High-Property Anode Catalyst Compositing Co-Based Perovskite and NiFe-Layered Double Hydroxide for Alkaline Seawater Splitting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Materials
2.2. Material Characterizations
2.3. Electrochemical Measurements,
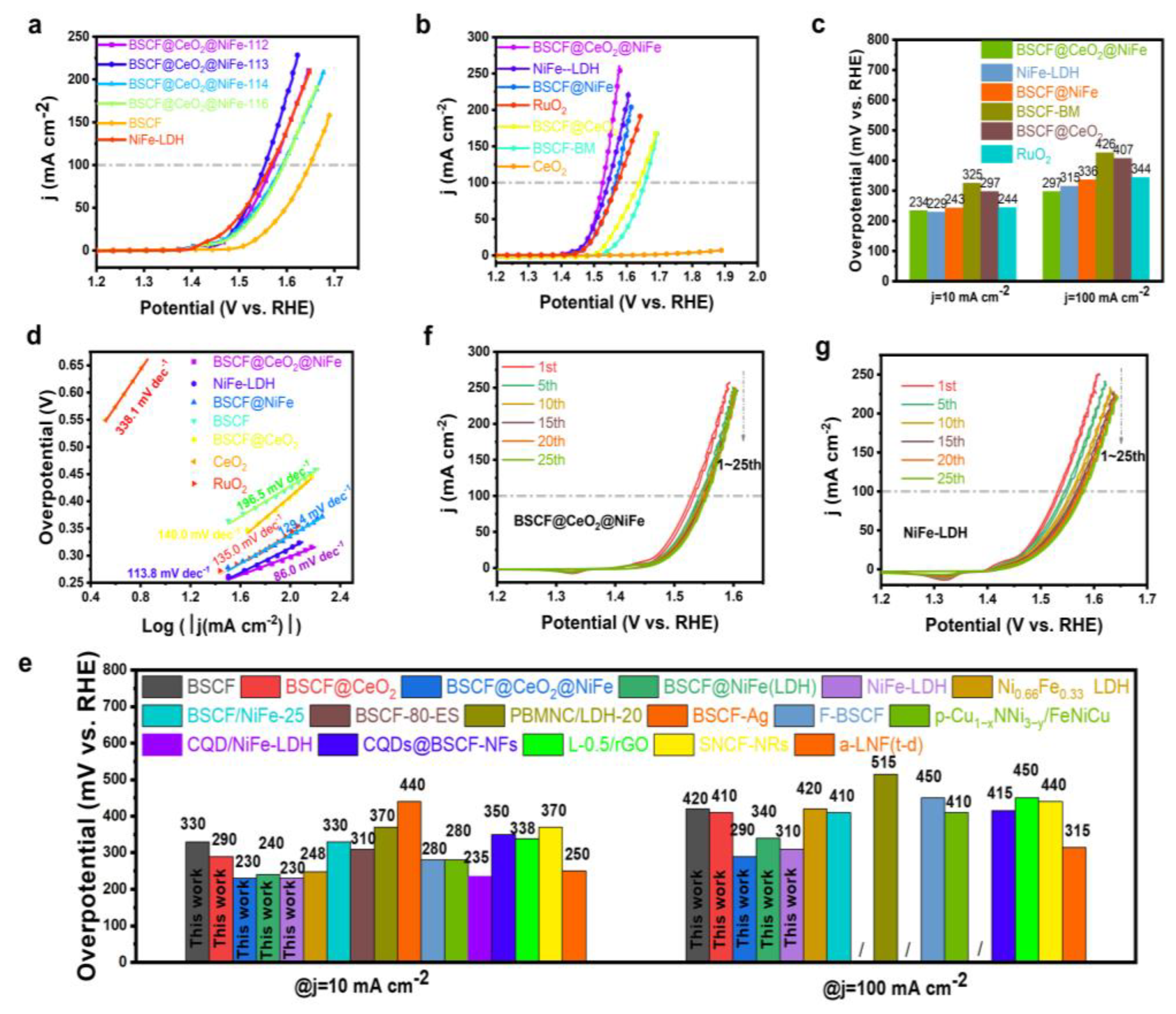
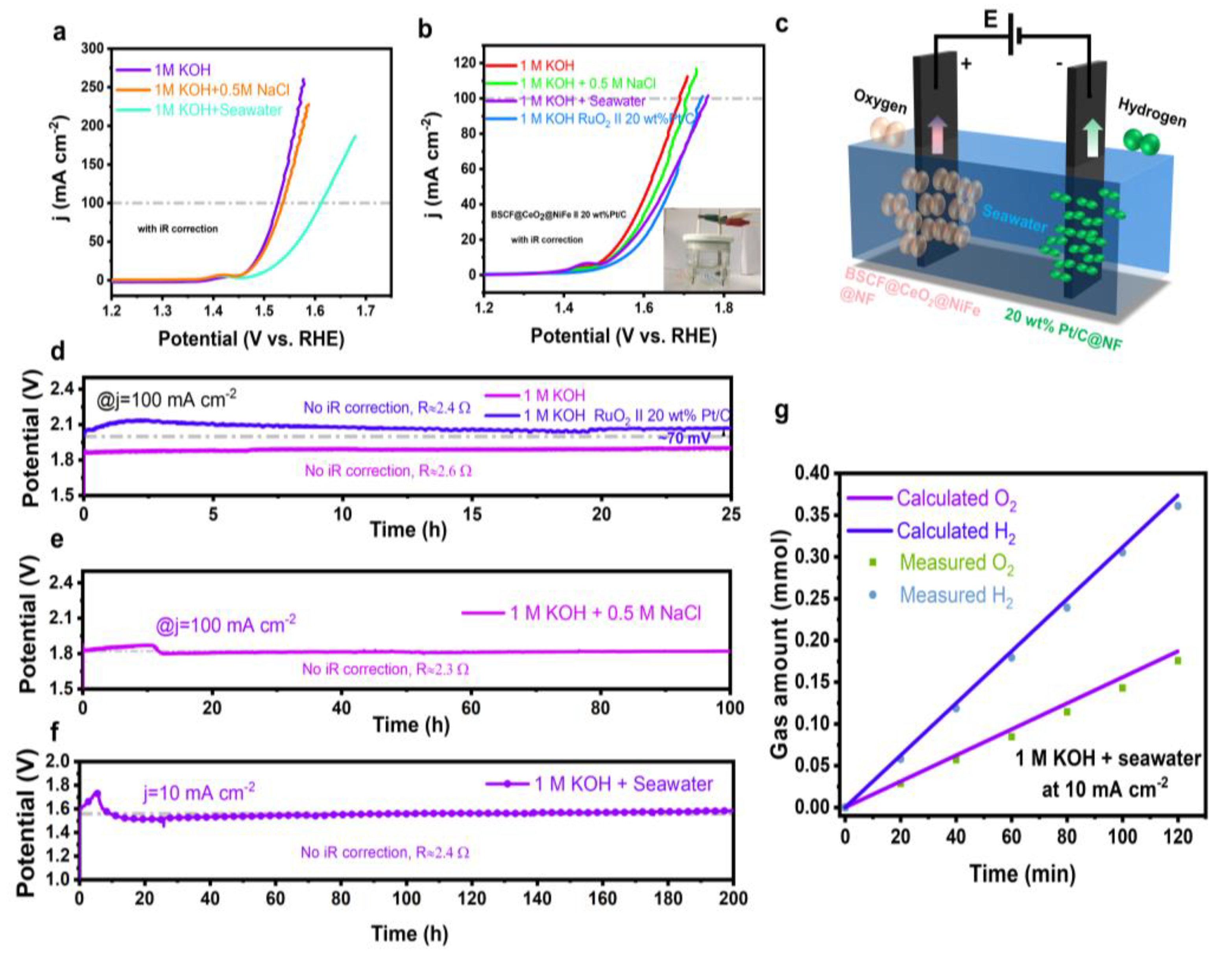
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, L.; Zhu, Q.; Song, S.; McElhenny, B.; Wang, D.; Wu, C.; Qin, Z.; Bao, J.; Yu, Y.; Chen, S.; et al. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 2019, 10, 5106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.-Y.; Wu, C.-X.; Feng, X.-J.; Tan, H.-Q.; Yan, L.-K.; Liu, Y.; Kang, Z.-H.; Wang, E.-B.; Li, Y.-G. Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@C electrocatalyst superior to Pt/C. Energy Environ. Sci. 2017, 10, 788–798. [Google Scholar] [CrossRef]
- Kuang, Y.; Kenney, M.J.; Meng, Y.; Hung, W.H.; Liu, Y.; Huang, J.E.; Prasanna, R.; Li, P.; Li, Y.; Wang, L.; et al. Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels. Proc. Natl. Acad. Sci. USA 2019, 116, 6624–6629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Guio, C.G.; Stern, L.A.; Hu, X. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem. Soc. Rev. 2014, 43, 6555–6569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Wang, T.; Zhang, J.; Liu, P.; Sun, H.; Zhuang, X.; Chen, M.; Feng, X. Accelerated Hydrogen Evolution Kinetics on NiFe-Layered Double Hydroxide Electrocatalysts by Tailoring Water Dissociation Active Sites. Adv. Mater. 2018, 30, 1706279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, W.; Cao, S.; Piao, L. Recent progress for hydrogen production by photocatalytic natural or simulated seawater splitting. Nano Res. 2020, 13, 2313–2322. [Google Scholar] [CrossRef]
- Cui, B.; Hu, Z.; Liu, C.; Liu, S.; Chen, F.; Hu, S.; Zhang, J.; Zhou, W.; Deng, Y.; Qin, Z.; et al. Heterogeneous lamellar-edged Fe-Ni(OH)2/Ni3S2 nanoarray for efficient and stable seawater oxidation. Nano Res. 2020, 14, 1149–1155. [Google Scholar] [CrossRef]
- Vos, J.G.; Wezendonk, T.A.; Jeremiasse, A.W.; Koper, M.T.M. MnOx/IrOx as Selective Oxygen Evolution Electrocatalyst in Acidic Chloride Solution. J. Am. Chem. Soc. 2018, 140, 10270–10281. [Google Scholar] [CrossRef] [Green Version]
- Li, B.Q.; Zhang, S.Y.; Tang, C.; Cui, X.; Zhang, Q. Anionic Regulated NiFe (Oxy)Sulfide Electrocatalysts for Water Oxidation. Small 2017, 13, 1700610. [Google Scholar] [CrossRef]
