1. Introduction
At present, energy scarcity and environmental degradation are common problems faced by all countries [
1,
2,
3]. PCMs have good market application prospects due to certain advantages such as high heat storage density, low price, and stable phase transition temperature [
4]. Energy-saving technology with phase change heat storage as the core has become a current research hotspot. Phase change heat storage technology can be used in aerospace, construction, solar energy, refrigeration equipment industries [
5,
6,
7,
8,
9,
10].
Organic PCM and inorganic PCM are the two types of PCM available. Inorganic PCM has the characteristics of large phase transition temperature range, large enthalpy value, and unstable energy output, while organic PCM has the characteristics of low heat storage density, no corrosion, and no supercooling [
11]. The phase transition enthalpy of inorganic salts is large, and the phase transition temperature is stable, but the degree of supercooling during the phase transition is large, which is corrosive to the storage material and phase separation occurs with the increase of the number of cycles [
12].
There are several methods to solve the supercooling of PCM, such as nucleating agent [
13,
14,
15], ultrasonic vibration [
16,
17,
18,
19], and mechanical vibration [
20]. Adding nucleating agents and thickening agents to phase change materials is a widely and leading method adopted by researchers to reduce the degree of supercooling and phase separation [
21,
22,
23,
24,
25]. Cui et al. [
26] prepared a new type of CPCM through synergistic action using nano-SiO
2 as a nucleating agent, silicon carbide foam as a thermal conductivity enhancer, and carbon nanotubes (CNT) as photothermal enhancers. The results showed that 2 wt.% nano-SiO
2 can reduce the supercooling to about 1.4 °C and using silicon carbide foam can increase the thermal conductivity from 0.64 W/(m·K) to 1.54 W/(m·K). Sun et al. [
27] prepared CPCM composed of sodium acetate, stearic acid, and octadecanol by the melt blending method. On this basis, DSP was used as a nucleating agent and sodium carboxymethyl cellulose (CMC) was used as a thickening agent to improve the supercooling and phase separation of CPCM. The results showed that when DSP is used as a nucleating agent, the supercooling of CPCM can be reduced to less than 2 °C; when sodium carboxymethyl cellulose (CMC) is used as a thickening agent, the phase separation problem of CPCM can be well improved. However, there are still few studies on the simultaneous induction of supercooled salt hydrate crystallization by nucleating agents and mechanical vibration. Aiming at the problem of phase separation of phase change materials, the method of adding thickener is usually adopted. A certain proportion of sodium carboxymethyl cellulose can well reduce the separation of hydrated anhydrous salts during the phase change cycle [
28,
29]. Given the low thermal conductivity of phase change materials, researchers usually add expanded graphite [
30], graphite powder [
31] and graphene nanosheets [
32], and other good thermal conductivity material to improve the thermal conductivity of sodium acetate trihydrate.
The weaknesses of the existing literature are as follows: 1. Most researchers only use nucleating agents or external induction methods to improve supercooling, and do not use their synergistic methods, so the effect of improving supercooling is insufficient. 2. To solve the phase separation, most researchers have adopted the method of adding thickeners. Adding thickeners can solve the phase separation in a short time, but there is no literature on the phase separation problem of PCM that is in the molten state for a long time. 3. Many scholars believe that the greater the amount of thermal conductivity enhancer, the greater the increase in thermal conductivity, without considering the structural supercooling phenomenon caused by the content of thermal conductivity enhancer exceeding a certain percentage. Combining the deficiencies of the existing literature, we adopted the experiments of the synergistic effect of disodium hydrogen phosphate dodecahydrate (DSP), expanded graphite (EG), and physical perturbation.
We employed rotor agitation as a physical perturbation input source to improve the subcooling degree and phase separation of SAT in this work, and we comprehensively investigated the effects of stirring, DSP, CMC, and EG on SAT performance. Finally, we analyze the corrosion of SAT to common metals in this environment, providing a reference for the application of SAT in TES.
2. Materials and Experimental Equipment
Figure 1 shows the preparation process of CPCM, which is prepared by the synergy of DSP, EG, and physical disturbance with SAT as the matrix.
SAT, DSP, and CMC (analytical reagent grade, purity > 99%) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). EG foams with a pore size of 50 um and porosity of 0.998 were offered by Ze Sheng Technology Ltd. (Anhui, China).
The experimental apparatus consists of a synchronous thermal analyzer (DSC, 214 Polymer, Netzsch, Germany), scanning electron microscope (SEM, Hitachi S4800, Hitachi Inc., Tokyo, Japan), X-ray powder diffractometer (XRD, PW3040/60, PANalytical B.V., Almelo, Holland), thermal conductivity tester (TPS2500S, Hot Disk Inc., Uppsala, Sweden), data acquisition instrument (Agilgent34970A, Agilent Technologies Inc., Palo Alto, CA, USA), electronic balance (BSA124S, precision ± 0.1 mg, Sartorius Inc., Gottingen, Germany), computer (Dell, Austin, TX, USA), several beakers, test tubes, and Heraeus film resistor (PT100, M222A, precision ± 0.1 °C, Heraeus Inc., Hanau, Germany).
3. Experimental
3.1. Research on Subcooling and Phase Separation
3.1.1. Research on Reducing Subcooling
Subcooling refers to the phenomenon that the phase change material has not yet begun to solidify when the temperature is lower than the melting point, and the sub-cooling degree refers to the temperature difference between the two temperature inflection points on the step cooling curve [
4]. Many PCMs have supercooling properties, which is the driving force of PCM crystallization. The degree of undercooling is small and the solidification time is long. The grains have enough growth time to achieve the purpose of longer heat storage time [
16].
The solution to overcooling is divided into passive nucleation and active nucleation. Passive nucleation is also divided into two types, heterogeneous nucleation, and homogeneous nucleation. Adding phase change material itself is called homogeneous nucleation. The addition of other nucleating agents is called heterogeneous nucleation [
29]. No matter which method is used, the ultimate goal is to provide nucleation sites to achieve the effect of enhanced nucleation. Active nucleation is used to induce crystallization by applying an external force, such as ultrasonic vibration, electric vibration, stirring, shock wave, etc. [
33]. Mao, Jinfeng et al. used the heterogeneous nucleation method to stabilize the SAT subcooling at about 4 °C, and the exothermic time can also be maintained at about 20 min [
30]. Zhou, Guobing et al. found that the greater the external shock momentum of active nucleation, the shorter the induced crystallization time [
34].
The first experiment was to put 20 g SAT into a 100 mL beaker and seal it, labeled as SAT sample. The second one was to add different proportions (0.5–2 wt.%) of DSP nucleating agent to 20 g SAT to prepare composite phase change materials (CPCM) with different loadings. According to the amount of DSP, they were marked as SAT/0.5 wt.% DSP, SAT/1 wt.% DSP, SAT/1.5 wt.% DSP, and SAT/2 wt.% DSP, etc. the third experiment was to put a rotor with a mass of 2.753 g in 20 g SAT and seal the beaker, then placed it on a magnetic stirrer with a speed of 750 r/min, labeled as SAT/Stir sample; The above-classified samples were passed through multiple cold and heat cycle experiments to observe the supercooling and heat storage conditions.
