Mechanical Properties of Animal Tendons: A Review and Comparative Study for the Identification of the Most Suitable Human Tendon Surrogates
Abstract
:1. Introduction
- Finger extensors: extensor indicis (EI) and digitorum (ED);
- Finger flexors: digitorum superficialis (FDS), profundus (FDP), and pollicis longus (FPL);
- Long head of biceps (LHB);
- Anterior supraspinatus, middle supraspinatus, and posterior supraspinatus;
- Anterior tibialis tendon (ATT) and Achilles tendon (AT).
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search
2.3. Data Items
2.4. Additional Analysis
3. Results
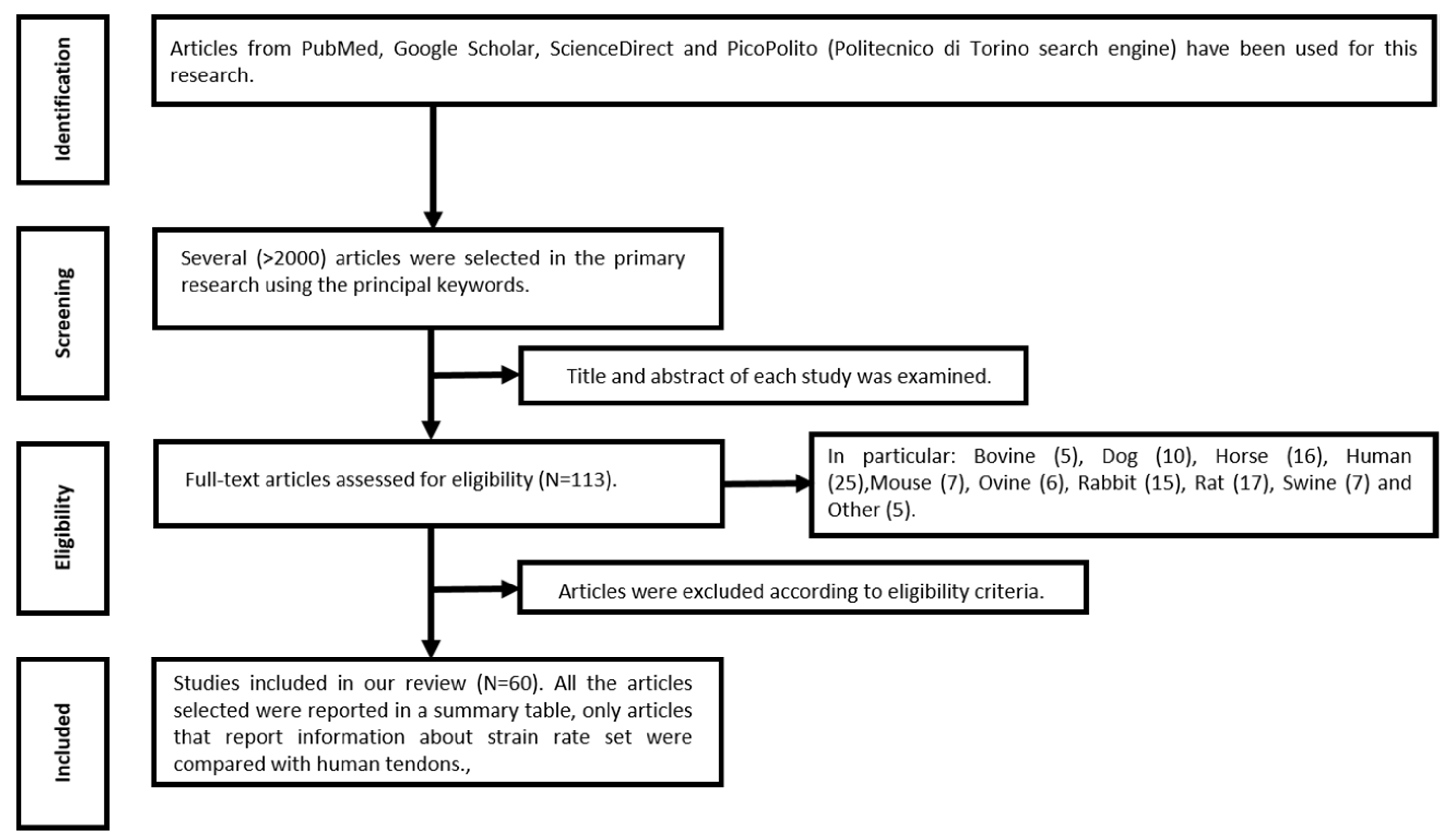
3.1. Study Selection
| Animal Species | Studies |
|---|---|
| Bovine | Domnick et al. (2016) [12], Legerlotz et al. (2010) [11] |
| Dog | Baker et al. (2012) [19], Dejardin et al. (2001) [20], Derwin et al. (2006) [21], Derwin et al. (2007) [5], Derwin et al. (2009) [22], Balogh et al. (2016) [23], Haut et al. (1992) [24], Liu et al. (2019) [4] |
| Horse | Batson et al. (2003) [25], Birch (2007) [15], Dowling and Dart. (2005) [26], Dowling et. al (2002) [27], Thorpe et al. (2010) [28] |
| Mouse | Rigozzi et al. (2010) [29], Dyment et al. (2012) [30], Lin et al. (2005) [31], Gilday et al. (2014) [32], Mikic et al. (2008) [33] |
| Ovine | Huri et al. (2013) [34], Santoni et al. (2010) [35], Gibbons et al. (1991) [36], Rumian et al. (2009) [37], Salehpour et al. (1995) [38] |
| Rabbit | Awad et al. (2003) [3], Saber et al. (2010) [39], Young et al. (1998) [40], Juncosa-Melvin et al. (2006) [41], Trudel et al. (2007) [42], Viidik (1969) [43], Yamamoto et al. (1992) [44] |
| Rat | Eliasson et al. (2007) [45], Lee et al. (2020) [46], Legerlotz et al. (2007) [47], Majewski et al. (2008) [48], Pietschmann et al. (2013) [49], Ferry et al. (2007) [50], Sahin et al. (2012) [51], Su et al. (2008) [52], Lavagnino et al. (2005) [53], Legerlotz et al. (2010) [11] |
| 11-months-old foal | Cherdchutham et al. (2001) [54] |
| Swine | Domnick et al. (2016) [12], Shadwick (1990) [16], Woo et al. (1981) [55], Smith et al. (1996) [56], Woo et al. (1980) [57], Diehl et al. (2006) [58] |
| Human | Birch (2007) [15], Carpenter et al. (2005) [59], Itoi et al. (1995) [60], Wren et al. (2001) [61], Lewis and Shaw (1997) [62], Butler et al. (1986) [63], Domnick et al. (2016) [12], Hashemi et al. (2005) [64], Weber et al. (2015) [65], Pring et al. (1985) [66] |
3.2. Synthesis of Results
3.3. Study Characteristics
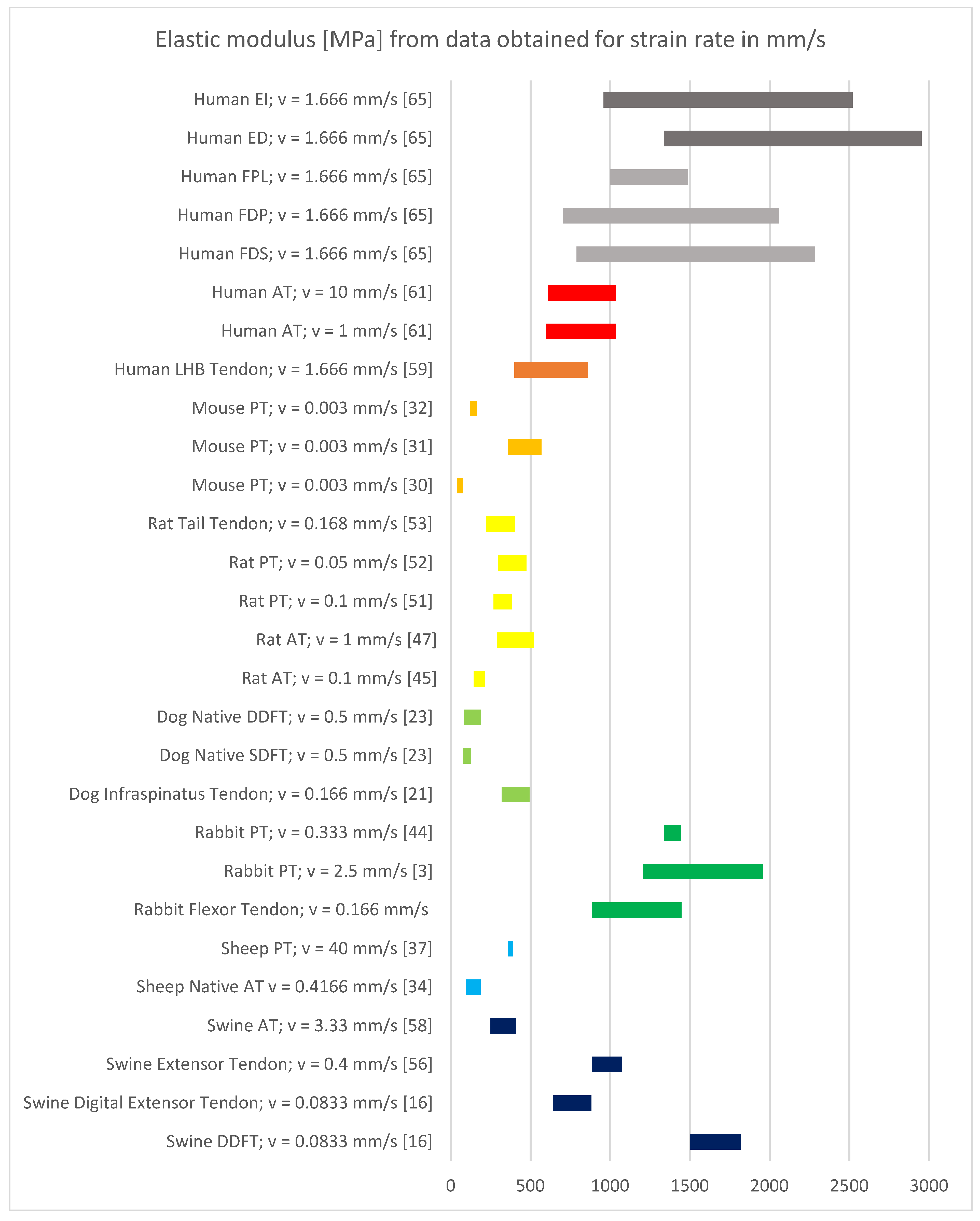
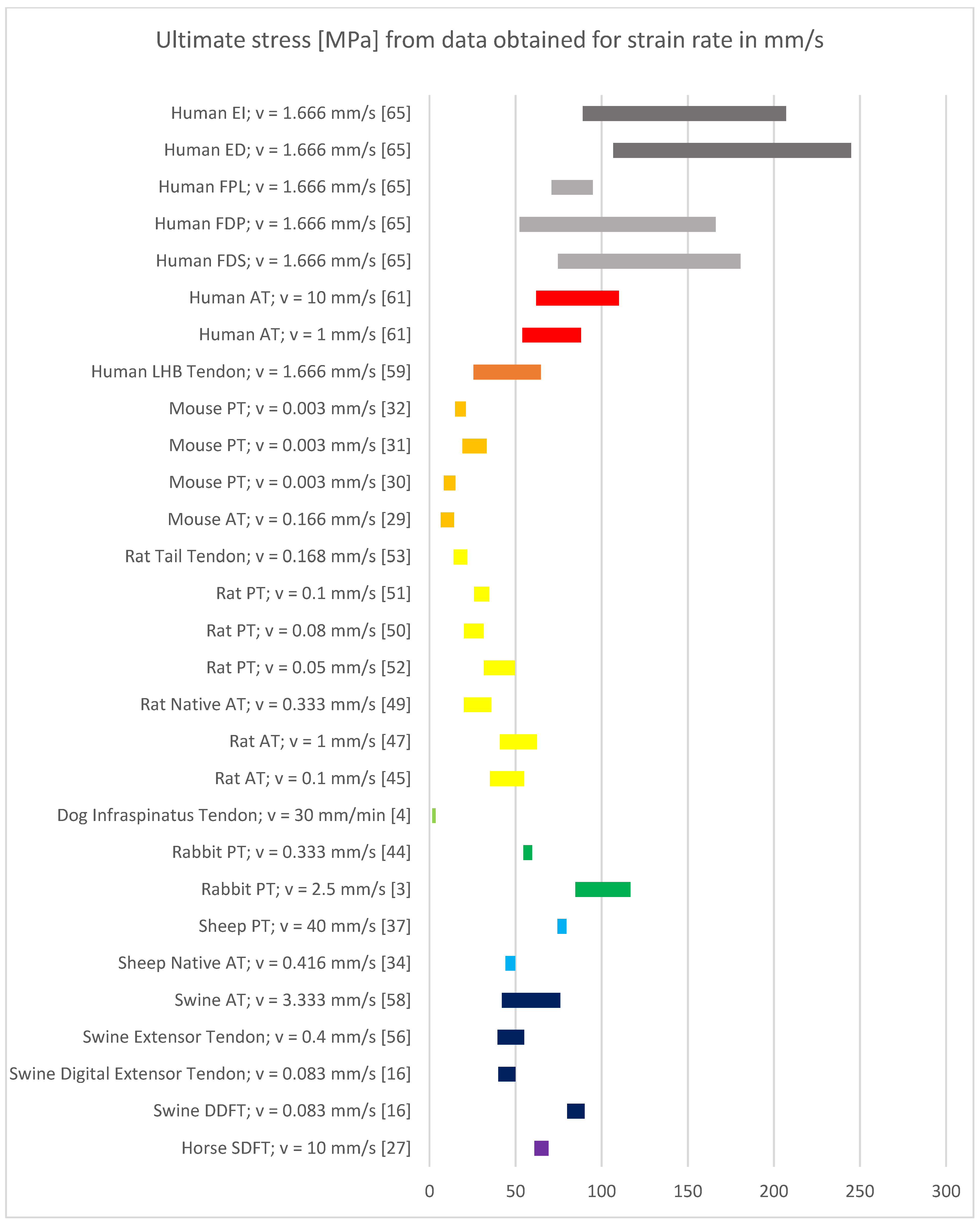
3.4. Results of Mechanical Property Evaluation in mm/s
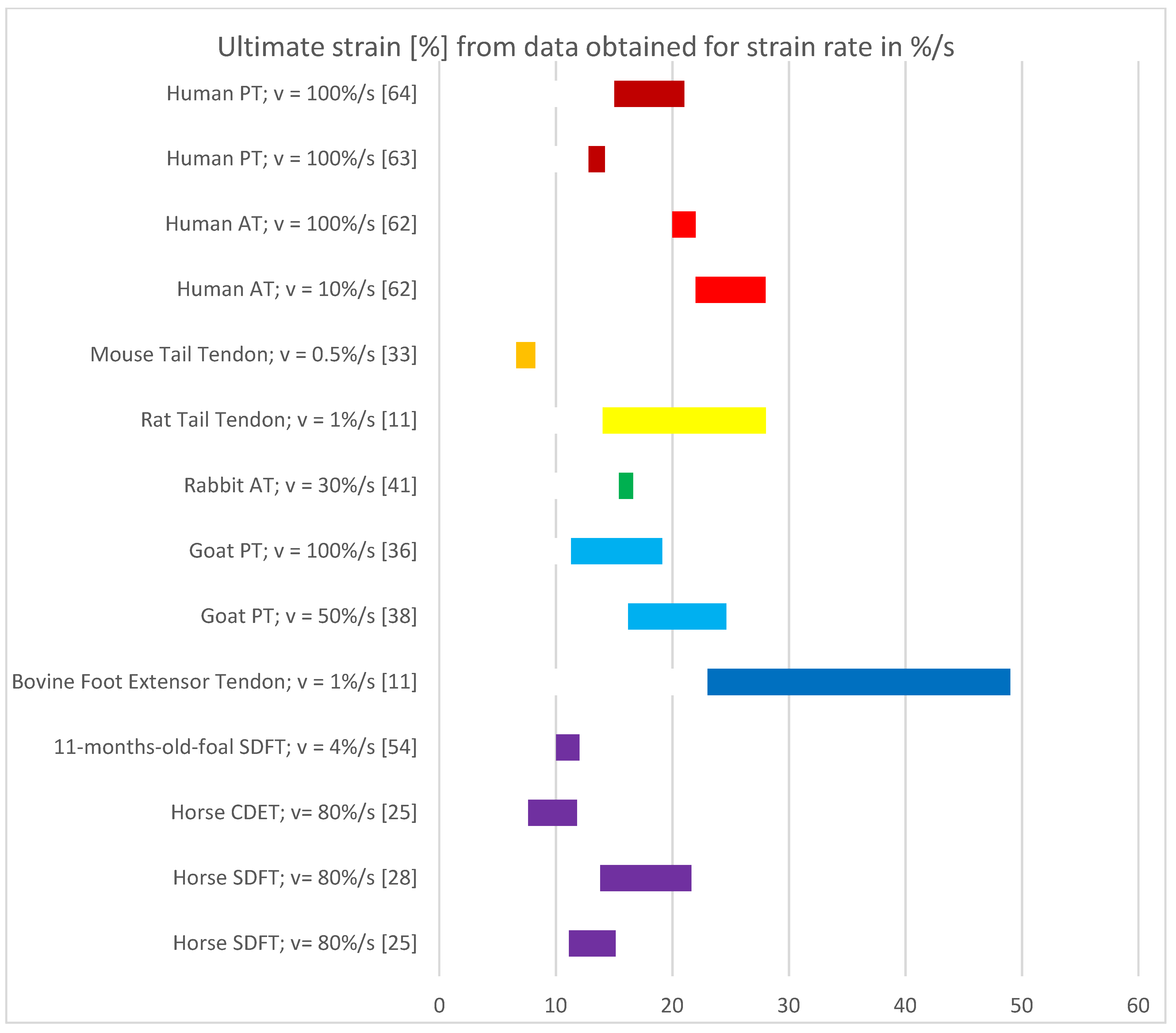
3.5. Results of Mechanical Property Evaluation in %/s
3.6. Results of Additional Analysis—Strain Rate Analysis
4. Discussion
4.1. Discussion of Results with Strain Rate in mm/s
4.2. Discussion of Results with Strain Rate in %/s
4.3. Discussion of Strain Rate Analysis
4.4. Limitations
5. Conclusions
- The flexor tendon of the hand (FPL, FDP, and FDS) shows partial similarities for Youngs’ modulus and ultimate stress with swine DDFT.
- The extensor tendon of the hand (EI and ED) has similarities with the rabbit PT.
- The LHB tendon shows similarities with the mechanical properties reported for the rat AT, rat PT, and swine digital extensor tendon.
- The human PT tendon has some similarities with the results obtained for the goat PT.
- The human AT tendon shows comparable mechanical properties with the goat PT, but there are other partial similarities.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.J.; Wang, C.J.; Yang, K.D.; Kuo, Y.R.; Huang, H.C.; Huang, Y.T.; Sun, Y.C.; Wang, F.S. Extracorporeal Shock Waves Promote Healing of Collagenase-Induced Achilles Tendinitis and Increase TGF-β1 and IGF-I Expression. J. Orthop. Res. 2004, 22, 854–861. [Google Scholar] [CrossRef]
