Extraction and Purification of (E)-Resveratrol from the Bark of Conifer Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Moisture Content
2.4. Total Phenolic Assay
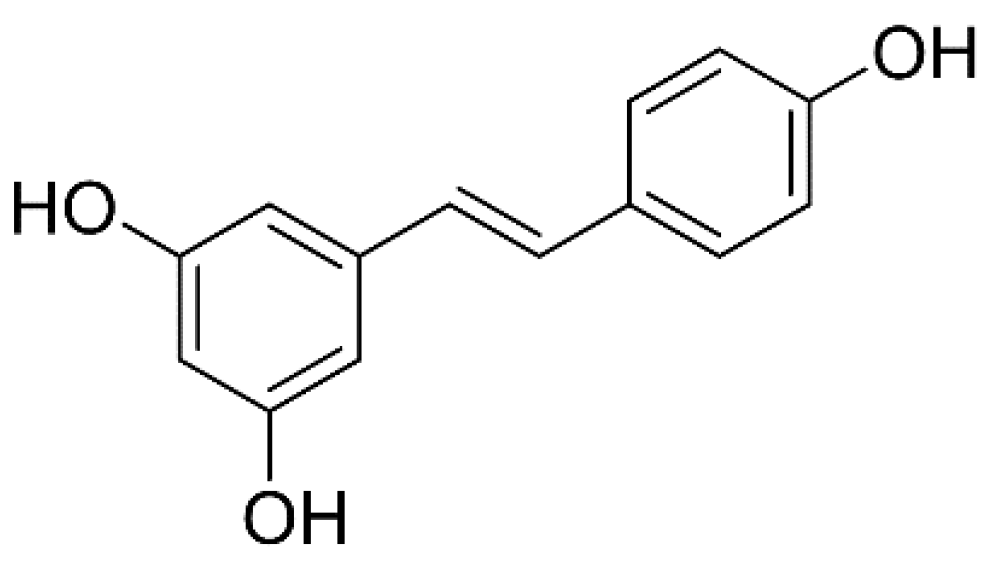
2.5. Quantification of (E)-Resveratrol
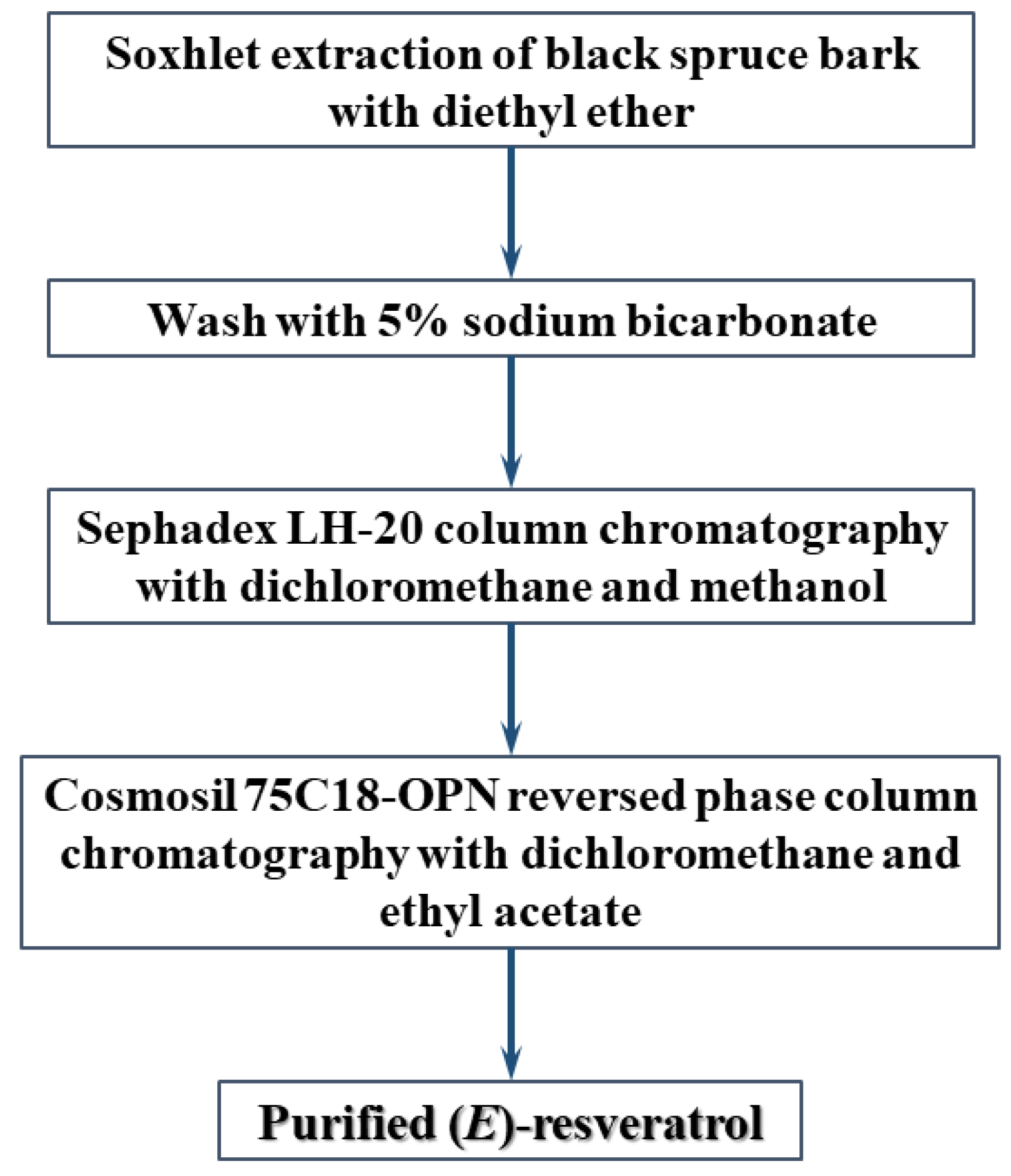
2.6. Extraction and Purification of (E)-Resveratrol
3. Results and Discussion
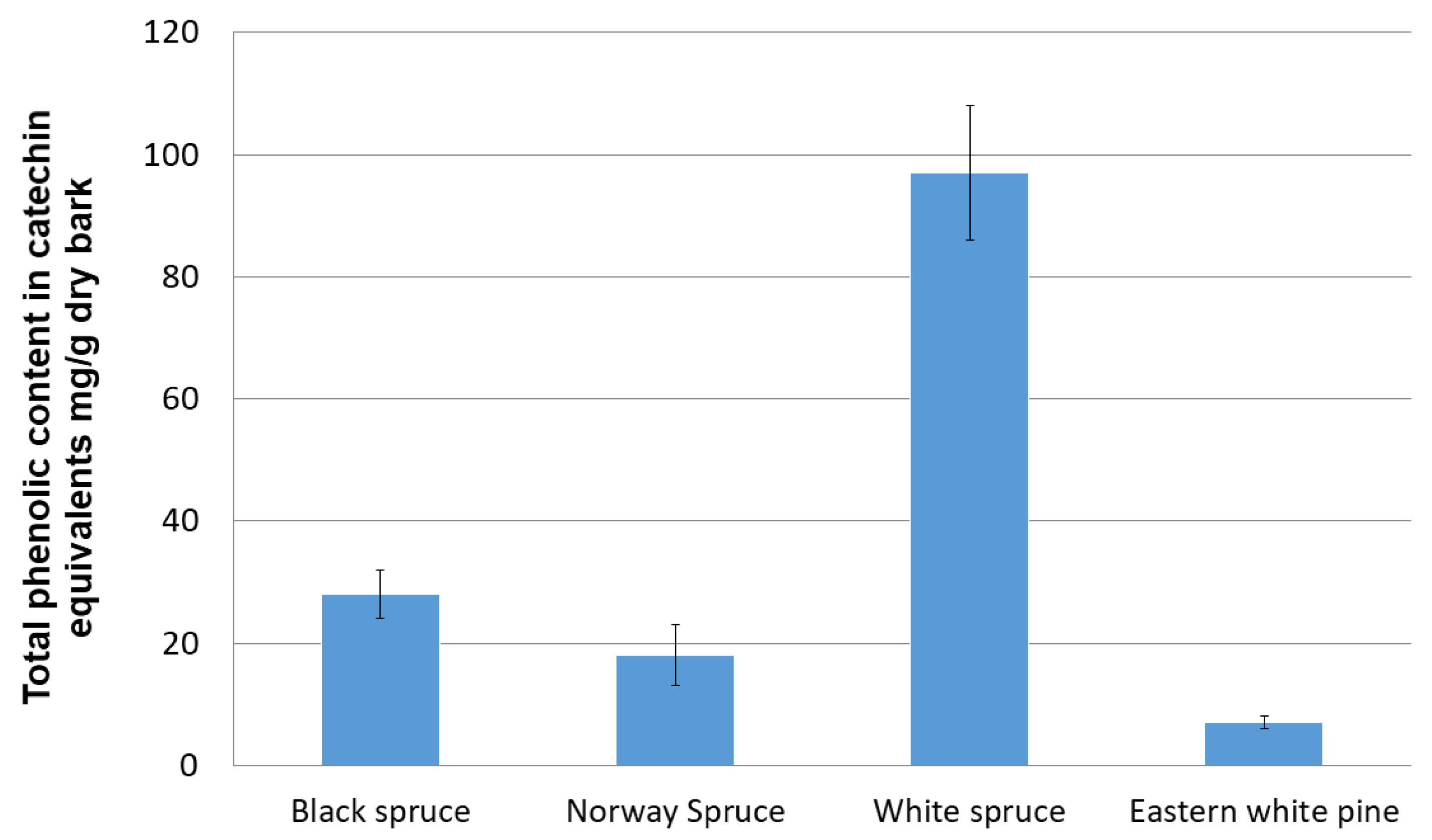
3.1. Total Phenolic Content and (E)-Resveratrol in Bark Extracts
3.2. A Method for the Extraction and Purification of (E)-Resveratrol from Black Spruce Bark
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. The world of resveratrol. Adv. Exp. Med. Biol. 2001, 492, 159–182. [Google Scholar] [PubMed]
- Shang, Y.J.; Qian, Y.P.; Liu, X.D.; Dai, F.; Shang, X.L.; Jia, W.Q.; Liu, Q.; Fang, J.G.; Zhou, B. Radical-scavenging activity and mechanism of resveratrol-oriented analogues: Influence of the solvent, radical, and substitution. J. Org. Chem. 2009, 74, 5025–5031. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, S.; Wolter, F.; Stein, J.M. Molecular mechanisms of the chemopreventive effects of resveratrol and its analogs in carcinogenesis. Mol. Nutr. Food Res. 2005, 49, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Holme, A.L.; Pervaiz, S. Resveratrol in cell fate decisions. J. Bioenerg. Biomembr. 2007, 39, 59–63. [Google Scholar] [CrossRef]
- Szkudelska, K.; Szkudelski, T. Resveratrol, obesity and diabetes. Eur. J. Pharmacol. 2010, 635, 1–8. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, J.; Shi, J. Anti-inflammatory activities of resveratrol in the brain: Role of resveratrol in microglial activation. Eur. J. Pharmacol. 2010, 636, 1–7. [Google Scholar] [CrossRef]
