A Review of the Dynamic Mathematical Modeling of Heavy Metal Removal with the Biosorption Process
Abstract
:1. Introduction
1.1. Biosorption Process
- (i)
- Diffusion of the contaminant ion from the bulk phase to the outer surface of the sorbent. The phenomenon of surface diffusion is accompanied by overcoming the mass transfer resistance offered by the double layer interface between the bulk phase and the outer surface of the adsorbent.
- (ii)
- The surface diffusion is followed by diffusion of the ions from the outer surface of the adsorbent into its internal pores. In short, the pore diffusion is directly correlated with the internal porosity of the biomass. However, in some cases (e.g., microbial biosorbents), the sorption process occurs at the sorbent surface without this step.
- (iii)
- Further, the interaction sites are available on the inner pore walls of the sorbent, and the chemisorption of the metal ions on the interaction sites of the substrate proceeds until they are completely saturated with the influent ions.
1.2. Adsorption Isotherm
1.3. Uni-Component Adsorption Models
1.4. Biosorption Kinetics Mechanism
2. Batch Models
2.1. Multicomponent Equilibrium Models
2.1.1. Isotherm Models
- ψ = spreading pressure for individual components;
- = adsorbed phase concentration of species I (mmol per unit mass of adsorbent);
- = adsorbed phase concentration of single-component i (mmol per unit mass of; adsorbent;
- = total amount of species adsorbed (mmol per unit mass of adsorbent).
- —spreading pressure of system;
- —spreading pressure of each component i;
- R—universal gas constant;
- T—temperature in K;
- A—external surface area per unit mass of adsorbent.
- d is the Langmuir coefficient of a species (L/mmol);
- w is the amount of adsorbent per liter of solution (g/L);
- W is the amount of contaminant adsorbed per unit mass of the adsorbent (mg/g);
- Ce is the equilibrium concentration of a contaminant in the bulk phase (mmol/L);
- Qm is the maximum adsorption capacity of a species (mg/g).
- D is a dimensionless collision probability factor.
2.1.2. Pore Diffusion Model
- Qm is the maximum adsorption capacity of a species (mg/g);
- d is the Langmuir coefficient (L/mmol);
2.2. Models for Sorption Kinetics
2.2.1. Single-Component Pseudo-First- and Second-Order Models
| Model | Type | Formula |
|---|---|---|
| Langmuir [90] | Isotherm model | |
| Langmuir with competition between two species i and j [91] | Isotherm model | |
| Langmuir with competition between q species [92] | Isotherm model | |
| Langmuir–Freundlich [90] | Isotherm model | |
| Generalized Langmuir [93] | Isotherm model | |
| Redlich–Peterson [94] | Isotherm model | |
| Toth [95] | Isotherm model | |
| Hinz [96] | Isotherm model | |
| First order [97] | Kinetic model | |
| nth order [97] | Kinetic model | |
| Langmuir kinetic [97] | Kinetic model | |
| Langmuir kinetic with competition between n species [98] | Kinetic model | |
| First order with partly pseudo-irreversible adsorption [99] | Kinetic model |
2.2.2. External Mass Transfer Model
2.2.3. Multicomponent Surface Excess Kinetic Model
- k1 is an adsorption constant when ;
- m1 is monolayer coverage of component 1 (solute) per unit mass of the adsorbent, (kg/kg);
- m2 is monolayer coverage of component 2 (solvent) per unit mass of the adsorbent (kg/kg);
- x1 is the mass fraction of the solute in the bulk phase;
- S is the selectivity factor, a dimensionless parameter (maximum value of unity).
2.2.4. Pore Diffusion Model
- —effective diffusivity;
- —bed porosity;
- r—distance of spherical particle from center of sphere;
- —Bed density;
- R—radius of sphere;
- CA—Concentration of species A;
- Boundary condition 1: At r = 0 → ;
- Boundary condition 2: At r = R → ;
- —surface diffusivity;
- —film diffusion coefficient;
- a—surface area of the pellet.
- (i)
- Macro pore diffusion
- (ii)
- Micro pore diffusion
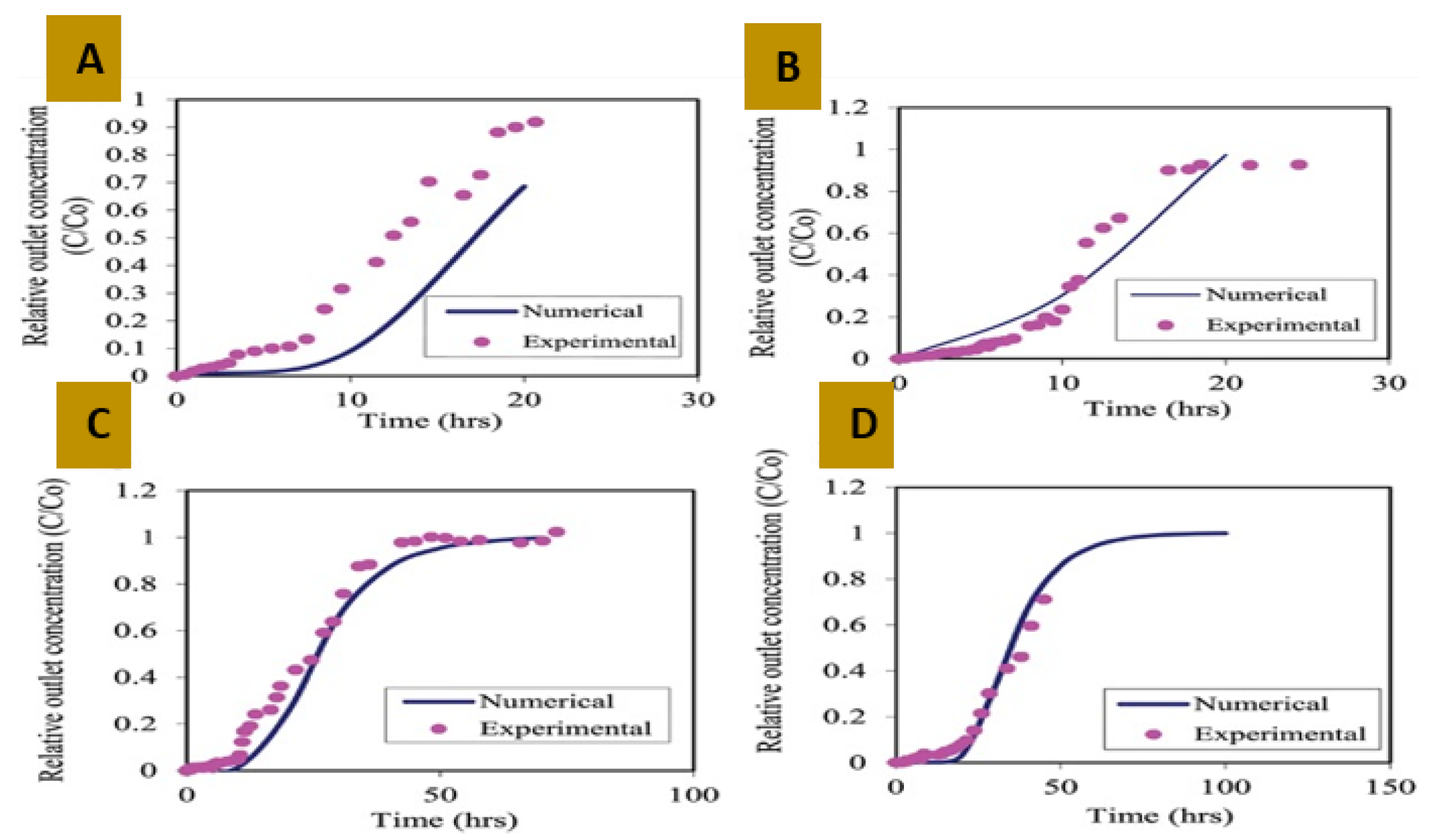
3. Fixed-Bed Dynamic Model
3.1. Uni Component Model
Limitations of the Abovementioned Models
3.2. Multicomponent Dynamic Models
3.2.1. Entrapment Model
3.2.2. Two-Dimensional Dispersion Model
3.2.3. Pore Diffusion Model
- (i)
- Equilibrium occurs between the pore liquid and particle interior; i.e., the solution flow to the pores is much faster than its uptake at sorption sites.
- (ii)
- Mass transfer in the pores is solely based on molecular diffusion; i.e., it follows Fick’s first law and is measured by the effective pore diffusion coefficient, Deff.
- (iii)
- Solute concentration in the pore liquid is very small relative to that in the adsorbed phase and can therefore be neglected.
