Mechanistic Investigations of the Synthesis of Lactic Acid from Glycerol Catalyzed by an Iridium–NHC Complex
Abstract
:1. Introduction
2. Computational Details and Models
3. Results and Discussion
3.1. Reaction Mechanism
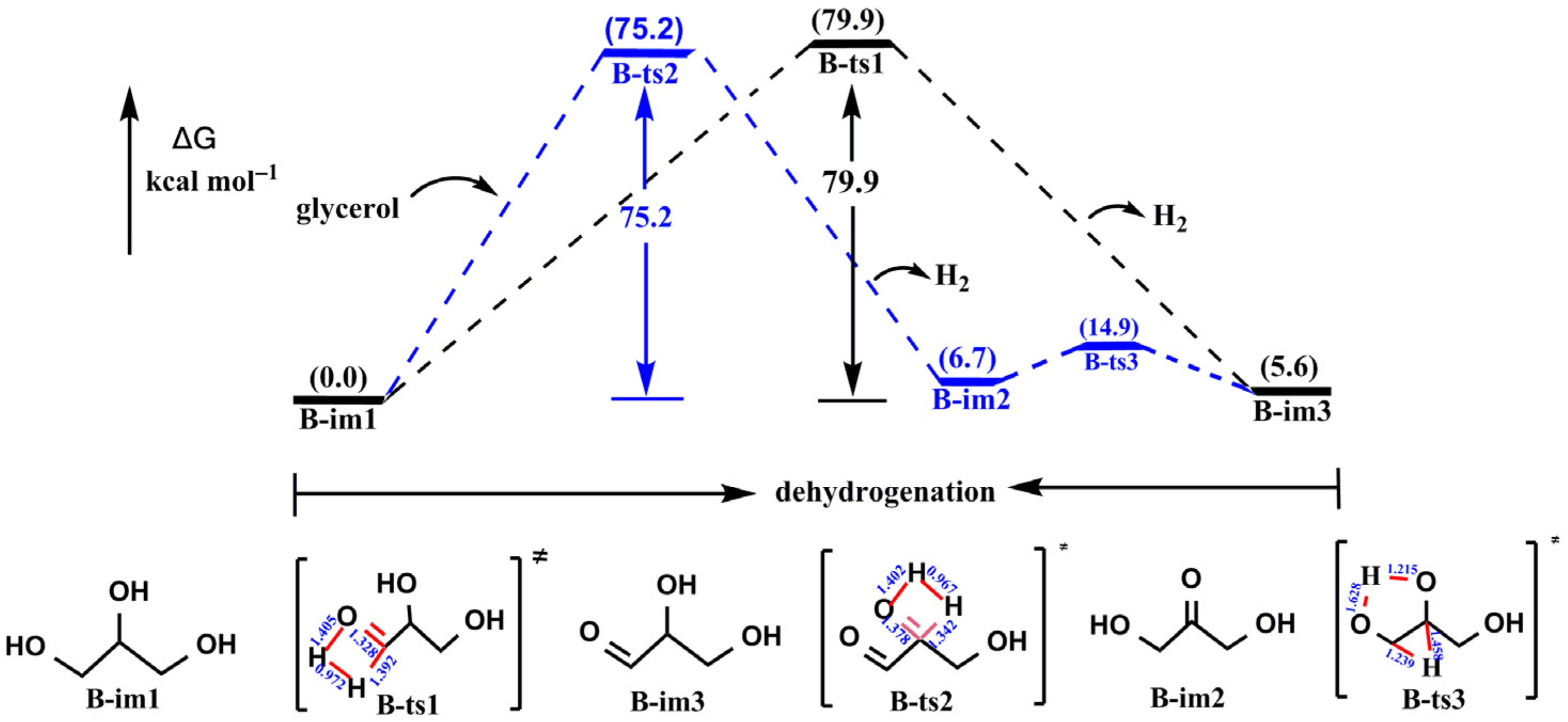
3.1.1. The Background Reaction of Glycerol to 2,3-Dihydroxypropanal
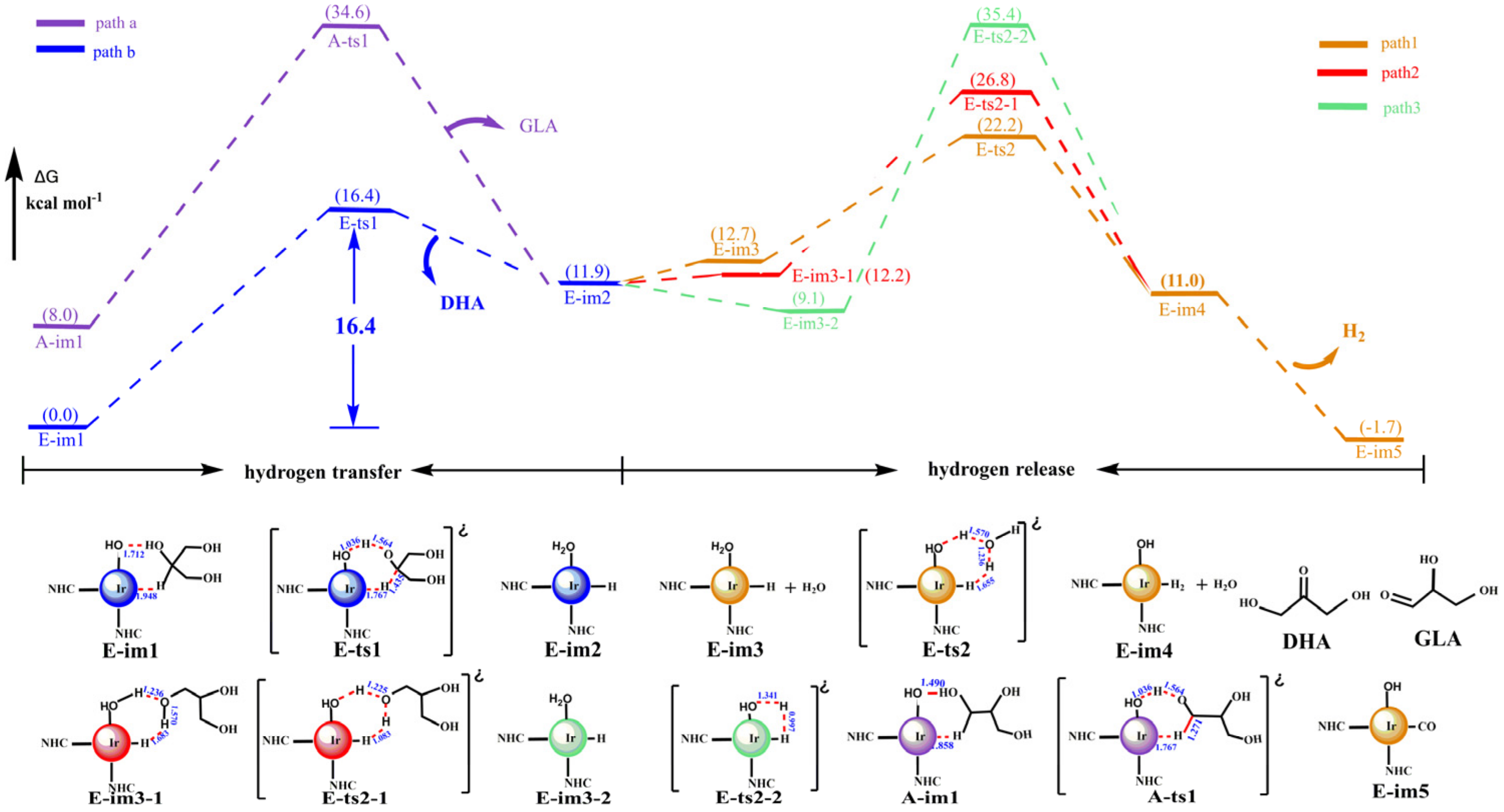
