Abstract
Background: Psoriasis is one of the most commonly recognized dermatological diseases, characterized by distinct structural changes, hyperproliferation and inflammation. The aim of the study was quantitative comparisons of psoriatic skin with skin without psoriatic lesions by non-invasive imaging methods. Methods: 71 patients diagnosed with psoriasis vulgaris underwent non-invasive imaging of skin at the site of the psoriatic lesion and at the site without such lesion. Skin density, epidermis thickness and subepidermal low-echogenic band (SLEB) thickness were measured by high-resolution ultrasound (HFU). Blood perfusion was assessed using laser speckle contrast analysis (LASCA) and skin temperature was measured by thermal imaging camera. Hyperspectral camera was used to obtain spectral reflectance profiles in psoriatic lesion and skin without psoriatic changes. Results: The greatest differences in skin density and epidermal thickness between psoriatic and unchanged skin were observed on the forearms. The skin covered with psoriatic plaques was 80% less dense, and the epidermis in this area was 121% thicker. The greatest thickness of SLEB was observed in the knee area (Me = 0.389 mm). Skin with psoriatic lesions is characterized by a higher temperature (Me = 33.6 vs. Me = 31) and blood perfusion than skin without psoriasis (Me = 98.76 vs. Me = 50.65). Skin without psoriasis shows lower reflectance than psoriatic lesion from 623 nm to 1000 nm; below this value, skin without psoriatic lesion shows higher reflectance. Conclusions: Skin density and epidermis thickness, skin blood perfusion, temperature and reflectance can be useful parameters for monitoring the course of psoriasis and its treatment, especially since the examination of psoriatic skin with proposed methods is non-invasive, quantitative and easy to perform in clinical conditions.
1. Introduction
Psoriasis is one of the most commonly recognized dermatological diseases. It affects 1–4% of the general population. The aetiology of psoriasis is not fully understood; its symptoms are known to be influenced by genetic, environmental and immunological factors. It is characterized by a chronic, unpredictable, inflammatory course with multiple relapses and no possibility of complete recovery [1,2,3,4]. A characteristic lesion is related to the increased epidermal proliferation and abnormal differentiation of keratinocytes, which is manifested by the presence of psoriatic plaques on the skin surface (Figure 1). Their location may vary, most often they occur on elbows, knees, back and hairy scalp [5,6].
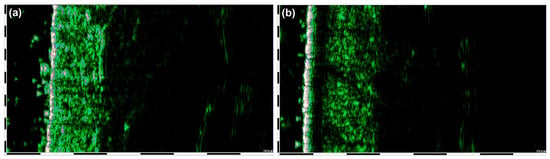
Figure 1.
HFU of the skin without psoriatic plaques (a) and skin covered with psoriatic plaques (b).
Epidermal proliferation is disturbed during the course of psoriasis. The passage time of keratinocytes from the basal layer to the stratum corneum is reduced eightfold. Physiologically, this process takes 30 days, and in patients with psoriasis it is shortened to 3–6 days [7,8,9]. The effect of the accelerated migration of keratinocytes is parakeratosis, which is the presence of nuclei in the cells of the stratum corneum. The pathomorphological image also shows agranulosis (atrophy of the granular layer), acanthosis (hypertrophy of the spinous layer) and papillomatosis (elongation of epidermal icicles). In addition, inflammatory cells are infiltrated and Munro micro-nodes are formed [5,10].
A red-brown papule covered with shiny, silvery scales, clearly demarcated from the surrounding area, constitutes the primary eruption. The circumferentially spreading lesions merge with each other to form dense ring-shaped structures. The skin around small eruptions sometimes turns white as a result of vasoconstriction forming the so-called Woronoff ring [11,12,13].
The lesions in the epidermis and dermis are accompanied by increased angiogenesis. Angiogenesis in psoriasis may be an inducer of psoriasis development. The number of vascular loops within psoriatic lesions grows significantly and blood flow increases. The vessels are twisted, serpentine and lignified. Additionally, gaps are formed in the vascular endothelium, which enables migration of inflammatory cells. The diseased vessels return to their physiological state within 1–9 months after the disappearance of psoriatic eruptions [6,14]. A comparison of healthy skin with skin lesions in the course of psoriasis is presented in Table 1.

Table 1.
List of skin lesions in psoriasis.