4. Overall Review of the Batch and Dynamic Models
- (i)
- Mathematical models.
- (ii)
- Bench scale experimental models
- (iii)
- Numerical and experimental (unscaled) procedures.
- (iv)
- Scaled experimental models (prototypes).
- (v)
- Experimental, scaled and numerical models [42].
5. Conclusions and Recommendations
- (i)
- More complete mathematical models (2D with axial and radial dispersion) and computation techniques should be used to accurately model the complex physical and chemical phenomena.
- (ii)
- Multicomponent biosorption modeling with respect to batch simulations is directly correlated to the concentration of all the species in the bulk phase in interaction with each specific functional group existing on the heterogeneous substrate and the availability of the interaction sites on the adsorbent. However, very few studies have been reported where the effect of process parameters on sorption efficiency has been studied though sensitivity analysis.
- (iii)
- More experimental and modeling studies on the fixed-bed dynamics columns are needed to accurately predict performance on an industrial scale.
- (iv)
- It is high time that the major focus of biosorption research shifts to pilot-scale studies with real wastewater rather than on laboratory experiments with metal solutions so that the technology can be practically applied. Research on the performance of the adsorbent materials is essential for the design of equipment employing the adsorption process.
- (v)
- In a fixed-bed column, it is essential to characterize three different types of unknown parameters. In the first group are the ones that are experimentally obtained (i.e., mass of adsorbent, porosity). The ones that are determined according to correlations in the literature are considered to be in the second group. This includes parameters such as the atomic weight of cations. The third group is represented by parameters such as dispersivity, which are fitted from the breakthrough intervals.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rani, L.; Kaushal, J.; Srivastav, A.L.; Mahajan, P. A critical review on recent developments in MOF adsorbents for the elimination of toxic heavy metals from aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 44771–44796. [Google Scholar] [CrossRef] [PubMed]
- Hanandeh, E.A.; Mahdi, Z.; Imtiaz, M.S. Modelling of the adsorption of Pb, Cu and Ni ions from single and multi-component aqueous solutions by date seed derived biochar: Comparison of six machine learning approaches. Environ. Res. 2021, 192, 110338. [Google Scholar] [CrossRef] [PubMed]
- Güçoğlu, M.; Şatıroğlu, N. Adsorption of Pb(II), Cu(II), Cd(II), Ni(II), and Co(II) ions by newly synthesized 2-(2′-Hydroxyphenyl)Benzothiazole-functionalized silica. J. Mol. Liq. 2022, 348, 118388. [Google Scholar] [CrossRef]
- Abass, M.R.; El-Kenany, W.M.; El-Masry, E.H. High efficient removal of lead(II) and cadmium(II) ions from multi-component aqueous solutions using polyacrylic acid acrylonitrile talc nanocomposite. Environ. Sci. Pollut. Res. 2022, 1–17. [Google Scholar] [CrossRef]
- Ramos, B.P.; Perez, I.D.; Aliprandini, P. Cu2+, Cr3+, and Ni2+ in mono- and multi-component aqueous solution adsorbed in passion fruit peels in natura and physicochemically modified: A comparative approach. Environ. Sci. Pollut. Res. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mama, C.N.; Nwonu, D.C.; Akanno, C.C.; Chukwuemeka, O.E. Adsorption capacity of composite bio-modified geopolymer for multi-component heavy metal system: Optimisation, equilibrium and kinetics study. Environ. Monit. Assess. 2022, 194, 134. [Google Scholar] [CrossRef]
- Spognardi, S.; Bravo, I.; Beni, C.; Menegoni, P.; Pietrelli, L.; Papetti, P. Arsenic accumulation in edible vegetables and health risk reduction by groundwater treatment using an adsorption process. Environ. Sci. Pollut. Res. 2019, 26, 32505–32516. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Y.; Zhao, X.; Gao, X.; Zheng, Y.; Zuo, H.; Wei, Z. Roles of different humin and heavy-metal resistant bacteria from composting on heavy metal removal. Bioresour. Technol. 2020, 296, 122375. [Google Scholar] [CrossRef]
- Bayo, J.; Esteban, G.; Castillo, J. The use of native and protonated grapefruit biomass (Citrus paradisi L.) for cadmium(II) biosorption: Equilibrium and kinetic modelling. Environ. Technol. 2012, 33, 761–772. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Eletta, O.A.A. Recent advances in the biosorption of pollutants by fish scales: A mini-review. Chem. Eng. Commun. 2020, 208, 1301–1312. [Google Scholar] [CrossRef]