- Li, P.; Wang, S.; Samo, I.A.; Zhang, X.; Wang, Z.; Wang, C.; Li, Y.; Du, Y.; Zhong, Y.; Cheng, C.; et al. Common-Ion Effect Triggered Highly Sustained Seawater Electrolysis with Additional NaCl Production. Research 2020, 2020, 2872141. [Google Scholar] [CrossRef]
- Liu, G.; Xu, Y.; Yang, T.; Jiang, L. Recent advances in electrocatalysts for seawater splitting. Nano Mater. Sci. 2020. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, X.; Meng, X. Recent advances on electrocatalytic and photocatalytic seawater splitting for hydrogen evolution. Int. J. Hydrog. Energy 2021, 46, 9087–9100. [Google Scholar] [CrossRef]
- Hausmann, J.N.; Schlögl, R.; Menezes, P.W.; Driess, M. Is direct seawater splitting economically meaningful? Energy Environ. Sci. 2021, 14, 3679–3685. [Google Scholar] [CrossRef]
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Sadeghi Erami, R.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of low-grade and saline surface water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- Dresp, S.; Thanh, T.N.; Klingenhof, M.; Brückner, S.; Hauke, P.; Strasser, P. Efficient direct seawater electrolysers using selective alkaline NiFe-LDH as OER catalyst in asymmetric electrolyte feeds. Energy Environ. Sci. 2020, 13, 1725–1729. [Google Scholar] [CrossRef]
- Barreca, D.; Carraro, G.; Gasparotto, A.; Maccato, C.; Warwick, M.E.A.; Kaunisto, K.; Sada, C.; Turner, S.; Gönüllü, Y.; Ruoko, T.-P.; et al. Fe2O3-TiO2Nano-heterostructure Photoanodes for Highly Efficient Solar Water Oxidation. Adv. Mater. Interfaces 2015, 2, 1500313. [Google Scholar] [CrossRef]
- Duan, Y.; Yu, Z.Y.; Hu, S.J.; Zheng, X.S.; Zhang, C.T.; Ding, H.H.; Hu, B.C.; Fu, Q.Q.; Yu, Z.L.; Zheng, X.; et al. Scaled-Up Synthesis of Amorphous NiFeMo Oxides and Their Rapid Surface Reconstruction for Superior Oxygen Evolution Catalysis. Angew Chem. Int. Ed. Engl. 2019, 58, 15772–15777. [Google Scholar] [CrossRef]
- Gorlin, M.; Chernev, P.; Ferreira de Araujo, J.; Reier, T.; Dresp, S.; Paul, B.; Krahnert, R.; Dau, H.; Strasser, P. Oxygen Evolution Reaction Dynamics, Faradaic Charge Efficiency, and the Active Metal Redox States of Ni-Fe Oxide Water Splitting Electrocatalysts. J. Am. Chem. Soc. 2016, 138, 5603–5614. [Google Scholar] [CrossRef]
- Cox, C.R.; Lee, J.Z.; Nocera, D.G.; Buonassisi, T. Ten-percent solar-to-fuel conversion with nonprecious materials. Proc. Natl. Acad. Sci. USA 2014, 111, 14057–14061. [Google Scholar] [CrossRef] [Green Version]
- Oh, N.K.; Seo, J.; Lee, S.; Kim, H.J.; Kim, U.; Lee, J.; Han, Y.K.; Park, H. Highly efficient and robust noble-metal free bifunctional water electrolysis catalyst achieved via complementary charge transfer. Nat. Commun. 2021, 12, 4606. [Google Scholar] [CrossRef]
