Structural Changes of Alkali Lignin under Ozone Treatment and Effect of Ozone-Oxidized Alkali Lignin on Cellulose Digestibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Purification of Alkali Lignin
2.3. Pretreatment of Alkali Lignin by Ozone
2.4. Enzymatic Hydrolysis of Avicel with Lignin Samples
2.5. Cellulase Adsorption Isotherm on Alkali Lignin
2.6. Characterization of Alkali Ligni
2.7. Analytical Methods
3. Results and Discussion
3.1. SEM Microscopy
3.2. Elemental Analysis
3.3. Fourier Transform Infrared Spectrum (FTIR) Analysis
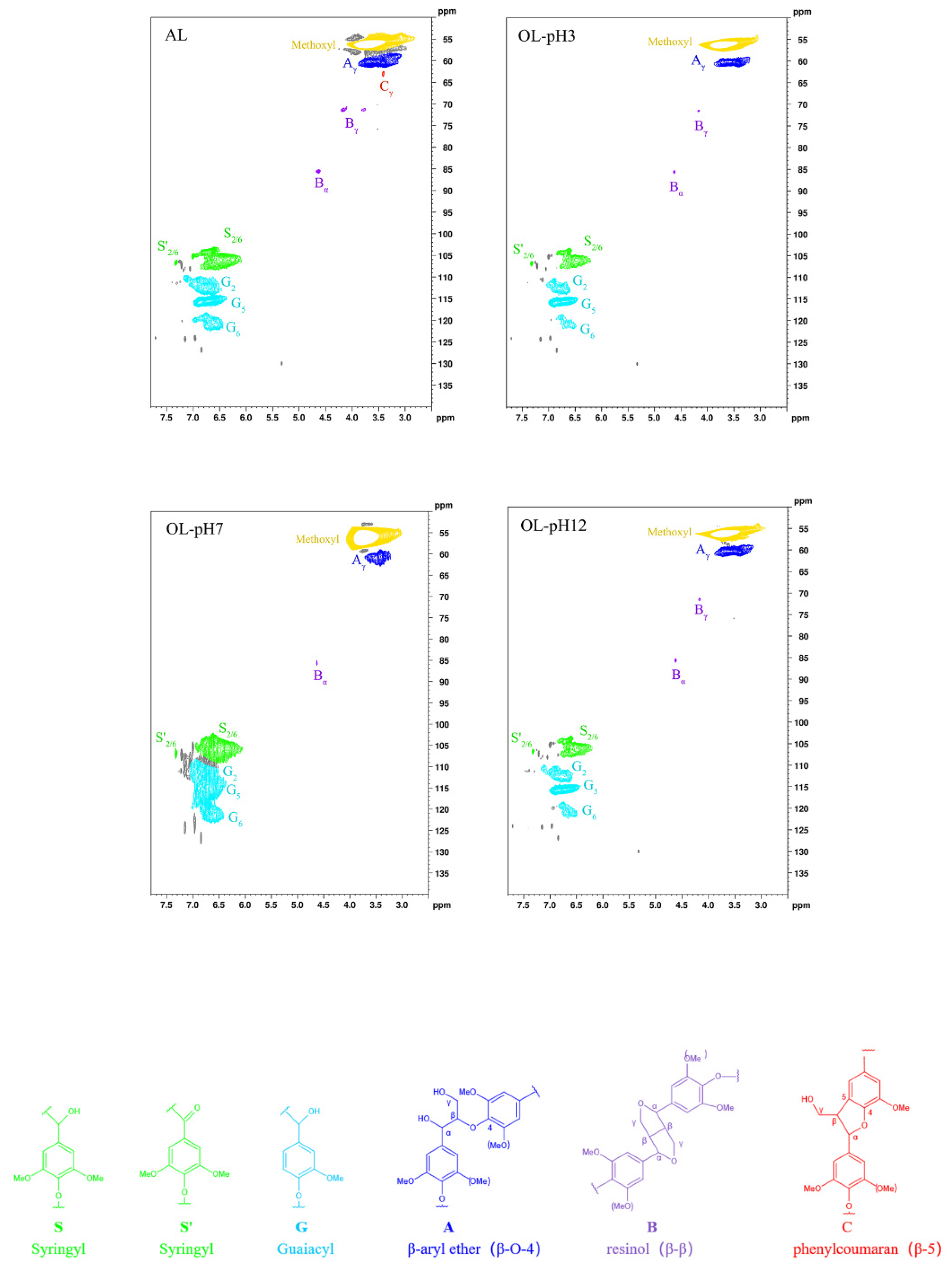
3.4. 1H-13C HSQC NMR Spectral Analysis
3.5. Gel Permeation Chromatography (GPC) Analysis
3.6. Surface Properties of Lignin Preparations
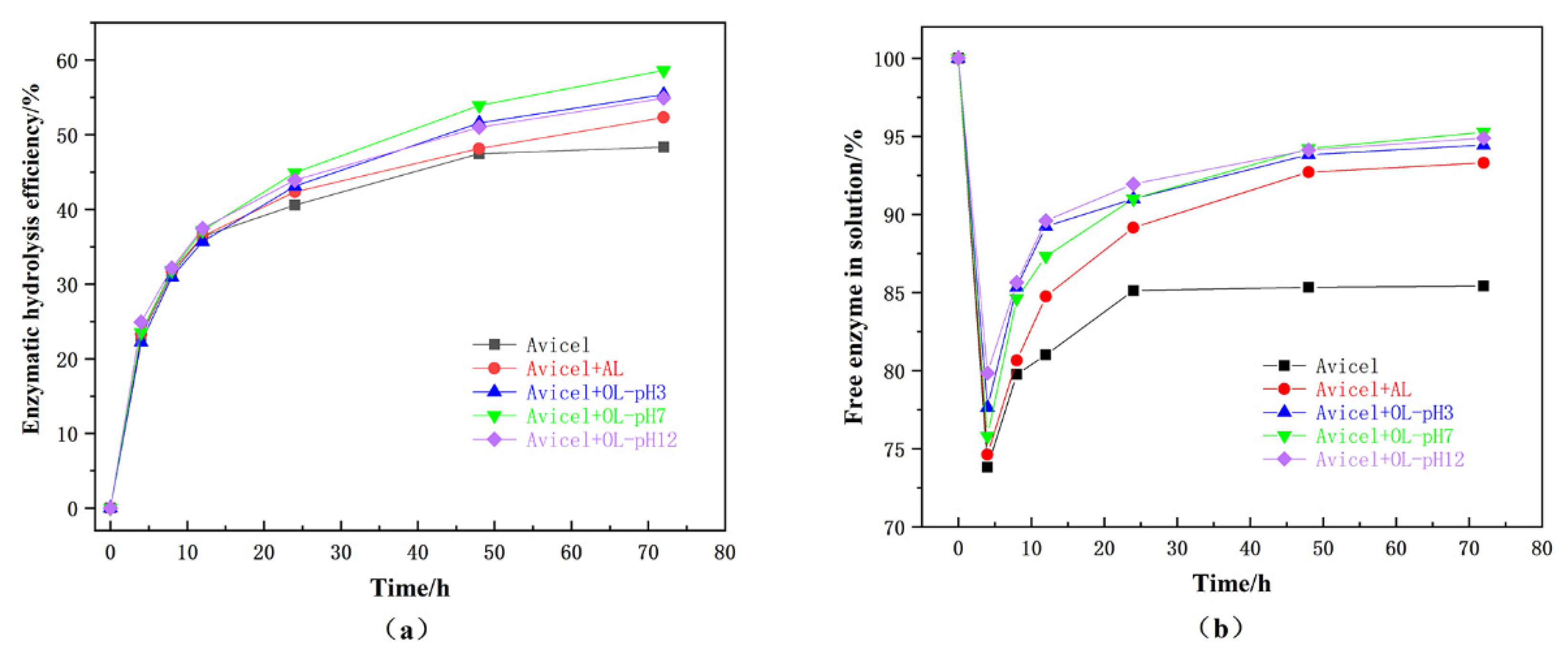
3.7. Effects of Alkaline Lignin Modification on Nonproductive Adsorption of Cellulase
3.8. Effects of Alkaline Lignin Modification on Glucose Yield and Cellulase Distribution in Enzymatic Saccharification of Avicel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhade, S.A.; Singh, N.; Gutierrez, O.Y.; Lopez-Ruiz, J.; Wang, H.; Holladay, J.D.; Liu, Y.; Karkamkar, A.; Weber, R.S.; Padmaperuma, A.B.; et al. Electrocatalytic Hydrogenation of Biomass-Derived Organics: A Review. Chem. Rev. 2020, 120, 11370–11419. [Google Scholar] [CrossRef]
- Rahikainen, J.L.; Martin-Sampedro, R.; Heikkinen, H.; Rovio, S.; Marjamaa, K.; Tamminen, T.; Rojas, O.J.; Kruus, K. Inhibitory effect of lignin during cellulose bioconversion: The effect of lignin chemistry on non-productive enzyme adsorption. Bioresour Technol. 2013, 133, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.K.; Patel, A.K.; Adsul, M.; Singhania, R.R. Cellulase adsorption on lignin: A roadblock for economic hydrolysis of biomass. Renew. Energy 2016, 98, 29–42. [Google Scholar] [CrossRef]
- Szalaty, T.J.; Klapiszewski, Ł.; Jesionowski, T. Recent developments in modification of lignin using ionic liquids for the fabrication of advanced materials–A review. J. Mol. Liq. 2020, 301, 112417. [Google Scholar] [CrossRef]