- Kumar, V.; Pandey, A.; Jahan, S.; Shukla, R.K.; Kumar, D.; Srivastava, A.; Singh, S.; Rajpurohit, C.S.; Yadav, S.; Khanna, V.K.; et al. Differential responses of trans-resveratrol on proliferation of neural progenitor cells and aged rat hippocampal neurogenesis. Sci. Rep. 2016, 6, 28142. [Google Scholar] [CrossRef]
- Bru, R.; Sellés, S.; Casado-Vela, J.; Belchí-Navarro, S.; Pedreño, M.A. Modified cyclodextrins are chemically defined glucan inducers of defense responses in grapevine cell cultures. J. Agric. Food Chem. 2006, 54, 65–71. [Google Scholar] [CrossRef]
- Shiraishi, M.; Chijiwa, H.; Fujishima, H.; Muramoto, K. Resveratrol production potential of grape flowers and green berries to screen genotypes for gray mold and powdery mildew resistance. Euphytica 2010, 176, 371–381. [Google Scholar] [CrossRef]
- Naiker, M.; Anderson, S.; Johnson, J.B.; Mani, J.S.; Wakeling, L.; Bowry, V. Loss of trans-resveratrol during storage and ageing of red wines. Aust. J. Grape Wine Res. 2020, 26, 385–387. [Google Scholar] [CrossRef]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Murrell, C.; Gerber, E.; Krebs, C.; Parepa, M.; Schaffner, U.; Bossdorf, O. Invasive knotweed affects native plants through allelopathy. Am. J. Bot. 2011, 98, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Fallopia japonica (Japanese knotweed): Invasive Species Compendium. Available online: https://www.cabi.org/isc/datasheet/23875 (accessed on 12 May 2018).
- Srinivas, G.; Babykutty, S.; Sathiadevan, P.P.; Srinivas, P. Molecular mechanism of emodin action: Transition from laxative ingredient to an antitumor agent. Med. Res. Rev. 2007, 27, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Berchová-Bímová, K.; Soltysiak, J.; Vach, M. Role of different taxa and cytotypes in heavy metals absorption in knotweeds (Fallopia). Sci. Agric. Bohem. 2014, 45, 11–18. [Google Scholar] [CrossRef]