The aim of the study was to develop new methods of quantifying the severity of changes in the course of psoriasis. The present research focused on skin parameters which follow from the pathogenesis of psoriasis. Quantitative assessment of psoriatic lesion in the course of psoriasis may allow for: (i) more effective selection of the optimal therapy, (ii) more effective evaluation of the therapeutic methods, (iii) assessment of changes regardless of the observer, (iv) assessment of changes with high reproducibility, (v) verification of the dynamics of lesions, even of slight intensity (slight difference). Moreover, the quantitative assessment of changes may contribute to attempts to correlate the intensity of the psoriatic lesion in quantitative terms with the currently used qualitative and semi-quantitative scales (e.g., PASI(Psoriasis Area Severity Index)). It should be noted that, so far, previous publications have not contained information on the use of hyperspectral imaging and LASCA perfusion measurement.
2. Materials and Methods
2.1. Participants
Seventy-one patients (38 men, mean age 44 ± 16 and 33 women, mean age 43 ± 17) were selected for the study. The inclusion conditions were as follows: active psoriasis vulgaris, no other related autoimmune diseases, pre-treatment status or post-treatment remission.
2.1.1. Skin Density and Epidermis Thickness Measurements
DUB SkinScanner high-frequency ultrasonography in B-scan projection with a 33 MHz probe with a maximum skin penetration of 6 mm and an axial resolution of 42 µm was used to examine skin density and epidermis thickness (entrance echo). Patients were subjected to high-frequency ultrasound (HFUS) imaging of skin within active lesions as well as within surrounding skin areas without any signs of psoriatic lesions on the elbows, calves and back (Figure 1). Among the images of the affected skin, the sub-epidermal low-echogenic band (SLEB) was also measured. Epidermal thickness was defined as the distance from the outermost epidermis to the interface between the epidermis and the dermis on B-mode imaging. Skin density measures were determined by using the “region of interest” (ROI) function in the DUB SkinScanner software. Three ROI of equal dimensions were delineated to encompass the largest area possible of the dermis and then the average result was used for calculations. SLEB thickness was measured by designation on the ultrasound image line between the beginning and the end of that structure.
2.1.2. Blood Perfusion Measurements
PeriCam PSI System with near infrared (NIR) laser (785 nm) used to provide non-invasive, real-time measurements of blood perfusion (Figure 2). PeriCam PSI based on laser speckle contrast analysis (LASCA) technology, otherwise known as laser speckle contrast imaging. Data analysis was carried out using the PimSoft software version 1.5 (Perimed AB, Järfälla, Sweden). The PSI parameter was set as follows: Image acquisition rate—21 frames per second; spatial resolution—0.12 mm; head working distance—12 ± 1 cm.
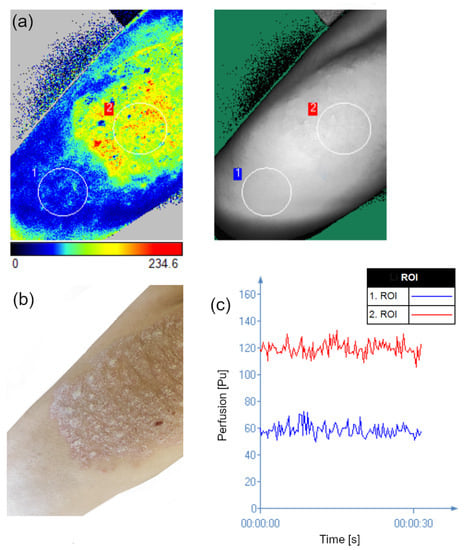
Figure 2.
LASCA perfusion map representing the skin without psoriatic lesion (ROI 1) and psoriatic lesion (ROI 2) (a). Clinical photography corresponding to the measured area (b) time evolution of the perfusion in the two ROI shown in image a (c).
2.1.3. Skin Temperature Measurements
Thermographic analysis was carried out with a thermal imaging camera FLIR T420, of the FLIR Systems Company, Sweden (Figure 3). The thermal resolution of the camera was <0.045 °C; the wavelength range was 7.5–13 μm; the resolution of the obtained image was 320 × 240 pixels. FLIR ResearchIR software, version 3.5 (FLIR® Systems, Inc., Wilsonville, OR, USA) was used for data analysis. Measurements were taken for the emissivity of the skin, which equals 0.98. The room temperature during the experiment was controlled at about 21 ± 1 °C and relative humidity was maintained at 50–60%.
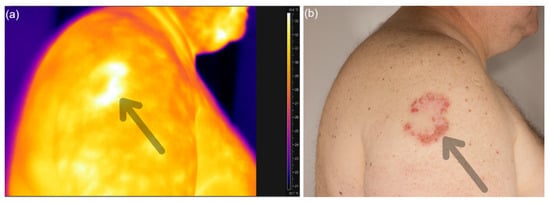
Figure 3.