- Huang, C.; He, J.; Min, D.; Lai, C.; Yong, Q. Understanding the Nonproductive Enzyme Adsorption and Physicochemical Properties of Residual Lignins in Moso Bamboo Pretreated with Sulfuric Acid and Kraft Pulping. Appl. Biochem. Biotechnol. 2016, 180, 1508–1523. [Google Scholar] [CrossRef] [PubMed]
- Yarbrough, J.M.; Mittal, A.; Mansfield, E.; Taylor, L.E., 2nd; Hobdey, S.E.; Sammond, D.W.; Bomble, Y.J.; Crowley, M.F.; Decker, S.R.; Himmel, M.E.; et al. New perspective on glycoside hydrolase binding to lignin from pretreated corn stover. Biotechnol. Biofuels 2015, 8, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Meng, X.; Ragauskas, A.J.; Lai, C.; Ling, Z.; Huang, C.; Yong, Q. Unlocking the secret of lignin-enzyme interactions: Recent advances in developing state-of-the-art analytical techniques. Biotechnol. Adv. 2021, 54, 107830. [Google Scholar] [CrossRef]
- Yoo, C.G.; Li, M.; Meng, X.; Pu, Y.; Ragauskas, A.J. Effects of organosolv and ammonia pretreatments on lignin properties and its inhibition for enzymatic hydrolysis. Green Chem. 2017, 19, 2006–2016. [Google Scholar] [CrossRef]
- Wu, K.; Ying, W.; Shi, Z.; Yang, H.; Zheng, Z.; Zhang, J.; Yang, J. Fenton reaction-oxidized bamboo lignin surface and structural modification to reduce nonproductive cellulase binding and improve enzyme digestion of cellulose. ACS Sustain. Chem. Eng. 2018, 6, 3853–3861. [Google Scholar] [CrossRef]
- Nakagame, S.; Chandra, R.P.; Kadla, J.F.; Saddler, J.N. The isolation, characterization and effect of lignin isolated from steam pretreated Douglas-fir on the enzymatic hydrolysis of cellulose. Bioresour Technol. 2011, 102, 4507–4517. [Google Scholar] [CrossRef]
- Luo, L.; Yuan, X.; Zhang, S.; Wang, X.; Li, M.; Wang, S. Effect of Pretreatments on the Enzymatic Hydrolysis of High-Yield Bamboo Chemo-Mechanical Pulp by Changing the Surface Lignin Content. Polymers 2021, 13, 787. [Google Scholar] [CrossRef]
- Romero-Borbón, E.; Oropeza-González, A.E.; González-García, Y.; Córdova, J. Thermochemical and Enzymatic Saccharification of Water Hyacinth Biomass into Fermentable Sugars. Processes 2022, 10, 210. [Google Scholar] [CrossRef]