- Ansorge, H.L.; Beredjiklian, P.K.; Soslowsky, L.J. CD44 Deficiency Improves Healing Tendon Mechanics and Increases Matrix and Cytokine Expression in a Mouse Patellar Tendon Injury Model. J. Orthop. Res. 2009, 27, 1386–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, H.A.; Boivin, G.P.; Dressler, M.R.; Smith, F.N.L.; Young, R.G.; Butler, D.L. Repair of Patellar Tendon Injuries Using a Cell-Collagen Composite. J. Orthop. Res. 2003, 21, 420–431. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Y.; Reisdorf, R.L.; Qi, J.; Lu, C.K.; Berglund, L.J.; Amadio, P.C.; Moran, S.L.; Steinmann, S.P.; An, K.N.; et al. Engineered Tendon-Fibrocartilage-Bone Composite and Bone Marrow-Derived Mesenchymal Stem Cell Sheet Augmentation Promotes Rotator Cuff Healing in a Non-Weight-Bearing Canine Model. Biomaterials 2019, 192, 189–198. [Google Scholar] [CrossRef]
- Derwin, K.A.; Baker, A.R.; Codsi, M.J.; Iannotti, J.P. Assessment of the Canine Model of Rotator Cuff Injury and Repair. J. Shoulder Elb. Surg. 2007, 16, 140–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soslowsky, L.J.; Carpenter, J.E.; DeBano, C.M.; Banerji, I.; Moalli, M.R. Development and Use of an Animal Model for Investigations on Rotator Cuff Disease. J. Shoulder Elb. Surg. 1996, 5, 383–392. [Google Scholar] [CrossRef]
- Otarodifard, K.; Wong, J.; Preston, C.F.; Tibone, J.E.; Lee, T.Q. Relative Fixation Strength of Rabbit Subscapularis Repair Is Comparable to Human Supraspinatus Repair at Time. Clin. Orthop. Relat. Res. 2014, 472, 2440–2447. [Google Scholar] [CrossRef] [Green Version]
- Jenson, P.W.; Lillich, J.D.; Roush, J.K.; Gaughan, E.M. Ex Vivo Strength Comparison of Bioabsorbable Tendon Plates and Bioabsorbable Suture in a 3-Loop Pulley Pattern for Repair of Transected Flexor Tendons from Horse Cadavers. Vet. Surg. 2005, 34, 565–570. [Google Scholar] [CrossRef]
- Duffy, D.J.; Chang, Y.-J.; Fisher, M.B.; Chambers, A.R.; Moore, G.E. Effect of Epitendinous Suture Caliber on the Tensile Strength of Repaired Canine Flexor Tendons. Am. J. Vet. Res. 2021, 82, 510–515. [Google Scholar] [CrossRef]
- Hausmann, J.-T.; Vekszler, G.; Bijak, M.; Benesch, T.; Vécsei, V.; Gäbler, C. Biomechanical Comparison of Modified Kessler and Running Suture Repair in 3 Different Animal Tendons and in Human Flexor Tendons. J. Hand Surg. 2009, 34, 93–101. [Google Scholar] [CrossRef]
- Legerlotz, K.; Riley, G.P.; Screen, H.R. Specimen Dimensions Influence the Measurement of Material Properties in Tendon Fascicles. J. Biomech. 2010, 43, 2274–2280. [Google Scholar] [CrossRef] [Green Version]
- Domnick, C.; Wieskötter, B.; Raschke, M.J.; Schulze, M.; Kronenberg, D.; Wefelmeier, M.; Langer, M.F.; Herbort, M. Evaluation of Biomechanical Properties: Are Porcine Flexor Tendons and Bovine Extensor Tendons Eligible Surrogates for Human Tendons in in Vitro Studies? Arch. Orthop. Trauma. Surg. 2016, 136, 1465–1471. [Google Scholar] [CrossRef]
- Benedict, J.V.; Walker, L.B.; Harris, E.H. Stress-Strain Characteristics and Tensile Strength of Unembalmed Human Tendon. J. Biomech. 1968, 1, 53–63. [Google Scholar] [CrossRef]
- Böl, M.; Ehret, A.; Leichsenring, K.; Ernst, M. Tissue-Scale Anisotropy and Compressibility of Tendon in Semi-Confined Compression Tests. J. Biomech. 2015, 48, 1092–1098. [Google Scholar] [CrossRef]
- Birch, H.L. Tendon Matrix Composition and Turnover in Relation to Functional Requirements. Int. J. Exp. Pathol. 2007, 88, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Shadwick, R.E. Elastic Energy Storage in Tendons: Mechanical Differences Related to Function and Age. J. Appl. Physiol. 1990, 68, 1033–1040. [Google Scholar] [CrossRef] [Green Version]
- Donahue, T.L.H.; Gregersen, C.; Hull, M.L.; Howell, S. Comparison of Viscoelastic, Structural, and Material Properties of Double-Looped Anterior Cruciate Ligament Grafts Made from Bovine Digital Extensor and Human Hamstring Tendons. J. Biomech. Eng. 2000, 123, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Robinson, P.S.; Lin, T.W.; Reynolds, P.R.; Derwin, K.A.; Iozzo, R.; Soslowsky, L.J. Strain-Rate Sensitive Mechanical Properties of Tendon Fascicles From Mice With Genetically Engineered Alterations in Collagen and Decorin. J. Biomech. Eng. 2004, 126, 252–257. [Google Scholar] [CrossRef]