3.1.2. The Dehydrogenation of Glycerol with the Iridium–NHC Complex
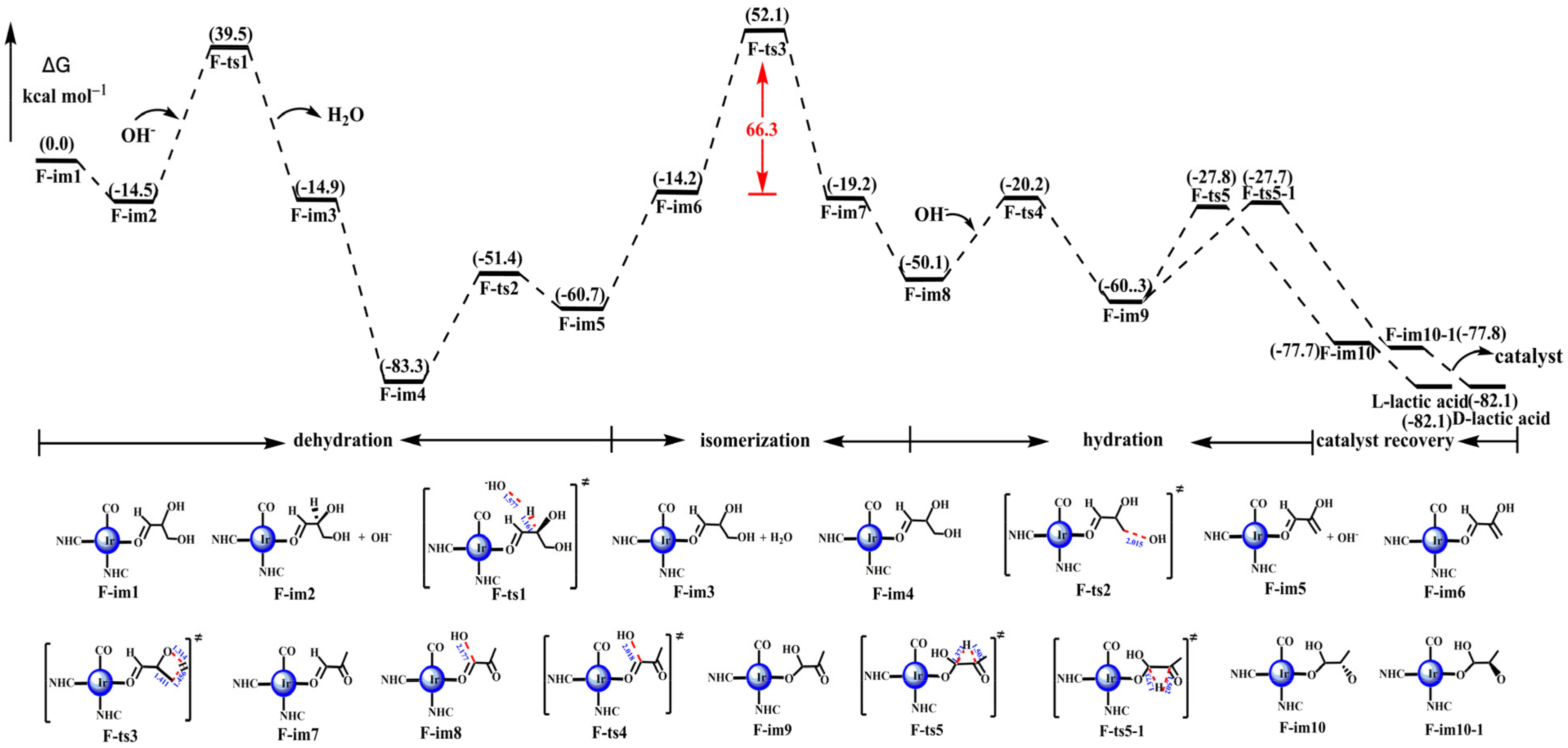
3.1.3. The Background Reactions of 2,3-Dihydroxypropanal to Lactic Acid
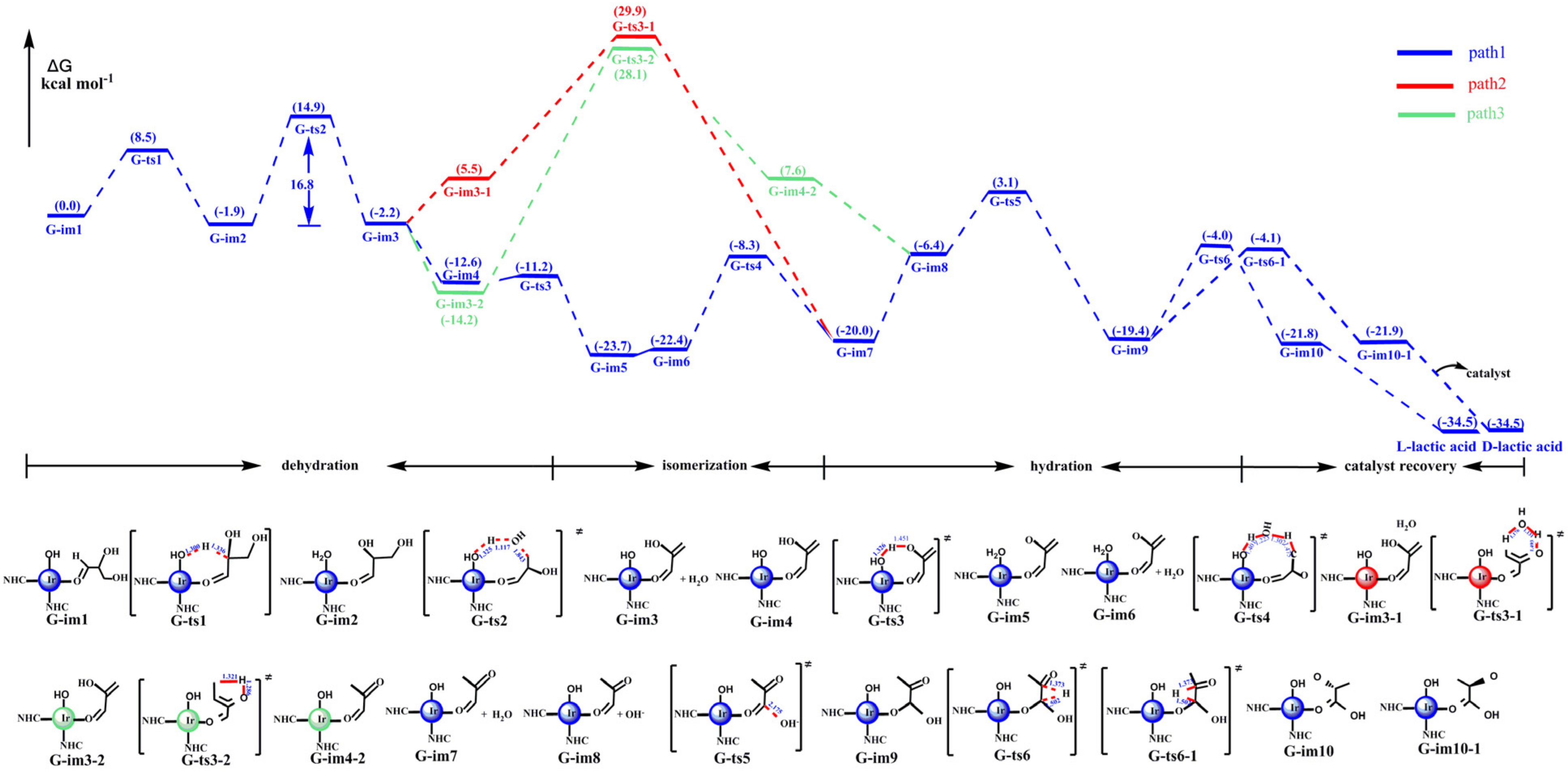
3.1.4. The Catalytic Reactions of 2,3-Dihydroxypropanal to Lactic Acid
3.2. Deep Insights on the Conversion of Glycerol to Lactic Acid
4. Conclusions
- (1)
- The reaction from glycerol to lactic acid went through dehydrogenation, dehydration, and hydration. In the noncatalytic reaction, the rate-determining step (RDS) was the dehydrogenation of glycerol. In addition, there was no obvious selectivity in the dehydrogenation reaction of glycerol that occurred on the first carbon or second carbon. They both had a high energy barrier (79.9 kcal mol−1 and 75.2 kcal mol−1, respectively).