- Tsai, H.W.; Su, Y.H. Double Perovskite LaFe1-xNixO3 Coated with Sea Urchin-like Gold Nanoparticles Using Electrophoresis as the Photoelectrochemical Electrode to Enhance H2 Production via Surface Plasmon Resonance Effect. Nanomaterials 2022, 12, 622. [Google Scholar] [CrossRef] [PubMed]
- Nandikes, G.; Gouse Peera, S.; Singh, L. Perovskite-Based Nanocomposite Electrocatalysts: An Alternative to Platinum ORR Catalyst in Microbial Fuel Cell Cathodes. Energies 2022, 15, 272. [Google Scholar] [CrossRef]
- Yu, L.; Wu, L.; Song, S.; McElhenny, B.; Zhang, F.; Chen, S.; Ren, Z. Hydrogen Generation from Seawater Electrolysis over a Sandwich-like NiCoN|NixP|NiCoN Microsheet Array Catalyst. ACS Energy Lett. 2020, 5, 2681–2689. [Google Scholar] [CrossRef]
- Peera, S.G.; Koutavarapu, R.; Liu, C.; Rajeshkhanna, G.; Asokan, A.; Reddy, C.V. Cobalt Nanoparticle-Embedded Nitrogen-Doped Carbon Catalyst Derived from a Solid-State Metal-Organic Framework Complex for OER and HER Electrocatalysis. Energies 2021, 14, 1320. [Google Scholar] [CrossRef]
- Wang, P.; Qi, J.; Chen, X.; Li, C.; Li, W.; Wang, T.; Liang, C. Three-Dimensional Heterostructured NiCoP@NiMn-Layered Double Hydroxide Arrays Supported on Ni Foam as a Bifunctional Electrocatalyst for Overall Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 4385–4395. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.; Ma, L.; Guo, Y.; Sun, J.; Zhang, N.; Jiang, R. Water-Induced Formation of Ni2 P-Ni12 P5 Interfaces with Superior Electrocatalytic Activity toward Hydrogen Evolution Reaction. Small 2021, 17, e2006770. [Google Scholar] [CrossRef]
- Zou, Y.; Xiao, B.; Shi, J.-W.; Hao, H.; Ma, D.; Lv, Y.; Sun, G.; Li, J.; Cheng, Y. 3D hierarchical heterostructure assembled by NiFe LDH/(NiFe)Sx on biomass-derived hollow carbon microtubes as bifunctional electrocatalysts for overall water splitting. Electrochim. Acta 2020, 348, 136339. [Google Scholar] [CrossRef]
- Liu, C.; Ma, H.; Yuan, M.; Yu, Z.; Li, J.; Shi, K.; Liang, Z.; Yang, Y.; Zhu, T.; Sun, G.; et al. (NiFe)S2 nanoparticles grown on graphene as an efficient electrocatalyst for oxygen evolution reaction. Electrochim. Acta 2018, 286, 195–204. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, X.-J.; Cao, X.; Huang, X.; Tan, C.; Tian, J.; Liu, H.; Wang, J.; Zhang, H. Ni3S2 nanorods/Ni foam composite electrode with low overpotential for electrocatalytic oxygen evolution. Energy Environ. Sci. 2013, 6, 2921–2924. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Yang, G.; Song, W.; Zhong, W.; Wang, X.; Wang, M.; Sun, T.; Tang, Y. Heterogeneous Bimetallic Mo-NiPx/NiSy as a Highly Efficient Electrocatalyst for Robust Overall Water Splitting. Adv. Funct. Mater. 2021, 31, 2101532. [Google Scholar] [CrossRef]