- Naja, G.; Mustin, C.; Volesky, B.; Berthelin, J. Stabilization of the initial electrochemical potential for a metal-based potentiometric titration study of a biosorption process. Chemosphere 2006, 62, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.S.; Basu, A.; Islam, M.R. The removal of As(III) and As(V) from aqueous solutions by waste materials. Bioresour. Technol. 2008, 99, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Basu, A.; Kashyap, V.; Roberts, D.J. Experimental and Numerical Analysis of Biological Regeneration of Perchlorate Laden Ion-Exchange Resins in Batch Reactors. Environ. Eng. Sci. 2010, 27, 75–84. [Google Scholar] [CrossRef]
- Yang, J.; Volesky, B. Cadmium Biosorption Rate in Protonated Sargassum Biomass. Environ. Sci. Technol. 1999, 33, 751–757. [Google Scholar] [CrossRef]
- Bădescu, I.S.; Bulgariu, D.; Ahmad, I.; Bulgariu, L. Valorisation possibilities of exhausted biosorbents loaded with metal ions—A review. J. Environ. Manag. 2018, 224, 288–297. [Google Scholar] [CrossRef]
- Ferreira, R.C.; Dias, D.; Fonseca, I.; Bernardo, M.; Willimann Pimenta, J.L.C.; Lapa, N.; de Barros, M.A.S.D. Multi-component adsorption study by using bone char: Modelling and removal mechanisms. Environ. Technol. 2022, 43, 789–804. [Google Scholar] [CrossRef]
- Abdulaziz, M.; Musayev, S. Multicomponent Biosorption of Heavy Metals from Aqueous Solutions: A Review. Pol. J. Environ. Stud. 2017, 26, 1433–1441. [Google Scholar] [CrossRef] [Green Version]
- Plazinski, W. Equilibrium and kinetic modeling of metal ion biosorption: On the ways of model generalization for the case of multicomponent systems. Adsorption 2013, 19, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Kumar, V.; Datta, S.; Dhanjal, D.S.; Sharma, K.; Samuel, J.; Singh, J. Current advancement and future prospect of biosorbents for bioremediation. Sci. Total Environ. 2020, 709, 135895. [Google Scholar] [CrossRef]
- Girish, C.R. Various isotherm models for multicomponent adsorption: A review. Int. J. Civ. Eng. Technol. 2017, 8, 80–86. [Google Scholar]
- Basu, A.; Rahaman, M.S.; Islam, M.R. Extension of the pore diffusion approach for modelling binary adsorption of lead and arsenic ions in a fixed-bed column packed with atlantic cod fish scales. Can. J. Chem. Eng. 2011, 89, 499–507. [Google Scholar] [CrossRef]
- Manjunath, S.V.; Kumar, M. Evaluation of single-component and multi-component adsorption of metronidazole, phosphate and nitrate on activated carbon from Prosopıs julıflora. Chem. Eng. J. 2018, 346, 525–534. [Google Scholar] [CrossRef]
- Basu, A.; Rahaman, M.S.; Mustafiz, S.; Islam, M.R. Batch studies of lead adsorption from a multi-component aqueous solution onto Atlantic cod fish scale (Gadus morhua) substrate. J. Environ. Eng. Sci. 2007, 6, 455–462. [Google Scholar] [CrossRef]
- Niu, C.H.; Volesky, B.; Cleiman, D. Biosorption of arsenic (V) with acid-washed crab shells. Water Res. 2007, 41, 2473–2478. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Mustafiz, S.; Islam, M.R.; Bjorndalen, N.; Rahaman, M.S.; Chaalal, O. A comprehensive approach for modeling sorption of lead and cobalt ions through fish scales as an adsorbent. Chem. Eng. Commun. 2006, 193, 580–605. [Google Scholar] [CrossRef]
- Schiewer, S.; Volesky, B. Modeling Multi-Metal Ion Exchange in Biosorption. Environ. Sci. Technol. 1996, 30, 2921–2927. [Google Scholar] [CrossRef]
- Assche, T.R.C.V.; Baron, G.; Denayer, J. An explicit multicomponent adsorption isotherm model: Accounting for the size-effect for components with Langmuir adsorption behavior. Adsorption 2018, 24, 517–530. [Google Scholar] [CrossRef]
- Steffen, V.; Silva, E.A.; Evangelista, L.R.; Cardozo-Filho, L. Debye–Hückel approximation for simplification of ions adsorption equilibrium model based on Poisson–Boltzmann equation. Surf. Interfaces 2018, 10, 144–148. [Google Scholar] [CrossRef]
- Patel, H. Fixed-bed column adsorption study: A comprehensive review. Appl. Water Sci. 2019, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Danish, M.; Hashim, R.; Ibrahim, M.N.M.; Rafatullah, M.; Sulaiman, O. Surface characterization and comparative adsorption properties of Cr(VI) on pyrolysed adsorbents of Acacia mangium wood and Phoenix dactylifera L. stone carbon. J. Anal. Appl. Pyrolysis 2012, 97, 19–28. [Google Scholar] [CrossRef]