- (2)
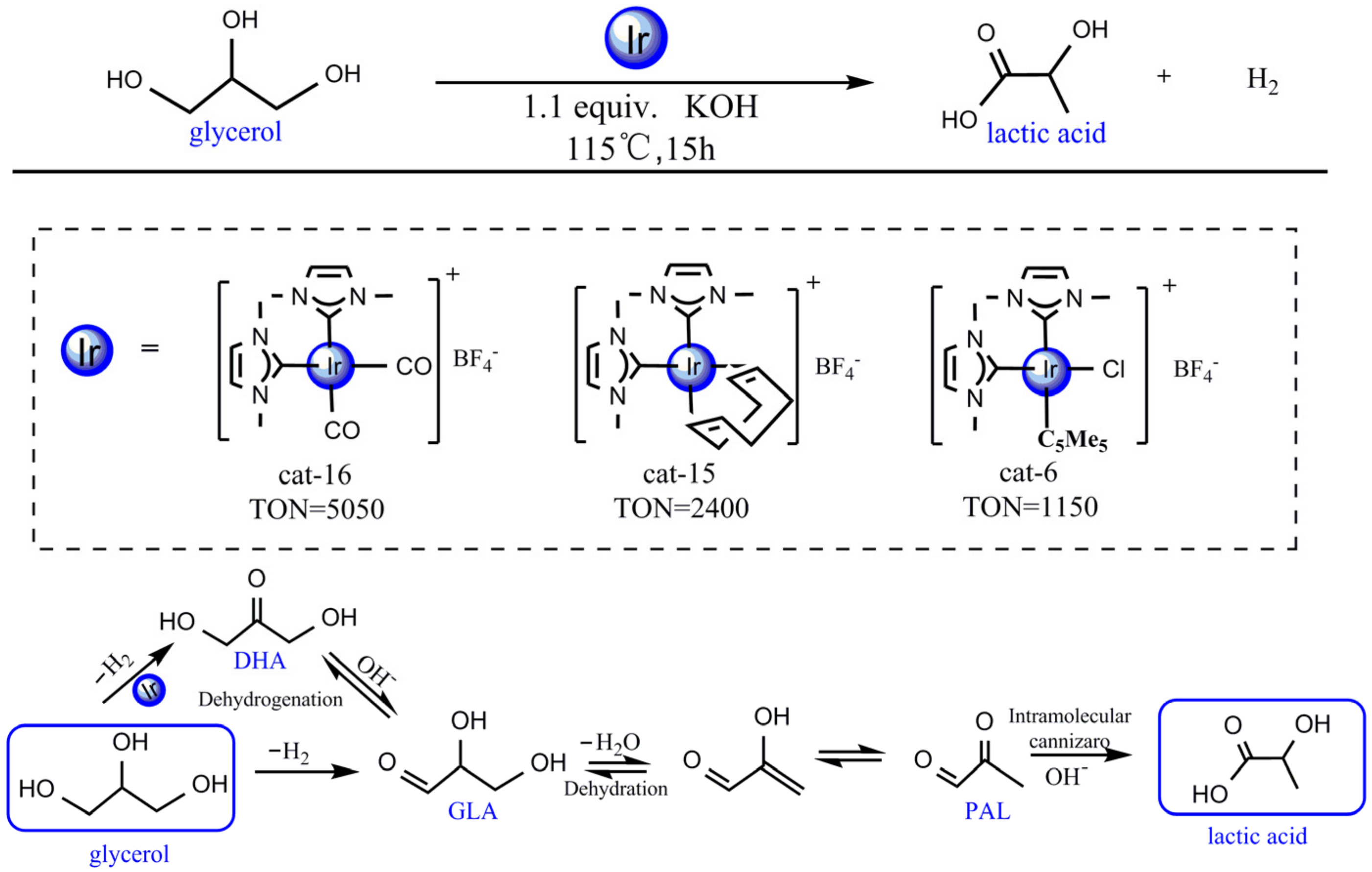
- With the catalysis of the iridium–NHC complex, the catalytic effect of cat-16 was performed by the iridium–NHC complex with the coordinated hydroxide. Glycerol was dehydrogenated to produce dihydroxyacetone, which was isomerized to 2,3-dihydroxypropanal. Then, 2,3-dihydroxypropanal was dehydrated and hydrated to produce lactic acid. The rate-determining step (RDS) of the catalytic reaction was the loss of the hydroxyl group of G-im2, and the energy barrier was much lower: 16.8 kcal mol−1 compared with 75.2 kcal mol−1 (the noncatalytic reaction).
- (3)
- With the iridium–NHC complex, glycerol would selectively dehydrogenate to dihydroxyacetone with a lower energy barrier (16.8 kcal mol−1), and the energy barrier of dehydrogenation to 2,3-dihydroxypropanal was 26.6 kcal mol−1. LUMO orbital analysis showed that the orbital energy of dehydrogenation to dihydroxyacetone was lower than that of dehydrogenation to 2,3-dihydroxypropanal. Consequently, the hydrogen anion on the second carbon was more easily pulled out by metal iridium to form hydrogen.