- Dionigi, F.; Strasser, P. NiFe-Based (Oxy)hydroxide Catalysts for Oxygen Evolution Reaction in Non-Acidic Electrolytes. Adv. Energy Mater. 2016, 6, 1600621. [Google Scholar] [CrossRef]
- Cheng, F.; Feng, X.; Chen, X.; Lin, W.; Rong, J.; Yang, W. Synergistic action of Co-Fe layered double hydroxide electrocatalyst and multiple ions of sea salt for efficient seawater oxidation at near-neutral pH. Electrochim. Acta 2017, 251, 336–343. [Google Scholar] [CrossRef]
- Cao, S.; Huang, H.; Shi, K.; Wei, L.; You, N.; Fan, X.; Yang, Z.; Zhang, W. Engineering superhydrophilic/superaerophobic hierarchical structures of Co-CH@NiFe-LDH/NF to boost the oxygen evolution reaction. Chem. Eng. J. 2021, 422, 130123. [Google Scholar] [CrossRef]
- Hunter, B.M.; Hieringer, W.; Winkler, J.R.; Gray, H.B.; Müller, A.M. Effect of interlayer anions on [NiFe]-LDH nanosheet water oxidation activity. Energy Environ. Sci. 2016, 9, 1734–1743. [Google Scholar] [CrossRef] [Green Version]
- Andronescu, C.; Seisel, S.; Wilde, P.; Barwe, S.; Masa, J.; Chen, Y.T.; Ventosa, E.; Schuhmann, W. Influence of Temperature and Electrolyte Concentration on the Structure and Catalytic Oxygen Evolution Activity of Nickel-Iron Layered Double Hydroxide. Chemistry 2018, 24, 13773–13777. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Yang, N.; Yang, Y.; Wang, Q.; Xie, X.; Sun-Waterhouse, D.; Shang, L.; Zhang, T.; Waterhouse, G.I.N. Atomic Cation-Vacancy Engineering of NiFe-Layered Double Hydroxides for Improved Activity and Stability towards the Oxygen Evolution Reaction. Angew Chem. Int. Ed. Engl. 2021, 60, 24612–24619. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, Y.; Tahini, H.A.; Lin, Q.; Chen, Y.; Guan, D.; Zhou, C.; Hu, Z.; Lin, H.J.; Chan, T.S.; et al. Single-phase perovskite oxide with super-exchange induced atomic-scale synergistic active centers enables ultrafast hydrogen evolution. Nat. Commun. 2020, 11, 5657. [Google Scholar] [CrossRef]
- Guan, D.; Ryu, G.; Hu, Z.; Zhou, J.; Dong, C.L.; Huang, Y.C.; Zhang, K.; Zhong, Y.; Komarek, A.C.; Zhu, M.; et al. Utilizing ion leaching effects for achieving high oxygen-evolving performance on hybrid nanocomposite with self-optimized behaviors. Nat. Commun. 2020, 11, 3376. [Google Scholar] [CrossRef]
