Abstract
Despite providing interesting solutions to reduce the number of synthetic steps, to decrease energy consumption or to generate less waste, therefore contributing to a more sustainable way of producing important chemicals, the expansion of the use of homogeneous catalysis in industrial processes is hampered by several drawbacks. One of the most important is the difficulty to recycle the noble metals generating potential high costs and pollution of the synthesized products by metal traces detrimental to their applications. Supporting the metals on abundant and cheap biosourced polymers has recently appeared as an almost ideal solution: They are much easier to recover from the reaction medium and usually maintain high catalytic activity. The present bibliographical review focuses on the development of catalysts based on group 10 transition metals (nickel, palladium, platinum) supported on biopolymers obtained from wood, such as cellulose, hemicellulose, lignin, and their derivatives. The applications of these catalysts in organic synthesis or depollution are also addressed in this review with examples of C-C couplings, oxidation, or hydrogenation reactions.
1. Introduction
Catalysis is one of the pillars of green chemistry. Currently, catalysis, particularly with transition metals, have found numerous applications for the production of fine chemicals. The usefulness of transition metal catalysis is obvious, as it is often a way to reduce the number of synthetic steps and to consume less energy in chemical processes. However, its development in industrial processes still struggles to expand and is hampered by several obstacles. Indeed, frequent use of high catalyst loadings combined to the difficulty to recycle homogeneous catalysts can contribute to an important consumption of noble metals, leading to an elevated cost for the process. Moreover, the toxicity of some metals or the effect of their presence in the final product represent a considerable problem for chemical industry because very low traces of metallic residues can usually be tolerated (particularly if the molecules are intended to pharmaceutical or electronic-organic applications). Therefore, it is necessary to investigate new catalytic systems both efficient and easy to recycle, with low metal leaching, in order to expand the use of catalysis and improve the catalytic processes. Heterogeneous catalysts have many advantages; they can in particular be easily recycled by filtration or centrifugation. With this goal in mind, the use of supported catalysts could represent an interesting solution. Supported catalysis combine some advantages of both types of catalysis: They are easy to recover like heterogeneous catalysts but generally have a higher catalytic activity than them (sometimes close to homogeneous catalysts). The catalytic mechanism of supported catalysts itself could be qualified homogeneous in some cases, in particular that of supported metal complexes [1].
The biopolymers contained in wood seem to be great candidate to act as support for catalysts. They are renewable, biodegradable, abundant so relatively cheap, chemically stable and quite insoluble in most solvents (which enables easy separation from the reaction medium). The chemical composition of wood not only varies from different tree species but it is also different between parts of the same tree [2]. Within a tree species, the composition is also influenced by external factors such as climate, nature of the soil, or geographic location for examples. However, despite these variations, wood generally contains around 50% of carbon, 44% of oxygen, and 6% of hydrogen, with trace amounts of metal cations. From a molecular point of view, wood is constituted of two major components: carbohydrate (65–75% of dry wood weight) and lignin (18–35%), plus other non-polymeric molecules or inorganic residues. Among the carbohydrates, cellulose is the most abundant as it represents 40–50% of the dry mass, with hemicellulose only accounting for 25–35%. Biopolymers extracted from wood, as well as wood itself, can act as support for catalysts [3].
The aim of this review is to offer to readers an overview of the catalysts based on group 10 transition metals (Ni, Pd, Pt) and supported on wood or biopolymers obtained from it. The preparation and characterization of the supported catalysts as well as their catalytic activities are highlighted. Group 10 transition metals have proven themselves to be almost indispensable catalysts for organic synthesis or for depollution purpose [4]. Indeed, they can promote various C-C couplings and although the use of palladium-catalyzed cross couplings in organic synthesis was rewarded with a Nobel prize a little over ten years ago, a lot of new catalysts more efficient, more selective or easier to recycle are still developed every year [5,6,7,8,9,10,11]. Group 10 transition metals are also very efficient redox catalysts with this property being exploited for depollution, in the total oxidation of organic residues for example [12,13] or in synthesis, when they catalyzed hydrogenation of unsaturated compounds [14,15,16]. All this diversity of catalytic reactions is illustrated in the present review.