- (4)
- The analyses of electrostatic potential (ESP), hardness, and f- Fukui function confirmed that the iridium–NHC complex acted as the hydrogen anion receptor and nucleophilic reaction center. The hydroxide performed catalytic effects compared to the noncatalyzed reaction, while the iridium–NHC complex with the coordinated hydroxide formed a new catalytic species that played a catalytic role in the dehydrogenation, dehydration, and hydration reactions of the glycerol.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, L.; Moteki, T.; Gokhale, A.A.; Flaherty, D.W.; Toste, F.D. Production of Fuels and Chemicals from Biomass: Condensation Reactions and Beyond. Chem 2016, 1, 32–58. [Google Scholar] [CrossRef] [Green Version]
- Bentsen, N.S.; Felby, C. Biomass for energy in the European Union—A review of bioenergy resource assessments. Biotechnol. Biofuels. 2012, 5, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Ng, J.-H.; Leong, S.K.; Lam, S.S.; Ani, F.N.; Chong, C.T. Microwave-assisted and carbonaceous catalytic pyrolysis of crude glycerol from biodiesel waste for energy production. Energy Convers. Manag. 2017, 143, 399–409. [Google Scholar] [CrossRef]
- Maneerung, T.; Kawi, S.; Dai, Y.; Wang, C.-H. Sustainable biodiesel production via transesterification of waste cooking oil by using CaO catalysts prepared from chicken manure. Energy Convers. Manag. 2016, 123, 487–497. [Google Scholar] [CrossRef]
- Mahabir, J.; Koylass, N.; Samaroo, N.; Narine, K.; Ward, K. Towards resource circular biodiesel production through glycerol upcycling. Energy Convers. Manag. 2021, 233, 113930. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Economics of biodiesel production: Review. Energy Convers. Manag. 2018, 168, 74–84. [Google Scholar] [CrossRef]
- Katryniok, B.; Kimura, H.; Skrzyńska, E.; Girardon, J.-S.; Fongarland, P.; Capron, M.; Ducoulombier, R.; Mimura, N.; Paul, S.; Dumeignil, F. Selective catalytic oxidation of glycerol: Perspectives for high value chemicals. Green Chem. 2011, 13, 1960. [Google Scholar] [CrossRef]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol as a potential renewable raw material for acrylic acid production. Green Chem. 2017, 19, 3186–3213. [Google Scholar] [CrossRef]
- Len, C.; Delbecq, F.; Corpas, C.C.; Ramos, E.R. Continuous Flow Conversion of Glycerol into Chemicals: An Overview. Synthesis 2018, 50, 723–741. [Google Scholar] [CrossRef]
- Varma, R.S.; Len, C. Glycerol valorization under continuous flow conditions-recent advances. Curr. Opin. Green Sustain. Chem. 2019, 15, 83–90. [Google Scholar] [CrossRef]