- She, S.; Zhu, Y.; Tahini, H.A.; Wu, X.; Guan, D.; Chen, Y.; Dai, J.; Chen, Y.; Tang, W.; Smith, S.C.; et al. Efficient Water Splitting Actualized through an Electrochemistry-Induced Hetero-Structured Antiperovskite/(Oxy)Hydroxide Hybrid. Small 2020, 16, e2006800. [Google Scholar] [CrossRef]
- Wu, X.; Miao, H.; Yin, M.; Hu, R.; Wang, F.; Zhang, H.; Xia, L.; Zhang, C.; Yuan, J. Biomimetic construction of bifunctional perovskite oxygen catalyst for zinc-air batteries. Electrochim. Acta 2021, 399, 139407. [Google Scholar] [CrossRef]
- Li, G.; Hou, S.; Gui, L.; Feng, F.; Zhang, D.; He, B.; Zhao, L. Carbon quantum dots decorated Ba0.5Sr0.5Co0.8Fe0.2O3- perovskite nanofibers for boosting oxygen evolution reaction. Appl. Catal. B Environ. 2019, 257, 117919. [Google Scholar] [CrossRef]
- Dai, Y.; Yu, J.; Zhang, Z.; Cheng, C.; Tan, P.; Shao, Z.; Ni, M. Interfacial La Diffusion in the CeO2/LaFeO3 Hybrid for Enhanced Oxygen Evolution Activity. ACS Appl. Mater. Interfaces 2021, 13, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Yu, Y.; Zhou, W.; Xu, X.; Zhu, Z. Highly defective CeO2 as a promoter for efficient and stable water oxidation. J. Mater. Chem. A 2015, 3, 634–640. [Google Scholar] [CrossRef]
- Wang, X.; Yan, H.; Zhang, J.; Hong, X.; Yang, S.; Wang, C.; Li, Z. Stamen-petal-like CeO2/NiMn layered double hydroxides composite for high-rate-performance supercapacitor. J. Alloy Compd. 2019, 810, 151911. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, H.; Xie, K. Activating Lattice Oxygen at the Twisted Surface in a Mesoporous CeO2 Single Crystal for Efficient and Durable Catalytic CO Oxidation. Angew. Chem. Int. Ed. Engl. 2021, 60, 5240–5244. [Google Scholar] [CrossRef]
- Wu, X.; Miao, H.; Hu, R.; Chen, B.; Yin, M.; Zhang, H.; Xia, L.; Zhang, C.; Yuan, J. A-site deficient perovskite nanofibers boost oxygen evolution reaction for zinc-air batteries. Appl. Surf. Sci. 2021, 536, 147806. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, F.; Zhang, R.; Evans, D.G.; Duan, X. Preparation of Layered Double-Hydroxide Nanomaterials with a Uniform Crystallite Size Using a New Method Involving Separate Nucleation and Aging Steps. Chem. Mater. 2002, 14, 4286–4291. [Google Scholar] [CrossRef]
- Xue, X.; Yu, F.; Peng, B.; Wang, G.; Lv, Y.; Chen, L.; Yao, Y.; Dai, B.; Shi, Y.; Guo, X. One-step synthesis of nickel–iron layered double hydroxides with tungstate acid anions via flash nano-precipitation for the oxygen evolution reaction. Sustain. Energy Fuels 2019, 3, 237–244. [Google Scholar] [CrossRef]
