Abstract
Valorization of lignocellulosic materials into value-added biobased chemicals is attracting increasing attention in the sustainable chemical industry. As an important building block, furoic acid has been commonly utilized to manufacture polymers, flavors, perfumes, bactericides, fungicides, etc. It is generally produced through the selective oxidation of furfural. In this study, we provide the results of the conversion of biomass-based xylose to furoic acid in a chemoenzymatic cascade reaction with the use of a heterogeneous chemocatalyst and a dehydrogenase biocatalyst. For this purpose, NaOH-treated waste shrimp shell was used as a biobased carrier to prepare high activity and thermostability of biobased solid acid catalysts (Sn-DAT-SS) for the dehydration of corncob-valorized xylose into furfural at 170 °C in 30 min. Subsequently, xylose-derived furfural and its derivative furfuryl alcohol were wholly oxidized into furoic acid with whole cells of E. coli HMFOMUT at 30 °C and pH 7.0. The productivity of furoic acid was 0.35 g furoic acid/(g xylan in corncob). This established chemoenzymatic process could be utilized to efficiently valorize biomass into value-added furoic acid.
1. Introduction
Lignocellulosic biomass, mostly from agricultural and forestry solid waste, is composed of three major components, lignin, cellulose, and hemicellulose, and has been valorized into high-value and sustainable products [1,2]. Furfural is a valuable biobased building material derived from C5-sugar in biomass, which is a versatile molecule for the production of resins, polymers, solvents, pharmaceuticals, bulk, and fine chemicals [3,4]. Various homogeneous and heterogeneous catalysts have been utilized for the valorization of lignocellulosic biomass into furfural [5,6,7]. Widely utilized heterogeneous chemocatalysts, including heteropolyacids, niobium-oxide, H-SAPO-34, MCM-41, zeolites, sulfonated montmorillonite, sulfonated kaolin, sulfonated perlite, and so on [3,4,8,9,10,11,12], exhibit excellent catalytic activity for catalyzing biomass, xylan, or xylose into furfural than homogeneous chemocatalysts and are easier to recover [13,14,15].
As an important furan biobased building block platform, furoic acid is commonly utilized to synthesize polymers, flavors, medicines, perfumes, bactericides, and fungicides [16,17]. Industrially, furoic acid is currently manufactured via a Cannizzaro reaction in an aqueous NaOH solution [18]. To avoid the excessive formation of undesirable byproducts, noble metal heterogeneous catalysts have been utilized for the oxidation of furfural into furoic acid [19]. Furfural was selectively oxidized to furoic acid over the Ag2O/CuO catalyst [20]. In order to synthesize furoic acid under mild conditions, biocatalytic synthesis has been developed due to high catalytic activity and selectivity [21]. B. cereus cells oxidized furfural into furoic acid with the yield of 95% at 30 °C [22]. Immobilized whole-cell biocatalysts transformed furfural to furoic acid at 30 °C in 24 h [23].
Sequential catalytic reactions play a pivotal role towards sustainable biomass valorization [24]. To efficiently valorize lignocellulosic biomass into furoic acid, the processes involving chemoenzymatically sequential conversion of biomass into furoic acid can been developed in a tandem reaction with catalysis of biomass into furfural by chemocatalysts and bio-oxidization of furfural into furoic acid by biocatalysts. In this work, one sulfonated tin-based Sn-DAT-SS was used as a heterogeneous chemocatalyst for the dehydration of biomass-derived xylose into furfural, and recombinant E. coli HMFOMUT cell was utilized as a dehydrogenase biocatalyst to transform xylose-valorized furfural into furoic acid. A green valorization of low-cost and renewable lignocellulosic materials into furoic acid was conducted in a tandem reaction with Sn-DAT-SS and HMFOMUT cells.
2. Materials and Methods
2.1. Materials and Reagents
Corncobs were collected from a village in Lianyungang City (Jiangsu province, China), which was composed of 34.1 wt % xylan. Shrimp shells (SSs) were obtained from local seafood market in Changzhou City (Jiangsu province, China). Sodium hydroxide (NaOH), ammonia, SnCl4·5H2O, sulfuric acid (H2SO4), and ethanol were of chemical grade and bought from Sinopharm Group Chemical Reagent Co., Ltd. (Shanghai, China).