- Baker, A.R.; McCarron, J.A.; Tan, C.D.; Iannotti, J.P.; Derwin, K.A. Does Augmentation with a Reinforced Fascia Patch Improve Rotator Cuff Repair Outcomes? Clin. Orthop. Relat. Res. 2012, 470, 2513–2521. [Google Scholar] [CrossRef] [Green Version]
- Dejardin, L.M.; Arnoczky, S.P.; Ewers, B.J.; Haut, R.C.; Clarke, R.B. Tissue-Engineered Rotator Cuff Tendon Using Porcine Small Intestine Submucosa. Am. J. Sports Med. 2001, 29, 175–184. [Google Scholar] [CrossRef]
- Derwin, K.A.; Baker, A.R.; Spragg, R.K.; Leigh, D.R.; Iannotti, J.P. Commercial Extracellular Matrix Scaffolds for Rotator Cuff Tendon Repair. J. Bone Jt. Surg. 2006, 88, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Derwin, K.A.; Codsi, M.J.; Milks, R.A.; Baker, A.R.; McCarron, J.A.; Iannotti, J.P. Rotator Cuff Repair Augmentation in a Canine Model with Use of a Woven Poly-L-Lactide Device. J. Bone Jt. Surg. 2009, 91, 1159–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balogh, D.G.; Biskup, J.J.; O’Sullivan, M.G.; Scott, R.M.; Groschen, D.; Evans, R.B.; Conzemius, M.G. Biochemical, Histologic, and Biomechanical Characterization of Native and Decellularized Flexor Tendon Specimens Harvested from the Pelvic Limbs of Orthopedically Normal Dogs. Am. J. Vet. Res. 2016, 77, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Haut, R.C.; Lancaster, R.L.; DeCamp, C.E. Mechanical Properties of the Canine Patellar Tendon: Some Correlations with Age and the Content of Collagen. J. Biomech. 1992, 25, 163–173. [Google Scholar] [CrossRef]
- Batson, E.L.; Paramour, R.J.; Smith, T.J.; Birch, H.; Patterson-Kane, J.C.; Goodship, A.E. Are the Material Properties and Matrix Composition of Equine Flexor and Extensor Tendons Determined by Their Functions? Equine Vet. J. 2010, 35, 314–318. [Google Scholar] [CrossRef]
- Dowling, B.; Dart, A. Mechanical and Functional Properties of the Equine Superficial Digital Flexor Tendon. Vet. J. 2005, 170, 184–192. [Google Scholar] [CrossRef]
- Dowling, B.A.; Dart, A.J.; Hodgson, D.R.; Rose, R.J.; Walsh, W.R. Recombinant Equine Growth Hormone Does Not Affect the in Vitro Biomechanical Properties of Equine Superficial Digital Flexor Tendon. Vet. Surg. 2002, 31, 325–330. [Google Scholar] [CrossRef]
- Thorpe, C.T.; Stark, R.J.F.; Goodship, A.E.; Birch, H.L. Mechanical Properties of the Equine Superficial Digital Flexor Tendon Relate to Specific Collagen Cross-Link Levels. Equine Vet. J. 2010, 42, 538–543. [Google Scholar] [CrossRef]
- Rigozzi, S.; Müller, R.; Snedeker, J.G. Collagen Fibril Morphology and Mechanical Properties of the Achilles Tendon in Two Inbred Mouse Strains. J. Anat. 2010, 216, 724–731. [Google Scholar] [CrossRef] [Green Version]
- Dyment, N.A.; Kazemi, N.; Aschbacher-Smith, L.E.; Barthelery, N.J.; Kenter, K.; Gooch, C.; Shearn, J.T.; Wylie, C.; Butler, D.L. The Relationships among Spatiotemporal Collagen Gene Expression, Histology, and Biomechanics Following Full-Length Injury in the Murine Patellar Tendon. J. Orthop. Res. 2011, 30, 28–36. [Google Scholar] [CrossRef]
- Lin, T.W.; Cardenas, L.; Soslowsky, L.J. Tendon Properties in Interleukin-4 and Interleukin-6 Knockout Mice. J. Biomech. 2005, 38, 99–105. [Google Scholar] [CrossRef]
- Gilday, S.D.; Casstevens, E.C.; Kenter, K.; Shearn, J.T.; Butler, D.L. Murine Patellar Tendon Biomechanical Properties and Regional Strain Patterns during Natural Tendon-to-Bone Healing after Acute Injury. J. Biomech. 2013, 47, 2035–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikic, B.; Entwistle, R.; Rossmeier, K.; Bierwert, L. Effect of GDF-7 Deficiency on Tail Tendon Phenotype in Mice. J. Orthop. Res. 2008, 26, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Huri, G.; Biçer, S.; Özgözen, L.; Uçar, Y.; Garbis, N.G.; Hyun, Y.S. A Novel Repair Method for the Treatment of Acute Achilles Tendon Rupture with Minimally Invasive Approach Using Button Implant: A Biomechanical Study. Foot Ankle Surg. 2013, 19, 261–266. [Google Scholar] [CrossRef]