- Hu, C.; Sedghi, S.; Madani, S.H.; Silvestre-Albero, A.; Sakamoto, H.; Kwong, P.; Pendleton, P.; Smernik, R.J.; Rodríguez-Reinoso, F.; Kaneko, K.; et al. Control of the pore size distribution and its spatial homogeneity in particulate activated carbon. Carbon 2014, 78, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Cunningham, J.A.; Werth, C.J.; Reinhard, M.; Roberts, P.V. Effects of grain-scale mass transfer on the transport of volatile organics through sediments: 1. Model development. Water Resour. Res. 1997, 33, 2713–2726. [Google Scholar] [CrossRef]
- Saadi, R.; Saadi, Z.; Fazaeli, R.; Fard, N.E. Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J. Chem. Eng. 2015, 32, 787–799. [Google Scholar] [CrossRef]
- Nemeş, L.; Bulgariu, L. Optimization of process parameters for heavy metals biosorption onto mustard waste biomass. Open Chem. 2016, 14, 175–187. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Çelebi, O.; Üzüm, Ç.; Shahwan, T.; Erten, H.N. A radiotracer study of the adsorption behavior of aqueous Ba2+ ions on nanoparticles of zero-valent iron. J. Hazard. Mater. 2007, 148, 761–767. [Google Scholar] [CrossRef] [Green Version]
- Vijayaraghavan, K.; Padmesh, T.V.N.; Palanivelu, K.; Velan, M. Biosorption of nickel(II) ions onto Sargassum wightii: Application of two-parameter and three-parameter isotherm models. J. Hazard. Mater. 2006, 133, 304–308. [Google Scholar] [CrossRef]
- Brouers, F.; Al-Musawi, T.J. On the optimal use of isotherm models for the characterization of biosorption of lead onto algae. J. Mol. Liq. 2015, 212, 46–51. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Anu, N.; Giri Nandagopal, M.S.; Selvaraju, N. Relevance of isotherm models in biosorption of pollutants by agricultural byproducts. J. Environ. Chem. Eng. 2014, 2, 398–414. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Basu, A.; Islam, M.R. Scaling Up of Chemical Injection Experiments. Pet. Sci. Technol. 2009, 27, 654–665. [Google Scholar] [CrossRef]
- Faust, S.D.; Aly, O.M. Adsorption Models. In Adsorption Processes for Water Treatment; Faust, S.D., Aly, O.M., Eds.; Butterworth-Heinemann: Oxford, UK, 1987; pp. 25–64. [Google Scholar] [CrossRef]
- Li, H.; Yang, C. Nitrite Removal Using Ion Exchange Resin: Batch vs. Fixed Bed Performance. Sep. Sci. Technol. 2015, 50, 1721–1730. [Google Scholar] [CrossRef]
- Ng, J.C.Y.; Cheung, W.H.; McKay, G. Equilibrium studies for the sorption of lead from effluents using chitosan. Chemosphere 2003, 52, 1021–1030. [Google Scholar] [CrossRef]
- Nouri, L.; Ghodbane, I.; Hamdaoui, O.; Chiha, M. Batch sorption dynamics and equilibrium for the removal of cadmium ions from aqueous phase using wheat bran. J. Hazard. Mater. 2007, 149, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Ali Redha, A. Removal of heavy metals from aqueous media by biosorption. Arab. J. Basic Appl. Sci. 2020, 27, 183–193. [Google Scholar] [CrossRef]
- Kaushal, A.; Singh, S. Adsorption phenomenon and its application in removal of lead from waste water: A review. Int. J. Hydrol. 2017, 1, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Ponnusami, V.; Rajan, K.S.; Srivastava, S.N. Application of film-pore diffusion model for methylene blue adsorption onto plant leaf powders. Chem. Eng. J. 2010, 163, 236–242. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Saito, A.; Foley, H.C. Curvature and parametric sensitivity in models for adsorption in micropores. AIChE J. 1991, 37, 429–436. [Google Scholar] [CrossRef]
- Baig, K.S.; Doan, H.D.; Wu, J. Multicomponent isotherms for biosorption of Ni2+ and Zn2+. Desalination 2009, 249, 429–439. [Google Scholar] [CrossRef]
- Dissanayake, D.M.R.E.A.; Wijesinghe, W.M.K.E.H.; Iqbal, S.S.; Priyantha, N.; Iqbal, M.C.M. Isotherm and kinetic study on Ni(II) and Pb(II) biosorption by the fern Asplenium nidus L. Ecol. Eng. 2016, 88, 237–241. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Grassi, M.; Gudiño, E.; de Oliveira, P. A New Look To Non-Fickian Diffusion. Appl. Math. Model. 2015, 39, 194–204. [Google Scholar] [CrossRef]
- Abdel-Ghani, N.T.; Hegazy, A.K.; El-Chaghaby, G.A. Typha domingensis leaf powder for decontamination of aluminium, iron, zinc and lead: Biosorption kinetics and equilibrium modeling. Int. J. Environ. Sci. Technol. 2009, 6, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Sari, A.A.; Amriani, F.; Muryanto, M.; Triwulandari, E.; Sudiyani, Y.; Barlianti, V.; Narrij Lotulung, P.D.; Hadibarata, T. Mechanism, adsorption kinetics and applications of carbonaceous adsorbents derived from black liquor sludge. J. Taiwan Inst. Chem. Eng. 2017, 77, 236–243. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Hanafiah, M.A.K.M. Biosorption of copper ions from dilute aqueous solutions on base treatedrubber (Hevea brasiliensis) leaves powder: Kinetics, isotherm, and biosorption mechanisms. J. Environ. Sci. 2008, 20, 1168–1176. [Google Scholar] [CrossRef]