2.2. Synthesis of Furfural from Xylose Hydrolysates via Dehydration by Sn-DAT-SS
Using NaOH-treated waste shrimp shell (SS) powers as a carrier, a sulfonated Sn-DAT-SS catalyst was prepared as previously reported [25]. Xylose-rich hydrolysate (20.0 g/L xylose) from corncob (75 g/L) was prepared using oxalic acid (5.0 wt %) as catalyst as previously reported [26]. Synthesis of furfural was conducted in an autoclave (Dantu Huanqiu Electrical Instrument Co., Zhenjiang, P.R. China). In a typical experiment, 30 mL xylose-rich hydrolysate (20.0 g/L xylose, pH 1.9) was mixed with different loadings of the Sn-DAT-SS catalyst (1–4 wt %). Then, the autoclave was heated through a jacket and quickly raised to the required reaction temperature (160–180 °C) and maintained for a specified reaction duration (10–50 min) by stirring (500 rpm). After the dehydration of xylose, the whole autoclave was quickly placed into an ice-water bath. Furfural yields (%) were calculated according to Equation (1). Three parallel experiments were necessary, and the deviations were lower than 5%.
2.3. Biosynthesis of Furoic Acid
Recombinant E. coli HMFOMUT containing dehydrogenase activity were activated for 8 h at 30 °C on Luria-Bertani (LB) medium supplemented with kanamycin (50 mg/L). The cells were then cultured and harvested as previously reported [27].
For the biotransformation of xylose-derived furfural into furoic acid, corncob-derived xylose (20.0 g/L, 30 mL, pH 1.9) and Sn-DAT-SS (2 wt %) were blended together in a 100-mL autoclave. After dehydration for 30 min at 170 °C by stirring (500 rpm), the formed furfural solution was cooled down and further adjusted to pH 6–8 with NaOH solution (3 M). Subsequently, the furfural dehydrogenation reaction was conducted with HMFOMUT cells (50.0 g/L) at 25–45 °C.
For the biotransformation of commercial furfuryl alcohol into furoic acid, synthesis of furoic acid was conducted using commercial furfuryl alcohol (75.0 mM) with HMFOMUT cells (50.0 g/L) at 30 °C and pH 7.0.
The yield of furoic acid was defined as follows:
2.4. Analytical Methods
5-HMF and furoic acid were measured by LC-2030C HPLC (3D SHIMADZU, Kyoto, Japan) equipped with Discovery® C18 (25 cm × 4.6 mm, 5 μm) at column temperature of 60 °C, eluted with CH3CN:0.4% (NH4)2SO4 (5:95) at 0.6 mL/min. The detection wavelength was 268 nm. Furfural and furfuryl alcohol were measured by LC-2030C HPLC (3D SHIMADZU, Kyoto, Japan) using NovaPak®CNHP Waters (3.9 × 150 mm) at a column temperature of 60 °C, eluted with CH3OH:0.4% (NH4)2SO4 (5:95) at 0.8 mL/min. Furfural was detected at 254 nm, and furfuryl alcohol was detected at 210 nm.
3. Results and Discussion
3.1. Dehydration of Xylose-Rich Hydrolysate to Furfural by Sn-DAT-SS
Dehydration conditions have profound influences on the furfural generation [28,29,30]. To improve Sn-DAT-SS’s dehydration ability, three parameters (e.g., Sn-DAT-SS loading, dehydration temperature, and dehydration time) were investigated on the influence of furfural formation in the aqueous media. The yields of furfural increased when the loadings of Sn-DAT-SS were raised from 1 to 2 wt %. Using 2 wt % loading of Sn-DAT-SS as a catalyst, the yield of furfural was 41.3%. Upon increasing Sn-DAT-SS’s loading from 2 to 4 wt %, no significant change was observed on furfural yields (Figure 1a). An excessive Sn-DAT-SS dose did not increase the acidity of catalytic media, which could not promote the furfural formation. Hence, the optimum Sn-DAT-SS loading was 2 wt %. The dehydration temperature and duration also have key roles in the valorization of biomass-derived xylose into furfural (Figure 1b). At different dehydration temperatures (160, 170, and 180 °C), the highest furfural yields were obtained as follows: Yield (170 °C, 30 min) = 41.3% > Yield (180 °C, 30 min) = 39.3% > Yield (160 °C, 40 min) = 31.0%. The optimum dehydration temperature and time were 170 °C and 30 min, respectively. A lower dehydration temperature might not supply sufficient energy to dehydrate xylose into furfural. At higher dehydration temperatures, it is evident that furfural shows signs of degrading, resulting in lower yields and the generation of unwanted byproducts [31]. In summary, the optimum Sn-DAT-SS loading, dehydration temperature, and time were 2 wt %, 170 °C, and 30 min, respectively. Under the optimum dehydration conditions, corncob-derived xylose (20.0 g/L) could be dehydrated to furfural (80.0 mM) with the yield of 41.3% (based on the xylan weight in corncob).