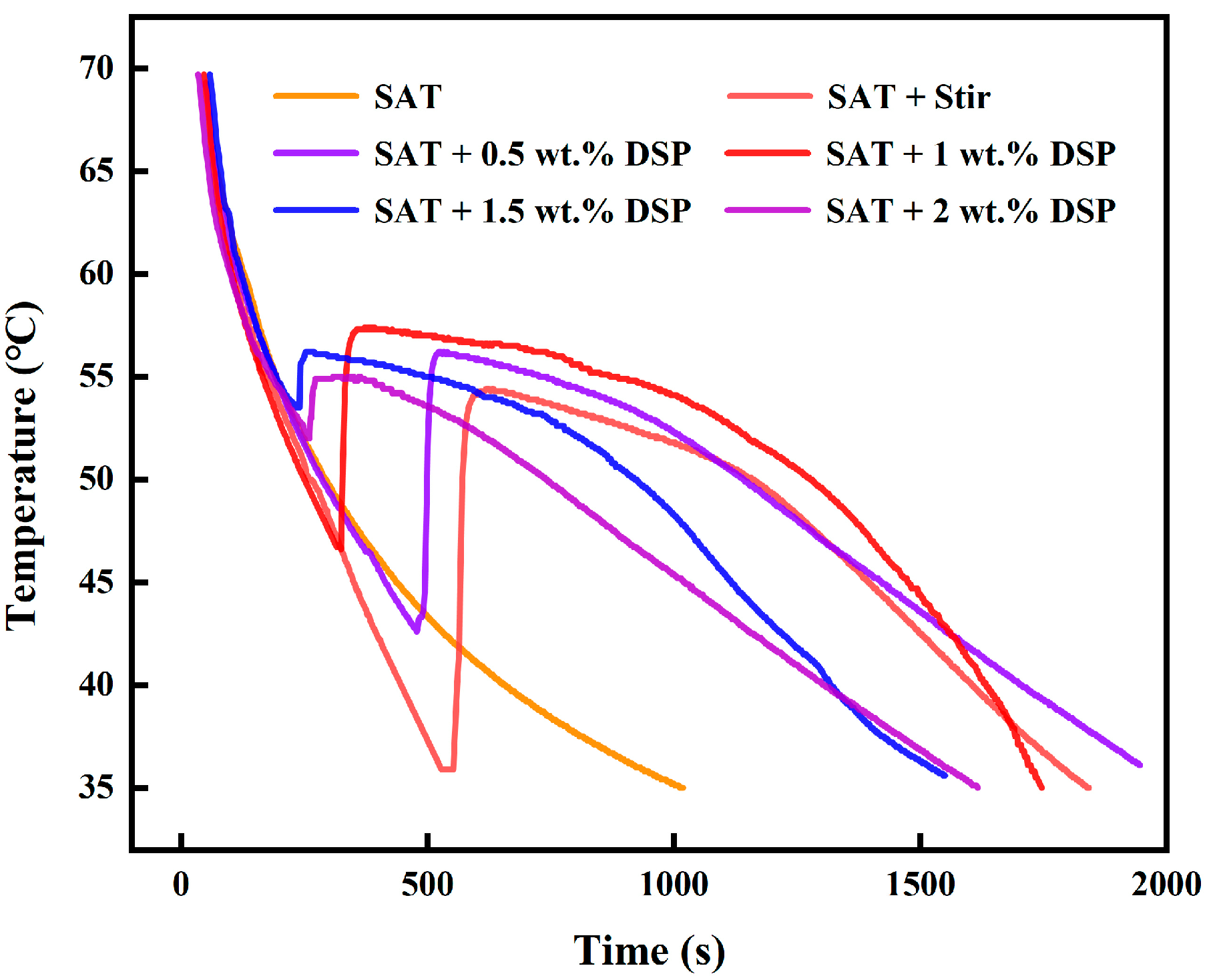
It can be seen from the supercooling curve in
Figure 2 that the supercooling of pure SAT was greater than 30 °C, and there was no crystallization heat release even if it was cooled to room temperature. Using agitated SAT, when the temperature dropped to 35 °C, the exothermic crystallization began, and the degree of subcooling was about 20 °C, which could not solve the problem of overcooling, and the phase change heat storage time was also very short. The addition of 1.5 wt.% DSP nucleating agent in SAT can reduce the degree of subcooling to 3 °C, but the degree of subcooling would increase when the amount of DSP exceeds 1.5 wt.%. Therefore, the optimum addition amount of DSP nucleating agent is 1.5 wt.%. Adding only stirring or DSP nucleating agents can reduce the degree of subcooling, but it is difficult to reduce the degree of subcooling below 1 °C. The phase change heat storage time is very short; almost no phase change heat storage platform is observed.
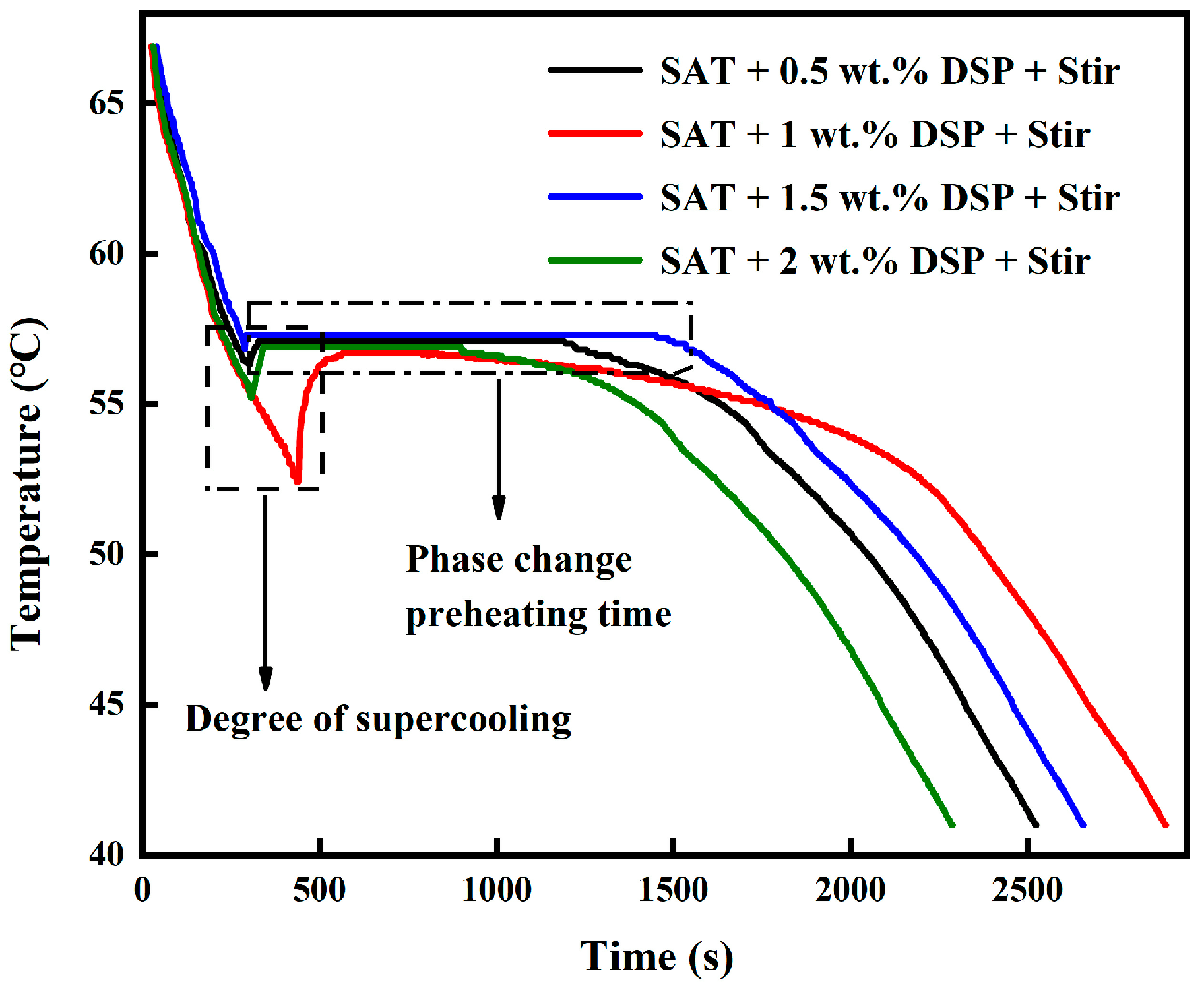
The second and the third methods were combined to prepare a set of experiments. The data was drawn into a cold curve after multiple cold and heat cycle experiments, as shown in
Figure 3. It was observed from the supercooling graph that the 1.5 wt.% DSP nucleating agent and stirring can reduce the degree of supercooling to 0.5 °C at the same time. It can store heat for 23 min at a constant temperature of about 57.3 °C.