Thermograms of patient with psoriatic lesion (a) and clinical photography of that area (b).
2.1.4. Hyperspectral Measurements
Hyperspectral camera Specim IQ (Spectral Imaging Ltd., Oulu, Finland) were used to compare psoriatic skin and skin without psoriatic plaque. We obtained images with a resolution of 512 × 512 pixels and 204 spectral bands across the wavelength range 400–1000 nm. Spectral image processing was performed using the method developed by Robert Koprowski et al. [15]; image analysis, conversion of raw data into a matrix and extraction of the selected features in the MATLAB program were carried out. Spectral characteristics were obtained for psoriatic lesion and skin without psoriatic changes (Figure 4).
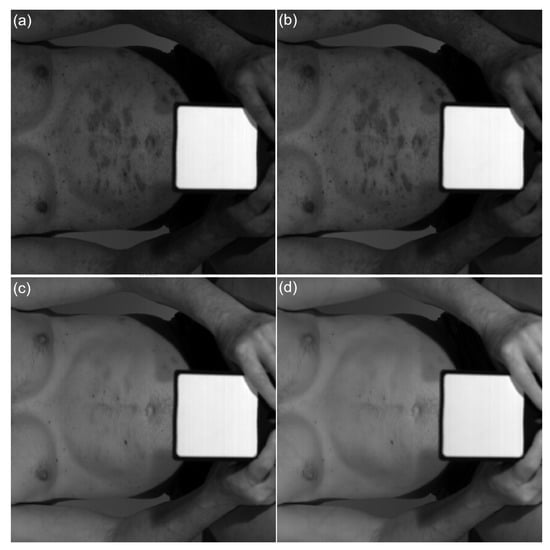
Figure 4.
Subchannel images captured by the hyperspectral camera from a psoriatic skin under halogen light illumination: (a) 574 nm, (b) 596 nm, (c) 636 nm, (d) 701 nm.
2.1.5. Statistical Analysis
Data analyses were performed using Statistica 13.3 ((TIBCO Software, Palo Alto, CA, USA) and OriginLab 2020 (OriginLab Corporation, MA, USA). Distribution normality was assessed using the Shapiro–Wilk W test and a quantile–quantile plot. The Wilcoxon signed-rank test was used to analyze perfusion differences between skin with and without psoriatic lesion. One-way analysis of variance (ANOVA) was applied to analyze data between the skin temperature in the area of psoriatic lesion and skin without psoriatic changes and to compare differences in the skin density and epidermal thickness. Possible correlations between skin density and epidermis thicknesses were analyzed by performing Spearman’s rank correlation coefficient. The level of statistical significance was assumed to be α = 0.05.
3. Results
Comparison of dermal echo-density between skin with psoriatic lesion and skin without any psoriatic changes showed that skin density was statistically significantly lower in psoriatic skin in all examined areas (p = 0.0003). The smallest difference in density between skin without psoriatic plaque and psoriatic skin was observed on the abdomen. On the abdomen, the skin covered with psoriatic plaques was 66% less dense than the skin without psoriasis. The highest difference in density between skin without psoriatic plaque and psoriatic skin was observed on the forearm, where psoriatic skin was 80% less dense than skin without psoriatic plaque (Figure 5).
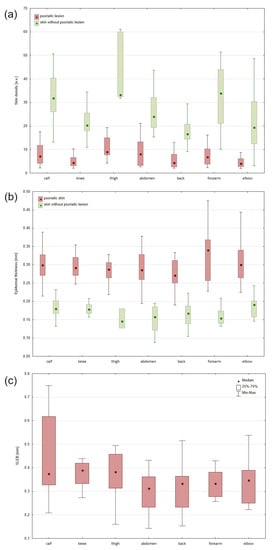
Figure 5.
Density of the skin without psoriatic plaques and psoriatic lesion (a). Thickness of the epidermis without psoriatic plaques and psoriatic skin: median, interquartile range, min–max (b). Thickness of the SLEB determined using high-resolution ultrasound: median, interquartile range, min–max (c).
The epidermal entrance echo thickness was statistically significantly higher in psoriatic skin than skin without psoriatic lesion in all sites (p = 0.2570). The smallest difference in thickness between skin without psoriatic plaque and psoriatic skin was observed on the elbow. On the elbow, the epidermal covered area with psoriatic plaques was 57% thicker than the skin without psoriasis. The highest difference in epidermal thickness between skin without psoriatic plaque and psoriatic skin was observed on the forearm. Epidermal on the forearm was 121% thicker on the area with psoriatic lesion than on the area without any changes.