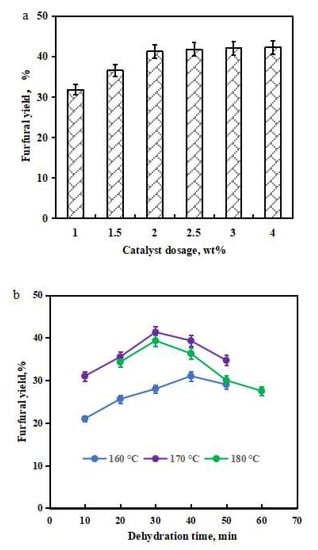
Figure 1.
Investigation of Sn-DAT-SS loading in the yield of furfural (30 mL corncob-derived xylose hydrolysate, Sn-DAT-SS 1–4 wt %, 170 °C, 30 min) (a). Investigation of dehydration temperature (160, 170, and 180 °C), and duration (10–60 min) in the furfural production (30 mL corncob-derived xylose, Sn-DAT-SS 2 wt %) (b).
The reuse of chemocatalysts is crucial in the industrial production of furfural [14,32]. In order to test the performance stability of shrimp shell-supported tin-based solid acid Sn-DAT-SS catalysts, recovered and repeated reuse of Sn-DAT-SS was carried out for seven batches. As depicted in Figure 2, furfural yield arrived at 41.3% in the first run. In the second run, no significant change was observed in the furfural yield. After the second run, the yields of furfural decreased. From the second to the seventh run, the yields decreased from 41.1% to 30.8%. From the first to the seventh run, the furfural concentration was in the range of 60–80 mM. SO42−/SnO2-attapulgite, SO42−/SnO2-DM, and Cl0.3-S-R could be reused for five batches [14,33,34]. CSUTS-CSW was reused for seven batches [35]. The Sn-DAT-150 SS demonstrated high stability for the dehydration of xylose to furfural under the synthesis conditions tested.
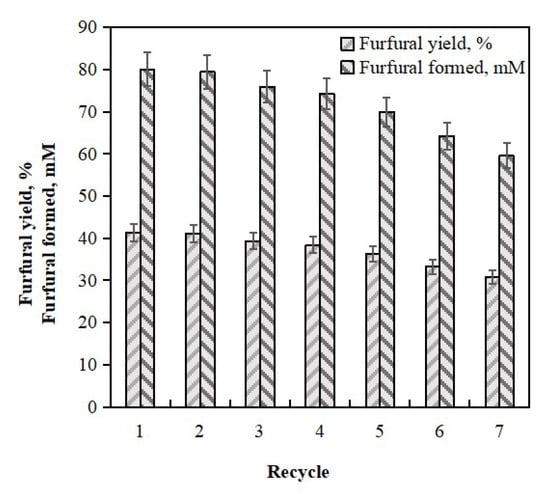
Figure 2.
Reuse of Sn-DAT-SS catalyst (30 mL corncob-derived xylose hydrolysate, Sn-DAT-SS 1–4 wt %, 170 °C, 30 min).
3.2. Bioconversion of Xylose-Valorized Furfural into Furoic Acid
Whole-cell biocatalysts provide unique advantages and have been widely utilized to efficiently synthesize value-added fine and bulk chemicals [36,37,38]. Biocatalytic temperature and pH have significant influences on the biocatalytic activity [39,40]. Using dilute xylose-derived furfural (75.0 mM, pH 7.0) as substrate, the influence of bioreduction temperatures (25–45 °C) on biocatalytic activity was illustrated in Figure 3a. It was observed that the reductase activity clearly increased with the increase in bioreduction temperature from 25 °C to 30 °C. Within 30 °C, a higher temperature could accelerate the biocatalytic activity. The highest reductase activity was observed at 30 °C. Above or below 30 °C, the reductase activity notably dropped (Figure 3a), possibly due to the thermal deactivation of reductase in the HMFOMUT cells during the bioreaction. Various bioreaction pH values in the range of 6–8 were examined for their effects on reductase activity. Low pH (≤6.5) gave a low reaction rate for the oxidation of furfural. Upon raising reaction pH from 6 to 7.0, biocatalytic activity increased. HMFOMUT cells displayed suitable furfural-oxidizing activities only within a narrow pH range (pH 6.5–7.0). The highest reductase activity was reached at pH 7.0 (Figure 3b). Over pH 7.0, a weak alkaline environment resulted in the decreased biocatalytic activity. Hence, the optimum bioreduction temperature and pH were 30 °C and 7.0, respectively.