- Galy, N.; Nguyen, R.; Yalgin, H.; Thiebault, N.; Luart, D.; Len, C. Glycerol in subcritical and supercritical solvents. J. Chem. Technol. Biotechnol. 2017, 92, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Ge, X.; Cui, S.; Li, Y. Value-added processing of crude glycerol into chemicals and polymers. Bioresour. Technol. 2016, 215, 144–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Jiang, H.; Zhu, X.; Lu, R.; Shi, L.; Lu, F. Effect of Tungsten Species on Selective Hydrogenolysis of Glycerol to 1,3-Propanediol. ChemSusChem 2021, 14, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Mangayil, R.; Efimova, E.; Konttinen, J.; Santala, V. Co-production of 1,3 propanediol and long-chain alkyl esters from crude glycerol. New Biotechnol. 2019, 53, 81–89. [Google Scholar] [CrossRef]
- Gabrysch, T.; Peng, B.X.; Bunea, S.; Dyker, G.; Muhler, M. The Role of Metallic Copper in the Selective Hydrodeoxygenation of Glycerol to 1,2-Propanediol over Cu/ZrO2. Chemcatchem 2018, 10, 1344–1350. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S. Coke-tolerant SiW20 -Al/Zr10 catalyst for glycerol dehydration to acrolein. Chin. J. Catal. 2017, 38, 1697–1710. [Google Scholar] [CrossRef]
- Liu, S.; Yu, Z.; Wang, Y.; Sun, Z.; Liu, Y.; Shi, C.; Wang, A. Catalytic dehydration of glycerol to acrolein over unsupported MoP. Catal. Today 2021, 379, 132–140. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. A review on glycerol valorization to acrolein over solid acid catalysts. J. Taiwan Inst. Chem. Eng. 2016, 67, 29–44. [Google Scholar] [CrossRef]
- Zhou, Y.; Shen, Y.; Xi, J.; Luo, X. Selective Electro-Oxidation of Glycerol to Dihydroxyacetone by Pt Ag Skeletons. ACS Appl. Mater. Interfaces 2019, 11, 28953–28959. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, D.; Wang, X.; Zhou, J.; Li, J.; Zhang, M.; Shen, Y.; Chu, H.; Qu, Y. Overcoming the Deactivation of Pt/CNT by Introducing CeO2 for Selective Base-Free Glycerol-to-Glyceric Acid Oxidation. ACS Catal. 2020, 10, 3832–3837. [Google Scholar] [CrossRef]
- Huang, L.-W.; Vo, T.-G.; Chiang, C.-Y. Converting glycerol aqueous solution to hydrogen energy and dihydroxyacetone by the BiVO4 photoelectrochemical cell. Electrochim. Acta 2019, 322, 134725. [Google Scholar] [CrossRef]
- Su, X.; Lin, W.; Cheng, H.; Zhang, C.; Wang, Y.; Yu, X.; Wu, Z.; Zhao, F. Metal-free catalytic conversion of CO2 and glycerol to glycerol carbonate. Green Chem. 2017, 19, 1775–1781. [Google Scholar] [CrossRef]
- Liu, J.; He, D. Transformation of CO2 with glycerol to glycerol carbonate by a novel ZnWO4-ZnO catalyst. J. CO2 Util. 2018, 26, 370–379. [Google Scholar] [CrossRef]
- Granados-Reyes, J.; Salagre, P.; Cesteros, Y. CaAl-layered double hydroxides as active catalysts for the transesterification of glycerol to glycerol carbonate. Appl. Clay Sci. 2016, 132–133, 216–222. [Google Scholar] [CrossRef]
- Sharninghausen, L.S.; Mercado, B.Q.; Crabtree, R.H.; Hazari, N. Selective conversion of glycerol to lactic acid with iron pincer precatalysts. Chem. Commun. 2015, 51, 16201–16204. [Google Scholar] [CrossRef]