- Vishan, I.; Saha, B.; Sivaprakasam, S.; Kalamdhad, A. Evaluation of Cd(II) biosorption in aqueous solution by using lyophilized biomass of novel bacterial strain Bacillus badius AK: Biosorption kinetics, thermodynamics and mechanism. Environ. Technol. Innov. 2019, 14, 100323. [Google Scholar] [CrossRef]
- Du, J.; Sun, P.; Feng, Z.; Zhang, X.; Zhao, Y. The biosorption capacity of biochar for 4-bromodiphengl ether: Study of its kinetics, mechanism, and use as a carrier for immobilized bacteria. Environ. Sci. Pollut. Res. 2016, 23, 3770–3780. [Google Scholar] [CrossRef]
- Lu, N.; Hu, T.; Zhai, Y.; Qin, H.; Aliyeva, J.; Zhang, H. Fungal cell with artificial metal container for heavy metals biosorption: Equilibrium, kinetics study and mechanisms analysis. Environ. Res. 2020, 182, 109061. [Google Scholar] [CrossRef]
- Ali, M.H.; Hussian, A.M.; Abdel-Satar, A.M.; Goher, M.E.; Napiórkowska-Krzebietke, A.; El-Monem, A.M.A. The isotherm and kinetic studies of the biosorption of heavy metals by non-living cells of Chlorella vulgaris. J. Elem. 2016, 21, 1263–1276. [Google Scholar] [CrossRef]
- Cui, H.; Yan, C.; Jia, P.; Cao, W. Adsorption and sensing behaviors of SF6 decomposed species on Ni-doped C3N monolayer: A first-principles study. Appl. Surf. Sci. 2020, 512, 145759. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P. Single- and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse—an agricultural waste. Water Res. 2002, 36, 2304–2318. [Google Scholar] [CrossRef]
- Torrik, E.; Soleimani, M.; Ravanchi, M.T. Application of Kinetic Models for Heavy Metal Adsorption in the Single and Multicomponent Adsorption System. Int. J. Environ. Res. 2019, 13, 813–828. [Google Scholar] [CrossRef]
- Radke, C.J.; Prausnitz, J.M. Thermodynamics of multi-solute adsorption from dilute liquid solutions. AIChE J. 1972, 18, 761–768. [Google Scholar] [CrossRef]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Jadhav, A.J.; Srivastava, V.C. Multicomponent adsorption isotherm modeling using thermodynamically inconsistent and consistent models. AIChE J. 2019, 65, e16727. [Google Scholar] [CrossRef]
- Jadhav, A.J.; Srivastava, V.C. Adsorbed solution theory based modeling of binary adsorption of nitrobenzene, aniline and phenol onto granulated activated carbon. Chem. Eng. J. 2013, 229, 450–459. [Google Scholar] [CrossRef]
- Noroozi, B.; Sorial, G.A. Applicable models for multi-component adsorption of dyes: A review. J. Environ. Sci. 2013, 25, 419–429. [Google Scholar] [CrossRef]
- Xu, Z.; Cai, J.-G.; Pan, B.-C. Mathematically modeling fixed-bed adsorption in aqueous systems. J. Zhejiang Univ. Sci. A 2013, 14, 155–176. [Google Scholar] [CrossRef] [Green Version]
- Karimi, S.; Tavakkoli Yaraki, M.; Karri, R.R. A comprehensive review of the adsorption mechanisms and factors influencing the adsorption process from the perspective of bioethanol dehydration. Renew. Sustain. Energy Rev. 2019, 107, 535–553. [Google Scholar] [CrossRef]
- Lee, S.M.; Davis, A.P. Removal of cu(II) and cd(II) from aqueous solution by seafood processing waste sludge. Water Res. 2001, 35, 534–540. [Google Scholar] [CrossRef]
- Chen, Q.; Tian, Y.; Li, P.; Yan, C.; Pang, Y.; Zheng, L.; Deng, H.; Zhou, W.; Meng, X. Study on Shale Adsorption Equation Based on Monolayer Adsorption, Multilayer Adsorption, and Capillary Condensation. J. Chem. 2017, 2017, 1496463. [Google Scholar] [CrossRef] [Green Version]
- Mustafiz, S.; Basu, A.; Islam, M.R.; Dewaidar, A.; Chaalal, O. A Novel Method for Heavy Metal Removal. Energy Sources 2002, 24, 1043–1051. [Google Scholar] [CrossRef]
- Ali, M.E.; Hoque, M.E.; Safdar Hossain, S.K.; Biswas, M.C. Nanoadsorbents for wastewater treatment: Next generation biotechnological solution. Int. J. Environ. Sci. Technol. 2020, 17, 4095–4132. [Google Scholar] [CrossRef]
- Esalah, J.O.; Weber, M.E.; Vera, J.H. Removal of lead, cadmium and zinc from aqueous solutions by precipitation with sodium Di-(n-octyl) phosphinate. Can. J. Chem. Eng. 2000, 78, 948–954. [Google Scholar] [CrossRef]
- Hayes, K.F.; Leckie, J.O. Modeling ionic strength effects on cation adsorption at hydrous oxide/solution interfaces. J. Colloid Interface Sci. 1987, 115, 564–572. [Google Scholar] [CrossRef]
- Schiewer, S. Modelling complexation and electrostatic attraction in heavy metal biosorption by Sargassum biomass. J. Appl. Phycol. 1999, 11, 79–87. [Google Scholar] [CrossRef]
- Yun, H.J.; Choi, Y.W.; Kim, N.J.; Sohn, D.W. Physicochemical properties of phosphatidylcholine (PC) monolayers with different alkyl chains, at the air/water interface. Bull. Korean Chem. Soc. 2003, 24, 377–383. [Google Scholar]