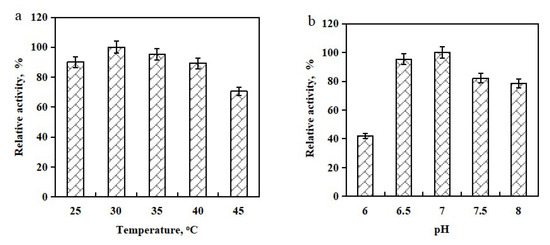
Figure 3.
Investigation of biocatalytic temperature (25, 30, 35, 40, and 45 °C) (a) and pH (6.0, 6.5, 7.0, 7.5, and 8.0) (b) on the oxidation of xylose-derived furfural.
HMFOMUT cells were utilized as biocatalysts for transforming dilute xylose-derived furfural (75.0 mM) into furoic acid. Within 5 h, the generation rate of furfuryl alcohol was quicker than that of furoic acid. In 5 h, furfuryl alcohol was achieved at the maximum (61.2 mM), while furoic acid was observed at 4.7 mM. After 5 h, furfuryl alcohol was biotransformed into furoic acid. Upon bioconversion for 60 h, furfural was converted into 72.0 mM furoic acid with the yield of 96% (based on the furfural) (Figure 4a). Upon biotransformation from 60 to 100 h, small amounts of furfural and furfural-derived furfuryl alcohol were oxidized gradually, and the yield of furoic acid increased slowly. In 100 h, furfural and furfural-derived furfuryl alcohol were wholly transformed into furoic acid. Furthermore, synthesis of furoic acid was conducted using commercial furfuryl alcohol (75.0 mM). Within 0–100 h, furfuryl alcohol was linearly transformed into furoic acid (Figure 4b). After 100 h, the furoic acid yield increased slowly. In 120 h, furfuryl alcohol was wholly oxidized into furoic acid. In the range of 20–100 h, furfural formed in the concentration of 1.2–6.3 mM. Furfural was further oxidized to furoic acid within 120 h. Industrially, furoic acid is produced from furfural via the Cannizzaro disproportionation reaction in alkali solution, which needs the addition of H2SO4 to neutralize the reaction mixture, obtaining furfuryl alcohol, furoic acid, and bisulfate salt [17]. Dehydrogenase from C. testosteroni, E. coli, N. corallina, and G. oxydans could be used as biocatalysts for the production of furoic acid under ambient conditions [18,21,27,41,42]. HMFOMUT cells could wholly convert furfural and furfuryl alcohol into furoic acid with high bioreduction activity and adequate selectivity. An attractive alternative exists in the biocatalytic transformation that performs reactions with much higher selectivity under ambient conditions. Efforts with regard to biocatalysis have been made to achieve selective oxidation of furfural to furoic acid.
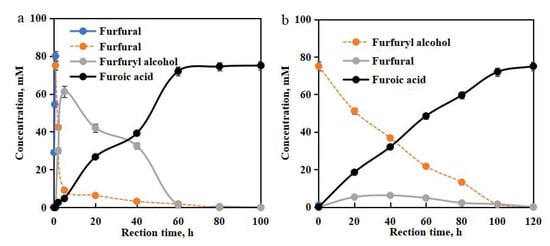
Figure 4.
Conversion of xylose-rich hydrolysate to furoic acid in a chemoenzymatic cascade reaction with chemocatalyst (Sn-DAT-SS 2 wt %, 170 °C, pH 1.9) and biocatalyst (HMFOMUT cell 50 g/L, 30 °C, pH 7.0) (a). Conversion of furfuryl alcohol into furoic acid (HMFOMUT cell 50 g/L, 30 °C, pH 7.0) (b).
3.3. Mass Balance from Corncob to Furans in Chemoenzymatic Cascade Reaction
The calculated mass balance from corncob to furans is illustrated in Figure 5. In 1 L deionized water containing oxalic acid (50.0 g), corncob powders (75.0 g, dry weight) composed of 25.58 g xylan were placed into an autoclave. After incubation for 40 min at 140 °C under the agitation of 500 rpm, the xylose-rich hydrolysate containing 20.0 g xylose was obtained and placed into a 3 L autoclave. The xylose hydrolysate was separated by simple filtration, and the corncob residual was collected. In this 3 L autoclave containing 1 L of xylose hydrolysate, Sn-DAT-SS powder (20.0 g) was added to dehydrate xylose into furfural at 170 °C within 30 min. The formed furfural solution composed of 7.68 g furfural was regulated to pH 7.0 and further bioconverted into 8.96 g furoic acid by HMFOMUT cells (50.0 g) at 30 °C for 100 h. The sustainable transformation of renewable and low-cost bioresource into furans has gained increased attention in recent years [43,44,45,46]. One efficient chemoenzymatic valorization of lignocellulosic biomass into furoic acid can be realized in a cascade reaction with a heterogeneous chemocatalyst and HMFOMUT cell biocatalysis. The productivity of furoic acid reached 0.35 g furoic acid/(g xylan in corncob).
