1. Introduction
Diabetes mellitus is a chronic disease considered a growing global public health problem [
1]. It is estimated that, in 2019, diabetes affected approximately 463.0 million people worldwide, and this number will grow exponentially to 700.2 million by 2045 [
2]. Diabetes is defined as a metabolic disease characterized by persistent hyperglycemia, arising from the ineffective production of insulin or in its action, or both mechanisms, generating complications [
1].
The most common complication of diabetes is diabetic peripheral neuropathy, which usually gives rise to the diabetic foot. According to the International Working Group on Diabetic Foot (2001) [
3], a diabetic foot is defined as an infection, ulceration, or destruction of soft tissues that affects the lower limbs, affecting approximately 40 to 60 million people globally [
2].
One of the strategies to avoid diabetic foot complications is related to straightforward measures, such as care for the feet and the use of adapted shoes, and the inclusion of orthopedic orthosis [
4,
5]. In this context, it is essential to choose the appropriate materials for producing the orthosis to ensure that its use is a success, since each material has particularities that can cause foot changes [
4,
5]. The term “orthosis” is derived from
ortho, which means “to straighten” or “correct.” An orthosis is a brace or another wearable device used to achieve one or more of the following goals: improved mobility, immobilization of an injured part of the body to facilitate recovery, prevention of injuries, correction of biomechanical misalignments, pain reduction and weight-bearing, rehabilitation, and correction of a deformity.
Therefore, the materials need to have characteristics that reduce the pressures imposed on the sole to avoid excessive loads and forces [
6], and the ability to absorb energy without deforming the material during the step [
7].
Other important properties to be observed in an orthosis are the hardness and stress of rupture since the diabetic individual has reduced foot sensitivity. Thus, the greater the hardness of a material, the greater its resistance, leading to more excellent foot protection [
4]. Moreover, if the material presents high values of rupture stress, it may offer the orthosis more resistance, contributing directly to the product’s longevity [
8].
According to Cordeiro (2010) [
8], the density and capacity to absorb the moisture in the feet are attributes that deserve to be highlighted in the choice of materials for producing an orthosis. In the first case, the lower the density of a material, the more lightness it will offer to the orthosis, giving a smoother and more adaptable footstep. The ability to absorb foot moisture, on the other hand, provides the material with an important characteristic, as it prevents the orthosis from facilitating the the proliferation of microorganisms, which can result in opportunistic infections.
Therefore, these aspects justify the increase in research on the subject. The physical, mechanical, and chemical properties suggested in the production of an orthopedic orthosis for individuals with diabetic foot are indispensable for the final success and its applicability [
7,
8]. The objectives of this work included the physical characterization (properties of elastic deformation, resistance, durability, lightness, energy absorption, resistance to high temperatures, and chemical composition) favorable to the physical and mechanical characteristics of four industrial materials, namely silicone, Evapod, Podadur, and latex—a pre-vulcanized latex extracted from the tree trunk of
Hevea brasiliensis—to characterize some materials used for producing orthopedic orthoses for diabetic foot.
2. Materials and Methods
The criteria used for choosing the materials were based on information contained in the literature [
8]. The following materials were chosen: silicone (Redelease
®, São Paulo, SP, Brazil); pre-vulcanized latex of Hevea brasiliensis (Epoxfiber
®, Rio de Janeiro, RJ, Brazil); Evapod (Podaly Palmilhas LTDA ME, Brusque, SC, Brazil); ethyl vinyl acetate foam—Podadur (Podaly Palmilhas LTDA ME, Brusque, SC, Brazil). The pre-vulcanized latex of
Hevea brasiliensis is extracted from tree trunks and is an Amazon state natural latex rubber with clinical potential for tissue reparation [
4,
9]. These materials’ benefits for orthosis include standard industrial production, easy obtention, and low cost. Specific ASTM International rules were adopted to standardize all the tests (astm.org) (accessed on 20 April 2022).
2.1. Tensile Testing
The tensile tests (tension and deformation at rupture) were carried out in a universal testing machine, EMIC model DL3000 (Instron Brasil Equipamentos Científicos LTDA, São José dos Pinhais, PR, Brazil). The methodology used was based on the ASTM D882 [
10] standard for thin films. The test bodies (C.P.) were cut to dimensions of 12.6 cm × 1.2 cm (approximately) and adjusted to the claws of the equipment, whose initial separation was fixed at 10 cm. The traction speed was 12.5 mm/minute, and the load cell used was 500 N or 50 kgf. Five C.P.s were evaluated for each test. Due to the composition of the material and its specificities, latex used 10 C.P. for analysis.