- Purushothaman, R.K.P.; van Haveren, J.; van Es, D.S.; Melián-Cabrera, I.; Meeldijk, J.D.; Heeres, H.J. An efficient one pot conversion of glycerol to lactic acid using bimetallic gold-platinum catalysts on a nanocrystalline CeO2 support. Appl. Catal. B Environ. 2014, 147, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Palacio, R.; Torres, S.; Lopez, D.; Hernandez, D. Selective glycerol conversion to lactic acid on Co3O4/CeO2 catalysts. Catal. Today 2018, 302, 196–202. [Google Scholar] [CrossRef]
- Oberhauser, W.; Evangelisti, C.; Liscio, A.; Kovtun, A.; Cao, Y.; Vizza, F. Glycerol to lactic acid conversion by NHC-stabilized iridium nanoparticles. J. Catal. 2018, 368, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Tao, M.; Li, Y.; Geletii, Y.V.; Hill, C.L.; Wang, X. Aerobic oxidation of glycerol catalyzed by M salts of PMo12O403−(M = K+, Zn2+, Cu2+, Al3+, Cr3+, Fe3+). Appl. Catal. A Gen. 2019, 579, 52–57. [Google Scholar] [CrossRef]
- Dusselier, M.; Wouwe, P.V.; Dewaele, A.; Makshina, E.; Sels, B.F. Lactic acid as a platform chemical in the biobased economy: The role of chemocatalysis. Energy Environ. Sci. 2013, 6, 1415–1442. [Google Scholar] [CrossRef]
- Djukić-Vuković, A.; Mladenović, D.; Ivanović, J.; Pejin, J.; Mojović, L. Towards sustainability of lactic acid and poly-lactic acid polymers production. Renew. Sustain. Energy Rev. 2019, 108, 238–252. [Google Scholar] [CrossRef]
- Slomkowski, S.; Penczek, S.; Duda, A. Polylactides-an overview. Polym. Adv. Technol. 2014, 25, 436–447. [Google Scholar] [CrossRef]
- Madhavan-Nampoothiri, K.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Direct lactic acid fermentation: Focus on simultaneous saccharification and lactic acid production. Biotechnol. Adv. 2009, 27, 145–152. [Google Scholar] [CrossRef]
- Tao, M.; Li, Y.; Zhang, X.; Li, Z.; Hill, C.L.; Wang, X. A Polyoxometalate-Based Microfluidic Device for Liquid-Phase Oxidation of Glycerol. ChemSusChem 2019, 12, 2550–2553. [Google Scholar] [CrossRef]
- Guérin, V.; Legault, Y.C. Synthesis of NHC-Iridium (III) Complexes Based on N-Iminoimidazolium Ylides and Their Use for the Amine Alkylation by Borrowing Hydrogen Catalysis. Organometallics 2021, 40, 408–417. [Google Scholar] [CrossRef]
- Reshi, N.U.D.; Bera, J.K. Recent advances in annellated NHCs and their metal complexes. Coord. Chem. Rev. 2020, 422, 213334. [Google Scholar] [CrossRef]
- Nasr, A.; Winkler, A.; Tamm, M. Anionic N-heterocyclic carbenes: Synthesis, coordination chemistry and applications in homogeneous catalysis. Coord. Chem. Rev. 2016, 316, 68–124. [Google Scholar] [CrossRef]
- Jacobsen, H.; Correa, A.; Poater, A.; Costabile, C.; Cavallo, L. Understanding the M(NHC) (NHC=N-heterocyclic carbene) bond. Coord. Chem. Rev. 2009, 253, 687–703. [Google Scholar] [CrossRef]