- Meng, X.; Bhagia, S.; Wang, Y.; Zhou, Y.; Pu, Y.; Dunlap, J.R.; Shuai, L.; Ragauskas, A.J.; Yoo, C.G. Effects of the advanced organosolv pretreatment strategies on structural properties of woody biomass. Ind. Crops Prod. 2020, 146, 112144. [Google Scholar] [CrossRef]
- Shi, F.; Xiang, H.; Li, Y. Combined pretreatment using ozonolysis and ball milling to improve enzymatic saccharification of corn straw. Bioresour Technol. 2015, 179, 444–451. [Google Scholar] [CrossRef]
- Travaini, R.; Martin-Juarez, J.; Lorenzo-Hernando, A.; Bolado-Rodriguez, S. Ozonolysis: An advantageous pretreatment for lignocellulosic biomass revisited. Bioresour Technol. 2016, 199, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Cubero, M.T.; Palacin, L.G.; Gonzalez-Benito, G.; Bolado, S.; Lucas, S.; Coca, M. An analysis of lignin removal in a fixed bed reactor by reaction of cereal straws with ozone. Bioresour Technol. 2012, 107, 229–234. [Google Scholar] [CrossRef]
- Panneerselvam, A.; Sharma-Shivappa, R.R.; Kolar, P.; Clare, D.A.; Ranney, T. Hydrolysis of ozone pretreated energy grasses for optimal fermentable sugar production. Bioresour Technol. 2013, 148, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Perrone, O.M.; Rossi, J.S.; Moretti, M.M.S.; Nunes, C.; Bordignon, S.E.; Gomes, E.; Da-Silva, R.; Boscolo, M. Influence of ozonolysis time during sugarcane pretreatment: Effects on the fiber and enzymatic saccharification. Bioresour Technol. 2017, 224, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Travaini, R.; Otero, M.D.; Coca, M.; Da-Silva, R.; Bolado, S. Sugarcane bagasse ozonolysis pretreatment: Effect on enzymatic digestibility and inhibitory compound formation. Bioresour Technol. 2013, 133, 332–339. [Google Scholar] [CrossRef]
- Andersen, S.L.F.; Castoldi, R.; Garcia, J.A.A.; Bracht, A.; Peralta, R.A.; de Lima, E.A.; Helm, C.V.; Moreira, R.D.F.P.M.; Peralta, R.M. Improving enzymatic saccharification of Eucalyptus grandis branches by ozone pretreatment. Wood Sci. Technol. 2018, 53, 49–69. [Google Scholar] [CrossRef]