- Reviving Resveratrol. Available online: http://cen.acs.org/articles/95/i10/Reviving-resveratrol.html (accessed on 15 May 2018).
- Hammerbacher, A.; Ralph, S.G.; Bohlmann, J.; Fenning, T.M.; Gershenzon, J.; Schmidt, A. Biosynthesis of the major tetrahydroxystilbenes in spruce, astringin and isorhapontin, proceeds via resveratrol and is enhanced by fungal infection. Plant Physiol. 2011, 157, 876–890. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Grigorchuk, V.P.; Ogneva, Z.V.; Suprun, A.R.; Dubrovina, A.S. Stilbene biosynthesis in the needles of spruce Picea jezoensis. Phytochemistry 2016, 131, 57–67. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Ogneva, Z.V.; Suprun, A.R.; Zhuravlev, Y.N. Expression of the stilbene synthase genes in the needles of spruce Picea jezoensis. Russ. J. Genet. 2016, 52, 1157–1163. [Google Scholar] [CrossRef]
- Jyske, T.; Kuroda, K.; Suuronen, J.; Pranovich, A.; Roig-Juan, S.; Aoki, D.; Fukushima, K. In planta localization of stilbenes within Picea abies phloem. Plant Physiol. 2016, 172, 913–928. [Google Scholar] [CrossRef]
- Zhuang, X.L.; Zhang, T.; Ma, S.J.; Dong, X.C. Selective on-line extraction of trans-resveratrol and emodin from Polygonum cuspidatum using molecularly imprinted polymer. J. Chromatogr. Sci. 2008, 46, 739–742. [Google Scholar] [CrossRef]
- Wang, D.G.; Liu, W.Y.; Chen, G.T. A simple method for the isolation and purification of resveratrol from Polygonum cuspidatum. J. Pharm. Anal. 2013, 3, 241–247. [Google Scholar] [CrossRef]