When the DSP nucleating agent and stirring work together, the friction between the rotor and the PCM increases the local solute concentration and the number of crystal collisions. As a result, the formation of crystal nuclei is accelerated, leading to more crystal nucleation sites being formed. Its long-lasting crystallization exotherm at about 57.3 °C is because the DSP nucleating agent can continuously provide nucleation sites. Secondly, the relative molecular mass of sodium acetate and disodium hydrogen phosphate is greater than that of water. Hence, the solid salt formed during melting will generally separate from the saturated aqueous solution under the action of gravity and deposit at the bottom of the beaker, resulting in a decrease in overall nucleation sites. Stirring in the cooling and heating cycle can fully mix the sodium acetate, disodium hydrogen phosphate, and water molecules, which can prevent phase separation and stabilize the supercooling effect. It was further confirmed from Herrick’s research that stirring the phase change material will suspend the precipitated particles in the solution, and when the phase change material cools, the separated salt and water will re-form salt hydrates [
35].
3.1.2. Research on Improving Phase Separation
Due to the density difference between liquid and solid during cold and heat cycles, anhydrous salts are gravitationally precipitated from saturated aqueous solutions upon melting [
33], resulting in the phenomenon that the anhydrous salt sticks to the test tube wall above the liquid surface. As the number of cooling and heating cycles increases, more and more anhydrous salt is precipitated [
36]. The anhydrous salt separated in the previous round cannot be completely mixed and crystallized with the liquid solution during the cooling and heating cycle to a large extent, which makes SAT unable to exert the advantages of high heat storage. To solve this problem, most researchers use thickeners to improve the phase separation of salt hydrates. According to Kumar, N. et al., when CMC thickener was added to SAT, due to the structural characteristics of CMC, the viscosity of the solution was increased, and the separation of anhydrous salt was well prevented [
33]. However, when the phase change material is in a molten state for a long time, even the addition of a thickener cannot prevent the precipitation of anhydrous salts well, thus recurring phase separation [
37,
38].
Figure 4a shows the phase separation of 10 g SAT after five cooling and heating cycles. As shown in
Figure 4b, the phase separation can be solved well when the amount of CMC thickener added reaches 2.5 wt.%. However, it should be noted that the water level in the water tank must be a few centimeters higher than the phase change material during the cooling and heating cycle, otherwise the PCM splashed on the wall of the test tube will hardly fall off into the liquid.
Figure 4c shows the phase change material after standing at a constant temperature of 70 °C for 24 h. When the phase change material is in the molten state for a long time, even if CMC is added, the phase separation cannot be solved well. On the contrary, the CMC thickener will agglomerate on the upper surface of the PCM. This is because the density of CMC is less than the density of SAT, so it precipitates from the solution and agglomerates on the surface of the solution, which does not play a role in thickening.
Adding thickeners to SAT that has been in a molten state for a long time has a poor effect and can also reduce the latent heat of SAT. To solve the problem of phase separation of SAT under long-term melting, we proposed a stirring method. The stirring was added to 10 g SAT in the molten state, and no phase separation occurred after 48 h, as shown in
Figure 4d. Stirring can make the dense anhydrous salt float, thereby uniformly dispersing the anhydrous salt and water molecules with different densities in the test tube, which prevents phase separation. However, the mouth of the test tube must be sealed while stirring, otherwise it will be difficult to develop the original properties of SAT after the evaporation of water molecules.
3.2. Research on Stirring Momentum and Induction Time
In this section, two examples were experimentally investigated to analyze the effect of the momentum generated by agitation on the induced crystallization. The first case is to use different mass rotors (1.520 g, 2.753 g, 3.919 g, 4.197 g) on a magnetic stirrer with a rotating speed of 750 r/min to study the induced crystallization time of composite phase change materials. The second one, when the rotor mass is 2.753 g, the influence of different speeds (250 r/min, 500 r/min, 750 r/min, 1000 r/min) on the induction crystallization time of the composite phase change material is tested.
Figure 5a,b are the temperature change diagrams of the composite phase change material after being taken out of the water bath and cooled at room temperature when the stirring function is turned on at 56.5 °C. The balance line that first appears in the two graphs represents the induction time. Under normal circumstances, even if the stirring function is turned on, the temperature continues to drop from 56.5 °C until the crystallization temperature rises. Therefore, the temperature change should be a quadratic function. However, for the convenience of the reader’s analysis, the induction time is replaced by a parallel line. Even at the same rotation speed and mass, the induction time is different each time, so each mass and rotation speed are repeated four times, and finally, the arithmetic average value is taken for analysis, as shown in
Figure 6a,b.
Through the above two experiments, the following conclusions were drawn: the greater the momentum of the stirring (the greater the rotor mass, the faster the rotor rotation speed), the more conducive to the solidification of the supercooled sodium acetate solution. With the increase of the applied momentum, the induction time is shortened. The practical motive is that the impact and friction generated by the rotation of the rotor will cause pressure and density fluctuations between the molecules of the composite phase change material, which provides the required energy and driving force for nucleation of some molecules, and increases the probability of nucleation. There are also two descriptions of auxiliary crystallization. Firstly, the composite phase change material crystals remaining on the inner wall of the beaker during the cooling and heating cycle fall off in the phase change material liquid with the impact of rotation, providing nucleation sites for the liquid phase change material, thereby further promoting crystallization. Second, as the rotor rotates, some anhydrous salt particles are thrown towards the side wall of the beaker and adhere to it under the centrifugal force generated by the rotor, providing nucleation points close to the inner wall surface. Thereby, the liquid phase change material near the wall is rapidly activated, and finally the crystallization of the whole system is promoted. No matter which reason is dominant, mechanical agitation is an effective way to induce the solidification and exotherm of supercooled salt hydrate.
3.3. EG’s Effect on Thermal Conductivity
Lower thermal conductivity is also a big problem of salt hydrates. Many salt hydrates have thermal conductivity between 0.5–1.5 w/m·k [
39]. The thermal conductivity of SAT used in this paper was 0.62 w/m·k. J. Mao et al. [
40,
41] found that when SAT is used as a phase change material, it is necessary to increase its thermal conductivity, and EG is a good thermal conductivity enhancer. Adding 10% expanded graphite to SAT can improve its thermal conductivity to 400% [
30]. The study by Hasan Babaei et al. showed that the introduction of carbon nanotubes and graphene into long-chain alkanes can significantly improve the thermal conductivity of PCM [
42].
