Low-Cost H-Grade Polyacrylamide Gel with High-Temperature Resistance
Abstract
:1. Introduction
2. Experimental Part
2.1. Experimental Materials
2.2. Synthesis of Cross-Linkers
2.3. Preparation of Gel Solution
- (1)
- A total of 1.5 g polyacrylamide was dissolved into 500 mL tap water. After strong stirring, a 0.3 wt% polyacrylamide solution was gained. This solution was hydrated under room temperature and placed away from light for 24 h.
- (2)
- A total of 50 mL of the solution was taken and added to 100 mL sample bottle. Then, 0.1 wt% of antioxidant (thiourea) and different concentrations of cross-linker were added and stirred for 25 min to obtain homogeneous gel solution. This stirring time and antioxidant dosage were determined according to the experiments.
- (3)
- The gel stood for 30 min to remove air bubbles. Then, the sample bottle was put into the oven at 120 °C to form the gel. This part of the experiments also used a balance with an accuracy of ±0.0001 g, as in the previous section, so that the composition of the gel solution could be precisely controlled.
2.4. Measurement of Gel-Forming Time and Dosage Optimization of Cross-Linking Agent
2.5. Gel Temperature Resistance Test
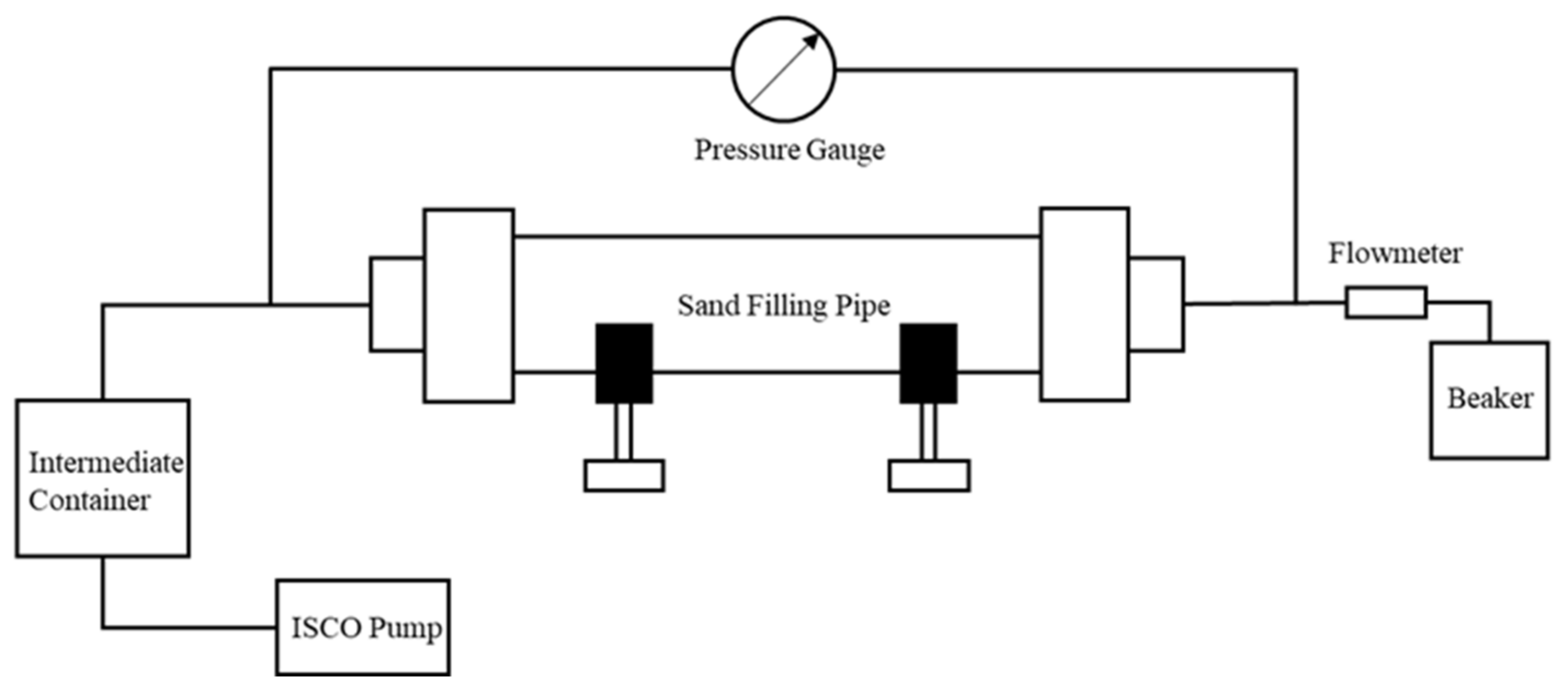