- Güder, A.; Korkmaz, H.; Gökce, H.; Alpaslan, Y.B.; Alpaslan, G. Isolation, characterization, spectroscopic properties and quantum chemical computations of an important phytoalexin resveratrol as antioxidant component from Vitis labrusca L. and their chemical compositions. Spectrochim. Acta Mol. Biomol. Spectrosc. 2014, 133, 378–395. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Zhang, Q.; Zhang, D.; Shi, Y.; Jiang, C.; Shi, X. Preliminary separation and purification of resveratrol from extract of peanut (Arachis hypogaea) sprouts by macroporous adsorption resins. Food Chem 2014, 145, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Miyaichi, Y.; Nunomura, N.; Kawata, Y.; Kizu, H.; Tomimori, T.; Watanabe, T.; Takano, A.; Malla, K.J. Studies on Nepalese crude drugs: Chemical constituents of Bhote Khair, the underground parts of Eskemukerjea megacarpum Hara. Chem. Pharm. Bull. 2006, 54, 136–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- García-Pérez, M.E.; Royer, M.; Herbette, G.; Desjardins, Y.; Pouliot, R.; Stevanovic, T. Picea mariana bark: A new source of trans-resveratrol and other bioactive polyphenols. Food Chem. 2012, 135, 1173–1182. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Marshall, D.A.; Stringer, S.J.; Spiers, J.D. Stilbene, Ellagic Acid, Flavonol, and Phenolic content of muscadine grape (Vitis rotundifolia Michx.) cultivars. Pharm. Crops 2012, 3, 69–77. [Google Scholar] [CrossRef]
- Prior, R.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the folin−ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Kanda, H.; Oishi, K.; Machmudah, S.; Diono, W.; Goto, M. Ethanol-free extraction of resveratrol and its glycoside from Japanese knotweed rhizome by liquefied dimethyl ether without pretreatments. Asia-Pac. J. Chem. Eng. 2021, 16, e2600. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Sato, M.; Kato, H.; Kureshiro, H.; Takagi, N. Nanoscale analysis of pharmacologically active catechins in body fluids by HPLC using borate complex extraction pretreatment. Talanta 1998, 46, 717–726. [Google Scholar] [CrossRef]