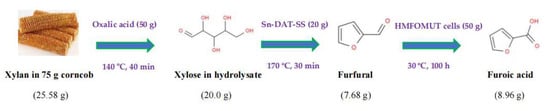
Figure 5.
Mass balance from corncob to furoic acid.
4. Conclusions
Various homogeneous and heterogeneous catalysts have been widely utilized for the production of furfural. Because of their stability, low corrosion, high catalytic activity, and easy recovery, heterogeneous catalysts have potential in the industrial production of furfural. As a value-added furfural derivative, biobased furoic acid has a market in the pharmaceutical and agrochemical fields. It can be prepared via a biocatalytic process by taking advantage of the biocatalytic oxidation of furfural. In this work, we reported a sustainable process for the chemoenzymatic cascade catalysis of corncob-valorized xylose into furoic acid, by first employing dehydration of xylose with a biocompatible heterogeneous chemocatalyst followed by bio-oxidation with dehydrogenase biocatalyst.
In this work, NaOH-treated waste shrimp shell (SS) was utilized as a biobased carrier to prepare the high activity and thermostability of the Tin-based heterogeneous catalyst Sn-DAT-SS. In the aqueous media, Sn-DAT-SS (2 wt %) could dehydrate corncob-valorized xylose (20.0 g/L xylose) into furfural (80.0 mM) with 41.3% yield (based on the xylan weight in corncob) at 170 °C in 30 min. Using corncob-derived xylose as substrate, the Sn-DAT-SS catalyst could be recovered and reused for seven runs. From the first to the seventh run, the formed furfural could reach 60–80 mM. Using whole cells of E. coli HMFOMUT as biocatalysts, furfural could be wholly oxidized into furoic acid at 30 °C and pH 7.0. During the bioconversion of furfural, a furfural derivative, furfuryl alcohol, was observed. Furfuryl alcohol could be further oxidized into furoic acid. Using 75.0 mM of furfuryl alcohol as substrate, furoic acid could be obtained with 100% yield. A green, low-cost valorization of renewable lignocellulosic materials into furoic acid was conducted in a tandem reaction with Sn-DAT-SS and HMFOMUTS cells. The productivity of furoic acid was 0.35 g furoic acid/g xylan (0.12 g furoic acid/g corncob).
Notably, the toxicity of furfural to the HNFOMUT cell would limit greater concentrations of furoic acid production. In addition, it is necessary to develop an efficient process for the recovery of furoic acid from the aqueous phase containing many different compounds. To synthesize furoic acid via the chemoenzymatic approach in a cost-effective manner, an important challenge is to seek out appropriate heterogeneous chemocatalysts and dehydrogenase biocatalysts. Related work is still ongoing in our laboratory that aims to provide an economic way for the valorization of biomass into value-added biobased chemicals.