- Santoni, B.G.; McGilvray, K.C.; Lyons, A.S.; Bansal, M.; Turner, A.S.; MacGillivray, J.D.; Coleman, S.H.; Puttlitz, C.M. Biomechanical Analysis of an Ovine Rotator Cuff Repair via Porous Patch Augmentation in a Chronic Rupture Model. Am. J. Sports Med. 2010, 38, 679–686. [Google Scholar] [CrossRef]
- Gibbons, M.J.; Butler, D.L.; Grood, E.S.; Bylski-Austrow, D.I.; Levy, M.S.; Noyes, F.R. Effects of Gamma Irradiation on the Initial Mechanical and Material Properties of Goat Bone-Patellar Tendon-Bone Allografts. J. Orthop. Res. 1991, 9, 209–218. [Google Scholar] [CrossRef]
- Rumian, A.P.; Draper, E.R.C.; Wallace, A.L.; Goodship, A.E. The Influence of the Mechanical Environment on Remodelling of the Patellar Tendon. J. Bone Jt. Surgery. Br. Vol. 2009, 91, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Salehpour, A.; Butler, D.L.; Proch, F.S.; Schwartz, H.E.; Feder, S.M.; Doxey, C.M.; Ratcliffe, A. Dose-Dependent Response of Gamma Irradiation on Mechanical Properties and Related Biochemical Composition of Goat Bone-Patellar Tendon-Bone Allografts. J. Orthop. Res. 1995, 13, 898–906. [Google Scholar] [CrossRef]
- Saber, S.; Zhang, A.Y.; Ki, S.H.; Lindsey, D.P.; Smith, R.L.; Riboh, J.; Pham, H.; Chang, J. Flexor Tendon Tissue Engineering: Bioreactor Cyclic Strain Increases Construct Strength. Tissue Eng. Part A 2010, 16, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- Young, R.G.; Butler, D.L.; Weber, W.; Caplan, A.; Gordon, S.L.; Fink, D.J. Use of Mesenchymal Stem Cells in a Collagen Matrix for Achilles Tendon Repair. J. Orthop. Res. 1998, 16, 406–413. [Google Scholar] [CrossRef]
- Juncosa-Melvin, N.; Boivin, G.P.; Galloway, M.T.; Gooch, C.; West, J.R.; Butler, D.L. Effects of Cell-to-Collagen Ratio in Stem Cell-Seeded Constructs for Achilles Tendon Repair. Tissue Eng. 2006, 12, 681–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trudel, G.; Koike, Y.; Ramachandran, N.; Doherty, G.; Dinh, L.; Lecompte, M.; Uhthoff, H.K. Mechanical Alterations of Rabbit Achilles’ Tendon After Immobilization Correlate with Bone Mineral Density But Not With Magnetic Resonance or Ultrasound Imaging. Arch. Phys. Med. Rehabil. 2007, 88, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Viidik, A. Tensile Strength Properties of Achilles Tendon Systems in Trained and Untrained Rabbits. Acta Orthop. Scand. 1969, 40, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.; Hayashi, K. Mechanical Properties of Collagen Fascicles from the Rabbit Patellar Tendon. J. Biomech. Eng. 1999, 121, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, P.; Fahlgren, A.; Pasternak, B.; Aspenberg, P. Unloaded Rat Achilles Tendons Continue to Grow, but Lose Viscoelasticity. J. Appl. Physiol. 2007, 103, 459–463. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.W.; Wang, C.; Bae, T.S.; Yang, I.; Liu, Y.; Park, C.W.; Kim, H.N. Tendon Regeneration after Partial-Thickness Peroneus Longus Tendon Harvesting: Magnetic Resonance Imaging Evaluation and in Vivo Animal Study. Am. J. Sports Med. 2020, 48, 2499–2509. [Google Scholar] [CrossRef]
- Legerlotz, K.; Schjerling, P.; Langberg, H.; Brüggemann, G.-P.; Niehoff, A. The Effect of Running, Strength, and Vibration Strength Training on the Mechanical, Morphological, and Biochemical Properties of the Achilles Tendon in Rats. J. Appl. Physiol. 2007, 102, 564–572. [Google Scholar] [CrossRef] [Green Version]
- Majewski, M.; Betz, O.; Ochsner, P.E.; Liu, F.; Porter, R.M.; Evans, C.H. Ex Vivo Adenoviral Transfer of Bone Morphogenetic Protein 12 (BMP-12) cDNA Improves Achilles Tendon Healing in a Rat Model. Gene Ther. 2008, 15, 1139–1146. [Google Scholar] [CrossRef] [Green Version]
- Pietschmann, M.F.; Frankewycz, B.; Schmitz, P.; Docheva, D.; Sievers, B.; Jansson, V.; Schieker, M.; Müller, P.E. Comparison of Tenocytes and Mesenchymal Stem Cells Seeded on Biodegradable Scaffolds in a Full-Size Tendon Defect Model. J. Mater. Sci. Mater. Med. 2012, 24, 211–220. [Google Scholar] [CrossRef]
- Ferry, S.T.; Dahners, L.E.; Afshari, H.M.; Weinhold, P.S. The Effects of Common Anti-Inflammatory Drugs on the Healing Rat Patellar Tendon. Am. J. Sports Med. 2007, 35, 1326–1333. [Google Scholar] [CrossRef]