- Sharninghausen, L.S.; Campos, J.; Manas, M.G.; Crabtree, R.H. Efficient selective and atom economic catalytic conversion of glycerol to lactic acid. Nat. Commun. 2014, 5, 5084. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Biju, A.T. Imines as acceptors and donors in N-heterocyclic carbene (NHC) organocatalysis. Chem. Commun. 2020, 56, 8537–8552. [Google Scholar] [CrossRef]
- Marian, C.M.; Heil, A.; Kleinschmidt, M. The DFT/MRCI method. WIREs Comput. Molec. Sci. 2018, 9, e1394. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision, D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys. 2006, 125, 194101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Koga, T.; Tatewaki, H.; Shimazaki, T. Chemical reliable uncontracted Gaussian-type basis sets for atoms H to Lr. Chem. Phys. Lett. 2000, 328, 473–482. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Weiner, P.K.; Langridge, R.; Blaney, J.M.; Schaefer, R.; Kollman, P.A. Electrostatic Potential Molecular-Surfaces. Proc. Natl. Acad. Sci. Biol. 1982, 79, 3754–3758. [Google Scholar] [CrossRef] [Green Version]
- Domingo, L.R.; Perez, P. Global and local reactivity indices for electrophilic/nucleophilic free radicals. Org. Biomol. Chem. 2013, 11, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Pearson, R.G. Absolute Hardness—Companion Parameter to Absolute Electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Ona, O.B.; Clercq, O.D.; Alcoba, D.R.; Torre, A.; Lain, L.; Van, N.D.; Bultinck, P. Atom and Bond Fukui Functions and Matrices: A Hirshfeld-I Atoms-in-Molecule Approach. Chemphyschem 2016, 17, 2881–2889. [Google Scholar] [CrossRef]
- Fukui, K.; Yonezawa, T.; Shingu, H. A Molecular Orbital Theory of Reactivity in Aromatic Hydrocarbons. J. Chem. Phys. 1952, 20, 722–725. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. Model 1996, 14, 33–38. [Google Scholar] [CrossRef]




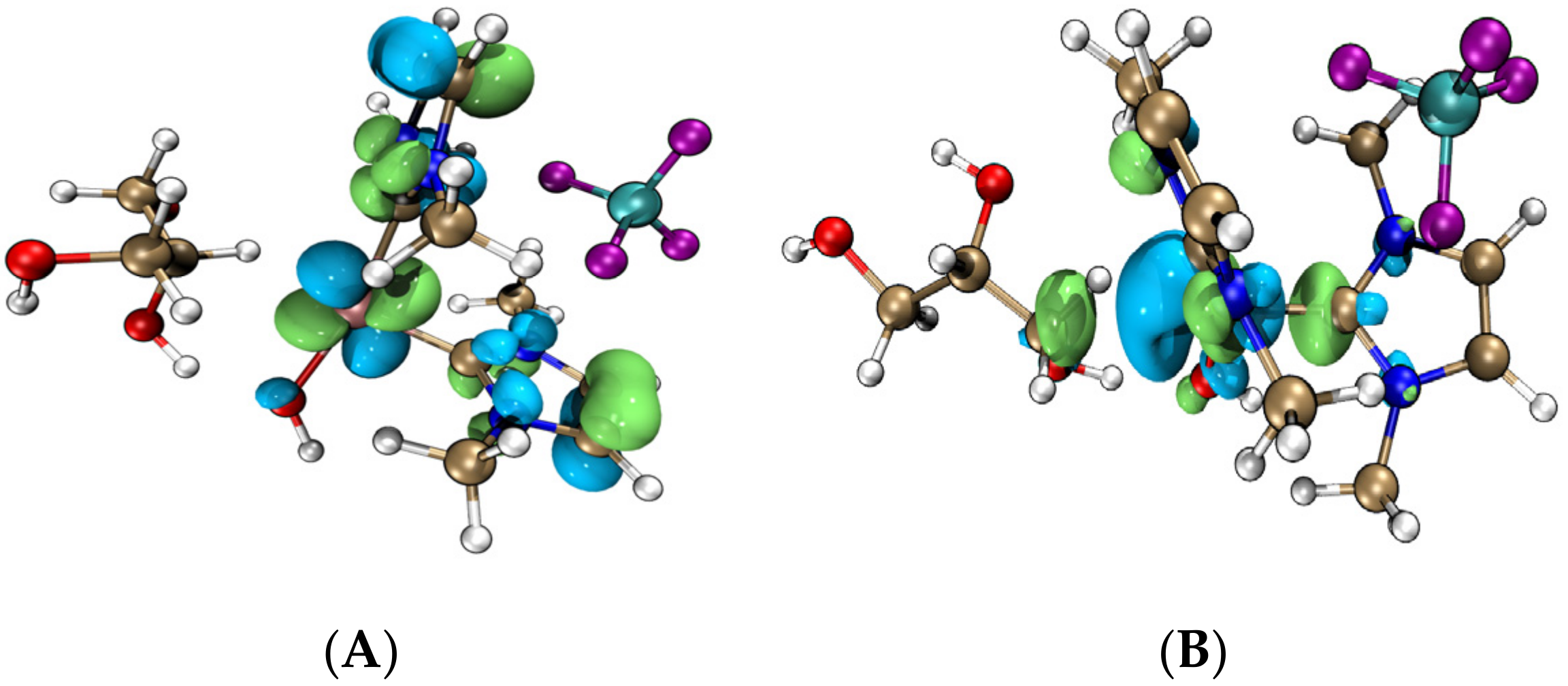
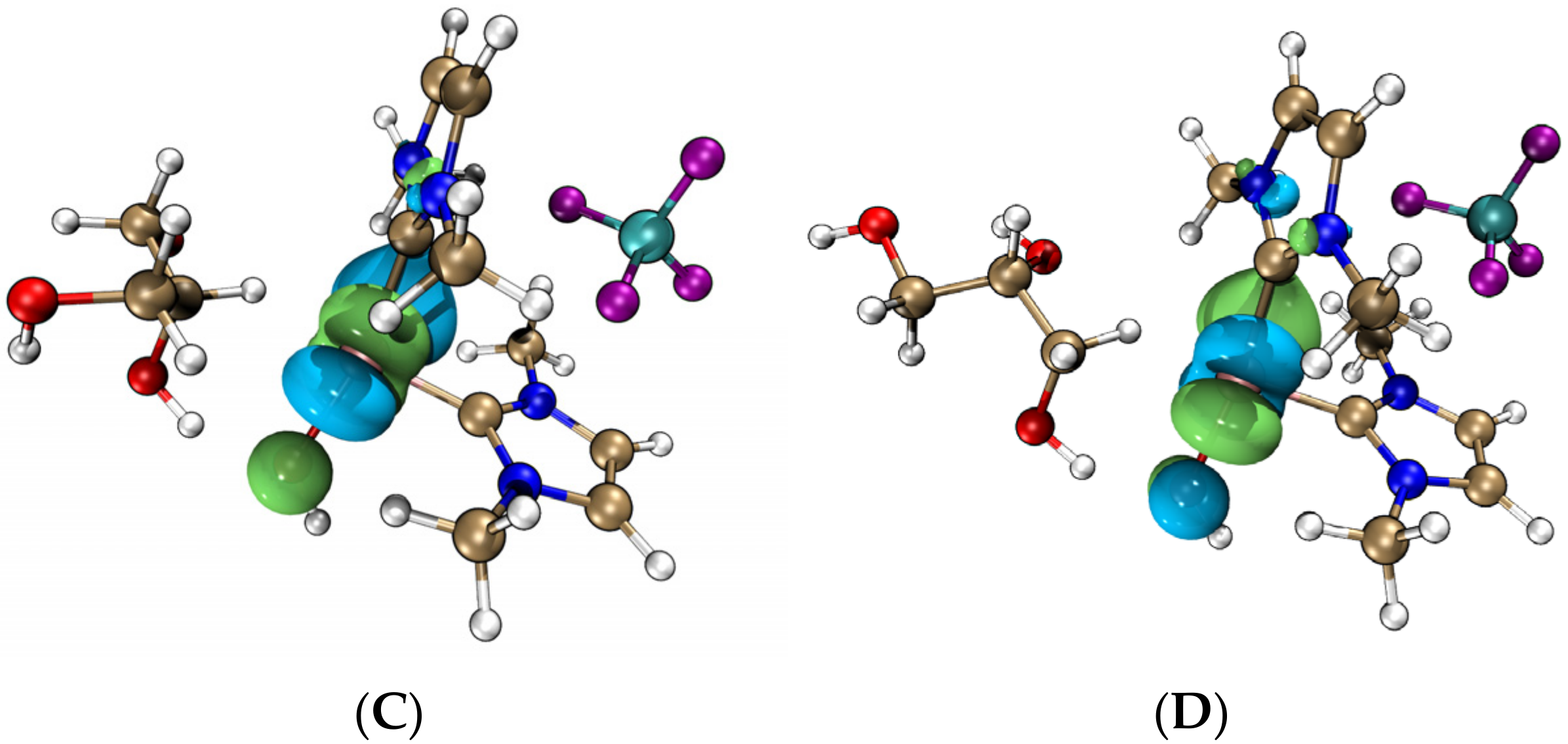
| Substance | Nucleophilicity | Hardness/eV |
|---|---|---|
| Cat-6 (Ir) | 0.27 | 5.1 |
| Cat-15 (Ir) | 0.29 | 6.8 |
| Cat-16 (Ir) | 0.39 | 7.6 |
| Glycerol | 12.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Xu, S.; Ge, C.; Hu, C. Mechanistic Investigations of the Synthesis of Lactic Acid from Glycerol Catalyzed by an Iridium–NHC Complex. Processes 2022, 10, 626. https://doi.org/10.3390/pr10040626
Chen S, Xu S, Ge C, Hu C. Mechanistic Investigations of the Synthesis of Lactic Acid from Glycerol Catalyzed by an Iridium–NHC Complex. Processes. 2022; 10(4):626. https://doi.org/10.3390/pr10040626
Chicago/Turabian StyleChen, Shiyao, Shuguang Xu, Chenyu Ge, and Changwei Hu. 2022. "Mechanistic Investigations of the Synthesis of Lactic Acid from Glycerol Catalyzed by an Iridium–NHC Complex" Processes 10, no. 4: 626. https://doi.org/10.3390/pr10040626
APA StyleChen, S., Xu, S., Ge, C., & Hu, C. (2022). Mechanistic Investigations of the Synthesis of Lactic Acid from Glycerol Catalyzed by an Iridium–NHC Complex. Processes, 10(4), 626. https://doi.org/10.3390/pr10040626