Author Contributions
Conceptualization, methodology and writing—original draft, W.H.; data curation, software, supervision, and writing—review and editing, Y.H. and J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the Postgraduate Research & Practical Innovation Program of Jiangsu Province (KYCX21-2867; SJCX21-1244; SJCX21-1245; SJCX21-1246).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of Biomass into Chemicals over Metal Catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef] [PubMed]
- Kalhor, P.; Ghandi, K. Deep Eutectic Solvents as catalysts for upgrading biomass. Catalysts 2021, 11, 178. [Google Scholar] [CrossRef]
- Di, J.; Gong, L.; Yang, D.; He, Y.; Tang, Z.; Ma, C. Enhanced conversion of biomass to furfurylamine with high productivity by tandem catalysis with sulfonated perlite and ω-transaminase whole-cell biocatalyst. J. Biotechnol. 2021, 334, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zha, J.; Pan, L.; Ma, C.; He, Y.-C. Highly efficient conversion of sunflower stalk-hydrolysate to furfural by sunflower stalk residue-derived carbonaceous solid acid in deep eutectic solvent/organic solvent system. Bioresour. Technol. 2022, 351, 126945. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Tong, X.; Yu, L.; Zhang, M.; Yan, Y.; Zhuang, X. A catalytic oxidative valorization of biomass-derived furfural with ethanol by copper/azodi-carboxylate system. Catal. Today 2019, 319, 100–104. [Google Scholar] [CrossRef]
- Choudhary, V.; Pinar, A.; Sandler, S.; Vlachos, D.; Lobo, R. Xylose isomerization to xylulose and its dehydration to furfural in aqueous media. ACS Catal. 2011, 1, 1724–1728. [Google Scholar] [CrossRef]
- Tau, L.-Y.; Nurabiyiah, M.; Nor, M.-M.-Y. Furfural production from oil Palm biomass using a biomass-derived supercritical ethanol solvent and formic acid catalyst. Procedia Eng. 2016, 148, 392–400. [Google Scholar]
- He, Y.; Jiang, C.; Chong, G.; Di, J.; Wu, Y.; Wang, B.; Xue, X.; Ma, C. Chemical-enzymatic conversion of corncob-derived xylose to furfuralcohol by the tandem catalysis with SO42−/SnO2-kaoline and E. coli CCZU-T15 cells in toluene–water media. Bioresour. Technol. 2017, 245, 841–849. [Google Scholar] [CrossRef]
- Garcia-Sancho, C.; Sadaba, I.; Moreno-Tost, R.; Merida-Robles, J.; Santamaria-Gonzalez, J.; Lopez-Granados, M.; Maireles-Torres, P. Dehydration of xylose to furfural over MCM-41-supported niobium-oxide catalysts. ChemSusChem 2013, 6, 635–642. [Google Scholar] [CrossRef]
- Huang, Y.; Liao, X.; Deng, Y.; He, Y. Co-catalysis of corncob with dilute formic acid plus solid acid SO42−/SnO2-montmorillonite under the microwave for enhancing the biosynthesis of furfuralcohol. Catal. Lett. 2019, 120, 38–41. [Google Scholar] [CrossRef]
- Kaiprommarat, S.; Kongparakul, S.; Reubroycharoen, P.; Guan, G.-Q.; Samar, C. Highly efficient sulfonic MCM-41 catalyst for furfural production: Furan-based biofuel agent. Fuel 2016, 174, 189–196. [Google Scholar] [CrossRef]
- Li, X.-Y.; Yang, J.-X.; Xu, R.; Lu, L.-F.; Kong, F.-K.; Liang, M.; Jiang, L.-J.; Nie, S.-X.; Si, C.-L. Kinetic study of furfural production from Eucalyptus sawdust using H-SAPO-34 as solid Brønsted acid and Lewis acid catalysts in biomass-derived solvents. Ind. Crop. Prod. 2019, 135, 196–205. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Li, Q.; Zhang, P.-Q.; Ma, C.-L.; Xu, J.-H.; He, Y.-C. Catalytic conversion of corncob to furfuryl alcohol in tandem reaction with in-loaded sulfonated zeolite and NADPH-dependent reductase biocatalyst. Bioresour. Technol. 2021, 320, 124267. [Google Scholar] [CrossRef]
- He, Y.-C.; Ding, Y.; Ma, C.-L.; Di, J.-H.; Jiang, C.-X.; Li, A.-T. One-pot conversion of biomass-derived xylose to furfuralcohol by a chemo-enzymatic sequential acid-catalyzed dehydration and bioreduction. Green Chem. 2017, 19, 3844–3850. [Google Scholar] [CrossRef]
- Kim, H.; Yang, S.; Kim, D. One-pot conversion of alginic acid into furfural using Amberlyst-15 as a solid acid catalyst in gamma-butyrolactone/water co-solvent system. Environ. Res. 2020, 187, 109667. [Google Scholar] [CrossRef]