- Miura, T.; Lee, S.H.; Inoue, S.; Endo, T. Combined pretreatment using ozonolysis and wet-disk milling to improve enzymatic saccharification of Japanese cedar. Bioresour Technol. 2012, 126, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Jameel, H.; Chang, H.M.; Park, S. The effect of delignification of forest biomass on enzymatic hydrolysis. Bioresour Technol. 2011, 102, 9083–9089. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wang, W.; Tong, S.; Zhang, C.; Liu, P. Evaluation of the Effects of Isolated Lignin on Cellulose Enzymatic Hydrolysis of Corn Stover Pretreatment by NaOH Combined with Ozone. Molecules 2018, 23, 1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Zhang, C.; Tong, S.; Cui, Z.; Liu, P. Enhanced Enzymatic Hydrolysis and Structural Features of Corn Stover by NaOH and Ozone Combined Pretreatment. Molecules 2018, 23, 1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osuna-Laveaga, D.R.; García-Depraect, O.; Vallejo-Rodríguez, R.; López-López, A.; León-Becerril, E. Integrated Ozonation-Enzymatic Hydrolysis Pretreatment of Sugarcane Bagasse: Enhancement of Sugars Released to Expended Ozone Ratio. Processes 2020, 8, 1274. [Google Scholar] [CrossRef]
- Yao, L.; Yang, H.; Yoo, C.G.; Chen, C.; Meng, X.; Dai, J.; Yang, C.; Yu, J.; Ragauskas, A.J.; Chen, X. A mechanistic study of cellulase adsorption onto lignin. Green Chem. 2021, 23, 333–339. [Google Scholar] [CrossRef]
- Szalaty, T.J.; Klapiszewski, L.; Stanisz, M.; Moszynski, D.; Skrzypczak, A.; Jesionowski, T. Catalyst-free activation of kraft lignin in air using hydrogen sulfate ionic liquids. Int. J. Biol. Macromol. 2018, 119, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, J.-Q.; Li, H.; Yuan, T.-Q.; Wang, Y.-Y.; Sun, R.-C. Heat Treatment of Industrial Alkaline Lignin and its Potential Application as an Adhesive for Green Wood–Lignin Composites. ACS Sustain. Chem. Eng. 2017, 5, 7269–7277. [Google Scholar] [CrossRef]
- Yao, L.; Chen, C.; Yoo, C.G.; Meng, X.; Li, M.; Pu, Y.; Ragauskas, A.J.; Dong, C.; Yang, H. Insights of ethanol organosolv pretreatment on lignin properties of Broussonetia papyrifera. ACS Sustain. Chem. Eng. 2018, 6, 14767–14773. [Google Scholar] [CrossRef]
