.; Deng, G. Hederacoside-C Inhibition of Staphylococcus aureus-Induced Mastitis via TLR2 & TLR4 and Their Downstream Signaling NF-κB and MAPKs Pathways In Vivo and In Vitro. Inflammation 2020, 43, 579–594. [Google Scholar]
- Schmidt, S.; Heimesaat, M.; Fischer, A.; Bereswill, S.; Melzig, M. Saponins increase susceptibility of vancomycin-resistant enterococci to antibiotic compounds. Eur. J. Microbiol. Immunol. 2014, 4, 204–212. [Google Scholar] [CrossRef] [Green Version]
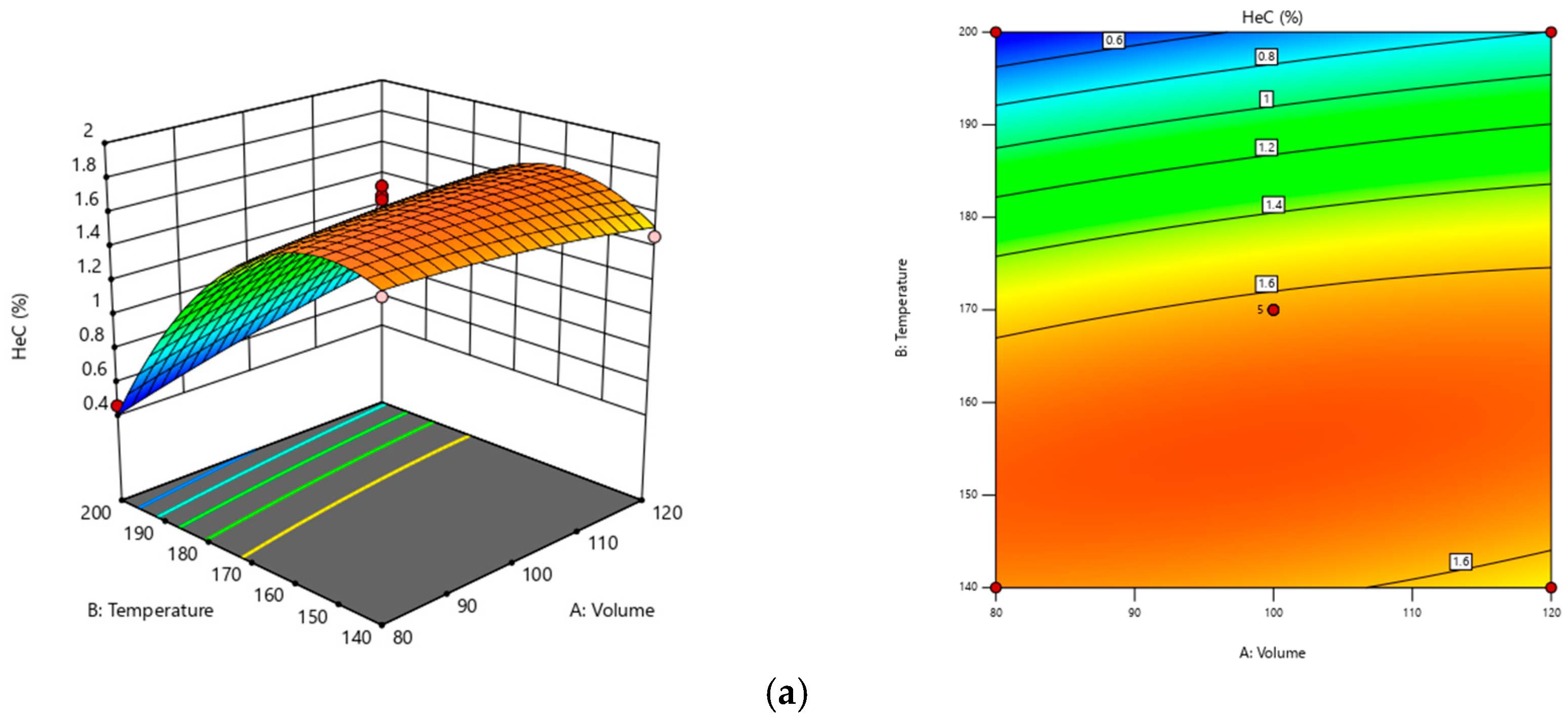
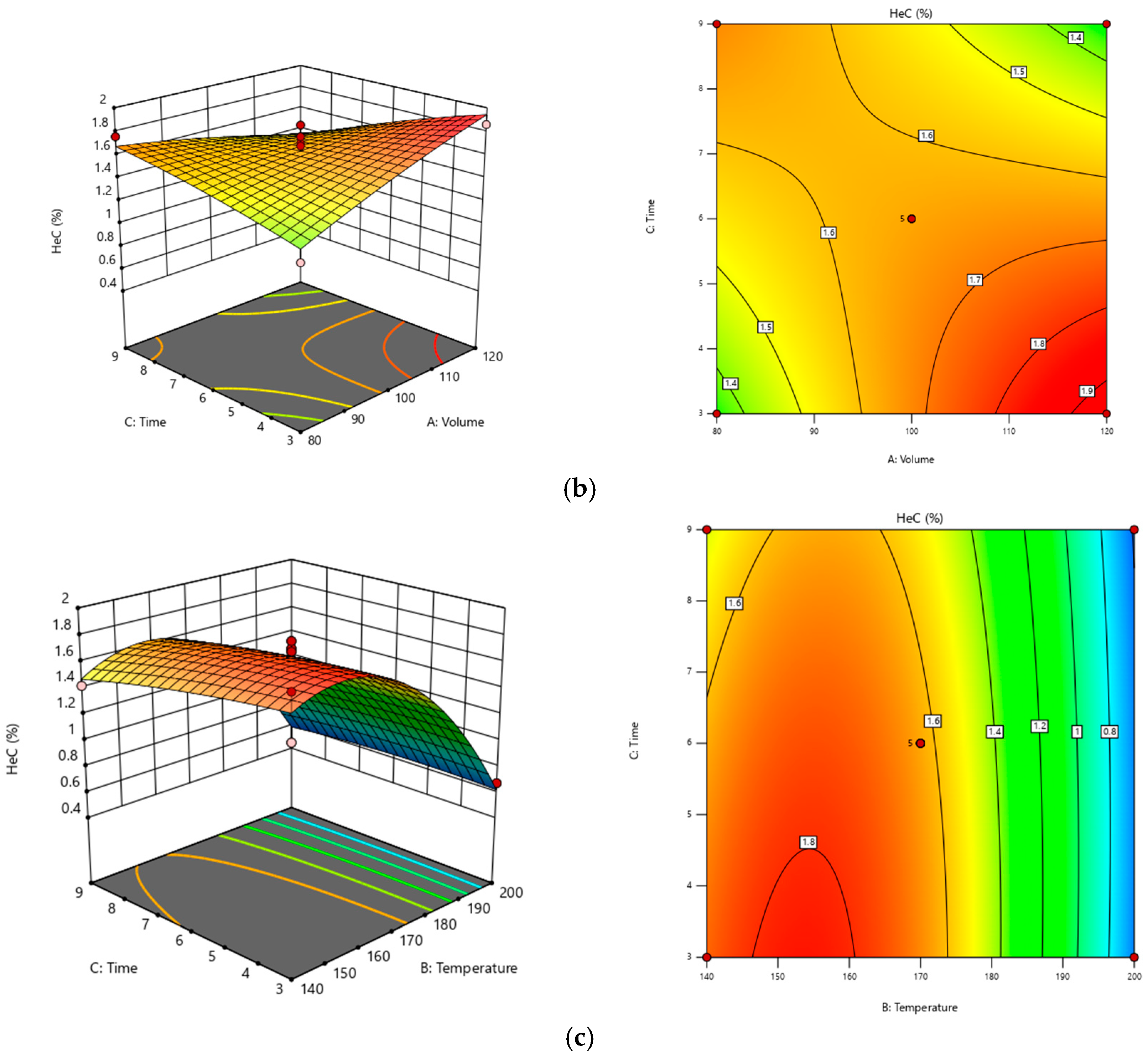
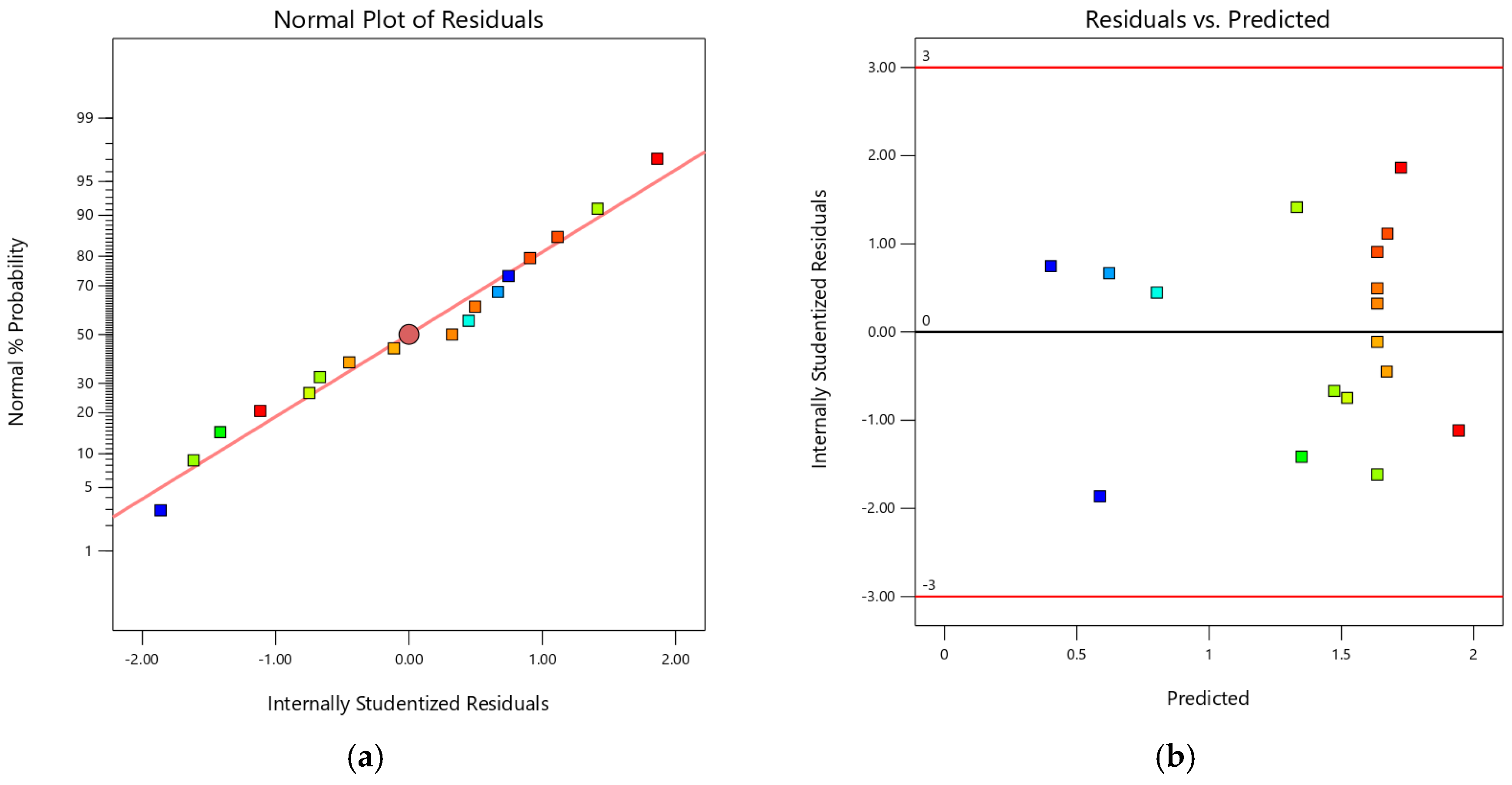
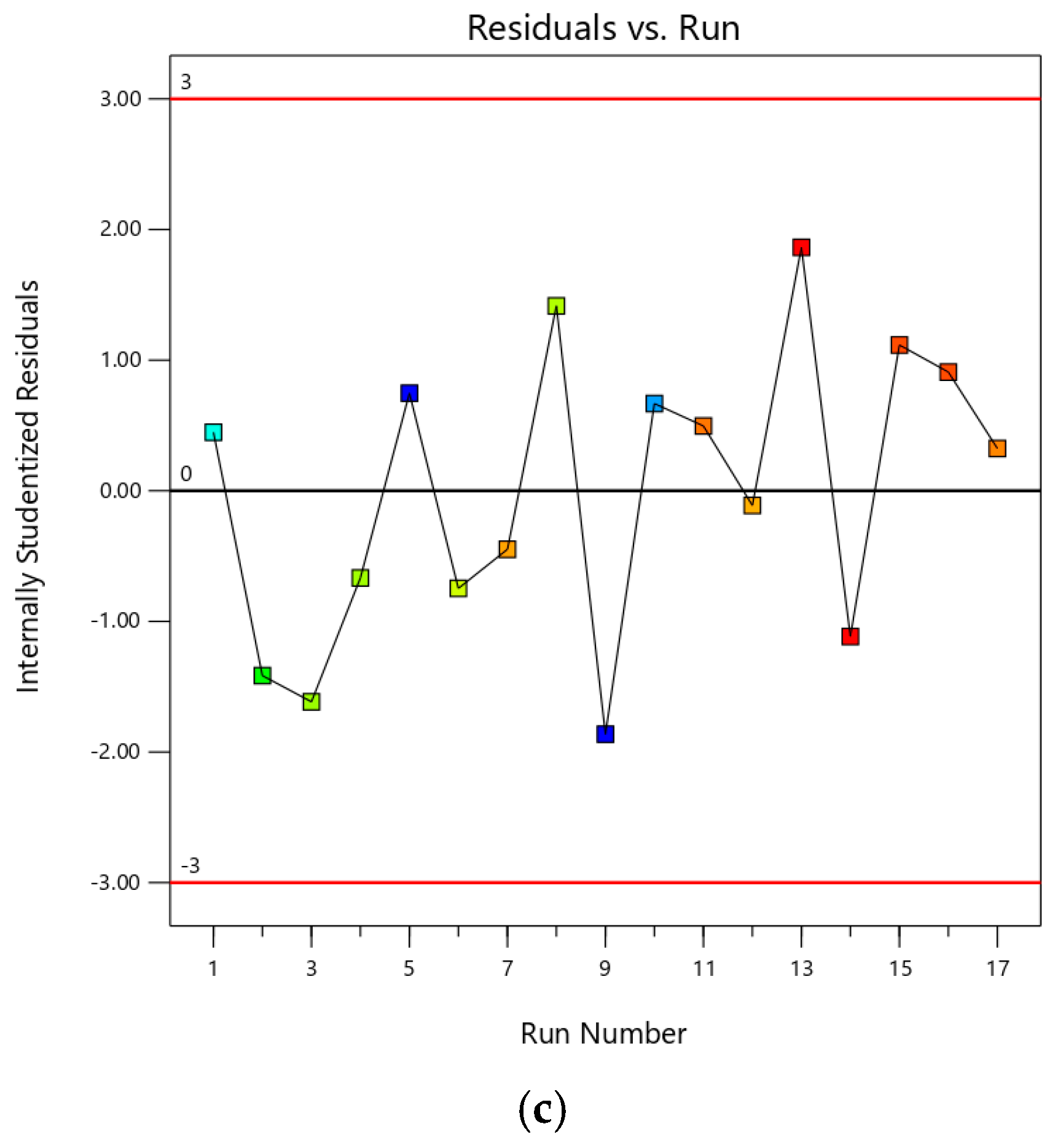
| Std | Run | Factor A: Volume (mL) | Factor B: Temperature (°C) | Factor C: Time (min) | Response: Yield of Saponin Contents (%) |
|---|---|---|---|---|---|
| 4 | 1 | 120 | 200 | 6 | 0.836 |
| 5 | 2 | 80 | 170 | 3 | 1.244 |
| 14 | 3 | 100 | 170 | 6 | 1.420 |