2.2. Shore A Hardness Test
Shore A hardness was tested according to the ASTM D 2240 [
11] standard with a Shore A LEP006-2 digital durometer (Woltest Industria, Comércio, Importação e Exportação LTDA, São Paulo, SP, Brazil) and a thickness gauge with a LEP007 dial indicator (ZAAS brand)
®, São Paulo, SP, Brazil). The obtained C.P. had a minimum thickness of 6.0 mm with a flat and smooth surface. The C.P. was supported on a hard, flat, horizontal surface, where the durometer was kept in an upright position and away from the C.P., with the pressure base parallel to its surface. At each C.P., at least three measurements were made at points at least 5 mm apart and, concerning the edges, at least 12 mm. The 1.0 kg mass was used for tests with Shore A in the upper part of the Shore durometer support. The thickness of the C.P. was within 6.00 ± 7.00 mm. The time for which the pressure base was in firm contact with the C.P. was 1 s. The temperature during the analysis was 23 °C, with 56% relative humidity. We used only 1 (one) C.P. for the tests.
2.3. Resilience Testing
Resilience was performed following DIN 53512 [
12]. Measurements were made on the brand’s LEP012 impact resilometer (Maqtest
® Automação e Controle Industrial L.T.D.A., Franca, SP, Brazil). For the measurement of C.P., an analog LEP010 caliper (Mitutoyo Sul Americana L.T.D.A., Suzano, SP, Brazil) was used. The C.P. had a thickness of 12.5 ± 0.5 mm and a diameter ranging from 29 to 53 mm. After positioning the C.P. on the fixing plate of the device, the pendulum was released from the horizontal position six times in a row to ensure that it fell in the same place each time. Resilience was measured with the last three shocks for each C.P., using three C.P.s for each sample.
2.4. Density Testing
2.4.1. Pycnometry Test
The pycnometry test was carried out according to the ASTM D 297 [
13] standard, with an LEP004 analytical balance (Gehaka, São Paulo, SP, Brazil) and LEP004 muffle furnace (Thermo Scientific, Waltham, MA, USA). A 50 mL pycnometer was used to immerse the C.P. in the liquid. The temperature used during the analysis was 23 °C, and the relative humidity was 55%. The fluid used to determine the specific mass was distilled water. The number of C.P.s used to perform the test was two for each material.
2.4.2. Liquid Immersion Test
The liquid immersion test followed the ASTM D 297 [
13] standard, with the LEP004 analytical balance (Gehaka, São Paulo, SP, Brazil). A 1 L beaker was used to immerse the C.P. into the liquid. The specific mass of the C.P. was obtained as a function of the dry weight and wet weight measured on the scale and the specific mass of the fluid used. During the experiment, the temperature was 23 °C, and the relative humidity was 55%. The fluid used to determine the specific mass was absolute alcohol. The number of C.P.s used to perform the test was six. The specific mass was obtained using the following formula: specific mass (g/cm
3) = [fluid specific mass (g/cm
3) × dry weight (g)]/[dry weight (g) − wet weight (g)].
2.5. Scanning Electron Microscopy
Scanning electron microscopy analyses were performed, and the C.P. were first assembled in stubs for metallization in gold. Metallization was performed in a metallizer (QT150 ES—Quorum®, Laughton, UK), where the samples were exposed to gold for 10 min at 20 nm. After the previous preparation of the C.P., they were analyzed in the SEM microscope (InspectTM 550-FEI Company, Hillsboro, OR, USA) in scans of 30, 100, 300, and 500 µm.
2.6. Water Absorption Test
According to the ASTM 3575 [
14] standard, the water absorption technique was performed. Five C.P.s were used for each material, with dimensions of 50 × 50 mm. Before immersion, the five C.P.s were individually weighed to obtain the dry mass value. Then, the C.P.s were immersed in a container with distilled water. After 24 h of immersion, each C.P. was weighed to quantify the wet mass and dipped again. After 48 h, the test started, and the C.P.s were again removed from the water for subsequent weighing. The water absorption value for either 24 or 48 h was calculated using the following formula: water absorption (kg/m
2) = mass (wet) − mass (dry)/surface area of the specimen.
2.7. X-ray Diffraction Test
For configuration in the laboratory, an Xpert Pro M.P.D. (Malvern Panalytical Spectris Company, Almelo, The Netherlands) was used, using CoKα radiation (λ = 0.17889 nm) at 40 kV and 40 mA with parallel beam geometry, and using a hybrid monochromator composed of a mirror and two Ge crystals cut in the direction (220). The height of the beam emerging from the monochromator was 1.2 mm. A 1/4° divergent slot and a Soller slot in the 0.02 rad diffracted beam were used to control axial divergence.