- Miao, H.; Chen, B.; Li, S.; Wu, X.; Wang, Q.; Zhang, C.; Sun, Z.; Li, H. All-solid-state flexible zinc-air battery with polyacrylamide alkaline gel electrolyte. J. Power Sources 2020, 450, 227653. [Google Scholar] [CrossRef]
- Han, Q.; Luo, Y.; Li, J.; Du, X.; Sun, S.; Wang, Y.; Liu, G.; Chen, Z. Efficient NiFe-based Oxygen Evolution Electrocatalysts and Origin of their Distinct Activity. Appl. Catal. B Environ. 2021, 304, 120937. [Google Scholar] [CrossRef]
- Dresp, S.; Dionigi, F.; Klingenhof, M.; Strasser, P. Direct Electrolytic Splitting of Seawater: Opportunities and Challenges. ACS Energy Lett. 2019, 4, 933–942. [Google Scholar] [CrossRef]
- Fabbri, E.; Nachtegaal, M.; Binninger, T.; Cheng, X.; Kim, B.J.; Durst, J.; Bozza, F.; Graule, T.; Schaublin, R.; Wiles, L.; et al. Dynamic surface self-reconstruction is the key of highly active perovskite nano-electrocatalysts for water splitting. Nat. Mater. 2017, 16, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Risch, M.; Grimaud, A.; May, K.J.; Stoerzinger, K.A.; Chen, T.J.; Mansour, A.N.; Shao-Horn, Y. Structural Changes of Cobalt-Based Perovskites upon Water Oxidation Investigated by EXAFS. J. Phys. Chem. C 2013, 117, 8628–8635. [Google Scholar] [CrossRef]
- Majee, R.; Islam, Q.A.; Bhattacharyya, S. Surface Charge Modulation of Perovskite Oxides at the Crystalline Junction with Layered Double Hydroxide for a Durable Rechargeable Zinc-Air Battery. ACS Appl. Mater. Interfaces 2019, 11, 35853–35862. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Chen, Z.G.; Chen, Y.; Su, C.; Tade, M.O.; Shao, Z. SrNb(0.1)Co(0.7)Fe(0.2)O(3-delta) perovskite as a next-generation electrocatalyst for oxygen evolution in alkaline solution. Angew Chem. Int. Ed. Engl. 2015, 54, 3897–3901. [Google Scholar] [CrossRef]
- Cheng, X.; Fabbri, E.; Kim, B.; Nachtegaal, M.; Schmidt, T.J. Effect of ball milling on the electrocatalytic activity of Ba0.5Sr0.5Co0.8Fe0.2O3 towards the oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 13130–13137. [Google Scholar] [CrossRef]
- May, K.J.; Carlton, C.E.; Stoerzinger, K.A.; Risch, M.; Suntivich, J.; Lee, Y.-L.; Grimaud, A.; Shao-Horn, Y. Influence of Oxygen Evolution during Water Oxidation on the Surface of Perovskite Oxide Catalysts. J. Phys. Chem. Lett. 2012, 3, 3264–3270. [Google Scholar] [CrossRef]