Highly significant negative correlations between epidermis thickness and skin density were found (p = 0.001).
In analyzed sonograms obtained from lesional skin, we found the presence of SLEB bordered between hyperechoic entrance echo and surrounding skin. The greatest thickness of SLEB was observed in the knee area (Me = 0.389 mm), and the smallest in the abdomen area (Me = 0.313 mm).
Analysis of the blood perfusion showed statistically significant differences between the skin without psoriatic lesion and lesional psoriatic skin (p < 0.001). Psoriatic skin was characterized by greater perfusion (Me = 98.76) than skin without psoriasis lesions (Me = 50.65) (Figure 6).
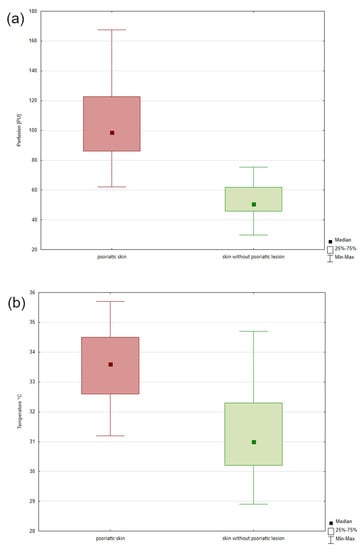
Figure 6.
The LASCA results of the blood perfusion of the psoriatic skin and skin without psoriatic lesion (a). The result of the measured skin temperature of psoriatic skin and skin without psoriatic lesion (b).
Analysis of the collected thermograms showed statistically significant differences between the image of the skin without psoriatic lesion and lesional psoriatic skin (p < 0.05). The skin with psoriasis was characterized by a higher temperature than the skin without psoriasis lesions. The difference between these areas was 2.6 °C (Figure 6).
Reflectance of the skin represents the proportion of incoming light reflected from the skin, and ranges from 0 (no reflection) to 1 (total reflection). Psoriatic skin and skin without psoriatic lesion reflectance level are plotted in Figure 7. The skin without psoriatic and psoriatic lesion spectra had absorption peaks in similar ranges. Upward-going peaks (at 520 nm, 570 nm and 720 nm) downward-going peaks (at 430 nm, 550 nm and 589 nm) can be observed. The reflectance spectra of psoriatic skin show greater dips at around 400 to 430 nm, 520 nm to 550 nm and 580 to 589 nm than skin without psoriatic lesion. Reflectance spectra of skin without psoriasis region >623 nm exhibit lower reflectance than psoriatic skin.
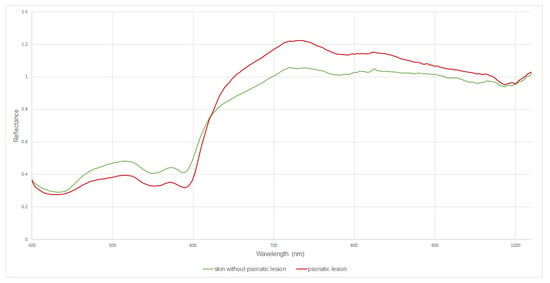
Figure 7.
Comparison of reflectance profiles in psoriatic lesion and skin without psoriatic changes in the spectral range 400–1000 nm.
4. Discussion
In recent years, great progress has been made in diagnostic methods used in dermatology. Scientists have been able to study skin using new imaging techniques [16]. However, high-frequency ultrasound of skin (HFUS), one of the oldest visualization methods used in dermatology, still enjoys unflagging popularity as a quick and relatively cheap method of in vivo testing. The possibility of real-time imaging, measurements of morphological and physiological parameters of the skin, safety, and the lack of contraindications to its use are other advantages of skin sonography. Ultrasound imaging is performed in order to evaluate healthy and diseased skin as well as to monitor the changes taking place in the tissue [17,18].
Psoriatic skin is characterized by distinct changes in structure, resulting from the underlying inflammation. Research to date shows increased thickness and reduced density of psoriatic skin compared to non-lesional skin [19]. When studying the healthy skin and psoriatic skin in the palm area, El Gammal et al. [20] observed clear differences between their entry echoes. It is less echogenic in healthy skin compared to psoriatic skin. Somlea et al. [21], in their study using 20-MHz HFUS, (Cortex Technology, Hadsund, Denmark)also confirmed that psoriatic skin is characterized by a greater epidermal thickness and lower dermal echogenicity than healthy skin, as well as the presence of SLEB, which is consistent with the results of our study.