- Hajahmadi, Z.; Younesi, H.; Bahramifar, N.; Khakpour, H.; Pirzadeh, K. Multicomponent isotherm for biosorption of Zn(II), CO(II) and Cd(II) from ternary mixture onto pretreated dried Aspergillus niger biomass. Water Resour. Ind. 2015, 11, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon: Part I. Two-parameter models and equations allowing determination of thermodynamic parameters. J. Hazard. Mater. 2007, 147, 381–394. [Google Scholar] [CrossRef]
- Walther, H.-J.; Buffle, J. Complexation Reactions in Aquatic Systems; Analytical Approach. Acta Hydrochim. Hydrobiol. 1989, 17, 230. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Unuabonah, E.I.; Oladoja, N.A. Competitive modeling for the biosorptive removal of copper and lead ions from aqueous solution by Mansonia wood sawdust. Bioresour. Technol. 2010, 101, 3844–3852. [Google Scholar] [CrossRef] [PubMed]
- McBride, M.B.; McBRIDE, M.B.A.; McBride, P.D.S.C.M.B. Environmental Chemistry of Soils; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Hikmat, N.A.; Qassim, B.B.; Khethi, M.T. Thermodynamic and Kinetic Studies of Lead Adsorption from Aquesous Solution onto Petiole and Fiber of Palm Tree. Am. J. Chem. 2014, 4, 116–124. [Google Scholar] [CrossRef]
- Jena, P.R.; Basu, J.K.; De, S. A generalized shrinking core model for multicomponent batch adsorption processes. Chem. Eng. J. 2004, 102, 267–275. [Google Scholar] [CrossRef]
- Nithya, K.; Sathish, A.; Kumar, P.S. Packed bed column optimization and modeling studies for removal of chromium ions using chemically modified Lantana camara adsorbent. J. Water Process Eng. 2020, 33, 101069. [Google Scholar] [CrossRef]
- Milot, C.; McBrien, J.; Allen, S.; Guibal, E. Influence of physicochemical and structural characteristics of chitosan flakes on molybdate sorption. J. Appl. Polym. Sci. 1998, 68, 571–580. [Google Scholar] [CrossRef]
- Kulkarni, R.M.; Vidya Shetty, K.; Srinikethan, G. Kinetic and equilibrium modeling of biosorption of nickel (II) and cadmium (II) on brewery sludge. Water Sci. Technol. 2019, 79, 888–894. [Google Scholar] [CrossRef]
- Jeppu, G.P.; Clement, T.P. A modified Langmuir-Freundlich isotherm model for simulating pH-dependent adsorption effects. J. Contam. Hydrol. 2012, 129–130, 46–53. [Google Scholar] [CrossRef]
- Nordstrand, J.; Dutta, J. Dynamic Langmuir Model: A Simpler Approach to Modeling Capacitive Deionization. J. Phys. Chem. C 2019, 123, 16479–16485. [Google Scholar] [CrossRef] [Green Version]
- Xiao, B.; Thomas, K.M. Competitive Adsorption of Aqueous Metal Ions on an Oxidized Nanoporous Activated Carbon. Langmuir 2004, 20, 4566–4578. [Google Scholar] [CrossRef]
- Keller, J.U.; Popernack, J.D.; Staudt, R. A generalization of langmuir′s adsorption isotherm to admolecules with interaction. In Adsorption Science and Technology; Hindawi: Brisbane, Australia, 2000; pp. 336–340. [Google Scholar] [CrossRef]
- Wu, F.-C.; Liu, B.-L.; Wu, K.-T.; Tseng, R.-L. A new linear form analysis of Redlich–Peterson isotherm equation for the adsorptions of dyes. Chem. Eng. J. 2010, 162, 21–27. [Google Scholar] [CrossRef]
- Kumar, K.V.; Monteiro de Castro, M.; Martinez-Escandell, M.; Molina-Sabio, M.; Rodriguez-Reinoso, F. A site energy distribution function from Toth isotherm for adsorption of gases on heterogeneous surfaces. Phys. Chem. Chem. Phys. 2011, 13, 5753–5759. [Google Scholar] [CrossRef] [PubMed]
- Selim, H.M. Chapter Five—Transport and Retention of Heavy Metal in Soils: Competitive Sorption. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 119, pp. 275–308. [Google Scholar]
- Islam, M.A.; Chowdhury, M.A.; Mozumder, M.S.I.; Uddin, M.T. Langmuir Adsorption Kinetics in Liquid Media: Interface Reaction Model. ACS Omega 2021, 6, 14481–14492. [Google Scholar] [CrossRef] [PubMed]
- Ucarli, O.; Yayintas, O.T.; Engin, M.S.; Cay, S.; Saglikoglu, G.; Yilmaz, S. Investigation of Competitive and Noncompetitive Adsorption of Some Heavy Metals Ions on Leucodon sciuroides (Hedw.) Schwägr. Langmuir 2020, 36, 8265–8271. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Azaiez, J.; Hill, J.M. Erroneous Application of Pseudo-Second-Order Adsorption Kinetics Model: Ignored Assumptions and Spurious Correlations. Ind. Eng. Chem. Res. 2018, 57, 2705–2709. [Google Scholar] [CrossRef]
- Kumar, D.; Pandey, L.K.; Gaur, J.P. Metal sorption by algal biomass: From batch to continuous system. Algal Res. 2016, 18, 95–109. [Google Scholar] [CrossRef]