- Shen, T.H.; Spillane, L.; Vavra, J.; Pham, T.H.M.; Peng, J.; Shao-Horn, Y.; Tileli, V. Oxygen Evolution Reaction in Ba0.5Sr0.5Co0.8Fe0.2O3-delta Aided by Intrinsic Co/Fe Spinel-Like Surface. J. Am. Chem Soc. 2020, 142, 15876–15883. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Zhong, Y.; Bu, Y.; Chen, X.; Zhong, Q.; Liu, M.; Shao, Z. A Perovskite Nanorod as Bifunctional Electrocatalyst for Overall Water Splitting. Adv. Energy Mater. 2017, 7, 1602122. [Google Scholar] [CrossRef]
- Yu, L.; Wu, L.; McElhenny, B.; Song, S.; Luo, D.; Zhang, F.; Yu, Y.; Chen, S.; Ren, Z. Ultrafast room-temperature synthesis of porous S-doped Ni/Fe (oxy)hydroxide electrodes for oxygen evolution catalysis in seawater splitting. Energy Environ. Sci. 2020, 13, 3439–3446. [Google Scholar] [CrossRef]
- Li, Y.; Luo, W.; Wu, D.; Wang, Q.; Yin, J.; Xi, P.; Qu, Y.; Gu, M.; Zhang, X.; Lu, Z. Atomic-level correlation between the electrochemical performance of an oxygen-evolving catalyst and the effects of CeO2 functionalization. Nano Res. 2021, 1–7. [Google Scholar] [CrossRef]
- Kim, N.-I.; Cho, S.-H.; Park, S.H.; Lee, Y.J.; Afzal, R.A.; Yoo, J.; Seo, Y.-S.; Lee, Y.J.; Park, J.-Y. B-site doping effects of NdBa0.75Ca0.25Co2O5+δ double perovskite catalysts for oxygen evolution and reduction reactions. J. Mater. Chem. A 2018, 6, 17807–17818. [Google Scholar] [CrossRef]
- Ye, X.; Song, S.; Li, L.; Chang, Y.-C.; Qin, S.; Liu, Z.; Huang, Y.-C.; Zhou, J.; Zhang, L.-j.; Dong, C.-L.; et al. A’–B Intersite Cooperation-Enhanced Water Splitting in Quadruple Perovskite Oxide CaCu3Ir4O12. Chem. Mater. 2021, 33, 9295–9305. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Cui, Z.; Li, Y.; Xin, S.; Zhou, J.; Long, Y.; Jin, C.; Goodenough, J.B. Exceptional oxygen evolution reactivities on CaCoO3 and SrCoO3. Sci. Adv. 2019, 5, eaav6262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Xia, M.; Zhu, C.; Chen, D.; Du, F. Perovskite With Tunable Active-Sites Oxidation State by High-Valence W for Enhanced Oxygen Evolution Reaction. Front. Chem. 2021, 9, 809111. [Google Scholar] [CrossRef]
- Xiong, J.; Zhong, H.; Li, J.; Zhang, X.; Shi, J.; Cai, W.; Qu, K.; Zhu, C.; Yang, Z.; Beckman, S.P.; et al. Engineering highly active oxygen sites in perovskite oxides for stable and efficient oxygen evolution. Appl. Catal. B Environ. 2019, 256. [Google Scholar] [CrossRef]
- Long, J.; Zhang, J.; Xu, X.; Wang, F. Crystalline NiFe layered double hydroxide with large pore volume as oxygen evolution electrocatalysts. Mater. Chem. Phys. 2020, 254, 123496. [Google Scholar] [CrossRef]
- Mondal, S.; Majee, R.; Arif Islam, Q.; Bhattacharyya, S. 2D Heterojunction Between Double Perovskite Oxide Nanosheet and Layered Double Hydroxide to Promote Rechargeable Zinc-Air Battery Performance. ChemElectroChem 2020, 7, 5005–5012. [Google Scholar] [CrossRef]