2. Cellulose
Cellulose is the most common and abundant biopolymer on Earth. It is found in some bacterial biofilms and some algae, but is mostly present as a structural component of green plant cell walls. It accounts for 35 to 55% of the biomass and up to 90% in cotton fibers. Paper is made of almost pure cellulose generally extracted from wood, which contains 40–50% of it. Herbaceous plants, such as hemp, flax, rape, canes, and bamboo are other interesting sources of cellulose, as are plant wastes (e.g., sugarcane bagasse, wheat straw, natural textiles) [17]. Many of the derivatives of cellulose are manufactured on an industrial scale for a very wide range of applications. The annual world production of cellulose in 2017 was 50 billion tons [18]. Cellulose is structured as a linear and fibrous chain of hundreds of D-glucose moieties linked by β-1,4-glycosidic bonds (Figure 1) [2,19]. The numerous hydroxyl groups establish a network of intra- and intermolecular H-bonding. Its formula is (C6H10O5)n with 200 < n < 7000, which makes it insoluble in water. Native cellulose fibers are made of grouped microfibrils exhibiting alternating crystalline and amorphous regions. Various allomorphs can be obtained with different extraction treatments and different plant species [17]. Cellulose is hydrophilic, biocompatible, non-toxic, environmentally-friendly, and easy to chemically modify [18,20].
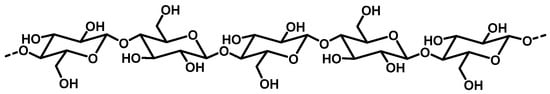
Figure 1.
Structure of cellulose biopolymer.
The amorphous regions of cellulose are removed by dilute acid hydrolysis to yield microcrystalline cellulose as rod-shaped particles in the microscale [21]. Nanostructured cellulose can be obtained along two processes. Cellulose nanofibrils (CNF) can be isolated by mechanical treatment of cellulose pulp assisted by chemical oxidation or enzymatic treatment; they are semi-flexible, 5–20 nm in diameter and several micrometers long [22]. Cellulose nanocrystal (CNC) are yielded by acid hydrolysis assisted by ultrasonic treatment; it is a highly crystalline material made of smaller rigid fibers (whisker-like, 100–1000 nm long and a few nm in diameter) [23]. Some water-soluble derivatives of cellulose are commonly produced by etherification reactions of the hydroxyl groups: methylcellulose, carboxymethyl cellulose (CMC), hydroxypropyl methylcellulose [18].
2.1. In C-C Coupling
2.1.1. In Suzuki–Miyaura Coupling
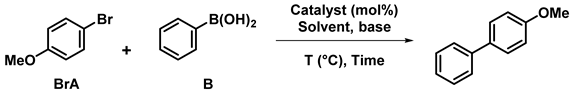
Suzuki–Miyaura cross-coupling reaction is amongst the most commonly used ways to create C-C bonds, a key step in organic synthesis with many industrial applications. Suzuki coupling reaction is catalyzed by transition metal species, generally palladium complexes, or nanoparticles (NPs).
Cellulose Derivatized with Functionalized Spacers
Several cellulose-supported palladium catalysts have been developed for Suzuki coupling reactions. N-heterocyclic carbenes (NHC) have been grafted onto cellulose in order to chelate palladium(II) ions. Wang et al. created sandwiched Pd(II) between N-methylimidazole moieties supported by two different cellulose chains [24]. Other teams have supposed that the modified cellulose acted as a polydentate ligand [25,26], the resulting complex being linked by the NHC, a hydroxyl group and a carboxyl group in the case of carboxymethylcellulose (CMC, Figure 2a). In all three cases, the Pd complex proved to efficiently catalyze Suzuki coupling reactions (up to 93% yield), with very little leaching of Pd in the medium thanks to the multiple capturing sites. However, the recycling of the catalyst was not viable after more than two runs due to an aggregation of palladium leading to a reduced catalytic activity (with less than 75% after 4 cycles).
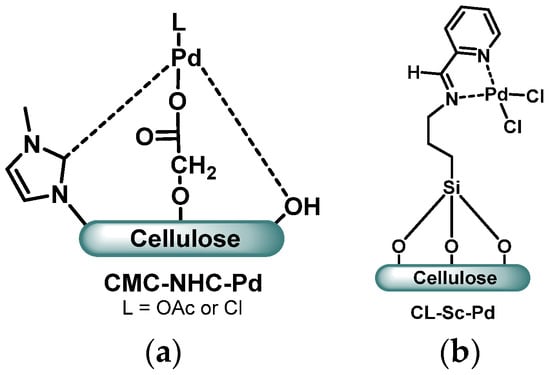
Figure 2.
(a) Pd(II) complex supported by carboxymethylcellulose derivatized with N-heterocyclic carbenes [25]; (b) cellulose Schiff base-supported Pd(II) catalyst [27].