2.6. Blocking Effect Test
2.7. Microstructure of the Gel
3. Results and Analysis
3.1. Optimal Concentration of Cross-Linking Agent and Gel-Forming Time
3.1.1. Phenolic Resin Cross-linker
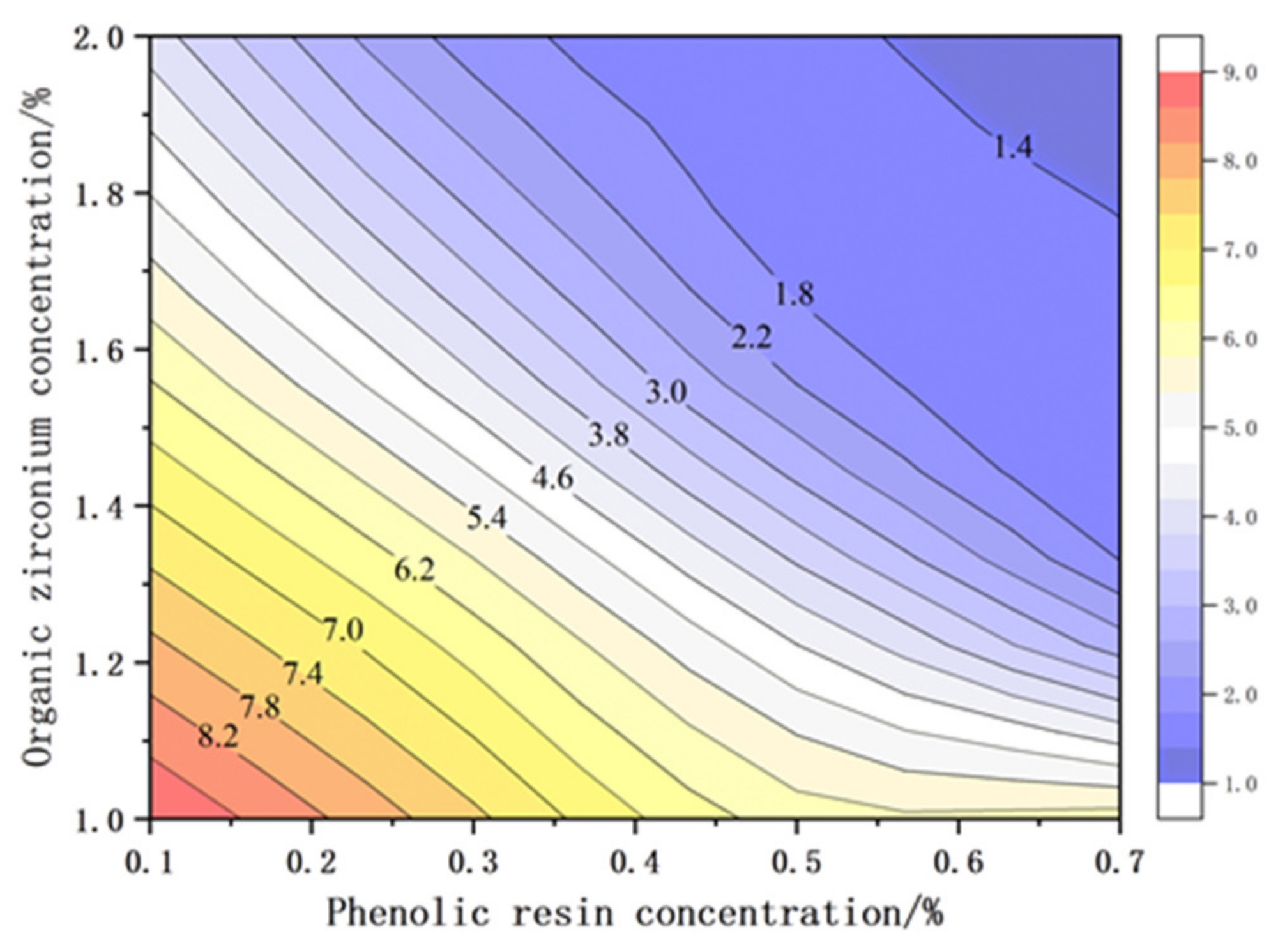
3.1.2. Organic Zirconium Secondary Cross-linker
3.2. Analysis of Rheological Results
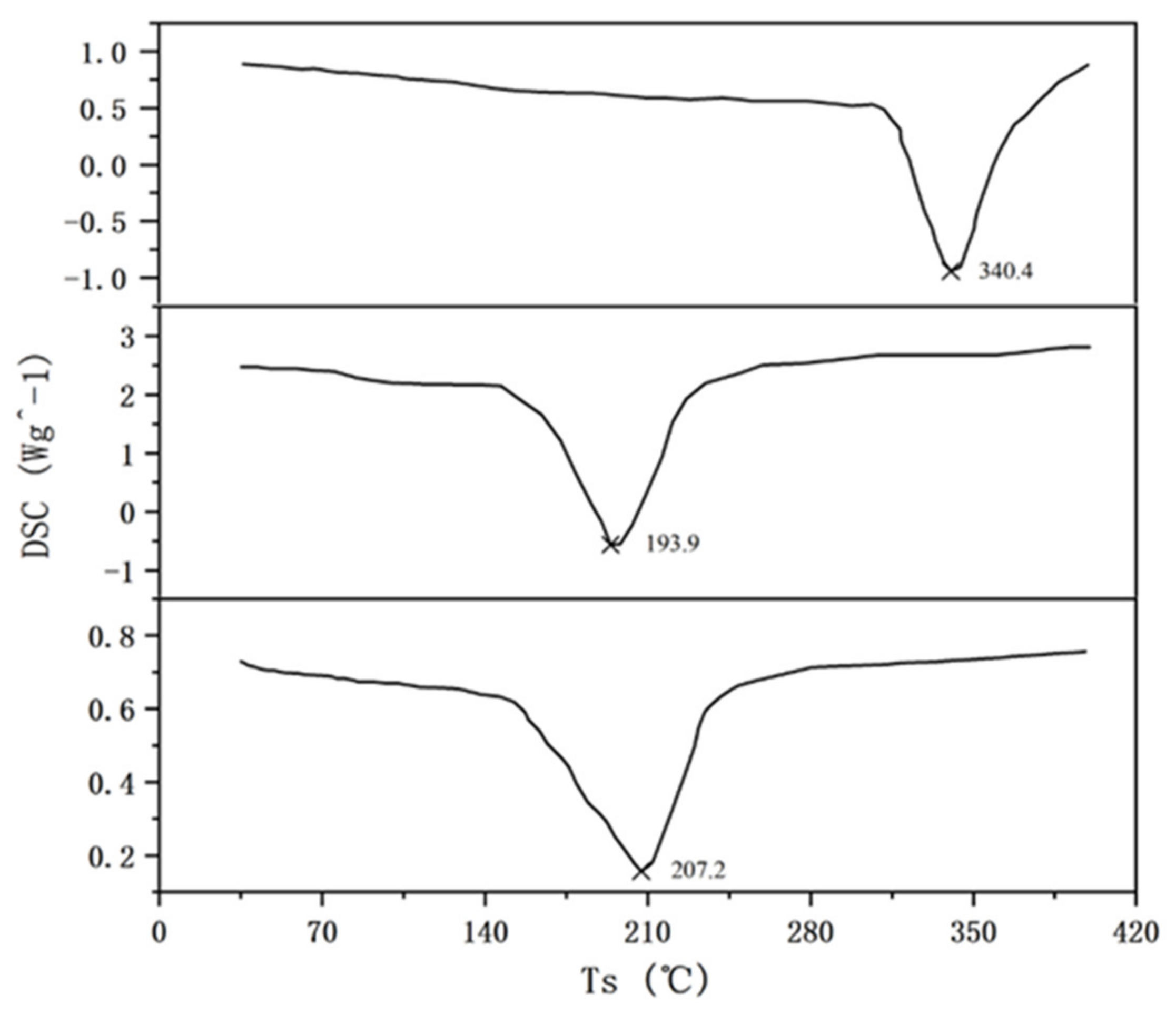
3.3. Thermal Stability of Gel
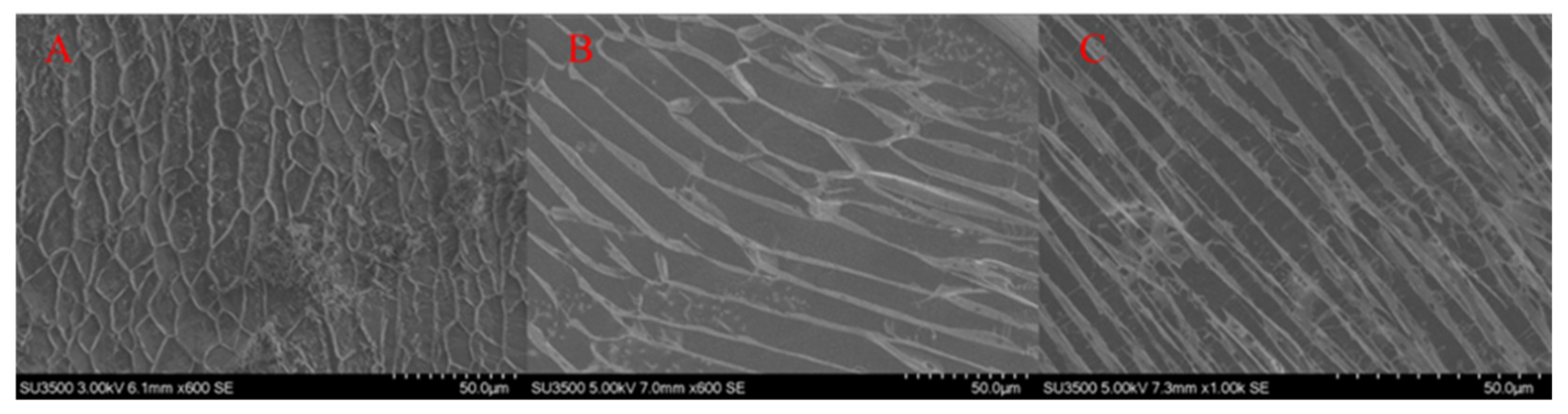
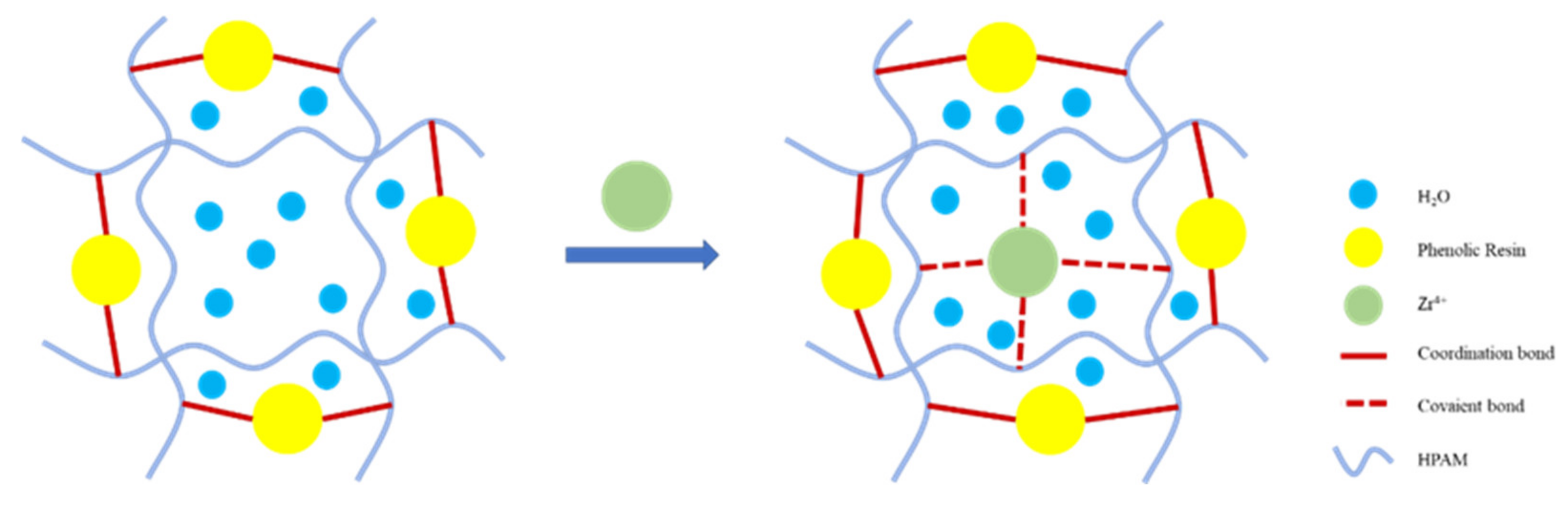
3.4. Gel Microstructure and Cross-linking Mechanism Analysis
3.5. Evaluation of Blocking Effect
4. Conclusions
- Under the economic dosage, 0.3 wt% polyacrylamide can form gels regardless of organic zirconium or phenolic resin cross-linker. However, the phenolic resin gel system had low strength compared with the organic zirconium gel system, which lacks good water-blocking performance. The organozirconium gel system had poor water-blocking capability and lacked temperature resistance ability.
- After initial cross-linking by phenolic resin (0.3%) and secondary cross-linking by organic zirconium (1.6%), a JM186 gel system can be obtained with temperature resistance up to 120 °C. The average elastic modulus and viscous modulus can reach 32.33 Pa and 3.25 Pa, which are significantly greater than those of the single cross-linker systems.
- By changing the dosage of cross-linking agents, the JM186 gel system gel-forming time can range from 1 h to 9 h, and the strength of the gel can reach H grade. The water-blocking efficiency of the sand-filled pipe can reach 96.03%.
- After secondary cross-linking by organic zirconium, the microscopic grid structure of the JM186 was denser and more thickened compared with the gel system of the single cross-linker, which is the reason why JM186 had the good thermal stability and greater gel strength.