The C.P.s measured in the Xpert Pro M.P.D.—Panalytical were prepared on a zero-background silicon plate whose dimensions were 25 mm in diameter and 2 mm in thickness, containing a cavity with a diameter of 10 mm and depth of 0.2 mm. The diffraction patterns were obtained in the range of 2θ = 10° to 100°, with a step of 0.013° and a total measurement time of 70 min.
Panalytical’s HighScore Plus software [
15] (Malvern Panalytical Spectris Company, Almelo, Netherlands) was used to adjust the theoretical curve to the experimental pattern to obtain the width at half height (F.W.H.M.) of the diffraction peaks. This peak width was calculated using the Split-PseudoVoigt function, which considers asymmetries and uses parameters on the left and right sides of the peak. Thus, the width at half height was calculated by the equation F.W.H.M. = F.W.H.M. (left) + F.W.H.M. (right)/2, where F.W.H.M. (left) and F.W.H.M. (right) represent the widths at half height to the left and right of the peak, respectively. In the function of the amorphous structure of latex C.P., it was not possible to perform this quantification by this method. The simulated characteristics of the most intense C.P. peaks were as follows: intensity—Podadur (483), Evapod (610), and silicone (48); position 2θ—Podadur (34.4), Evapod (34.5), and silicone (82.1); F.W.H.M. (degrees)—Podadur (0.32), Evapod (0.27), and silicone (0.43).
According to the Scherrer equation [
16], the crystallite sizes are inversely proportional to the width at half the height of the diffraction peaks. Thus, the widths of the most intense peaks presented indicate that the crystallite sizes are of a nanometric order for this family of crystallographic planes, as they are significant peaks.
2.8. Thermogravimetry Test (TGA)
The thermal behavior of the materials was tested using the thermogravimetry method. The tests were performed using the STA 6000 simultaneous thermal analyzer (Perkin Elmer, Waltham, MA, USA) using a heating rate of 25 °C/min to 700 °C under a 50.0 mL/min nitrogen atmosphere.
2.9. X-ray Fluorescence Test
A ZSXMini II X-ray fluorescence spectrometer (Rigaku Corporation, The Woodlands, TX, USA) operating at 40 kV and 1.2 mA with a Palladium (Pd) tube for X-ray fluorescence was used. The analyses were semi-quantitative, with the ability to quantify elements with an atomic number equal to or greater than that of Fluorine to Uranium. The C.P. was placed between two layers of mylar, and this, in turn, was fixed by a cylinder with a diameter of 45 mm and a height of 25 mm. In this way, the C.P. was subjected to radiation.
2.10. Statistical Analysis
The mean ± standard error of the mean was used for the statistical analysis, comparing the four materials. The one-way analysis of variance (ANOVA) was used to analyze the physical and mechanical tests, with the Tukey post-hoc test. The two-way ANOVA was used for the water absorption test, with the Tukey post-hoc test. Statistical significance level of p < 0.05 was considered for all tests. The analyses were performed with the GraphPad Prism 7.00 software (GraphPad Software, La Jolla, CA, USA).
4. Discussion
Diabetic foot is an abnormal foot condition that can cause the loss of neural skin tissue, ulcerations, and even lower-limb amputations [
2]. Depending on the peculiarities of the patient, the materials may have advantages and disadvantages in specific properties. However, all physical, mechanical, and chemical characteristics present in the materials used in selecting the orthosis are indispensable for their applicability [
17]. In this context, the present study analyzed four different materials used to produce orthopedic orthoses for diabetic foot.
The latex composite material presented the highest rupture stress value among all materials—see
Table 1—providing the orthosis with high resistance to wear and durability, resisting the demands imposed on the material, a characteristic that directly influences the product’s longevity [
7].
Evapod and Podadur were the materials that stood out in terms of the elastic modulus, as presented in
Table 1, emphasizing Podadur, which showed a significant difference from the other materials. This property gives the material a greater capacity for elastic deformation, quickly adapting to changes in the environment, which helps in the oscillation changes that the foot undergoes during the step. It is important to note that Evapod and Podadur are materials with the same composition (ethylene vinyl acetate—EVA). The difference between these materials is found in their compaction. Podadur is a more compact material, while Evapod is a non-compact material with perforations throughout its plate, influencing its physical and mechanical properties.