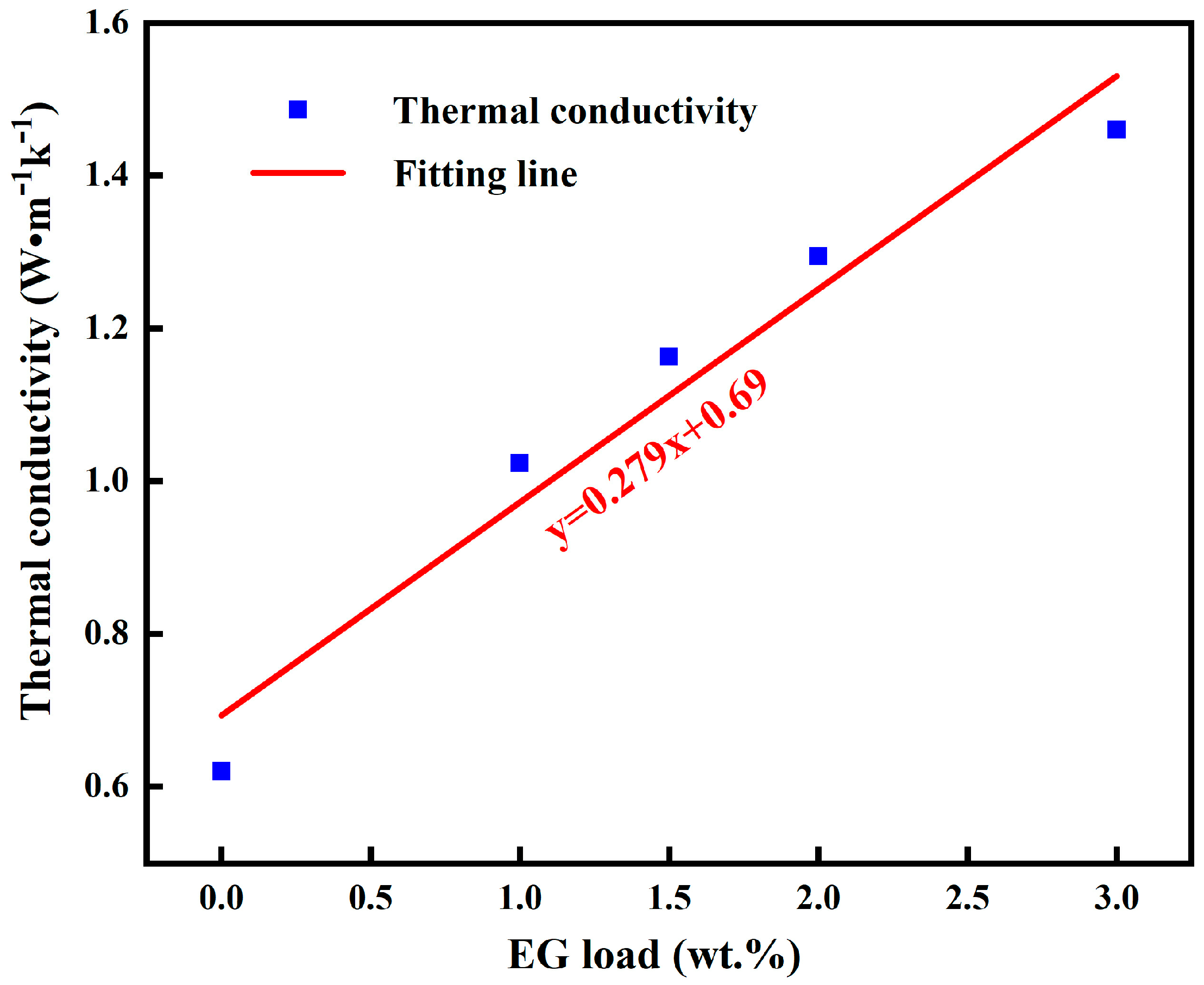
As shown in
Figure 7, the EG used in this article has a good layered and void structure from the scanning electron microscope and can be a good carrier. As shown in
Figure 8, when the EG addition amount is 0 wt.%, 1 wt.%, 1.5 wt.%, 2 wt.%, and 3 wt.%, the thermal conductivity is 0.62 w·m
−1k
−1, 1.0232 w·m
−1k
−1, 1.1626 w·m
−1k
−1, 1.2940 w·m
−1k
−1, and 1.46 w·m
−1k
−1, respectively. It can be seen that with the increase of EG contents, the thermal conductivity of the composite phase change material also increases, and the added amount of EG and the thermal conductivity of CPCM can be fitted to the line y = 0.279x + 0.69 (R
2 = 0.97). This rule has been reported in previous studies [
22,
43]. Another study has shown that as the percentage of EG increases, the network between carbon atoms becomes tighter, which leads to higher thermal conductivity [
44].
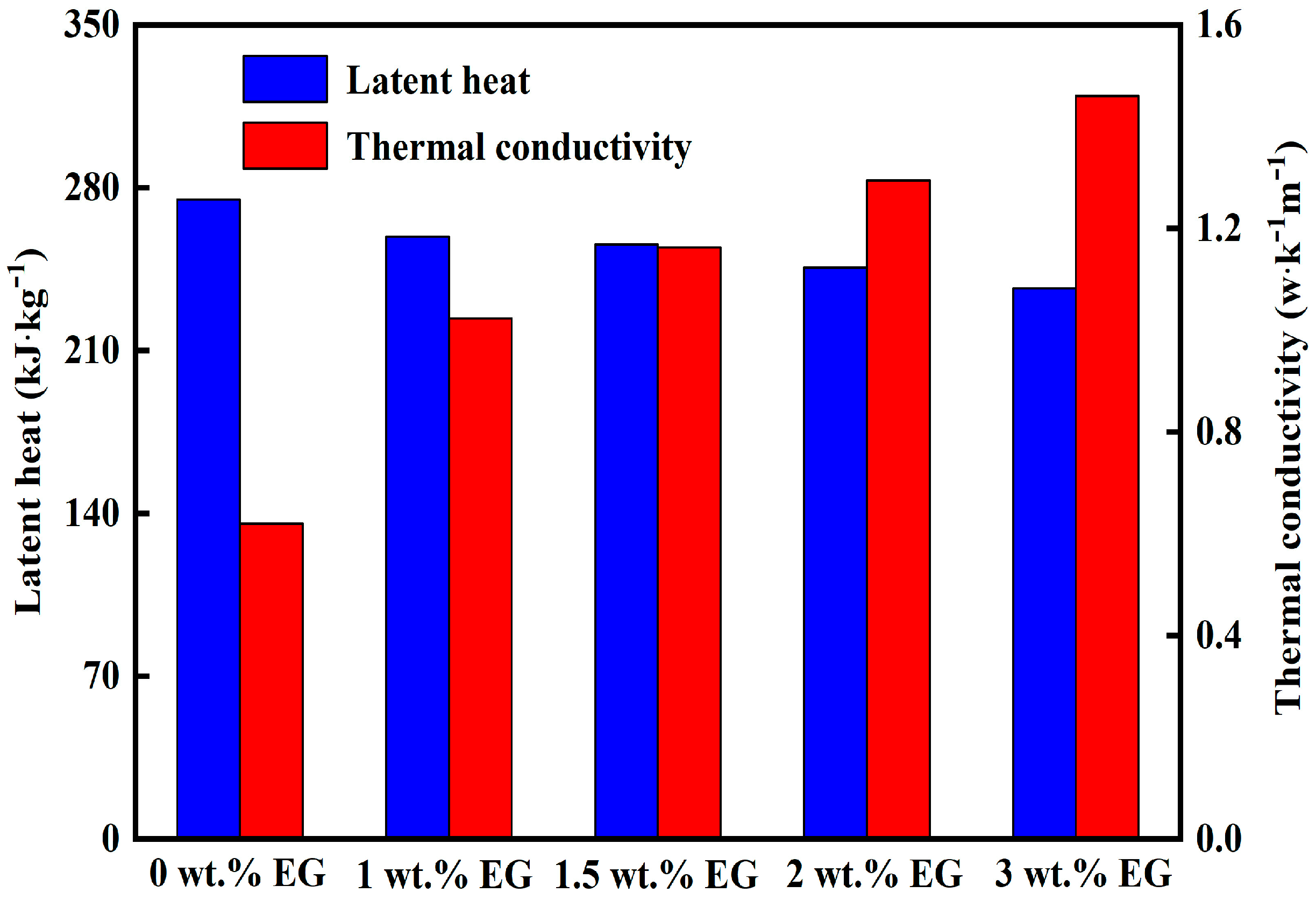
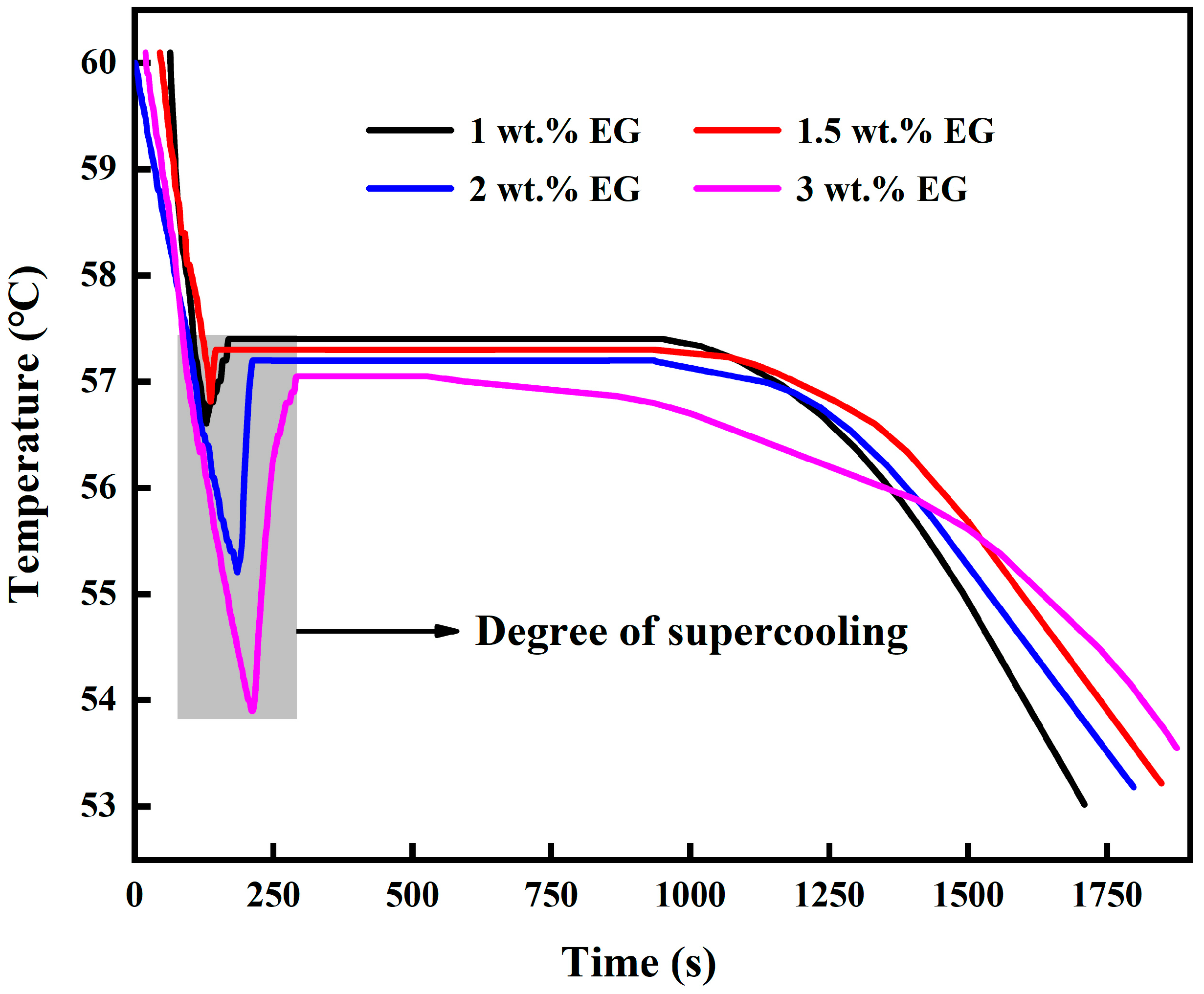
The DSC curve measured in
Figure 9 is a curve with the rate of heat absorption of the sample as the ordinate and the temperature as the abscissa. The latent heat value was obtained by integrating the peak area on this curve. The latent heat values from top to bottom in the DSC graph are 265 kJ/kg, 260.45 kJ/kg, 258.98 kJ/kg, 255.64 kJ/kg, 245.74 kJ/kg, 236.65 kJ/kg, and 223.48 kJ/kg, respectively. In accordance with
Figure 9 and