Yazdanparast et al. [22] showed statistically significant epidermis thickness and skin density differences between the skin without psoriatic plaques and lesional psoriatic skin. The density of lesional psoriatic skin was 65% lower and epidermis thickness was 87% higher than skin without any signs of psoriatic lesions. The epidermis in the psoriatic plaques area is hyperkeratotic and does not exfoliate properly. The sub-epidermal low-echogenic band can be observed in ultrasound images, which is the result of swelling and inflammatory infiltration observed in inflammatory diseases.
There is a significant correlation between the ultrasonic thickness and the histometric thickness of acanthosis with the infiltration of the dermis [20]. High-frequency ultrasound image results correlate with skin biopsy results. This conclusion by El Gammal et al. [20] was confirmed by the research of Khlebnikova et al. [23]. They conducted a comparative study of the basal cell carcinoma spread depth measured with 30 MHz and 75 MHz HFUS and histomorphometry. This allowed assessment of the effectiveness of determining the size and depth of penetration of the basal cell carcinoma [23]. This means that high-frequency ultrasound examinations can replace skin biopsy. As a result, it will be possible to frequently monitor the skin and the progress of therapy, which is impossible in the case of skin biopsy (invasive procedure).
In inflammatory skin diseases (eczema, atopic dermatitis, psoriasis), SLEB of various thicknesses and reduced echogenicity of other layers of the dermis can be observed. The lower skin echogenicity mainly results from skin swelling and infiltration of inflammatory cells. Skin swelling, due to the presence of water, increases the distance between collagen fibres, reducing tissue density [17]. The increase in SLEB in psoriatic lesions indicates the intensity of the inflammatory basis, and is not a specific marker of psoriasis.
Ultrasound images of sites affected by psoriatic lesions showed a thickened entry echo and streaked shadows perpendicular to the entry echo, probably caused by air bubbles trapped among psoriatic plaques. In addition, the total skin thickness within the psoriatic plaque is increased, and its reduction under the influence of applied therapy can be objectively visualized [17]. The epidermis is thickened and hyperechoic because the superficial scales produce a hyper-reflective epidermal band [24].
Abnormalities in the superficial capillary vessels present in psoriatic lesions contribute to the difference in blood flow. Blood flow is elevated in skin with psoriatic plaques compared to non-lesional skin. The raised blood flow is considered to be a result of an increase in the vessel number, their diameter and their length within plaques of psoriasis. Previous measurements of blood fusion within psoriatic lesions have been performed using the Laser Doppler Imaging (LDI) or Laser Doppler Perfusion Imaging (LDPI) methods. A. G. M. Hendriks et al. used LDPI methods to monitor the course of psoriatic topical treatment by calcipotriol-betamethasone dipropionate [25]. A. K. Murray et al. [26] investigated different components of the microcirculation in the skin with and without psoriatic lesion, using dual wavelength Laser Doppler Imaging (LDI). Their results suggest an area of increased blood perfusion for deeper (large) and superficial (small) vessels in skin adjacent to plaques compared with nonadjacent skin. In our study, we used the LASCA method to measure skin perfusion in psoriatic skin and skin without psoriatic lesion. That provided an opportunity to thoroughly monitor the blood flow perfusion and any given point of the process providing us with an image view as well as a blood flow curve. LDPI works in scanning mode, taking up to several minutes for acquisition of a full perfusion map, while the LASCA method is simpler and cheaper compared to the LDPI technique, and could be used for full-field monitoring of skin perfusion [27,28].
Elevated blood flow in a psoriatic lesion causes an increase in skin temperature in their area. Raman et al. [29], using thermal imaging and GLCM feature extraction, showed mean average temperature difference between the normal sort and psoriasis was found to be 2.91 °C on the upper limb region. Zalewska et al. [30] evaluated the usefulness of thermography in the estimation of psoriatic lesion activity. Montero-Vilchez et al. [31] measured a skin temperature using SkinThermometer (ST 500, Mirocaya, Bilbao, Spain) and compared temperature between healthy skin and psoriatic skin. The thermal imaging camera used in our study provides a more accurate result than the skin temperature measurement with the SkinThermometer and additionally provides information on skin temperature distribution.
The absorption of light in the epidermis is mainly due to the melanin concentration; in the dermis, it is influenced by the blood-borne pigments (oxy- and deoxy-hemoglobin) and water. Transportation of light through the skin tissue incurs numerous diffusions from the tissue surface and absorption in the chromophores [32]. A change in the level of chromophores can be detected during the reflectance determination. Analysis of the reflectance spectra may provide the data providing information on tissue activities associated with human skin chromophores [33]. Skin disorders like psoriasis, related to inflammation and the accumulation of melanin and hemoglobin, can be monitored using hyperspectral imaging.