- Yang, H.; Yoo, C.G.; Meng, X.; Pu, Y.; Muchero, W.; Tuskan, G.A.; Tschaplinski, T.J.; Ragauskas, A.J.; Yao, L. Structural changes of lignins in natural Populus variants during different pretreatments. Bioresour Technol. 2020, 295, 122240. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, S.; Wang, W.; Linhardt, R.J.; Ragauskas, A.J. Preparation of Highly Reactive Lignin by Ozone Oxidation: Application as Surfactants with Antioxidant and Anti-UV Properties. ACS Sustain. Chem. Eng. 2019, 8, 22–28. [Google Scholar] [CrossRef]
- Wen, J.L.; Sun, S.L.; Yuan, T.Q.; Xu, F.; Sun, R.C. Structural elucidation of lignin polymers of Eucalyptus chips during organosolv pretreatment and extended delignification. J. Agric. Food Chem. 2013, 61, 11067–11075. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Yang, H.; Yoo, C.G.; Meng, X.; Pu, Y.; Hao, N.; Ragauskas, A.J. Characteristics of Lignin Fractions from Dilute Acid Pretreated Switchgrass and Their Effect on Cellobiohydrolase from Trichoderma longibrachiatum. Front. Energy Res. 2018, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Huang, Y.; Sun, R.; Tu, M. The strong association of condensed phenolic moieties in isolated lignins with their inhibition of enzymatic hydrolysis. Green Chem. 2016, 18, 4276–4286. [Google Scholar] [CrossRef]
- Patri, A.S.; Mohan, R.; Pu, Y.; Yoo, C.G.; Ragauskas, A.J.; Kumar, R.; Kisailus, D.; Cai, C.M.; Wyman, C.E. THF co-solvent pretreatment prevents lignin redeposition from interfering with enzymes yielding prolonged cellulase activity. Biotechnol. Biofuels 2021, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Yang, H.; Yoo, C.G.; Meng, X.; Li, M.; Pu, Y.; Ragauskas, A.J.; Sykes, R.W. Adsorption of cellobiohydrolases I onto lignin fractions from dilute acid pretreated Broussonetia papyrifera. Bioresour Technol. 2017, 244, 957–962. [Google Scholar] [CrossRef]
- Yang, H.; Jin, Y.; Shi, Z.; Wang, D.; Zhao, P.; Yang, J. Effect of hydrothermal pretreated bamboo lignin on cellulose saccharification for bioethanol production. Ind. Crops Prod. 2020, 156, 112865. [Google Scholar] [CrossRef]