- Yao, C.; Chen, T. A film-diffusion-based adsorption kinetic equation and its application. Chem. Eng. Res. Des. 2017, 119, 87–92. [Google Scholar] [CrossRef]
- Song, F.Y.; Islam, M.R. Effect of salinity and rock type on sorption behavior of surfactants as applied in cleaning of petroleum contaminants. J. Pet. Sci. Eng. 1994, 10, 321–336. [Google Scholar] [CrossRef]
- Sarwar, M.; Islam, M.R. A non-fickian surface excess model for chemical transport through fractured porous media. Chem. Eng. Commun. 1997, 160, 1–34. [Google Scholar] [CrossRef]
- Rahman, M.H.; Wasiuddin, N.M.; Islam, M.R. Experimental and Numerical Modeling Studies of Arsenic Removal with Wood Ash from Aqueous Streams. Can. J. Chem. Eng. 2004, 82, 968–977. [Google Scholar] [CrossRef]
- Ghorbani, F.; Younesi, H.; Ghasempouri, S.M.; Zinatizadeh, A.A.; Amini, M.; Daneshi, A. Application of response surface methodology for optimization of cadmium biosorption in an aqueous solution by Saccharomyces cerevisiae. Chem. Eng. J. 2008, 145, 267–275. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Alomá, I.; Martín-Lara, M.A.; Rodríguez, I.L.; Blázquez, G.; Calero, M. Removal of nickel (II) ions from aqueous solutions by biosorption on sugarcane bagasse. J. Taiwan Inst. Chem. Eng. 2012, 43, 275–281. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Wan Daud, W.M.A.; Shamiri, A. A review of mathematical modeling of fixed-bed columns for carbon dioxide adsorption. Chem. Eng. Res. Des. 2014, 92, 961–988. [Google Scholar] [CrossRef]
- Yüksel, Ş.; Orhan, R. The Removal of Cr(VI) from Aqueous Solution by Activated Carbon Prepared from Apricot, Peach Stone and Almond Shell Mixture in a Fixed-Bed Column. Arab. J. Sci. Eng. 2019, 44, 5345–5357. [Google Scholar] [CrossRef]
- Suzaki, P.Y.R.; Munaro, M.T.; Triques, C.C.; Kleinübing, S.J.; Fagundes Klen, M.R.; Bergamasco, R.; de Matos Jorge, L.M. Phenomenological mathematical modeling of heavy metal biosorption in fixed-bed columns. Chem. Eng. J. 2017, 326, 389–400. [Google Scholar] [CrossRef]
- Amiri, M.J.; Khozaei, M.; Gil, A. Modification of the Thomas model for predicting unsymmetrical breakthrough curves using an adaptive neural-based fuzzy inference system. J. Water Health 2019, 17, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-G.; Kim, J.-H.; Kang, J.-K.; Kim, S.-B.; Park, S.-J.; Lee, S.-H.; Choi, J.-W. Comparative analysis of fixed-bed sorption models using phosphate breakthrough curves in slag filter media. Desalination Water Treat. 2015, 55, 1795–1805. [Google Scholar] [CrossRef]
- Biswas, S.; Mishra, U. Continuous Fixed-Bed Column Study and Adsorption Modeling: Removal of Lead Ion from Aqueous Solution by Charcoal Originated from Chemical Carbonization of Rubber Wood Sawdust. J. Chem. 2015, 2015, 907379. [Google Scholar] [CrossRef] [Green Version]
- Steiakakis, E.; Gamvroudis, C.; Alevizos, G. Kozeny-Carman Equation and Hydraulic Conductivity of Compacted Clayey Soils. Geomaterials 2012, 2, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Saadat, S.; Hekmatzadeh, A.A.; Karimi Jashni, A. Mathematical modeling of the Ni(II) removal from aqueous solutions onto pre-treated rice husk in fixed-bed columns: A comparison. Desalination Water Treat. 2016, 57, 16907–16918. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Omi, F.R.; Basu, A. Experimental and numerical modelling of arsenic adsorption in fixed-bed dynamic columns packed with atlantic cod fish scales. Can. J. Chem. Eng. 2015, 93, 2024–2030. [Google Scholar] [CrossRef]
- Coskuner, G.; Bentsen, R.G. Prediction of Instability for Miscible Displacements in a Hele-Shaw Cell. Oil Gas Sci. Technol.—Rev. IFP 1987, 42, 151–162. [Google Scholar] [CrossRef]
- Aksu, Z.; Çağatay, Ş.Ş.; Gönen, F. Continuous fixed bed biosorption of reactive dyes by dried Rhizopus arrhizus: Determination of column capacity. J. Hazard. Mater. 2007, 143, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Nwabanne, J.T.; Igbowe, P.K. Adsorption Performance of Packed Bed Column for the removal of Lead (ii) using oil Palm Fibre. Int. J. Appl. Sci. Technol. 2012, 2, 106–115. [Google Scholar]
- Dawood, S.; Sen, T.K.; Phan, C. Performance and dynamic modelling of biochar and kaolin packed bed adsorption column for aqueous phase methylene blue (MB) dye removal. Environ. Technol. 2019, 40, 3762–3772. [Google Scholar] [CrossRef]
- Charola, S.; Yadav, R.; Das, P.; Maiti, S. Fixed-bed adsorption of Reactive Orange 84 dye onto activated carbon prepared from empty cotton flower agro-waste. Sustain. Environ. Res. 2018, 28, 298–308. [Google Scholar] [CrossRef]