| 11 | 4 | 100 | 140 | 9 | 1.423 |
| 3 | 5 | 80 | 200 | 6 | 0.458 |
| 2 | 6 | 120 | 140 | 6 | 1.466 |
| 1 | 7 | 80 | 140 | 6 | 1.638 |
| 8 | 8 | 120 | 170 | 9 | 1.437 |
| 12 | 9 | 100 | 200 | 9 | 0.448 |
| 10 | 10 | 100 | 200 | 3 | 0.672 |
| 15 | 11 | 100 | 170 | 6 | 1.702 |
| 13 | 12 | 100 | 170 | 6 | 1.621 |
| 9 | 13 | 100 | 140 | 3 | 1.865 |
| 6 | 14 | 120 | 170 | 3 | 1.860 |
| 7 | 15 | 80 | 170 | 9 | 1.757 |
| 16 | 16 | 100 | 170 | 6 | 1.757 |
| 17 | 17 | 100 | 170 | 6 | 1.679 |
| Response 1: Yield. | ||||||
|---|---|---|---|---|---|---|
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
| Model | 3.46 | 9 | 0.3841 | 17.22 | 0.0006 | significant |
| A-Volume | 0.0316 | 1 | 0.0316 | 1.41 | 0.2731 | |
| B-Temperature | 1.98 | 1 | 1.98 | 88.66 | <0.0001 | |
| C-Time | 0.0414 | 1 | 0.0414 | 1.86 | 0.2154 | |
| AB | 0.0758 | 1 | 0.0758 | 3.40 | 0.1078 | |
| AC | 0.2191 | 1 | 0.2191 | 9.82 | 0.0165 | |