Figure 10, as the mass fraction of EG increases, the thermal conductivity becomes higher and higher, but the latent heat value becomes lower and lower. As shown in
Figure 11, when the mass fraction of EG is less than 1.5 wt.%, there is almost no change in the degree of subcooling, which can be well maintained at about 0.5 °C; When the mass fraction is greater than 1.5 wt.%, with the increase of EG mass fraction, the degree of supercooling increases and the unit latent heat decreases. The reasons for the analysis are as follows: the unit latent heat of CPCM is determined by SAT. With the increase of EG percentage, the percentage of SAT in unit volume decreases, which leads to the decrease of unit latent heat value. The increase in supercooling degree may be due to the increase of EG contents, which leads to the formation of colloidal suspension in CPCM. While the thermal conductivity of colloids is higher, the number of frozen phases decreases with decreasing particle size for a fixed time, resulting in an increase in supercooling [
45,
46]. Combining
Figure 9,
Figure 10 and
Figure 11, it is difficult to obtain a high thermal conductivity CPCM without affecting the latent heat and subcooling. According to the balance principle of low subcooling degree, high latent heat, and high thermal conductivity, the optimal state is when the addition amount of EG is 1.5 wt.%. At this time, the latent heat value is as high as 258.98 kJ/kg, the subcooling degree is also around 0.5 °C, and the thermal conductivity of the most important CPCM is increased by 187.5%.