Psoriatic lesions increase the reflectance from about 623 nm. Below this value, unchanged skin shows higher reflectance. This may indicate that hypercatotic lesions (psoriasis plaque) make the radiation in the wavelength range 400–623 nm penetrate deeper into the skin more efficiently, while from about 623 nm the psoriatic lesions reverse this tendency. In view of the above, changes in skin reflectance in this case may be related to two factors: changes in the content of skin chromophores within psoriatic lesions (e.g., increased hemoglobin content due to inflammation) and/or changes in the skin structure determining differences in scattering radiation on the surface of the skin. According to the Rayleigh scattering law, the intensity of light scattering varies inversely as a quarter of the wavelength of the height (~ λ−4). Compared to longer wavelengths, shorter wavelengths are diffused more efficiently and thus can be reflected more effectively. This indicates that skin without psoriatic skin diffuses (reflects) radiation more efficiently than psoriatic skin but only up to a wavelength of about 623 nm. From 623 nm to 1000 nm, psoriatic skin scatters (reflects) radiation more efficiently. This may be related, as mentioned earlier, to two parallel phenomena. For lower wavelengths, there is increased scattering according to Rayleigh’s law, where the scattering factor ks is equal to:
where
- m—the number of scattering particles
- d—particles diameter
- n—refractive index
- λ—wavelength
According to the above formula, as the scattering particle size increases, the scattering coefficient increases. Thus, the increase in the size of cells in the course of psoriatic lesions (above 623 nm) will probably be responsible for part of the changes in the hyperspectral spectrum; keratinocytes in the course of psoriasis are larger and have a cell nucleus. However, the difference in reflectance for healthy and diseased skin in the spectrum range between 400 and 630 nm may be caused by the phenomenon of more effective absorption of radiation by skin chromophores accumulated in lesions—all hemoglobin.
Our research proves that skin without psoriatic lesion shows higher reflectance spectra of 400 nm to 623 nm than psoriatic lesion, which indicates that psoriatic lesion has a higher amount of hemoglobin compared to normal skin.
Limitations of This Study
- This study includes only people of the Polish population;
- The study did not include patients with scalp psoriasis.
5. Conclusions
In conclusion, HFUS, LASCA technology and thermal imaging are important non-invasive methods, which complete the clinical diagnosis in dermatosis. HFUS allows monitoring of psoriasis, which will provide a real morphological image of psoriatic skin lesions. Ultrasonography enables monitoring of the course of the disease, its severity and the skin response to treatment. Additionally, it is a non-invasive method, positively correlating with biopsy. It is worth using high-frequency ultrasound in everyday practice because it is fast, safe and relatively cheap. The blood flow in psoriatic skin is different from that in healthy skin. LASCA technology makes it possible to measure the microvascularization of a skin area and allows analysis of cutaneous microvascular hemodynamics. LASCA technology studies and follows the inflammation through the duration which may be important in the course of psoriasis therapy. Observation of changes in blood perfusion may help in selecting the appropriate dermatological treatment and enable doctors to react before changes are visible on skin. Thermal imaging can detect skin inflammation and increased heat from a localized area would suggest pathology. Hyperspectral imaging can provide data about skin reflectance and may be useful in monitoring the effectiveness of the psoriasis therapy. It could be envisioned as a complementary diagnostic method to assess the development of the psoriatic skin.
Author Contributions
All authors were responsible for the concept and design of the study, provided critical feedback and helped shape the research. W.O. conceived the study, contributed to the interpretation of the results, analyzed the data, wrote the manuscript and designed the figures; A.D. and D.W.-D. conceived and planned the experiments, worked on the manuscript, J.Z. analyzed the data, verified the analytical methods and designed the diagrams, and S.W. and A.L.-T. worked on the manuscript, B.B.-F. carried out the experiments, supervised the findings of this work and drafted the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported by the Medical University of Silesia (PCN-1-199/N/0/K and PCN-2-061/N/1/O).