- Lawson, S.; Adebayo, B.; Robinson, C.; Al-Naddaf, Q.; Rownaghi, A.A.; Rezaei, F. The effects of cell density and intrinsic porosity on structural properties and adsorption kinetics in 3D-printed zeolite monoliths. Chem. Eng. Sci. 2020, 218, 115564. [Google Scholar] [CrossRef]
- Volesky, B. Biosorption process simulation tools. Hydrometallurgy 2003, 71, 179–190. [Google Scholar] [CrossRef]
- Ali, M.A.; Islam, M.R. The Effect of Asphaltene Precipitation on Carbonate-Rock Permeability: An Experimental and Numerical Approach. SPE Prod. Facil. 1998, 13, 178–183. [Google Scholar] [CrossRef]
- Ali, S.S.; Asif, M. Fluidization of nano-powders: Effect of flow pulsation. Powder Technol. 2012, 225, 86–92. [Google Scholar] [CrossRef]
- Wakao, N. A Proposal of Calculational Model for Pressure Swing Adsorption Based on the Pore Diffusion. J. Chem. Eng. Jpn. 2001, 34, 1443–1448. [Google Scholar] [CrossRef]
- Biesova, Z.; Miller, M.A.; Schneerson, R.; Shiloach, J.; Green, K.Y.; Robbins, J.B.; Keith, J.M. Preparation, characterization, and immunogenicity in mice of a recombinant influenza H5 hemagglutinin vaccine against the avian H5N1 A/Vietnam/1203/2004 influenza virus. Vaccine 2009, 27, 6234–6238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
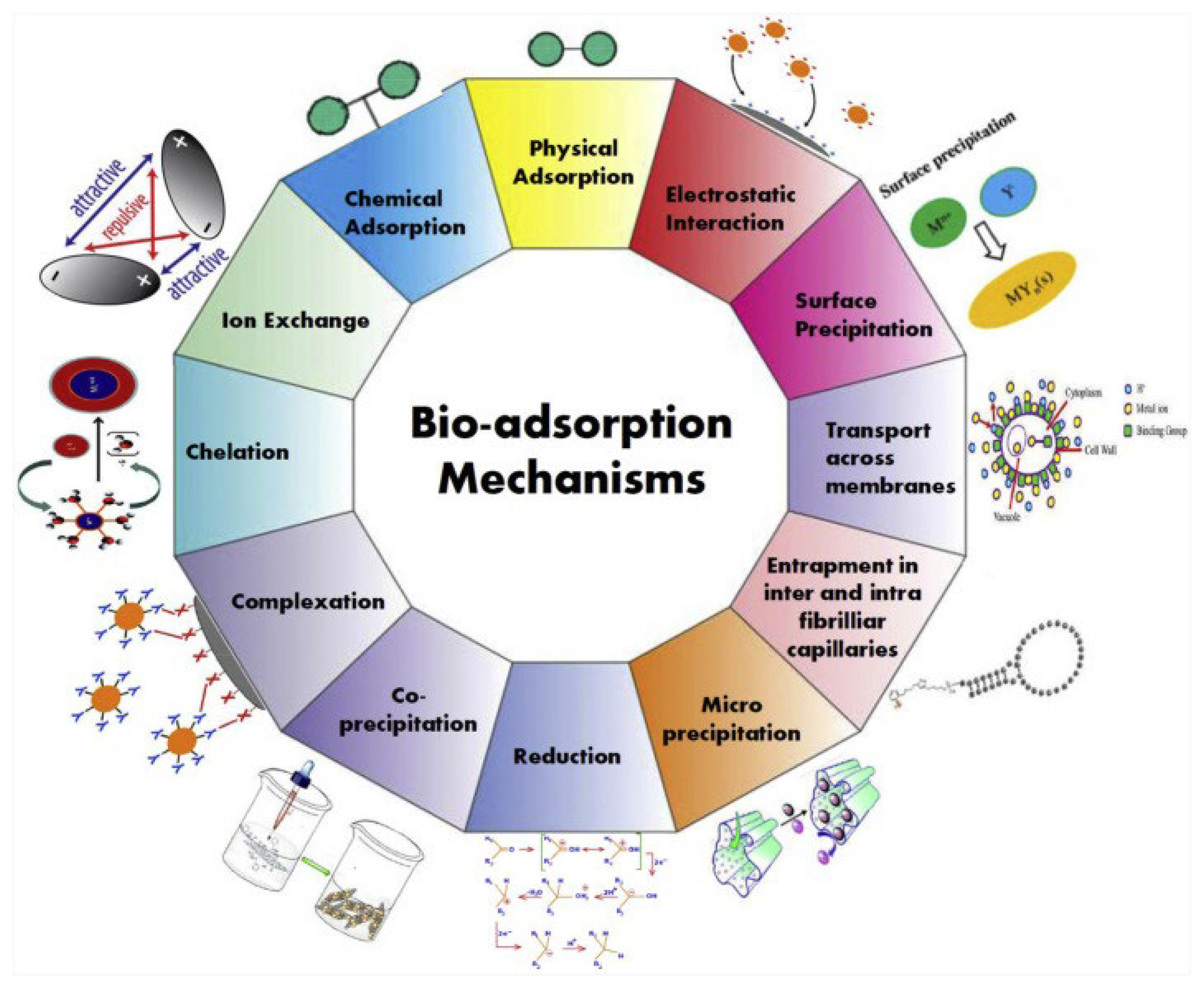

| Isotherms | Equation | Applications | Parameters Involved |
|---|---|---|---|
| Langmuir [44] | Homogeneous substrate. | 2 | |
| Freundlich [44] | Heterogeneous substrates. | 2 | |
| Hill–Debboers [36] | Mobile adsorption. Interaction of adsorbed species. | 2 | |
| Temkim [36] | Indirect adsorbate/adsorbate interaction. | 2 | |
| Sipps [36] | Heterogeneous substrates with high adsorbate concentration. | 3 | |
| Redlich–Peterson [36] | Nonideal monolayer adsorption. | 3 | |
| Kahn [36] | Bioadsorption from pure dilute solutions. | 3 | |
| Langmuir–Freundlich [36] | Applicable to distribution of adsorption energy on heterogeneous substrates. | 3 | |
| Dubinin–Radushkevich [36] | Non-isothermal conditions. | 3 |
| Applied Reaction Kinetics Model | Biosorbent | Reference |
|---|---|---|
| Pseudo-second-order | Typha domingensis leaf powder | Ghani et al., 2009 [55] |
| Pseudo-second-order | Carbonaceous adsorbent from black liquor sludge | Sari et al., 2017 [56] |
| Pseudo-second-order | Base-treated ber (Hevea brasiliensis) | Ngah and Hanafiah, 2008 [57] |
| Pseudo-second-order | Bacillus Badius AK | Vishan et al., 2019 [58] |
| Pseudo-first-order | Biochar | Du et al., 2016 [59] |
| Pseudo-first- and second-order | Fungal Cell | Lu et al., 2020 [60] |
| Model | Expression | Reference |
|---|---|---|
| Multicomponent Langmuir Isotherm | [70] | |
| [71] | ||
| Langmuir kinetic with competition between n species | [71] | |
| Multicomponent Langmuir–Freundlich | [71] |
| MODELS | Equation | Parameters Involved |
|---|---|---|
| Linearized Thomas model [111] | : Adsorption constant
: Adsorption capacity m: mass of adsorbent | |
| Adams–Bohart model [112] | : reaction rate constant (mL/mg.min)
: solubility (mg/L) z: bed height (cm) : flowrate (cm/min) | |
| Yoon–Nelson model [113] | qB = Qv(Co − Cb/2)tB/madsorbent | Qv: flowrate (L min−1) Co: influent metal ion concentration Cb: breakthrough metal ion concentration qB breakthrough adsorption capacity (mg/g) |
| Carman equation [114] |
a
: mass specific transfer area (cm−1)
: Reynold’s number of particle : packed bed pressure drop (Pa) : wall effect factor (−/−) f: friction parameter Q1: flowrate (L min−1) A1: outlet cross-sectional area h: bed height (cm) ε: porosity of bed µ: kinematic viscosity (cm2/min) | |
| Fate contaminant model [115] | C—ion concentration in the column void; u—pore velocity, cm s−1; D—axial dispersion, cm s−2; ρb—bulk density, g cm−3. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basu, A.; Ali, S.S.; Hossain, S.S.; Asif, M. A Review of the Dynamic Mathematical Modeling of Heavy Metal Removal with the Biosorption Process. Processes 2022, 10, 1154. https://doi.org/10.3390/pr10061154
Basu A, Ali SS, Hossain SS, Asif M. A Review of the Dynamic Mathematical Modeling of Heavy Metal Removal with the Biosorption Process. Processes. 2022; 10(6):1154. https://doi.org/10.3390/pr10061154
Chicago/Turabian StyleBasu, Avijit, Syed Sadiq Ali, SK Safdar Hossain, and Mohammad Asif. 2022. "A Review of the Dynamic Mathematical Modeling of Heavy Metal Removal with the Biosorption Process" Processes 10, no. 6: 1154. https://doi.org/10.3390/pr10061154
APA StyleBasu, A., Ali, S. S., Hossain, S. S., & Asif, M. (2022). A Review of the Dynamic Mathematical Modeling of Heavy Metal Removal with the Biosorption Process. Processes, 10(6), 1154. https://doi.org/10.3390/pr10061154