The hardness of the material is a relevant parameter to be analyzed to offer the individual superior foot protection. Podadur showed the highest hardness value—see
Table 1—compared to the other materials. The higher the hardness, the greater the product’s ability to resist external damage such as perforation since the diabetic individual has a significant reduction in their foot sensitivity [
4]. The combinations of plastazote foam (PLZ), silicone gel (SG), and ethyl vinyl acetate foam (EVA) layers (from top to bottom) are recognized as the best among three-layer insoles. Moreover, the best three-layer insole is more effective in promoting a favorable stress and strain distribution than single-layer insoles, especially in dynamic mode [
18].
The decrease in the foot sensitivity of the diabetic individual may result in the absence of the perception of loads imposed on the feet, establishing an overload of localized energy in certain areas. Thus, material resilience plays an essential role in absorbing the energy generated during the step. The lower the resilience values, the greater the capacity of a material to absorb energy [
5]. In the present study, it was observed that Podadur was the material with the lowest resilience value—see
Table 1—with a higher capacity for absorbing energy, which can result in decreased plantar pressure.
The density parameter is another fundamental property to be analyzed when choosing a material for an orthopedic orthosis. The material density depends significantly on its chemical composition, the additives used, and the type of cell present in its structure [
8]. This property is directly related to the lightness of the product. The lower the value, the lighter the material. The density values found in the analyzed materials ranged from 0.9 to 1.5 g/cm
3 (
Table 1). Evapod and Podadur showed the lowest density values, respectively, demonstrating that these materials can give the orthosis more lightness.
According to the International Working Group on Diabetic Foot (2001) [
3], autonomic neuropathy, standard in diabetic conditions, leads to a decrease or the total absence of sweat secretion. Foss-Freitas, Marques Júnior, and Foss (2008) [
19] report that distal anhidrosis mainly affects the extremities of the lower limbs, which are dried out, creating cracks and fissures, associated with a reduction in thermoregulatory capacity and vasomotor abnormalities.
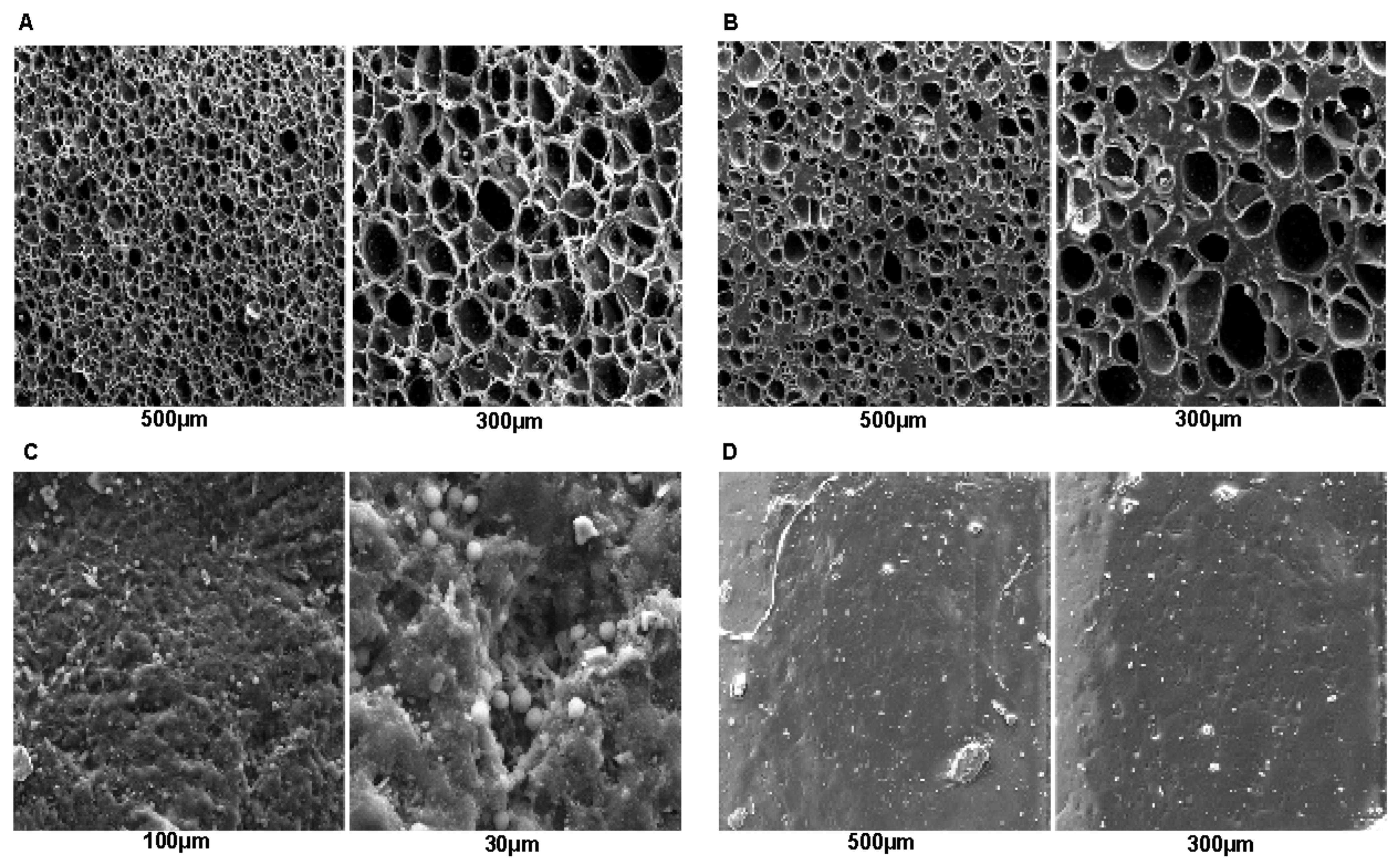
Suppose that there is no effective perspiration (sweat) in the feet. A material with open cells, such as Evapod (
Figure 1A), Podadur (
Figure 1B), or latex (
Figure 1C), does not show advantages in removing moisture from the feet. However, this characteristic makes these materials favorable in liquid absorption, corroborating the findings in
Table 2. It is worth noting that a material that absorbs many liquid types in its structure is more likely to have increased dimensions and weight, with a greater probability of causing surface microfractures and damage, compromising its mechanical properties [
20]. As shown in
Figure 1D, silicone, on the other hand, has an advantage in its morphological structure concerning the other materials due to the absence of open cells, which favors the absorption of liquid, corroborating the findings in
Table 2 and making it a better choice in orthosis production.