- The JM186 gel system is expected to achieve low cost and high water-blocking efficiency in high-temperature reservoirs. It will increase the wave efficiency of water injection and thus enhance the oil recovery rate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Dai, C.; Wang, K.; Zhao, M.; Gao, M.; Yang, Z.; Fang, J.; Wu, Y. Investigation on Preparation and Profile Control Mechanisms of the Dispersed Particle Gels (DPG) Formed from Phenol-Formaldehyde Cross-linked Polymer Gel. Ind. Eng. Chem. Res. 2016, 55, 6284–6292. [Google Scholar] [CrossRef]
- Seright, R.S.; Lane, R.H.; Sydansk, R.D. A strategy for attacking excess water production. SPE Prod. Facil. 2003, 18, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Bai, B.; Wang, Y. Applied Technologies and Prospects of Conformance Control Treatments in China. Oil Gas Sci. Technol. 2010, 65, 859–878. [Google Scholar] [CrossRef]
- Jia, H.; Pu, W.F.; Zhao, J.Z.; Liao, R. Experimental Investigation of the Novel PhenolFormaldehyde Cross-Linking HPAM Gel System: Based on the Secondary Cross-Linking Method of Organic Cross-Linkers and Its Gelation Performance Study after Flowing through Porous Media. Energy Fuels 2011, 25, 727–736. [Google Scholar] [CrossRef]
- Zhu, D.; Hou, J.; Meng, X.; Zheng, Z.; Wei, Q.; Chen, Y.; Bai, B. Effect of Different Phenolic Compounds on Performance of Organically Cross-Linked Terpolymer Gel Systems at Extremely High Temperatures. Energy Fuels 2017, 31, 8120–8130. [Google Scholar] [CrossRef]
- Karimi, S.; Esmaeilzadeh, F.; Mowla, D. Identification and selection of a stable gel polymer to control or reduce water production in gas condensate fields. Nat. Gas Sci. Eng. 2014, 21, 940–950. [Google Scholar] [CrossRef]
- He, S.; Bo, Y.; Yu, T.; Xie, Z.; Dai, C.; Zhang, H. Strength and temperature resistance of nano diatomaceous earth enhanced polyacrylamide gel plugging agent. Sci. Technol. Eng. 2018, 18, 65–67l. [Google Scholar]
- Xiao, X.; Luo, Y.; Yu, X. Preparation and properties of nano-titanium dioxide enhanced polyacrylamide/polyethyleneimine gels. Synth. Chem. 2019, 27, 823–826. [Google Scholar]
- Li, Q.; Yu, X.; Xiao, X.; Luo, Y.; Yang, H. Preparation and properties of nano-SiO2 enhanced PAM/PEI jelly gel. Fine Chem. 2021, 38, 200–205. [Google Scholar]
- Al-Muntasheri, G.A.; Zitha, P. Gel under Dynamic Stress in Porous Media: New Insights using Computed Tomography. In Proceedings of the SPE Saudi Arabia Section Technical Symposium, Al-Khobar, Saudi Arabia, 9–11 May 2009. [Google Scholar]
- Fu, M.; Zhao, X.; Wang, Z. Development and performance evaluation of high temperature resistant modified phenolic resin composite plugging agent system. Adv. Fine Petrochem. 2013, 14, 8–10. [Google Scholar]
- Zhu, D.; Bai, B.; Hou, J. Polymer gel systems for water management in high-temperature petroleum reservoirs: A chemical review. Energy Fuels 2017, 31, 13063–13087. [Google Scholar] [CrossRef]
- Wu, J.Y.; Qian, X.L.; Yu, P.Z. Synthesis and Performances of Water-Soluble Phenolic Resin. Adv. Mater. Res. 2014, 1049–1050, 129–132. [Google Scholar] [CrossRef]
- Dai, C.; Ge, J.; Zhang, G.; Zhao, F. Study on the factors affecting the freeze-formation of zirconium jelly. Oilfield Chem. 2001, 228–231. [Google Scholar]