Institutional Review Board Statement
The research was conducted after receiving a positive opinion of the Bioethics Committee of the SUM No. PCN/0022/KB1/12/I/20 on 19 May 2020. All volunteers gave informed consent to participate in the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McCormick, T.; Ayala-Fontanez, N.; Soler, D. Current knowledge on psoriasis and autoimmune diseases. Psoriasis 2016, 6, 7–32. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [PubMed]
- Tampa, M.; Sarbu, M.-I.; Mitran, M.-I.; Mitran, C.-I.; Matei, C.; Georgescu, S.-R. The Pathophysiological Mechanisms and the Quest for Biomarkers in Psoriasis, a Stress-Related Skin Disease. Dis. Markers 2018, 2018, 5823684. [Google Scholar] [CrossRef] [PubMed]
- Gudjonsson, J.E.; Kabashima, K.; Eyerich, K. Mechanisms of skin autoimmunity: Cellular and soluble immune components of the skin. J. Allergy Clin. Immunol. 2020, 146, 8–16. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Kimmel, G.W.; Lebwohl, M. Psoriasis: Overview and Diagnosis BT—Evidence-Based Psoriasis: Diagnosis and Treatment; Bhutani, T., Liao, W., Nakamura, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–16. [Google Scholar]
- Huang, T.-H.; Lin, C.-F.; Alalaiwe, A.; Yang, S.-C.; Fang, J.-Y. Apoptotic or Antiproliferative Activity of Natural Products against Keratinocytes for the Treatment of Psoriasis. Int. J. Mol. Sci. 2019, 20, 2558. [Google Scholar] [CrossRef]
- Georgescu, S.R.; Tampa, M.; Caruntu, C.; Sarbu, M.I.; Mitran, C.I.; Mitran, M.I.; Matei, C.; Constantin, C.; Neagu, M. Advances in understanding the immunological pathways in Psoriasis. Int. J. Mol. Sci. 2019, 20, 739. [Google Scholar] [CrossRef]
- Orsmond, A.; Bereza-Malcolm, L.; Lynch, T.; March, L.; Xue, M. Skin barrier dysregulation in psoriasis. Int. J. Mol. Sci. 2021, 22, 10841. [Google Scholar] [CrossRef]
- Murphrey, M.B.; Miao, J.H.; Zito, P.M. Histology, Stratum Corneum; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Kaliyadan, F. The Dermoscopic Auspitz Sign. Indian Derm. Online J. 2018, 9, 290–291. [Google Scholar] [CrossRef]
- Galluzzo, M.; Talamonti, M.; Di Stefani, A.; Chimenti, S. Linear psoriasis following the typical distribution of the sciatic nerve. J. Dermatol. Case Rep. 2015, 9, 6–11. [Google Scholar] [CrossRef]
- Sarac, G.; Koca, T.T.; Baglan, T. A brief summary of clinical types of psoriasis. Nothern Clin. Istanb. 2016, 3, 79–82. [Google Scholar]
- Heidenreich, R.; Röcken, M.; Ghoreschi, K. Angiogenesis drives psoriasis pathogenesis. Int. J. Exp. Pathol. 2009, 90, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Koprowski, R.; Wilczyński, S.; Wróbel, Z.; Błońska-Fajfrowska, B. Calibration and segmentation of skin areas in hyperspectral imaging for the needs of dermatology. BioMed. Eng. Online 2014, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Hindelang, B.; Aguirre, J.; Schwarz, M.; Berezhnoi, A.; Eyerich, K.; Ntziachristos, V.; Biedermann, T.; Darsow, U. Non-invasive imaging in dermatology and the unique potential of raster-scan optoacoustic mesoscopy. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Polańska, A.; Dańczak-Pazdrowska, A.; Jałowska, M.; Żaba, R.; Adamski, Z. Current applications of high-frequency ultrasonography in dermatology. Postepy Dermatol. Alergol. 2017, 34, 535–542. [Google Scholar] [CrossRef]
- Muralidharan, E.; Malhotra, S.; Singh, A. Can dermoscopy and ultrasonography be considered a prognostic tool in management of psoriasis? Indian J. Dermatol. 2021, 66, 704. Available online: http://www.e-ijd.org/text.asp?2021/66/6/704/336634 (accessed on 6 April 2022). [CrossRef]
- Marina, M.E.; Botar Jid, C.; Roman, I.I.; Mihu, C.M.; Tătaru, A.D. Ultrasonography in psoriatic disease. Med. Ultrason. 2015, 17, 377–382. [Google Scholar] [CrossRef][Green Version]