| BC | 0.0118 | 1 | 0.0118 | 0.5292 | 0.4906 | |
| A2 | 0.0043 | 1 | 0.0043 | 0.1916 | 0.6747 | |
| B2 | 1.07 | 1 | 1.07 | 48.03 | 0.0002 | |
| C2 | 0.0036 | 1 | 0.0036 | 0.1615 | 0.6997 | |
| Residual | 0.1561 | 7 | 0.0223 | |||
| Lack of Fit | 0.0884 | 3 | 0.0295 | 1.74 | 0.2969 | Not significant |
| Pure Error | 0.0677 | 4 | 0.0169 | |||
| Cor Total | 3.61 | 16 | ||||
| Fit Statistics | ||||||
| Std. Dev. | 0.1493 | R2 | 0.9568 | |||
| Mean | 1.37 | Adjusted R2 | 0.9012 | |||
| C.V.% | 10.90 | Predicted R2 | 0.5794 | |||
| Adeq Precision | 13.4584 | |||||
| Microorganism | Zone of Inhibition (mm) | ||||
|---|---|---|---|---|---|
| Control | Hedera nepalensis Extract | Amoxicillin | Cefotaxime | ||
| Gram (+) | S. aureus | - | - | 29.23 ± 0.03 | ND |
| S. pneumoniae | - | 19.33 ± 0.09 | 45.60 ± 0.06 | ND | |
| S. pyogenes | - | 18.60 ± 0.06 | 32.10 ± 0.06 | ND | |
| Gram (−) | H. influenzae | - | 12.63 ± 0.19 | 15.83 ± 0.09 | ND |