- Sahin, H.; Tholema, N.; Petersen, W.; Raschke, M.J.; Stange, R. Impaired Biomechanical Properties Correlate with Neoangiogenesis as Well as VEGF and MMP-3 Expression during Rat Patellar Tendon Healing. J. Orthop. Res. 2012, 30, 1952–1957. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-R.; Chen, H.-H.; Luo, Z.-P. Effect of Cyclic Stretching on the Tensile Properties of Patellar Tendon and Medial Collateral Ligament in Rat. Clin. Biomech. 2008, 23, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Lavagnino, M.; Arnoczky, S.P.; Frank, K.; Tian, T. Collagen Fibril Diameter Distribution Does Not Reflect Changes in the Mechanical Properties of in Vitro Stress-Deprived Tendons. J. Biomech. 2005, 38, 69–75. [Google Scholar] [CrossRef]
- Cherdchutham, W.; Meershoek, L.S.; van Weeren, P.R.; Barneveld, A. Effects of Exercise on Biomechanical Properties of the Superficial Digital Flexor Tendon in Foals. Am. J. Vet. Res. 2001, 62, 1859–1864. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.-Y.; Gomez, M.A.; Amiel, D.; Ritter, M.A.; Gelberman, R.H.; Akeson, W.H. The Effects of Exercise on the Biomechanical and Biochemical Properties of Swine Digital Flexor Tendons. J. Biomech. Eng. 1981, 103, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.W.; Young, I.S.; Kearney, J.N. Mechanical Properties of Tendons: Changes with Sterilization and Preservation. J. Biomech. Eng. 1996, 118, 56–61. [Google Scholar] [CrossRef]
- Woo, S.L.-Y.; Ritter, M.A.; Amiel, D.; Sanders, T.M.; Gomez, M.A.; Kuei, S.C.; Garfin, S.R.; Akeson, W.H. The Biomechanical and Biochemical Properties of Swine Tendons—Long Term Effects of Exercise on the Digital Extensors. Connect. Tissue Res. 1980, 7, 177–183. [Google Scholar] [CrossRef]
- Diehl, P.; Steinhauser, E.; Gollwitzer, H.; Heister, C.; Schauwecker, J.; Milz, S.; Mittelmeier, W.; Schmitt, M. Biomechanical and Immunohistochemical Analysis of High Hydrostatic Pressure-Treated Achilles Tendons. J. Orthop. Sci. 2006, 11, 380–385. [Google Scholar] [CrossRef]
- Carpenter, J.E.; Wening, J.D.; Mell, A.G.; Langenderfer, J.E.; Kuhn, J.E.; Hughes, R.E. Changes in the Long Head of the Biceps Tendon in Rotator Cuff Tear Shoulders. Clin. Biomech. 2005, 20, 162–165. [Google Scholar] [CrossRef]
- Itoi, E.; Berglund, L.J.; Grabowski, J.J.; Schultz, F.M.; Growney, E.S.; Morrey, B.F.; An, K.-N. Tensile Properties of the Supraspinatus Tendon. J. Orthop. Res. 1995, 13, 578–584. [Google Scholar] [CrossRef]
- Wren, T.A.; Yerby, S.A.; Beaupre, G.; Carter, D.R. Mechanical Properties of the Human Achilles Tendon. Clin. Biomech. 2001, 16, 245–251. [Google Scholar] [CrossRef]
- Lewis, G.; Shaw, K.M. Tensile Properties of Human Tendo Achillis: Effect of Donor Age and Strain Rate. J. Foot Ankle Surg. 1997, 36, 435–445. [Google Scholar] [CrossRef]
- Butler, D.L.; Kay, M.D.; Stouffer, D.C. Comparison of Material Properties in Fascicle-Bone Units from Human Patellar Tendon and Knee Ligaments. J. Biomech. 1986, 19, 425–432. [Google Scholar] [CrossRef]
- Hashemi, J.; Chandrashekar, N.; Slauterbeck, J. The Mechanical Properties of the Human Patellar Tendon Are Correlated to Its Mass Density and Are Independent of Sex. Clin. Biomech. 2005, 20, 645–652. [Google Scholar] [CrossRef]
- Weber, J.F.; Agur, A.M.R.; Fattah, A.Y.; Gordon, K.D.; Oliver, M.L. Tensile Mechanical Properties of Human Forearm Tendons. J. Hand Surg. 2015, 40, 711–719. [Google Scholar] [CrossRef]
- Pring, D.J.; Amis, A.A.; Coombs, R.R.H. The Mechanical Properties of Human Flexor Tendons in Relation to Artificial Tendons. J. Hand Surg. 1985, 10, 331–336. [Google Scholar] [CrossRef]
- Smith, R.; Birch, H.; Goodman, S.; Heinegård, D.; Goodship, A. The Influence of Ageing and Exercise on Tendon Growth and Degeneration—Hypotheses for the Initiation and Prevention of Strain-Induced Tendinopathies. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 133, 1039–1050. [Google Scholar] [CrossRef]
- Yamamoto, E.; Hayashi, K.; Yamamoto, N. Effects of Stress Shielding on the Transverse Mechanical Properties of Rabbit Patellar Tendons. J. Biomech. Eng. 2000, 122, 608–614. [Google Scholar] [CrossRef]