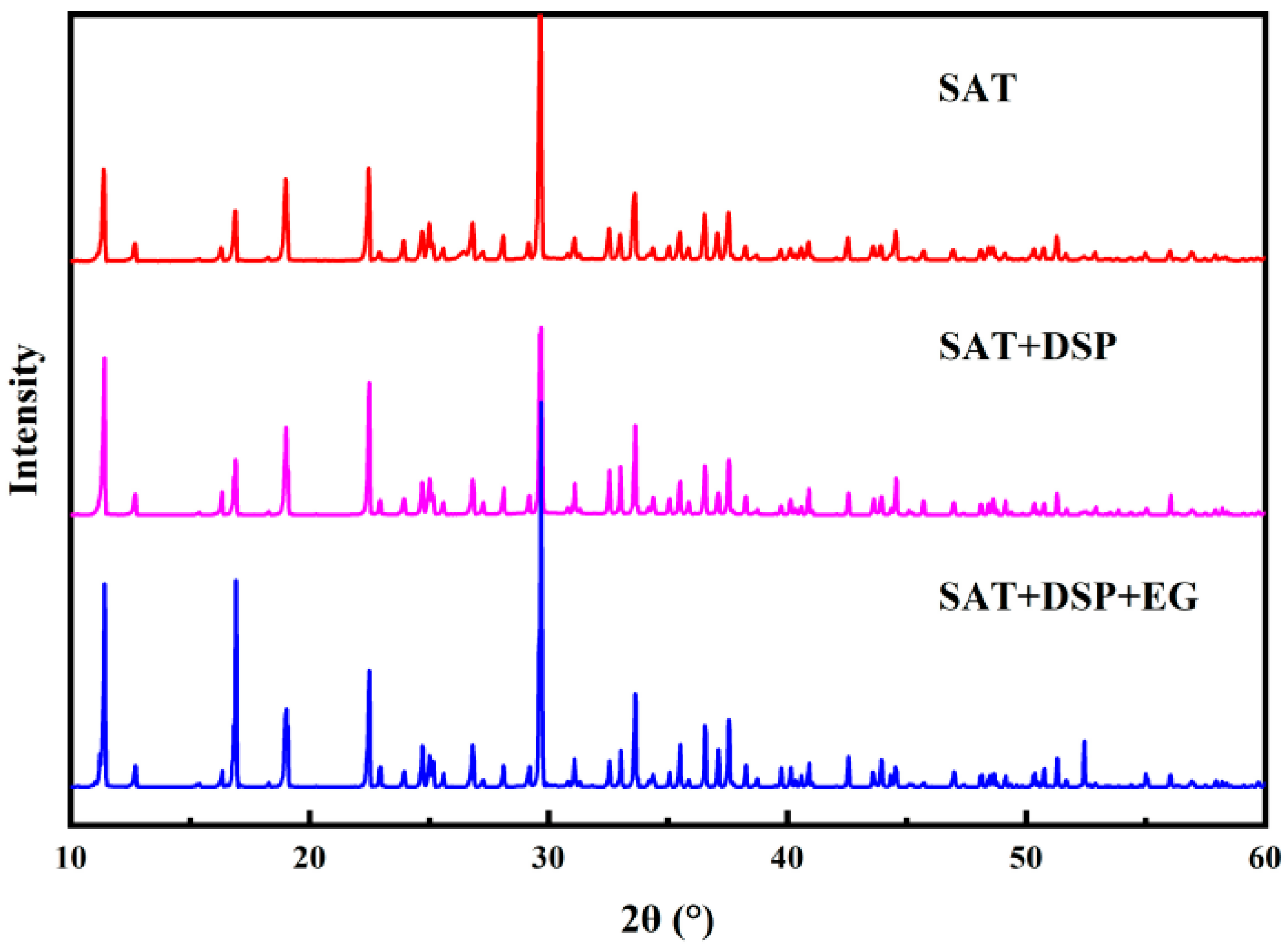
To demonstrate the absence of chemical reactions in the improved CPCM, XRD characterizations were performed on SAT, SAT/DSP, and SAT/DSP/EG, as shown in
Figure 12. The three materials have strong diffraction peaks at 11.38°, 16.9°, 19°, 22.48°, 29.66°, 33.62°, 36.54°, 37.54°, and the highest diffraction peak at 29.66°. The comparison of diffraction peaks shows that the diffraction peaks before and after the reaction corresponds well. Therefore, it is concluded that the three materials are simply mixed physically and no chemical reaction took place. However, the diffraction peak after the reaction is higher, indicating that the crystallinity of the improved CPCM material has increased significantly.
3.4. CPCM and Metal Coexist
When applying CPCM, a corresponding packaging container should be designed to contain, protect, and enhance heat transfer for CPCM [
47]. The packaging container needs to have a good service life to ensure safety and economy [
48,
49].
Since the thermal conductivity of the improved CPCM is not very high, it is better to have a higher thermal conductivity of the packaging container. This section studied the long-term coexistence of high thermal conductivity metals (iron, copper, aluminum, and 304 stainless steel) and CPCM. The CPCM used has the properties of strong alkali and weak acid salts, so it is initially predicted to corrode metal materials [
50]. In
Figure 13, the colors of iron, copper, aluminum, and 304 stainless steel before the reaction were silver-gray, red copper, white, and silver-white, respectively. The four metals have a good metallic luster and stripes observed by SEM.
First, all typical metals were sequentially added to four beakers containing CPCM and sealed. After that, a constant temperature water bath with a magnetic stirring function was prepared, and the beaker was immersed in the water bath once the constant temperature reached 80 °C. After the CPCM is melted, the magnetic stirring function is turned on and the constant temperature is maintained for 30 days. The working state of the water bath and the stirring of the materials are observed every day. Four typical metals were removed after 30 days, and the surfaces were cleaned with deionized water. By observing the surface color change of the metal material and the appearance under SEM, it can be judged whether the metal reacts with the CPCM.
The surface color and micromorphology of typical metals after reaction are shown in
Figure 14. Copper and aluminum lost their metallic luster after being immersed in a composite phase change material for 30 days, and their surface colors were dark brown and dark gray. It was observed by SEM that the surface becomes rough after the reaction of copper particles and aluminum particles. It was difficult to observe metal streaks, and there was obvious corrosion. The color of iron does not change significantly before and after the reaction, but the appearance under SEM is slightly different from that before the reaction, which hints towards the lack of vacuum upon sealing the beakers. Iron reacts to a certain extent with the oxygen and water in the beaker. This statement was confirmed as mentioned in previous studies [
51]. The color of 304 stainless steel has not changed after being immersed in the composite phase change material for 30 days, and no corroded features have been observed through SEM-scanning electron microscope images. According to previous studies, the corrosion resistance of stainless steel to CPCM can be well verified [
52,
53]. In the experiment, copper, aluminum, and iron are somewhat oxidized. Considering the long life of the packaging material in practical applications, it is recommended to use 304 stainless steel as the packaging material.
4. Conclusions and Future Work
In order to improve the problems of high subcooling degree, serious phase separation and low thermal conductivity of sodium acetate trihydrate (SAT) in TES system. In this paper, different proportions of disodium hydrogen phosphate dodecahydrate (DSP), expanded graphite (EG), and physical perturbations are used to synergize the experiments. After obtaining the optimal CPCM, the coexistence of CPCM and typical metals was tested. The conclusions are as follows:
- (1)
When only adding DSP nucleating agent or using physical disturbance to improve SAT supercooling, the best case can reduce the supercooling to less than 3 °C. When DSP nucleating agent and stirring act synergistically, the supercooling can be reduced to less than 0.5 °C. Therefore, it is concluded that the improvement effect of DSP nucleating agent and stirring on supercooling is better than that of the two alone; the greater the momentum of the physical disturbance, the shorter the induced crystallization time of CPCM.
- (2)
When the PCM is in the molten state for a long time, the thickening effect of CMC is not ideal, and it also has the disadvantages of reducing the unit latent heat of PCM. However, the PCM that is in the molten state for a long time under physical disturbance will not undergo phase separation, and it will not affect the latent heat of the PCM.