- Springsteen, G.; Wang, B. A detailed examination of boronic acid-diol complexation. Tetrahedron 2002, 58, 5291–5300. [Google Scholar] [CrossRef]
- Kolouchova-Hanzlikova, I.; Melzoch, K.; Filip, V.; Smidrkal, J. Rapid method for resveratrol determination by HPLC with electrochemical and UV detections in wines. Food Chem. 2004, 87, 151–158. [Google Scholar] [CrossRef]
- Figueiras, T.S.; Neves-Petersen, M.T.; Petersen, S.B. Activation energy of light induced isomerization of resveratrol. J. Fluoresc. 2011, 21, 1897–1906. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piyaratne, P.S.; LeBlanc, R.; Myracle, A.D.; Cole, B.J.W.; Fort, R.C., Jr. Extraction and Purification of (E)-Resveratrol from the Bark of Conifer Species. Processes 2022, 10, 647. https://doi.org/10.3390/pr10040647
Piyaratne PS, LeBlanc R, Myracle AD, Cole BJW, Fort RC Jr. Extraction and Purification of (E)-Resveratrol from the Bark of Conifer Species. Processes. 2022; 10(4):647. https://doi.org/10.3390/pr10040647
Chicago/Turabian StylePiyaratne, Panduka S., Regan LeBlanc, Angela D. Myracle, Barbara J. W. Cole, and Raymond C. Fort, Jr. 2022. "Extraction and Purification of (E)-Resveratrol from the Bark of Conifer Species" Processes 10, no. 4: 647. https://doi.org/10.3390/pr10040647
APA StylePiyaratne, P. S., LeBlanc, R., Myracle, A. D., Cole, B. J. W., & Fort, R. C., Jr. (2022). Extraction and Purification of (E)-Resveratrol from the Bark of Conifer Species. Processes, 10(4), 647. https://doi.org/10.3390/pr10040647