- Ying, W.; Shi, Z.; Yang, H.; Xu, G.; Zheng, Z.; Yang, J. Effect of alkaline lignin modification on cellulase-lignin interactions and enzymatic saccharification yield. Biotechnol. Biofuels 2018, 11, 214. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y. Lignin-enzyme interaction: Mechanism, mitigation approach, modeling, and research prospects. Biotechnol. Adv. 2017, 35, 466–489. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y.; Du, J.; Yang, Y.; Jin, Y. Influence of lignin addition on the enzymatic digestibility of pretreated lignocellulosic biomasses. Bioresour Technol. 2015, 181, 7–12. [Google Scholar] [CrossRef]
- Wu, K.; Shi, Z.; Yang, H.; Liao, Z.; Yang, J. Effect of ethanol organosolv lignin from bamboo on enzymatic hydrolysis of avicel. ACS Sustain. Chem. Eng. 2017, 5, 1721–1729. [Google Scholar] [CrossRef]
- Nakagame, S.; Chandra, R.P.; Saddler, J.N. The effect of isolated lignins, obtained from a range of pretreated lignocellulosic substrates, on enzymatic hydrolysis. Biotechnol. Bioeng. 2010, 105, 871–879. [Google Scholar] [CrossRef] [PubMed]
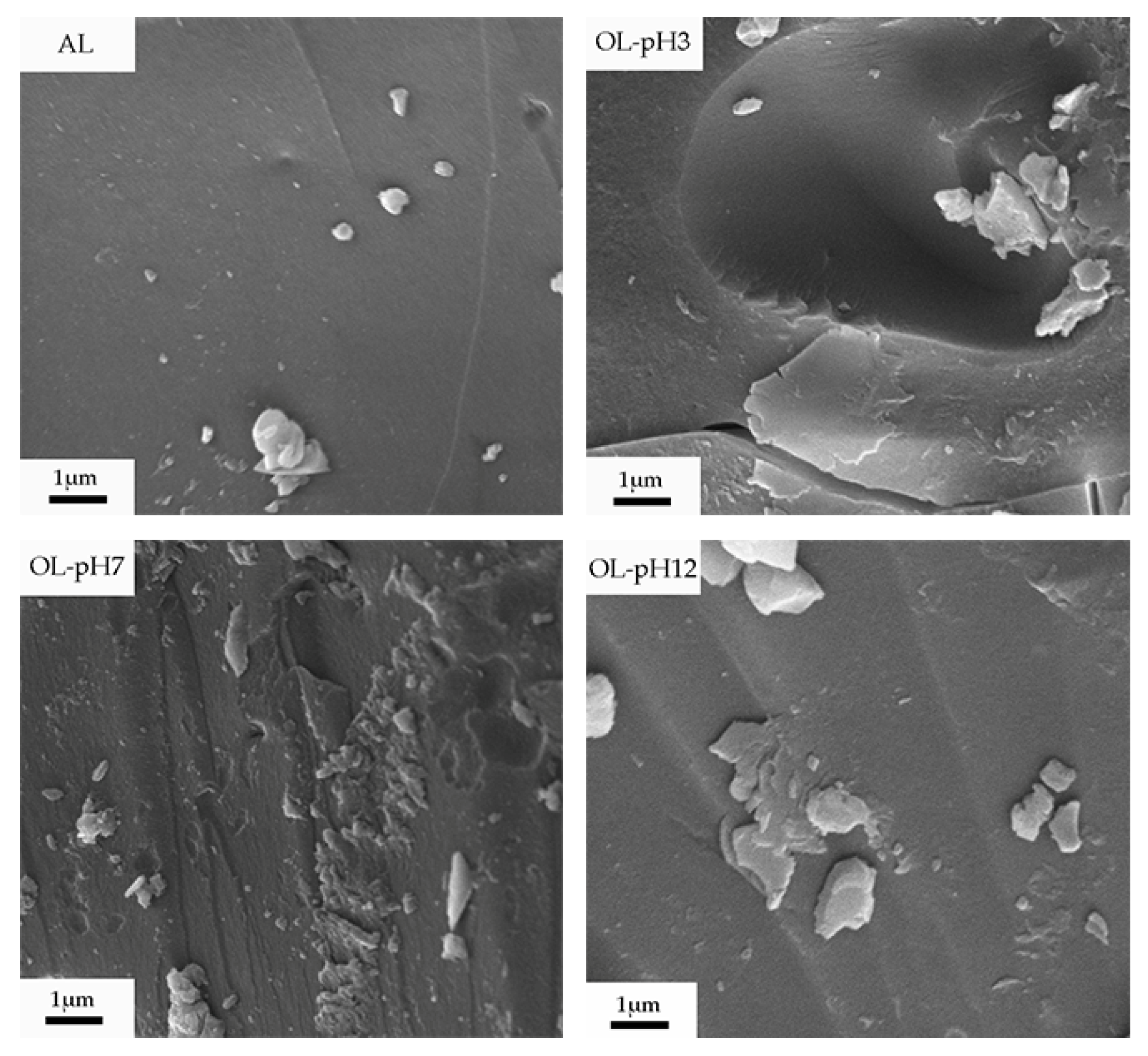

| Samples | C (%) | H (%) | O (%) | C900 Formula | Degree of Unsaturation |
|---|---|---|---|---|---|
| AL | 58.41 | 5.51 | 34.39 | C900H1019O397 | 391 |
| OL-pH3 | 61.65 | 5.59 | 31.76 | C900H979O348 | 411 |
| OL-pH7 | 61.63 | 5.52 | 31.97 | C900H967O350 | 417 |
| OL-pH12 | 64.15 | 5.74 | 29.07 | C900H966O306 | 418 |
| Assignment | Wavenumber cm−1 | AL | OL-pH3 | OL-pH7 | OL-pH12 |
|---|---|---|---|---|---|
| Hydroxyl group | 3452 | 0.88 | 0.95 | 1.09 | 1.76 |
| C-H stretching | 2937 | 0.57 | 0.38 | 0.46 | 0.22 |
| methoxy C-H | 2840 | 0.39 | 0.23 | 0.27 | 0.15 |
| carbonyl group | 1703 | 0.82 | 0.95 | 0.95 | 1.13 |
| Aromatic ring | 1608 | 0.98 | 1.03 | 1.05 | 1.31 |
| Aromatic ring | 1514 | 1.00 | 1.00 | 1.00 | 1.00 |
| C-H deformation | 1460 | 0.91 | 0.93 | 0.93 | 0.91 |
| Aromatic ring | 1426 | 0.77 | 0.80 | 0.81 | 0.77 |
| C-O vibration of syringyl | 1325 | 0.75 | 0.77 | 0.76 | 0.68 |
| Guaiacyl C-O units | 1269 | 0.92 | 0.91 | 0.89 | 0.85 |
| C-O vibration of guaiacyl | 1214 | 1.04 | 1.00 | 0.98 | 0.97 |
| Aromatic C-H deformation in syringyl | 1113 | 1.04 | 0.90 | 0.89 | 0.88 |
| C-O-C stretching | 1029 | 0.60 | 0.62 | 0.61 | 0.60 |
| Aromatic C-H deformation out of plane | 815 | 0.10 | 0.13 | 0.14 | 0.17 |