- (3)
EG can improve the thermal conductivity of SAT very well, but the addition amount should not be too much, otherwise it will have adverse effects, such as reducing latent heat and increasing supercooling. EG can improve the thermal conductivity of SAT very well, but the addition amount should not be too much, otherwise it will have adverse effects, such as reducing latent heat and increasing supercooling. In this paper, considering factors such as latent heat and subcooling, it was concluded that the optimal state of EG is 1.5 wt.%. At this time, the thermal conductivity of PCM can be increased from 0.62 w/m·k to 1.1625 w/m·k.
- (4)
The XRD test found that the peaks before and after the reaction were well fitted, and no new substances were produced. There is no chemical reaction and no change in the characteristics of the original phase change material in the studies to verify the synergistic impact of DSP, EG, and physical disruption.
- (5)
Through the reaction of CPCM with typical metals, it was concluded that copper, aluminum, and iron are all corroded, but stainless steel can coexist with CPCM well. It is highly recommended to use stainless steel as the packaging material. Iron can be used in the short term in the absence of stainless steel, but copper and aluminum are strongly not recommended.
In this paper, the performance of SAT is improved by the synergistic effect of DSP, EG and physical perturbation, but whether this synergistic method is useful for other materials has not been studied, and further research can be done on other PCMs in the future. Additionally, the methods herein can be used on direct contact regenerators for application in TES.
Author Contributions
Conceptualization, S.W.; methodology, S.W.; software, S.W. and C.W.; formal analysis, S.W. and M.B.H.; investigation, S.W. and X.C.; data curation, X.C. and Z.W.; writing—original draft preparation, S.W.; writing—review and editing, S.W. and C.W.; visualization, S.W.; supervision, X.C. and Z.W.; project administration, X.C.; funding acquisition, X.C. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Shandong Province Natural Science Foundation (NO. ZR2020ME190) and Shandong Key Research and Development Plan (NO. 2020CXGC011402).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Shandong Province Natural Science Foundation (NO. ZR2020ME190) and Shandong Key Research and Development Plan (NO. 2020CXGC011402) for the financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Y.; Cheng, X.; Wang, Z.; Tahir, M.H.; Wang, Z.; Wang, X.; Wang, C. Full recycling of high-value resources from cabbage waste by multi-stage utilization. Sci. Total Environ. 2022, 804, 149951. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, W.; Zhai, X.; Qian, C.; Chen, X. Feasibility and performance study of the hybrid ground-source heat pump system for one office building in Chinese heating dominated areas. Renew. Energy 2017, 101, 1131–1140. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, K.; Li, H.; Cao, G.; Wu, D.; Shi, Y. Exploring the potential relationship between indoor air quality and the concentration of airborne culturable fungi: A combined experimental and neural network modeling study. Environ. Sci. Pollut. Res. Int. 2018, 25, 3510–3517. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xu, C.; Kong, W.; Englmair, G.; Fan, J.; Wei, G.; Furbo, S. Review on sodium acetate trihydrate in flexible thermal energy storages: Properties, challenges and applications. J. Energy Storage 2021, 40, 102780. [Google Scholar] [CrossRef]
- Osterman, E.; Tyagi, V.V.; Butala, V.; Rahim, N.A.; Stritih, U. Review of PCM based cooling technologies for buildings. Energy Build. 2012, 49, 37–49. [Google Scholar] [CrossRef]
- Pomianowski, M.; Heiselberg, P.; Zhang, Y. Review of thermal energy storage technologies based on PCM application in buildings. Energy Build. 2013, 67, 56–69. [Google Scholar] [CrossRef]
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Zhi, C.; Lei, C.; Feng, S.; Fang, G. Preparation and characteristics of microencapsulated stearic acid as composite thermal energy storage material in buildings. Energy Build. 2013, 62, 469–474. [Google Scholar]
- Tay, N.; Belusko, M.; Bruno, F. Designing a PCM storage system using the effectiveness-number of transfer units method in low energy cooling of buildings. Energy Build. 2012, 50, 234–242. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Q.; Medina, M.A.; Liu, Y.; Liao, S. A study on the use of phase change materials (PCMs) in combination with a natural cold source for space cooling in telecommunications base stations (TBSs) in China. Appl. Energy 2014, 117, 95–103. [Google Scholar] [CrossRef]
- Swa, B.; Tya, B.; Zka, B.; Wpa, B. Thermal conductivity enhancement on phase change materials for thermal energy storage: A review. Energy Storage Mater. 2020, 25, 251–295. [Google Scholar]
- Wang, Q.; Wang, J.; Chen, Y.; Zhao, C.Y. Experimental investigation of barium hydroxide octahydrate as latent heat storage materials. Sol. Energy 2019, 177, 99–107. [Google Scholar] [CrossRef]
- Hu, P.; Lu, D.J.; Fan, X.Y.; Zhou, X.; Chen, Z.S. Phase change performance of sodium acetate trihydrate with AlN nanoparticles and CMC. Sol. Energy Mater. Sol. Cells 2011, 95, 2645–2649. [Google Scholar] [CrossRef]
- Garay Ramirez, B.M.L.; Glorieux, C.; San Martin Martinez, E.; Flores Cuautle, J.J.A. Tuning of thermal properties of sodium acetate trihydrate by blending with polymer and silver nanoparticles. Appl. Therm. Eng. 2014, 62, 838–844. [Google Scholar] [CrossRef]
- Wei, L.L.; Ohsasa, K. Supercooling and solidification behavior of phase change material. ISIJ Int. 2010, 50, 1265–1269. [Google Scholar] [CrossRef] [Green Version]
- Inada, T.; Zhang, X.; Yabe, A.; Kozawa, Y. Active control of phase change from supercooled water to ice by ultrasonic vibration 1. Control of freezing temperature. Int. J. Heat Mass Transf. 2001, 44, 4523–4531. [Google Scholar] [CrossRef]
- Zhang, X.; Inada, T.; Yabe, A.; Kozawa, Y. Active control of phase change from supercooled water to ice by ultrasonic vibration 2. Generation of ice slurries and effect of bubble nuclei. Int. J. Heat Mass Transf. 2001, 44, 4533–4539. [Google Scholar] [CrossRef]
- Miyasaka, E.; Takai, M.; Hidaka, H.; Kakimoto, Y.; Hirasawa, I. Effect of ultrasonic irradiation on nucleation phenomena in a Na2HPO4•12H2O melt being used as a heat storage material. Ultrason. Sonochem. 2006, 13, 308–312. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, L.; Zhongjie, S.U.; Ping, C.; Zhong, Y. Effects of ultrasound on phase separation and crystallization of sodium acetate trihydrate. CIESC J. 2010, 61, 104–108. (In Chinese) [Google Scholar]
- Pan, L.H.; Huang, L.W.; Qiao, Y.; Zhao, J.; Jiang, M. Influence of vibration on the supercooling relex of inorganic salt solution as a phase change material. J. Zhejiang Univ. Technol. 2008, 36, 655–658. [Google Scholar]
- Fang, Y.; Jin, C.; Liang, X.; Gao, X.; Zhang, Z. Preparation and performance of sodium acetate trihydrate/formamide composite phase change material. CIESC J. 2015, 66, 5142–5148. (In Chinese) [Google Scholar]