- Ma, L.; Yang, T.; Li, X.; Zhang, T.; Tan, J.; Zhao, G.; Dai, C. Study on the reaction process, microstructure and freeze-forming influencing factors of zirconium freeze gel. Oilfield Chem. 2015, 32, 48–52. [Google Scholar]
- Yao, E.; Yu, G.; Li, B.; Zhao, L.; Li, Y.; Bai, H.; Zhou, F. High-Temperature-Resistant, Low-Concentration Water-Controlling Composite Cross-Linked Polyacrylamide Weak Gel System Prepared from Oilfield Sewage. ACS Omega 2022, 7, 12570–12579. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, J.; Gu, Y.; Zhao, Q.; Yang, R.; Yang, Y. Polyacrylamide gel formed by Cr(III) and phenolic resin for water control in high-temperature reservoirs. J. Pet. Sci. Eng. 2020, 194, 107423. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, C.; You, Q.; Zou, C.; Gao, M.; Zhao, M. Experimental Investigation on a Novel Organic-Inorganic Crosslinked Polymer Gel for Water Control in Ultra-High Temperature Reservoirs. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, Jakarta, Indonesia, 17–19 October 2017. [Google Scholar]
- Sydansk, R.D. Acrylamide-polymer/chromium (III)-carboxyl-ategels for near wellbore matrix treatments. SPE Adv. Technol. Ser. 1993, 1, 146–152. [Google Scholar] [CrossRef]
- Dongwen, L.; Yuqin, W.; Lei, B. Application of deep profile control technology to Karamay conglomerate reservoirs. Xinjiang Pet. Geol. 2011, 32, 208–210. [Google Scholar]
- Liu, X.; Yaxin, S.; Shilin, D. Experimental study of Co/Fe/Al2O3/cordierite catalyzed selective reduction of NO by C3H6. J. Fuel Chem. 2018, 46, 743–753. [Google Scholar]
- Xinye, Y.; Juhong, M.; Shixin, P.E.I. Comprehensive experimental design of Ba(Zn_(1/3)Nb_(2/3))O_3 microwave dielectric ceramics prepared by sol-gel method. Exp. Technol. Manag. 2020, 37, 93–97. [Google Scholar]
- Jianping, Z. Preparation of Heterogeneous Gel Polymer Electrolytes with Silicon Cross-Linked Structure and its Performance Study; Soochow University: Taipei, Taiwan, 2019. [Google Scholar]
- Zhao, F. Oilfield Chemistry; China University of Petroleum Press: Dongying, China, 2010; pp. 132–134. [Google Scholar]
- Tang, X.C.; Yang, H.B.; Gao, Y.B. Preparation of a mielon-size silica·reinforced polymer mierosphere and evaluation of its properties as a blocking agent. Colloids Surf. A 2018, 547, 8–18. [Google Scholar] [CrossRef]
- Esmaeilnezhad, E.; Choi, H.J.; Schaffie, M.; Gholizadeh, M.; Ranjbar, M. Polymer coated magnetite-based magnetorheological fluid and its potential clean procedure applications to oil production. J. Clean. Prod. 2018, 171, 45–56. [Google Scholar] [CrossRef]
- Chenghua, L.; Yi, Z.; Qinhua, Z.; Wei, X. Principle and application of differential scanning calorimeter. Anal. Instrum. 2015, 4, 88–94. [Google Scholar]
- Yikun, L.; Yuxiang, L.; Yang, P.; Yang, Y. Water blocking and dissection in China in the 1960s. Pet. Drill. Technol. 2019, 41, 773–787. [Google Scholar]
- Hua, Z.; Lin, M.; Dong, Z.; Li, M.; Zhang, G.; Yang, J. Study of deep profile control and oil displacement technologies with nanoscale polymer microspheres. J. Colloid Interface Sci. 2014, 424, 67–74. [Google Scholar] [CrossRef] [PubMed]




| Strength Code | Gel Name | Corresponding Intensity Description |
|---|---|---|