- Papanikolaou, G.; Lanzafame, P.; Perathoner, S.; Centi, G.; Cozza, D.; Giorgianni, G.; Migliori, M.; Giordano, G. High performance of Au/ZTC based catalysts for the selective oxidation of bio-derivative furfural to 2-furoic acid. Catal. Commun. 2021, 149, 106234. [Google Scholar] [CrossRef]
- Douthwaite, M.; Huang, X.; Iqbal, S.; Miedziak, P.; Brett, G.; Kondrat, S.; Edwards, J.; Sankar, M.; Knight, D.; Bethell, D.; et al. The controlled catalytic oxidation of furfural to furoic acid using AuPd/MgOH2. Catal. Sci. Technol. 2017, 7, 5284–5293. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Zhang, X.; Zong, M.; Wang, C.; Li, N. Selective synthesis of 2-furoic acid and 5-hydroxymethyl-2-furancarboxylic acid from bio-based furans by recombinant Escherichia coli cells. J. Mol. Catal. 2019, 469, 68–74. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, S.; Li, Q.; Zhou, G.; Xia, H. Recent advances in the conversion of furfural into bio-chemicals through chemo- and bio-catalysis. RSC Adv. 2021, 11, 27042–27058. [Google Scholar] [CrossRef]
- Tian, Q.; Shi, D.; Sha, Y. CuO and Ag2O/CuO catalyzed oxidation of aldehydes to the corresponding carboxylic acids by molecular oxygen. Molecules 2008, 13, 948–957. [Google Scholar] [CrossRef]
- Peng, B.; Ma, C.; Zhang, P.; Wu, C.; Wang, Z.; Li, A.; He, Y.; Yang, B. An effective hybrid strategy for converting rice straw to furoic acid by tandem catalysis via Sn-sepiolite combined with recombinant E coli whole cells harboring horse liver alcohol dehydrogenase. Green Chem. 2019, 21, 5914–5923. [Google Scholar] [CrossRef]
- Mitsukura, K.; Sato, Y.; Yoshida, T.; Nagasawa, T. Oxidation of heterocyclic and aromatic aldehydes to the corresponding carboxylic acids by Acetobacter and Serratia strains. Biotechnol. Lett. 2004, 26, 1643–1648. [Google Scholar] [CrossRef]
- Ma, Z.; Liao, Z.; Ma, C.; He, Y.; Gong, C.; Yu, X. Chemoenzymatic conversion of Sorghum durra stalk into furoic acid by a sequential microwave-assisted solid acid conversion and immobilized whole-cells biocatalysis. Bioresour. Technol. 2020, 311, 123474. [Google Scholar] [CrossRef]
- Bu, C.; Yan, Y.; Zou, L.; Zheng, Z.; Ouyang, J. One-pot biosynthesis of furfuryl alcohol and lactic acid via a glucose coupled biphasic system using single Bacillus coagulans NL01. Bioresour. Technol. 2020, 313, 123705. [Google Scholar] [CrossRef]
- Ni, J.; Li, Q.; Gong, L.; Liao, X.; Zhang, Z.; Ma, C.; He, Y. Highly efficient chemoenzymatic cascade catalysis of biomass into furfurylamine by a heterogeneous shrimp shell-based chemocatalyst and an ω-transaminase biocatalyst in deep eutectic solvent–water. ACS Sustain. Chem. Eng. 2021, 9, 13084–13095. [Google Scholar] [CrossRef]
- Gong, L.; Xu, Z.-Y.; Dong, J.-J.; Li, H.; Han, R.-Z.; Xu, G.-C.; Ni, Y. Composite coal fly ash solid acid catalyst in synergy with chloride for biphasic preparation of furfural from corn stover hydrolysate. Bioresour. Technol. 2019, 293, 122065. [Google Scholar] [CrossRef]
- Wang, Z.-W.; Gong, C.-J.; He, Y.-C. Improved biosynthesis of 5-hydroxymethyl-2-furancarboxylic acid and furoic acid from biomass valorized furans with high substrate tolerance of recombinant Escherichia coli HMFOMUT whole-cells. Bioresour. Technol. 2020, 303, 122930. [Google Scholar] [CrossRef]
- Qi, X.; Guo, H.; Li, L.; Smith, R. Acid-catalyzed dehydration of fructose into 5-hydroxymethylfurfural by cellulose-derived amorphous carbon. ChemSusChem 2012, 5, 2215–2220. [Google Scholar] [CrossRef]
- Qu, Y.; Huang, C.; Song, Y.; Zhang, J.; Chen, B. Efficient dehydration of glucose to 5-hydroxymethylfurfural catalyzed by the ionic liquid, 1-hydroxyethyl-3-methylimidazolium tetrafluoroborate. Bioresour. Technol. 2012, 121, 462–466. [Google Scholar] [CrossRef]
- Mittal, A.; Black, K.S.; Vinzant, B.T.; O’Brien, M.; Tucker, P.M.; Johnson, D.K. Production of Furfural from Process-Relevant Biomass-Derived Pentoses in a Biphasic Reaction System. ACS Sustain. Chem. Eng. 2017, 5, 5694–5701. [Google Scholar] [CrossRef]