- El Gammal, S.; El Gammal, C.; Kaspar, K.; Pieck, C.; Altmeyer, P.; Vogt, M.; Ermert, H. Sonography of the Skin at 100 MHz Enables In Vivo Visualization of Stratum Corneum and Viable Epidermis in Palmar Skin and Psoriatic Plaquesy1. J. Invest. Dermatol. 1999, 113, 821–829. [Google Scholar] [CrossRef]
- Șomlea, M.; Boca, A.; Pop, A.; Ilieș, R.; Vesa, S.; Buzoianu, A.; Tătaru, A. High-frequency ultrasonography of psoriatic skin: A non-invasive technique in the evaluation of the entire skin of patients with psoriasis: A pilot study. Exp. Ther. Med. 2019, 18, 4981–4986. [Google Scholar] [CrossRef]
- Yazdanparast, T.; Yazdani, K.; Humbert, P.; Khatami, A.; Ahmad Nasrollahi, S.; Hassanzadeh, H.; Ehsani, A.H.; Firouzabadi, L.I.; Firooz, A. Comparison of biophysical, biomechanical and ultrasonographic properties of skin in chronic dermatitis, psoriasis and lichen planus. Med. J. Islamic Repub. Iran 2018, 32, 108. [Google Scholar] [CrossRef]
- Khlebnikova, A.; Molochkov, V.; Selezneva, E.; Belova, L.; Bezugly, A.; Sedova, T.; Molochkov, A. Basal cell carcinoma invasion depth determined with 30 and 75 MHz high-frequency ultrasound and histopathology—A comparative study. Med. Ultrason. 2020, 22, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Mandava, A.; Ravuri, P.R.; Konathan, R. High-resolution ultrasound imaging of cutaneous lesions. Indian J. Radiol. Imaging 2013, 23, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, A.G.M.; van de Kerkhof, P.C.M.; de Jonge, C.S.; Lucas, M.; Steenbergen, W.; Seyger, M.M.B. Clearing of psoriasis documented by laser Doppler perfusion imaging contrasts remaining elevation of dermal expression levels of CD31. Skin Res. Technol. 2015, 21, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.K.; Herrick, A.L.; Moore, T.L.; King, T.A.; Griffiths, C.E.M. Dual wavelength (532 and 633 nm) laser Doppler imaging of plaque psoriasis. Br. J. Dermatol. 2005, 152, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Briers, D.; Duncan, D.D.; Hirst, E.; Kirkpatrick, S.J.; Larsson, M.; Steenbergen, W.; Stromberg, T.; Thompson, O.B. Laser speckle contrast imaging: Theoretical and practical limitations. J. Biomed. Opt. 2013, 18, 066018. Available online: http://biomedicaloptics.spiedigitallibrary.org/article.aspx?doi=10.1117/1.JBO.18.6.066018 (accessed on 6 April 2022). [CrossRef] [PubMed]
- Briers, J.D.; McNamara, P.M.; O’Connell, M.L. Laser Speckle Contrast Analysis (LASCA) for Measuring Blood Flow. Microcirc. Imaging 2012, 147–163. [Google Scholar] [CrossRef]
- Raman, M.S.; Snekhalatha, U.; Nelufer, K.; Srivastava, S.; Narasimhan, M. Thermal imaging method in the evaluation of psoriasis in upper limb region. IOP Conf. Ser. Mater. Sci. Eng. 2020, 912, 062026. Available online: https://iopscience.iop.org/article/10.1088/1757-899X/912/6/062026 (accessed on 6 April 2022). [CrossRef]
- Zalewska, A.; Wiecek, B.; Sysa-Jedrzejowska, A.; Gralewicz, G.; Owczarek, G. Qualitative thermograhic analysis of psoriatic skin lesions. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; pp. 1192–1195. Available online: http://ieeexplore.ieee.org/document/1403381/ (accessed on 6 April 2022).
- Montero-Vilchez, T.; Segura-Fernández-Nogueras, M.-V.; Pérez-Rodríguez, I.; Soler-Gongora, M.; Martinez-Lopez, A.; Fernández-González, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin Barrier Function in Psoriasis and Atopic Dermatitis: Transepidermal Water Loss and Temperature as Useful Tools to Assess Disease Severity. J. Clin. Med. 2021, 10, 359. [Google Scholar] [CrossRef]
- Rehman, A.U.; Qureshi, S.A. A review of the medical hyperspectral imaging systems and unmixing algorithms’ in biological tissues. Photodiagnosis Photodyn. Ther. 2021, 33, 102165. [Google Scholar] [CrossRef]
- Nishidate, I.; Maeda, T.; Niizeki, K.; Aizu, Y. Estimation of melanin and hemoglobin using spectral reflectance images reconstructed from a digital RGB image by the Wiener estimation method. Sensors 2013, 13, 7902–7915. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).