| A | Non-detecting gel | The viscosity of the system is equivalent to the viscosity of the polymer, and the formation of gel cannot be observed with the naked eye |
| B | High flow gel | The viscosity of the gel system is slightly higher than the viscosity of the polymer |
| C | Flowable gel | The viscosity of the gel system is slightly higher than the viscosity of the polymer |
| D | Medium flow gel | When the glass bottle is turned over, a small amount of gel (mass fraction <15%) can not flow to the other end, often in the shape of a tongue |
| E | Hardly flowing gel | When the glass bottle is turned over, a few gels can slowly flow to the other end, and most of them (mass fraction > 15%) are not fluid |
| F | High deformation non-flowing gel | The gel cannot flow to the mouth of the bottle when the glass bottle is turned over |
| G | Medium deformation non-flowing gel | When turned over, it can only flow to the middle of the glass bottle |
| H | Slightly deformed non-flowing gel | When flipped, only the surface of the gel is deformed |
| I | Rigid gel | When turned over, the surface of the gel does not deform |
| J | Ringing gel | When shaking the glass bottle, you can feel the mechanical vibration like a tuning fork |
| Polyacrylamide Concentration/wt% | Antioxidant Concentration/wt% | Cross-Linker Concentration/% | Gel Grade | Gel-Forming Time/h | Dehydration |
|---|---|---|---|---|---|
| 0.3 | 0.1 | 0.1 | B | 12 | No |
| 0.2 | B | 10 | |||
| 0.3 | C | 9 | |||
| 0.4 | C | 8 | |||
| 0.5 | C | 8 | |||
| 0.6 | D | 7 | |||
| 0.7 | D | 6 | Yes |
| Polyacrylamide Concentration/wt% | Antioxidant Concentration/wt% | Phenolic Resin Concentration/% | Organic Zirconium Concentration/% | Gel Grade | Dehydration |
|---|---|---|---|---|---|
| 0.3 | 0.1 | 0.1 | 1.0 | F | No |
| 1.3 | F | ||||
| 1.6 | G | ||||
| 2.0 | G | Yes | |||
| 0.3 | 1.0 | F | No | ||
| 1.3 | G | ||||
| 1.6 | H | ||||
| 2.0 | I | Yes | |||
| 0.5 | 1.0 | F | No | ||
| 1.3 | G | ||||
| 1.6 | H | Yes | |||
| 2.0 | I | ||||
| 0.7 | 1.0 | F | Yes | ||
| 1.3 | G | ||||
| 1.6 | H | ||||
| 2.0 | I |
| No | Polyacrylamide Concentration/wt% | Antioxidant Concentration/wt% | Phenolic Resin Concentration/% | Organic Zirconium Concentration/% |
|---|---|---|---|---|
| JM186 | 0.3 | 0.1 | 0.3 | 1.6 |
| #1 | 0 | 1.6 | ||
| #2 | 0.3 | 0 |
| Sample No. | Permeability before Injection (mD) | Permeability after Gel Injection (mD) | Blocking Rate (%) |
|---|---|---|---|
| JM186 | 167.564 | 6.367 | 96.2 |
| 208.643 | 10.849 | 94.8 | |
| 230.724 | 6.691 | 97.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, E.; Li, B. Low-Cost H-Grade Polyacrylamide Gel with High-Temperature Resistance. Processes 2022, 10, 1165. https://doi.org/10.3390/pr10061165
Yao E, Li B. Low-Cost H-Grade Polyacrylamide Gel with High-Temperature Resistance. Processes. 2022; 10(6):1165. https://doi.org/10.3390/pr10061165
Chicago/Turabian StyleYao, Erdong, and Bojun Li. 2022. "Low-Cost H-Grade Polyacrylamide Gel with High-Temperature Resistance" Processes 10, no. 6: 1165. https://doi.org/10.3390/pr10061165
APA StyleYao, E., & Li, B. (2022). Low-Cost H-Grade Polyacrylamide Gel with High-Temperature Resistance. Processes, 10(6), 1165. https://doi.org/10.3390/pr10061165