- Abrahams, M. Mechanical Behaviour of Tendon. Med. Biol. Engng. 1967, 5, 433–443. [Google Scholar] [CrossRef]
- Herrick, W.C.; Kingsbury, H.B.; Lou, D.Y.S. A Study of the Normal Range of Strain, Strain Rate, and Stiffness of Tendon. J. Biomed. Mater. Res. 1978, 12, 877–894. [Google Scholar] [CrossRef]



| Type of Tendon | Species/Breed | Population (n. of Tendons) | Preconditioning | Loading Rate (mm∙s−1; mm∙min−1; %∙s−1) | Young’s Modulus (MPa) | Maximal Load (N) | Ultimate Stress (MPa) | Ultimate Strain (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Bovine | |||||||||
| Extensor digitorum tendon | na | 12 | Yes | 200 mm∙min−1 | na | 1.739 ± 254 | na | na | Domnick et al., 2016 [12] |
| Foot extensor tendon | na | 5 | na | 1%∙s−1 | 714 ± 120 | na | na | 36 ± 13 | Legerlotz et al., 2010 [11] |
| Dog | |||||||||
| Infraspinatus tendon (g) | Mongrel | 8 | Yes | 30 mm∙min−1 | na | 1.595 ± 285 | na | na | Baker et al., 2012 [19] |
| Infraspinatus tendon (b) | na | 6 | na | 25 mm∙s−1 | na | 187 ± 31 | na | na | Dejardin et al., 2001 [20] |
| Infraspinatus tendon (h) | na | 9 | Yes | 10 mm∙min−1 | 405.3 ± 86.4 | na | na | na | Derwin et al., 2006 [21] |
| Infraspinatus tendon (h) | Mongrel | 8 | Yes | 6 mm∙min−1 | na | 1.349 ± 181 | na | na | Derwin et al., 2007 [5] |
| Infraspinatus tendon (g) | Mongrel | 8 | Yes | 30 mm∙min−1 | na | 1.595 ± 285 | na | na | Derwin et al., 2009 [22] |
| Infraspinatus tendon (b) | Mixed breed | 14 | na | 30 mm∙min−1 | na | 163.20 ± 61.21 | 2.60 ± 0.97 | na | Liu et al., 2019 [4] |
| SDFT | na | 6 | Yes | 0.5 mm∙s−1 | 101.3 ± 24.0 | 1721.3 ± 729.9 | na | na | Balogh et al., 2016 [23] |
| DDFT | na | 5 | Yes | 0.5 mm∙s−1 | 136.4 ± 52.9 | 2014.3 ± 229.5 | na | na | Balogh et al., 2016 [23] |
| PT | Medium and large breeds | 27 | Yes | 100%∙s−1 | 474 ± 101 | na | na | na | Haut et al., 1992 [24] |
| Horse | |||||||||
| SDFT | na | 26 | Yes | 80%∙s−1 | 1086 ± 261 | (10 ± 3) ∙103 | 110 ± 33 | 13.1 ± 2.0 | Batson et al., 2003 [25] |
| SDFT | na | 12 | na | na | 970.8 ± 60.4 | 10.465 ± 410 | 115.74 ± 4.38 | 25.98 ± 1.44 | Birch et al., 2007 [15] |
| SDFT | na | na | na | na | 1189 ± 63 | (12.4 ± 1.3) ∙103 | 109.4 ± 8.4 | 12.5 ± 1.7 | Dowling and Dart, 2005 [26] |
| SDFT | Standardbred | 3 | No | 10 mm∙s−1 | na | 7.553 ± 881 | 65 ± 4.1 | 17.3 ± 1.2 | Dowling et al., 2002 [27] |
| SDFT | na | 38 | Yes | 80%∙s−1 | 1217.0 ± 199.4 | 12.379 ± 2 494 | 128.1 ± 74.7 | 17.7 ± 3.9 | Thorpe et al., 2010 [28] |
| CDET | na | 26 | Yes | 80%∙s−1 | 1586 ± 279 | (3 ± 1) ∙103 | 128 ± 42 | 9.7 ± 2.1 | Batson et al., 2003 [25] |
| CDET | na | 12 | na | na | 1236 ± 209.6 | 3756 ± 241 | 136.94 ± 10.44 | 20.45 ± 1.60 | Birch et al., 2007 [15] |
| Mouse | |||||||||
| AT (q) | na | 10 | Yes | 10 mm∙min−1 | na | 6.0 ± 2.3 | 10.4 ± 3.9 | 36 ± 17 | Rigozzi et al., 2010 [29] |
| PT (n) | na | 15 | Yes | 0.003 mm∙s−1 | 56.51 ± 18.29 | 4.13 ± 0.87 | 11.68 ± 3.38 | na | Dyment et al., 2012 [30] |
| PT (b) | na | 12 | Yes | 0.003 mm∙s−1 | 462.8 ± 104.0 | na | 26.1 ± 7.0 | na | Lin et al., 2005 [31] |
| PT (n) | na | 10 | Yes | 0.003 mm∙s−1 | 140.04 ± 19.60 | 4.73 ± 1.03 | 17.96 ± 3.09 | 10.80 ± 2.52 | Gilday et al., 2014 [32] |
| Tail tendon (r) | na | 20 | na | 0.5%∙s−1 | 526 ± 97 | na | 33 ± 7 | 7.4 ± 0.81 | Mikic et al., 2008 [33] |
| Ovine | |||||||||
| (Sheep) Native AT (b) | Merino wether | 9 | na | 25 mm∙min−1 | (139.6 ± 46.7) ∙103 | 704.5 ± 85.8 | 44.2 ± 5.6 | na | Huri et al., 2013 [34] |
| (Sheep) Infraspinatus tendon (e) | Rambouillet–Columbia cross | 9 | Yes | 0.5 %∙s−1 | na | 3516.39 ± 279.61 | na | na | Santoni et al., 2010 [35] |
| (Goat) PT (f) | Mixed breed | 24 | Yes | 100%∙s−1 | 1639.1 ± 435.9 | 1406.1 ± 363.8 | 126.8 ± 20.8 | 15.2 ± 3.9 | Gibbons et al., 1991 [36] |
| (Sheep) PT (b) | Welsh mule | 12 | Yes | 40 mm∙s−1 | 373 ± 16.7 | (2.92 ± 0.075) ∙103 | 76.9 ± 2.66 | 28.1 ± 0.80 | Rumian et al., 2009 [37] |
| (Goat) PT (b) | Mixed breed | 21 | Yes | 50%∙s−1 | 529.5 ± 109.7 | 1338.7 ± 463.2 | 81.4 ± 22.7 | 20.4 ± 4.2 | Salehpour et al., 1995 [38] |