| K. pneumoniae | - | - | - | 24.10 ± 0.10 | |
| P. aeruginosa | - | - | - | 10.60 ± 0.10 | |
| Microorganism | MIC | ||
|---|---|---|---|
| Hedera nepalensis Extract (mg/mL) | Amoxicillin (μg/mL) | ||
| Gram (+) | S. pneumoniae | 5 | 0.0312 |
| S. pyogenes | >5 | <0.0312 | |
| Gram (−) | H. influenzae | >5 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duong, H.T.; Trieu, L.H.; Linh, D.T.T.; Duy, L.X.; Thao, L.Q.; Minh, L.V.; Hiep, N.T.; Khoi, N.M. Optimization of Subcritical Fluid Extraction for Total Saponins from Hedera nepalensis Leaves Using Response Surface Methodology and Evaluation of Its Potential Antimicrobial Activity. Processes 2022, 10, 1268. https://doi.org/10.3390/pr10071268
Duong HT, Trieu LH, Linh DTT, Duy LX, Thao LQ, Minh LV, Hiep NT, Khoi NM. Optimization of Subcritical Fluid Extraction for Total Saponins from Hedera nepalensis Leaves Using Response Surface Methodology and Evaluation of Its Potential Antimicrobial Activity. Processes. 2022; 10(7):1268. https://doi.org/10.3390/pr10071268
Chicago/Turabian StyleDuong, Hoang Thanh, Ly Hai Trieu, Do Thi Thuy Linh, Le Xuan Duy, Le Quang Thao, Le Van Minh, Nguyen Tuan Hiep, and Nguyen Minh Khoi. 2022. "Optimization of Subcritical Fluid Extraction for Total Saponins from Hedera nepalensis Leaves Using Response Surface Methodology and Evaluation of Its Potential Antimicrobial Activity" Processes 10, no. 7: 1268. https://doi.org/10.3390/pr10071268
APA StyleDuong, H. T., Trieu, L. H., Linh, D. T. T., Duy, L. X., Thao, L. Q., Minh, L. V., Hiep, N. T., & Khoi, N. M. (2022). Optimization of Subcritical Fluid Extraction for Total Saponins from Hedera nepalensis Leaves Using Response Surface Methodology and Evaluation of Its Potential Antimicrobial Activity. Processes, 10(7), 1268. https://doi.org/10.3390/pr10071268