- Göl, E.Y.; Aytekin, A.; Özkahraman, E.E.; Karabudak, E. Investigation of oxygen evolution reaction performance of silver doped Ba0.5Sr0.5Co0.8Fe0.2O3-δ perovskite structure. J. Appl. Electrochem. 2020, 50, 1037–1043. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, G.; Zhong, Y.; Chen, Y.; Ma, N.; Zhou, W.; Shao, Z. A surface-modified antiperovskite as an electrocatalyst for water oxidation. Nat. Commun. 2018, 9, 2326. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Liu, J.; Wu, X.; Liu, R.; Han, X.; Han, Y.; Huang, H.; Liu, Y.; Kang, Z. Carbon quantum dot/NiFe layered double-hydroxide composite as a highly efficient electrocatalyst for water oxidation. ACS Appl. Mater. Interfaces 2014, 6, 7918–7925. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.; Li, M.; Zhang, Y.-Q.; Sun, Y.-F.; Luo, J.-L. All-In-One Perovskite Catalyst: Smart Controls of Architecture and Composition toward Enhanced Oxygen/Hydrogen Evolution Reactions. Adv. Energy Mater. 2017, 7, 1700666. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, Y.; Chen, H.M.; Hu, Z.; Hung, S.F.; Ma, N.; Dai, J.; Lin, H.J.; Chen, C.T.; Zhou, W.; et al. An Amorphous Nickel-Iron-Based Electrocatalyst with Unusual Local Structures for Ultrafast Oxygen Evolution Reaction. Adv. Mater. 2019, 31, e1900883. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, R.; Liu, F.; Qiu, H.; Miao, H.; Wang, Q.; Zhang, H.; Wang, F.; Yuan, J. High-Property Anode Catalyst Compositing Co-Based Perovskite and NiFe-Layered Double Hydroxide for Alkaline Seawater Splitting. Processes 2022, 10, 668. https://doi.org/10.3390/pr10040668
Hu R, Liu F, Qiu H, Miao H, Wang Q, Zhang H, Wang F, Yuan J. High-Property Anode Catalyst Compositing Co-Based Perovskite and NiFe-Layered Double Hydroxide for Alkaline Seawater Splitting. Processes. 2022; 10(4):668. https://doi.org/10.3390/pr10040668
Chicago/Turabian StyleHu, Ruigan, Fuyue Liu, Haoqi Qiu, He Miao, Qin Wang, Houcheng Zhang, Fu Wang, and Jinliang Yuan. 2022. "High-Property Anode Catalyst Compositing Co-Based Perovskite and NiFe-Layered Double Hydroxide for Alkaline Seawater Splitting" Processes 10, no. 4: 668. https://doi.org/10.3390/pr10040668
APA StyleHu, R., Liu, F., Qiu, H., Miao, H., Wang, Q., Zhang, H., Wang, F., & Yuan, J. (2022). High-Property Anode Catalyst Compositing Co-Based Perovskite and NiFe-Layered Double Hydroxide for Alkaline Seawater Splitting. Processes, 10(4), 668. https://doi.org/10.3390/pr10040668