- Fu, W.; Zou, T.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Thermal properties and thermal conductivity enhancement of composite phase change material using sodium acetate trihydrate–urea/expanded graphite for radiant floor heating system. Appl. Therm. Eng. 2018, 138, 618–626. [Google Scholar] [CrossRef]
- Dongling, W.U.; Tingxian, L.I.; Feng, H.E.; Wang, R. Preparation and performance of modified sodium acetate trihydrate composite phase change material for thermal energy storage. CIESC J. 2018, 69, 2860–2868. (In Chinese) [Google Scholar]
- Jintian, L.; Jinfeng, M.; Weihua, L.; Jing, L.; Xiaoyan, X. Supercooling mechanism and experimental study of sodium acetate trihydrate. J. Refrig. 2009, 30, 32–35. (In Chinese) [Google Scholar]
- Jintian, L.; Jinfeng, M.; Jing, L.; Shiguang, Y. A selection and optimization experimental study of additives to thermal energy storage material sodium acetate trihydrate. J. Funct. Mater. 2011, 42, 144–147. (In Chinese) [Google Scholar]
- Cui, H.; Wang, P.; Yang, H.; Tang, W. Enhancing the heat transfer and photothermal conversion of salt hydrate phase change material for efficient solar energy utilization. J. Energy Storage 2022, 49, 104130. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, Z.; Liu, M. thermal properties and charging/discharging characteristics of sodium acetate/stearic acid/octadecyl alcohol composite as phase change materials. Appl. Therm. Eng. 2022, 206, 118143. [Google Scholar] [CrossRef]
- Ryu, H.W.; Woo, S.W.; Shin, B.C.; Sang, D.K. Prevention of supercooling and stabilization of inorganic salt hydrates as latent heat storage materials. Sol. Energy Mater. Sol. Cells 1992, 27, 161–172. [Google Scholar] [CrossRef]
- Safari, A.; Saidur, R.; Sulaiman, F.A.; Xu, Y.; Dong, J. A review on supercooling of Phase Change Materials in thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 905–919. [Google Scholar] [CrossRef]
- Mao, J.; Dong, X.; Hou, P.; Lian, H. Preparation research of novel composite phase change materials based on sodium acetate trihydrate. Appl. Therm. Eng. 2017, 118, 817–825. [Google Scholar] [CrossRef]
- Danneman, M.; Johansen, J.B.; Furbo, S. Solidification behavior and thermal conductivity of bulk sodium acetate trihydrate composites with thickening agents and graphite. Sol. Energy Mater. Sol. Cells 2016, 145, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Fashandi, M.; Leung, S.N. Sodium acetate trihydrate-chitin nanowhisker nanocomposites with enhanced phase change performance for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 178, 259–265. [Google Scholar] [CrossRef]
- Kumar, N.; Hirschey, J.; Laclair, T.J.; Gluesenkamp, K.R.; Graham, S. Review of stability and thermal conductivity enhancements for salt hydrates. J. Energy Storage 2019, 24, 100794. [Google Scholar] [CrossRef]
- Zhou, G.; Zhu, M.; Xiang, Y. Effect of percussion vibration on solidification of supercooled salt hydrate PCM in thermal storage unit. Renew. Energy 2018, 126, 537–544. [Google Scholar] [CrossRef]
- Herrick, C.S. Melt-freeze-cycle life-testing of Glauber’s salt in a rolling cylinder heat store. Sol. Energy 1982, 28, 99–104. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Mohamed, S.A.; AL-Sulaiman, F.A.; Ibrahim, N.I.; Zahir, M.H.; Al-Ahmed, A.; Saidur, R.; Yılbas, B.S.; Sahin, A.Z. A review on current status and challenges of inorganic phase change materials for thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 1072–1089. [Google Scholar] [CrossRef]
- Kumar, N.; Banerjee, D. Phase change materials. In Handbook of Thermal Science and Engineering; Springer International Publishing: Cham, Switzerland, 2018; pp. 2213–2275. [Google Scholar]
- Mayinger, F. Heat and Cold Storage with PCM; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Li, W.H.; Mao, J.F.; Wang, L.J.; Sui, L.Y. Effect of The Additive on Thermal Conductivity of The Phase Change Material. In Proceedings of the International Conference on Chemical, Material and Metallurgical Engineering (ICCMME 2011), PEOPLES R CHINA, Beihai, China, 23–25 December 2011; pp. 1302–1306. [Google Scholar]
- Ling, Z.; Chen, J.; Xu, T.; Fang, X.; Gao, X.; Zhang, Z. Thermal conductivity of an organic phase change material/expanded graphite composite across the phase change temperature range and a novel thermal conductivity model. Energy Convers. Manag. 2015, 102, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Babaei, H.; Keblinski, P.; Khodadadi, J.M. Thermal conductivity enhancement of paraffins by increasing the alignment of molecules through adding CNT/graphene. Int. J. Heat Mass Transf. 2013, 58, 209–216. [Google Scholar] [CrossRef]
- Huang, Z.; Gao, X.; Xu, T.; Fang, Y.; Zhang, Z. Thermal property measurement and heat storage analysis of LiNO3/KCl—Expanded graphite composite phase change material. Appl. Energy 2014, 115, 265–271. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y.; Yuan, Y.; Cao, X.; Yang, X. Effect of carbon nanotubes on the thermal behavior of palmitic–stearic acid eutectic mixtures as phase change materials for energy storage. Sol. Energy 2014, 110, 64–70. [Google Scholar] [CrossRef]
- El Hasadi, Y.M.F.; Khodadadi, J.M. Numerical Simulation of the Effect of the Size of Suspensions on the Solidification Process of Nanoparticle-Enhanced Phase Change Materials. J. Heat Transf. 2013, 135, 052901. [Google Scholar] [CrossRef]
- El Hasadi, Y.M.F.; Khodadadi, J.M. One-dimensional Stefan problem formulation for solidification of nanostructure-enhanced phase change materials (NePCM). Int. J. Heat Mass Transf. 2013, 67, 202–213. [Google Scholar] [CrossRef]
- Regin, A.F.; Solanki, S.C.; Saini, J.S. Heat transfer characteristics of thermal energy storage system using PCM capsules: A review. Renew. Sustain. Energy Rev. 2008, 12, 2438–2458. [Google Scholar] [CrossRef]
- Ferrer, G.; Solé, A.; Barreneche, C.; Martorell, I.; Cabeza, L.F. Corrosion of metal containers for use in PCM energy storage. Renew. Energy 2015, 76, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Cabeza, L.F.; Roca, J.; Nogues, M.; Mehling, H.; Hiebler, S. Immersion corrosion testson metal-salt hydrate pairs used for latent heat storage in the 48 to 58 °C temperature range. Mater. Corros. 2002, 53, 902–907. [Google Scholar] [CrossRef]
- Goyal, M.; Kumar, S.; Bahadur, I.; Verma, C.; Ebenso, E.E. Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: A review. J. Mol. Liq. 2018, 256, 565–573. [Google Scholar] [CrossRef]
- Atkins, P. The Death of Metal: Corrosion. In Reactions; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Jaya Krishna, D.; Kochar, S. The Metallographic Study of Corrosion of Metals with Latent Heat Storage Materials Suitable for Solar Hot Water System. Trans. Indian Ceram. Soc. 2017, 76, 133–141. [Google Scholar] [CrossRef]
- Devanuri, J.K.; Gaddala, U.M.; Kumar, V. Investigation on compatibility and thermal reliability of phase change materials for low-temperature thermal energy storage. Mater. Renew. Sustain. Energy 2020, 9, 24. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).