The atoms’ spatial ordering in each type of material also directly influences the morphological characteristics of the material. In the present study, it was found through the X-ray diffraction findings that the Evapod, Podadur, and silicone materials (
Figure 2A–C) present well-defined crystallographic planes, corroborating their morphological structures, and this may be one of the reasons that some materials have such favorable performance in physical and mechanical tests, as is the case of Evapod and Podadur [
21].
Regarding thermogravimetry (
Figure 2E), it is observed that the analyzed materials can withstand high temperatures before beginning their loss of mass or water; this is not a problem when producing an orthosis since the materials on the market present the characteristic of becoming moldable at temperatures between 50 and 80 °C, which gives these materials the ability to become resistant to ambient temperatures. Thus, the four analyzed materials have thermal characteristics favorable to their clinical applicability [
22].
The standard quality for a processed product is necessary to observe a material’s chemical composition, especially the verification of the heavy metal content [
23]. It can be seen in
Table 3 that all analyzed materials have many metals in their composition. Metals are widely used as a structural element to replace, reinforce, or stabilize rigid fabrics, which are generally subjected to tensile and compression loads [
24].
According to Groover (2014) [
25], metals are characterized by their properties of ductility, malleability, and good electrical and thermal conductivity. Latex was the material formed by the most significant number of metallic elements, which is the reason that this material performed well in the tensile test; see
Table 1. This was followed by Podadur and Evapod, which performed well in the hardness tests, Shore A test, and traction test. The presence of semi-metallic and non-metallic elements and gases is due to the addition of chemical substances during the material handling process.
The diabetic foot requires special attention. The proper distribution of weight on the feet is essential in preventing and treating injuries. Orthoses are useful tools in the care and rehabilitation of these patients, as indicated by the American Diabetes Association, in the presence of neuropathy, regardless of the degree of involvement. Therefore, changes in the protective and structural sensitivity of the foot are definitive indications for the materials for future clinical purposes. The orthoses allow the better distribution of the plantar load, relieve pressure points, which are especially harmful to diabetic feet, reduce the likelihood of pressure ulcers forming, and aid in wound healing by clearing wounded areas in cases of injured patients [
14,
15,
16,
17,
18]. The chosen materials were individually studied and characterized. However, using material multilayers could improve the cited advantages instead of a single monolayer orthosis.
The results showed that each material has its specificities (
Table 4), demonstrating its indication for different situations. Material characterization under the International Standard Test Methods is essential for optimizing orthopedic orthoses. The minimum material requirements and the professional’s expertise can produce a better product, tailored to the diabetic patient’s particularities.