| Rabbit | |||||||||
| Flexor tendon (g) | New Zealand | 15 | Yes | 10 mm∙min−1 | 1166 ± 281 | 72.97 ± 14.53 | na | na | Saber et al., 2010 [39] |
| Gastrocnemius tendon (h) | New Zealand | 5 | na | 20%∙s−1 | 337.5 ± 205.8 | 189.0 ± 26.8 | 41.6 ± 18.9 | na | Young et al., 1998 [40] |
| AT (h) | New Zealand | 8 | Yes | 30%∙s−1 | 180.0 ± 12.5 | 390.0 ± 50.0 | 33.0 ± 4.2 | 16.0 ± 0.6 | Juncosa-Melvin et al., 2019 [41] |
| AT (b) | New Zealand | 10 | Yes | 10 mm∙s−1 | na | 768 ± 16 | na | na | Trudel et al., 2007 [42] |
| AT (b) | na | 13 | na | na | na | 377.4 ± 17.5 | na | na | Viidik et al., 1969 [43] |
| PT (i) | New Zealand | 8 | na | 2.5 mm∙s−1 | 1.581.4 ± 374.9 | 470.7 ± 67.2 | 100.7 ± 16.0 | 7.4 ± 1.5 | Awad et al., 2003 [3] |
| PT (j) | Japanese | 14 | Yes | 20 mm∙min−1 | 1.390 ± 53 | 799 ± 40 | 57.1 ± 2.5 | 5.1 ± 0.2 | Yamamoto et al., 1992 [44] |
| Rat | |||||||||
| AT (k) | Sprague–Dawley | 26 | Yes | 0.1 mm∙s−1 | 179 ± 36 | 63 ± 5 | 45 ± 10 | na | Eliasson et al., 2007 [45] |
| AT (l) | Sprague–Dawley | 15 | Yes | 3 mm∙min−1 | na | 43.3 ± 9.6 | na | na | Lee et al., 2020 [46] |
| AT (m) | Sprague–Dawley | 20 | Yes | 1 mm∙s−1 | 405 ± 115 | 48.6 ± 9.7 | 51.6 ± 10.8 | 20.5 ± 5.5 | Legerlotz et al., 2007 [47] |
| AT (n) | Sprague–Dawley | 12 | No | 1000 mm∙min−1 | na | 60–80 | na | na | Majewski et al., 2008 [48] |
| AT (o) | Lewis | 6 | Yes | 20 mm∙min−1 | na | 52.6 ± 7.8 | 27.9 ± 8.0 | na | Pietschmann et al., 2013 [49] |
| PT (b) | Sprague–Dawley | 141 | Yes | 0.08 mm∙s−1 | na | na | 25.7 ± 5.7 | 31.2 ± 5.9 | Ferry et al., 2007 [50] |
| PT (o) | Wistar | 8 | na | 0.1 mm∙s−1 | 323.88 ± 56.48 | na | 30.24 ± 4.41 | na | Sahin et al., 2012 [51] |
| PT (p) | Sprague–Dawley | 10 | Yes | 0.05 mm∙s−1 | 386 ± 88 | 55.0 ± 13.6 | 40.5 ± 8.95 | 20.0 ± 3.3 | Su et al., 2008 [52] |
| Tail tendon (b) | Sprague–Dawley | 6 | Yes | 0.168 mm∙s−1 | 312.8 ± 89.5 | na | 17.95 ± 3.99 | 7.60 ± 0.99 | Lavagnino et al., 2005 [53] |
| Tail tendon | na | 5 | na | 1%∙s−1 | 1.000 ± 165 | na | na | 21 ± 7 | Legerlotz et al., 2010 [11] |
| 11-month-old foal | |||||||||
| SDFT (a) | Dutch warmblood | 6 | Yes | 4%∙s−1 | na | na | 100 ± 10 | 11 ± 1 | Cherdchutham et al., 2001 [54] |
| Swine | |||||||||
| Flexor digitorum profundus tendon | na | 12 | Yes | 200 mm∙min−1 | na | 1795 ± 191 | na | na | Domnick et al., 2016 [12] |
| DDFT | na | 9 | na | 5 mm∙min−1 | (1.66 ± 0.16) ∙103 | na | 80–90 | na | Shadwick et al., 1990 [16] |
| Digital flexor tendon (b) | Yucatan | 18 | Yes | 2 cm∙min−1 | na | (1.63 ± 0.07) ∙103 | na | na | Woo et al., 1981 [55] |
| Digital extensor tendon | na | 9 | na | 5 mm∙min−1 | (0.76 ± 0.12) ∙103 | na | 40–50 | na | Shadwick et al., 1990 [16] |
| Extensor tendon (c) | na | 12 | Yes | 0.4 mm∙s−1 | (0.980 ± 0.0943) ∙103 | na | 47.29 ± 7.69 | 6.65 ± 1.23 | Smith et al., 1996 [56] |
| Medial digital extensor tendon (b) | Yucatan | 18 | na | 2 cm∙min−1 | na | 200 ± 20 | na | na | Woo et al., 1980 [57] |
| Lateral digital extensor tendon (b) | Yucatan | 18 | na | 2 cm∙min−1 | na | 290 ± 20 | na | na | Woo et al., 1980 [57] |
| AT (d) | na | 10 | Yes | 200 mm∙min−1 | 248–409 | na | 42–76 | na | Diehl et al., 2006 [58] |
| Type of Tendon | Population | Preconditioning | Loading Rate (mm∙s−1; mm∙min−1; %∙s−1) | Young’s Modulus (MPa) | Maximal Load (N) | Ultimate Stress (MPa) | Ultimate Strain (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Human | ||||||||
| LHB tendon (s) | 7 | Yes | 100 mm∙min−1 | 629 ± 230 | 305 ± 96.9 | 45.1 ± 19.6 | 18 ± 13 | Carpenter et al., 2005 [59] |
| Semitendinosus tendon | 12 | Yes | 200 mm∙min−1 | na | 1 406 ± 216 | na | na | Domnick et al., 2016 [12] |
| Anterior supraspinatus tendon | 11 | na | 10 %∙s−1 | na | 411.1 ± 158.8 | 16.5 ± 7.1 | na | Itoi et al., 1995 [60] |
| Middle supraspinatus tendon | 11 | na | 10 %∙s−1 | na | 152.6 ± 87.5 | 6.0 ± 2.6 | na | Itoi et al., 1995 [60] |
| Posterior supraspinatus tendon | 11 | na | 10 %∙s−1 | na | 88.1 ± 32.1 | 4.1 ± 1.3 | na | Itoi et al., 1995 [60] |