- Schüth, F.; Rinaldi, R.; Meine, N.; Käldström, M.; Hilgert, J.; Rechulski, M.D.K. Mechanocatalytic depolymerization of cellulose and raw biomass and downstream processing of the products. Catal. Today 2014, 234, 24–30. [Google Scholar] [CrossRef]
- Bai, X.-W.; Li, J.; Jia, C.-X.; Shao, J.-G.; Yang, Q.; Chen, Y.-Q.; Yang, H.-P.; Wang, X.-H.; Chen, H.-P. Preparation of furfural by catalytic pyrolysis of cellulose based on nano Na/Fe-solid acid. Fuel 2019, 258, 116089. [Google Scholar] [CrossRef]
- Jiang, C.; Su, C.; Yang, S.; Ma, C.; He, Y. One-pot co-catalysis of corncob with dilute hydrochloric acid and tin-based solid acid for the enhancement of furfural production. Bioresour. Technol. 2018, 268, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Chen, D.; Li, W.; Zhang, H. Efficient conversion of corn stover to 5-hydroxymethylfurfural and furfural using a novel acidic resin catalyst in water-1, 4-dioxane system. Mol. Catal. 2021, 515, 111920. [Google Scholar] [CrossRef]
- Liang, J.; Zha, J.; Zhao, N.; Tang, Z.; He, Y.; Ma, C. Valorization of waste lignocellulose to furfural by sulfonated biobased heterogeneous catalyst using ultrasonic-treated chestnut shell waste as carrier. Processes 2021, 9, 2269. [Google Scholar] [CrossRef]
- Li, Y.M.; Zhang, X.Y.; Li, N.; Xu, P.; Lou, W.Y.; Zong, M.H. Biocatalytic reduction of HMF to 2,5-bis(hydroxymethyl)furan by HMF-tolerant whole cells. ChemSusChem 2017, 10, 372–378. [Google Scholar] [CrossRef]
- Xu, Z.; Cheng, A.; Xing, X.; Zong, M.; Bai, Y.; Li, N. Improved synthesis of 2,5-bis(hydroxymethyl)furan from 5-hydroxymethylfurfural using acclimatized whole cells entrapped in calcium alginate. Bioresour. Technol. 2018, 262, 177–183. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Li, N.-W.; Guo, Z.; Zong, M.-H.; Li, N. Furan carboxylic acids production with high productivity by cofactor-engineered whole-cell biocatalysts. ChemCatChem 2020, 12, 3257–3264. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, L.; Wang, J.; Song, G.; Liu, H.; Cheng, H.; Yang, Z. Improving whole-cell biocatalysis by addition of deep eutectic solvents and natural deep eutectic solvents. ACS Sustain. Chem. Eng. 2017, 5, 5713–5722. [Google Scholar] [CrossRef]
- Liang, J.; Ji, L.; He, J.; Tang, S.; He, Y. Chemoenzymatic conversion of biomass-derived D-xylose to furfuryl alcohol with corn stalk-based solid acid catalyst and reductase biocatalyst in a deep eutectic solvent-water system. Processes 2022, 10, 113. [Google Scholar] [CrossRef]
- Pérez, H.-I.; Manjarrez, N.; Solís, A.; Luna, H.; Ramírex, M.-A.; Cassani, J. Microbial biocatalytic preparation of 2-furoic acid by oxidation of 2-furfuryl alcohol and 2-furanaldehyde with Nocardia corallina. Afr. J. Biotechnol. 2009, 8, 2279–2282. [Google Scholar]
- Zhou, X.; Zhou, X.; Xu, Y.; Chen, R.-R. Gluconobacter oxydans (ATCC 621H) catalyzed oxidation of furfural for detoxification of furfural and bioproduction of furoic acid. J. Chem. Technol. Biotechnol. 2017, 92, 1285–1289. [Google Scholar] [CrossRef]
- Dalvand, K.; Rubin, J.; Gunukula, S.; Wheeler, M.-C.; Hunt, G. Economics of biofuels: Market potential of furfural and its derivatives. Biomass Bioenerg. 2018, 115, 56–63. [Google Scholar] [CrossRef]
- Li, Q.; Ma, C.; Zhang, P.; Li, Y.; Zhu, X.; He, Y. Effective conversion of sugarcane bagasse to furfural by coconut shell activated carbon-based solid acid for enhancing whole-cell biosynthesis of furfurylamine. Ind. Crop. Prod. 2021, 160, 113169. [Google Scholar] [CrossRef]
- Liu, F.; Barrault, J.; De Oliveira Vigier, K.; Jérôme, F. Dehydration of highly concentrated solutions of fructose to 5-hydroxymethylfurfural in a cheap and sustainable choline chloride/carbon dioxide system. ChemSusChem 2012, 5, 1223–1226. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sadaba, I.; Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).