| Lignin Substructure | AL | OL-pH3 | OL-pH7 | OL-pH12 | ||||
|---|---|---|---|---|---|---|---|---|
| % a | % b | % a | % b | % a | % b | % a | % b | |
| S | 40.0 | 34.2 | 35.5 | 34.2 | ||||
| G | 60.0 | 65.8 | 64.5 | 65.8 | ||||
| S/G | 0.64 | 0.52 | 0.55 | 0.52 | ||||
| β-O-4 | 27.9 | 93.1 | 31.6 | 80.6 | 8.6 | 71.4 | 31.1 | 93.6 |
| β-β | 1.6 | 5.3 | 7.0 | 17.7 | 3.5 | 28.6 | 2.1 | 6.4 |
| Β-5 | 0.5 | 1.6 | 0.7 | 1.7 | <0.1 | <0.1 | <0.1 | <0.1 |
| Samples | Mn (g/mol) | Mw (g/mol) | PDI |
|---|---|---|---|
| AL | 7146 | 14,116 | 1.98 |
| OL-pH3 | 5154 | 10,667 | 2.07 |
| OL-pH7 | 5384 | 12,474 | 2.32 |
| OL-pH12 | 5946 | 13,192 | 2.22 |
| Samples | Zeta Potential (mV) | Hydrophobicity (L/g) |
|---|---|---|
| AL | −7.3 | 0.137 |
| OL-pH3 | −23.9 | 0.040 |
| OL-pH7 | −25.7 | 0.043 |
| OL-pH12 | −17.1 | 0.073 |
| Substrates | Γmax (g/g) | K (mL/mg) | R (mL/g) | R2 |
|---|---|---|---|---|
| Avicel | 39.06 | 8.26 | 322.58 | 0.978 |
| AL | 27.55 | 5.86 | 161.29 | 0.972 |
| OL-pH3 | 25.91 | 0.64 | 16.67 | 0.991 |
| OL-pH7 | 14.49 | 0.96 | 13.87 | 0.986 |
| OL-pH12 | 12.84 | 3.43 | 44.05 | 0.935 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhao, L.; Ren, J.; He, B. Structural Changes of Alkali Lignin under Ozone Treatment and Effect of Ozone-Oxidized Alkali Lignin on Cellulose Digestibility. Processes 2022, 10, 559. https://doi.org/10.3390/pr10030559
Wang H, Zhao L, Ren J, He B. Structural Changes of Alkali Lignin under Ozone Treatment and Effect of Ozone-Oxidized Alkali Lignin on Cellulose Digestibility. Processes. 2022; 10(3):559. https://doi.org/10.3390/pr10030559
Chicago/Turabian StyleWang, Hongyuan, Lihong Zhao, Junli Ren, and Beihai He. 2022. "Structural Changes of Alkali Lignin under Ozone Treatment and Effect of Ozone-Oxidized Alkali Lignin on Cellulose Digestibility" Processes 10, no. 3: 559. https://doi.org/10.3390/pr10030559
APA StyleWang, H., Zhao, L., Ren, J., & He, B. (2022). Structural Changes of Alkali Lignin under Ozone Treatment and Effect of Ozone-Oxidized Alkali Lignin on Cellulose Digestibility. Processes, 10(3), 559. https://doi.org/10.3390/pr10030559