| ATT | na | na | na | 426 ± 269 | (1.54 ± 0.17) ∙103 | 60.60 ± 9.34 | na | Birch et al., 2007 [15] |
| AT | na | na | na | 212 ± 109 | (3.87 ± 1.61) ∙103 | 53.53 ± 19.77 | na | Birch et al., 2007 [15] |
| AT (t) | 11 | na | 1 mm∙s−1 | 816 ± 218 | 4 617 ± 1 107 | 71 ± 17 | 7.5 ± 1.1 | Wren et al., 2001 [61] |
| AT (t) | 11 | na | 10 mm∙s−1 | 822 ± 211 | 5 579 ± 1 143 | 86 ± 24 | 9.9 ± 1.9 | Wren et al., 2001 [61] |
| AT | 16 | Yes | 10 %∙s−1 | 529.5 ± 109.7 | na | 73 ± 13 | 25 ± 3 | Lewis et Shaw., 1997 [62] |
| AT | 16 | Yes | 100 %∙s−1 | 1639.1 ± 435.9 | na | 81 ± 14 | 21 ± 1 | Lewis et Shaw., 1997 [62] |
| PT | 3 | na | 100 %∙s−1 | 643.1 ± 53.0 | na | 68.5 ± 6.0 | 13.5 ± 0.7 | Butler et al., 1986 [63] |
| PT | 20 | Yes | 100 %∙s−1 | 507.4 ± 135.3 | na | 58.7 ± 16.3 | 18 ± 3 | Hashemi et al., 2005 [64] |
| FDS | 5 | Yes | 100 mm∙min−1 | 1535.5 ± 747.5 | na | 127.65 ± 53 | 10.25 ± 1.5 | Weber et al., 2015 [65] |
| FDP | 5 | Yes | 100 mm∙min−1 | 1381.5 ± 677.75 | na | 109.25 ± 57 | 10.5 ± 1.25 | Weber et al., 2015 [65] |
| FPL | 5 | Yes | 100 mm∙min−1 | 1242 ± 244 | na | 82.9 ± 12 | 10 ± 3 | Weber et al., 2015 [65] |
| ED | 5 | Yes | 100 mm∙min−1 | 2145.25 ± 808 | na | 175.75 ± 69 | 10 ± 1 | Weber et al., 2015 [65] |
| EI | 5 | Yes | 100 mm∙min−1 | 1739 ± 781 | na | 148 ± 59 | 10 ± 2 | Weber et al., 2015 [65] |
| Tendon | Loading Rate (mm∙s−1) | Young’s Modulus (MPa) | Ultimate Stress (MPa) | Maximal Load (N) | Ultimate Strain (%) | Reference |
|---|---|---|---|---|---|---|
| Rabbit PT | 0.33 | 1390 ± 53 | 57.1 ± 2,5 | 799 ± 40 | 5.1 ± 0.27 | Yamamoto et al., 1992 [44] |
| Rabbit PT | 2.5 | 1581.4 ± 374.9 | 100.7 ± 16 | 470.7 ± 67.2 | 7.4 ± 1.5 | Awad et al., 2003 [3] |
| Rat AT | 0.1 | 179 ± 36 | 45 ± 10 | 63 ± 5 | na | Eliasson et al., 2007 [45] |
| Rat AT | 1 | 405 ± 115 | 51.6 ± 10.8 | 48.6 ± 9.7 | 20.5 ± 5.5 | Legerlotz et al., 2007 [47] |
| Rat PT | 0.05 | 386 ± 88 | 40.5 ± 8.95 | 55 ± 13.6 | 20.0 ± 3.3 | Su et al., 2008 [52] |
| Rat PT | 0.1 | 323.88 ± 56.48 | 30.24 ± 4.41 | na | na | Sahin et al., 2012 [51] |
| Human AT | 1 | 816 ± 218 | 71 ± 17 | 4617 ± 1107 | 7.5 ± 1.1 | Wren et al., 2001 [61] |
| Human AT | 10 | 822 ± 211 | 86 ± 24 | 5579 ± 1143 | 9.9 ± 1.9 | Wren et al., 2001 [61] |
| Tendon | Loading Rate (%∙s−1) | Young’s Modulus (MPa) | Ultimate Stress (MPa) | Maximal Load (N) | Ultimate Strain (%) | Reference |
|---|---|---|---|---|---|---|
| Goat PT | 50 | 529.5 ± 109.7 | 81.4 ± 22.7 | 1338.7 ± 463.2 | 20.4 ± 4.2 | Salehpour et al., 1995 [38] |
| Goat PT | 100 | 1639.1 ± 435.9 | 126.8 ± 20.8 | 1406.1 ± 363.8 | 15.2 ± 3.9 | Gibbons et al., 1991 [36] |
| Human AT | 10 | 401 ± 59 | 73 ± 13 | na | 25 ± 3 | Lewis et al., 1997 [62] |
| Human AT | 100 | 545 ± 43 | 81 ± 14 | na | 21 ± 1 | Lewis et al., 1997 [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgio, V.; Civera, M.; Rodriguez Reinoso, M.; Pizzolante, E.; Prezioso, S.; Bertuglia, A.; Surace, C. Mechanical Properties of Animal Tendons: A Review and Comparative Study for the Identification of the Most Suitable Human Tendon Surrogates. Processes 2022, 10, 485. https://doi.org/10.3390/pr10030485
Burgio V, Civera M, Rodriguez Reinoso M, Pizzolante E, Prezioso S, Bertuglia A, Surace C. Mechanical Properties of Animal Tendons: A Review and Comparative Study for the Identification of the Most Suitable Human Tendon Surrogates. Processes. 2022; 10(3):485. https://doi.org/10.3390/pr10030485
Chicago/Turabian StyleBurgio, Vito, Marco Civera, Mariana Rodriguez Reinoso, Elena Pizzolante, Simona Prezioso, Andrea Bertuglia, and Cecilia Surace. 2022. "Mechanical Properties of Animal Tendons: A Review and Comparative Study for the Identification of the Most Suitable Human Tendon Surrogates" Processes 10, no. 3: 485. https://doi.org/10.3390/pr10030485
APA StyleBurgio, V., Civera, M., Rodriguez Reinoso, M., Pizzolante, E., Prezioso, S., Bertuglia, A., & Surace, C. (2022). Mechanical Properties of Animal Tendons: A Review and Comparative Study for the Identification of the Most Suitable Human Tendon Surrogates. Processes, 10(3), 485. https://doi.org/10.3390/pr10030485