Cellulose has also been derivatized by a Schiff base supported via a silyl spacer and completed by a pyridyl moiety [27,28]. Such structures create a bidentate site to complex Pd(II) between two nitrogen atoms, with the possible addition of a third chelating atom from a native cellulose hydroxyl group. Both catalysts could be directly reused after filtration or centrifugation and washings, but with a significant loss in the catalytic activity: After 5 runs, the yield dropped to less than 77%, probably due to palladium aggregations. Interestingly, the catalyst CL-Sc-Pd (Figure 2b) developed by Baran et al. [27] proved to be efficient to catalyze Suzuki coupling reactions with both iodo- and bromo-aryl substrates, optimal conditions being under microwave heating in a solvent-free medium.
Using the same amino silylated arm, that team [29] has also designed a cellulose Schiff base with a glyoxal moiety bridging two cellulose chains. This functional environment offered a very stable site to complex palladium(II) or platinum(II) ions. Both heterogeneous Pd2+- and Pt2+-complexes showed a coupling of a variety of aryl halides with phenylboronic acid in the presence of K2CO3 under microwave irradiations at 50 °C. After 7 min, 60–98% (resp. 54–96%) yields were reached using aryl iodides and the Pd2+ (resp. Pt2+) catalyst. The yields were slightly lower when the reaction occurred with aryl bromides. The reaction of aryl chlorides was also catalyzed although with a much more reduced catalytic activity. Both catalysts could be recycled for 7 runs but the final yield progressively decreased after each reuse (down to ca. 60%). A similar structure, obtained with an isatine moiety [30] or a 2-hydroxynaphthaldehyde moiety [31] instead of the pyridyl, is also an efficient recyclable catalyst. A palladium complex has been immobilized on cellulose via another Schiff base, more precisely a salen-type tridentate ligand, with no silyl spacer (Figure 3a) [32]. This green supported catalyst was found efficient for Suzuki coupling reaction of various aryl halides with phenylboronic acid in water-ethanol at 50 °C with K2CO3. Furthermore, the catalyst demonstrates high to excellent yields and is easily recycled by simple filtration for up to twelve cycles without any significant loss of catalytic activity.
Sarkar, Rahman, and their team have valorized cellulose extracted from corn cobs. They synthetized poly(hydroxamic acid) (Figure 3b) and poly(amidoxime) derivatives supported on such biomaterial [33,34]. Both nitrogen- and oxygen-containing moieties have been shown to chelate Pd(II), thus forming stable bridging complexes between two polymeric chains. Both cellulose-supported complexes act as catalysts in Suzuki coupling reactions. The poly(hydroxamic acid) Pd(II) complex [34] can be recycled and keeps a good catalytic activity even after 5 runs: from 96% initially, the yield drops to 88%. The poly(amidoxime) Pd(II) complex [33] proves to be much less efficient (with 67% yield). However, after reduction by hydrazine, the resulting cellulose-supported Pd(0) nanoparticles (NPs) exhibit a high catalytic activity using either arylbromide or arylchloride substrates (with up to 99% or 96% yields, respectively) and either phenylboronic acid or 2-naphthylboronic acid. Such a catalyst was successfully used 6 times in a row with filtration, washings, and drying between each run: The yield remained almost constant (from 97% to 93%). Very little Pd leaching is observed due to the multiple complexing bonds, but aggregation occurs in the complex while the NPs remain quite unchanged. Amidoxime-functionalized cellulose has also been used to support both palladium(II) acetate and Fe3O4 NPs. The catalyst obtained, efficient in various Suzuki reactions (up to 95% yield), could be magnetically retrieved and reused at least for 5 successive runs [35].
So as to support Pd(II) complexes, cellulose has been derivatized with other nitrogenated moieties. Cellulose-supported diethylenetriamine [36] and 2-aminopyridine [37] both offer polydentate ligands that stabilize Pd(II) complexes. Their utilization as catalysts in Suzuki reaction was successful. Both could be recycled by centrifugation and rinsing with almost no leaching (thanks to the powerful chelation) but with a progressive aggregation of palladium. The yield of the coupling between 4-bromoanisole and phenylboronic acid goes from 91% initially down to 62% after 4 runs with the diethylenetriamine-Pd complex and from 95% initially down to 42% after 5 runs with 2-aminopyridine-Pd complex.
A nitrogenated spacer, terminated by a trimethylammonium group, was also used to anchor palladium to cellulose. After reduction, Pd NPs were evenly distributed on the cationic nanocellulose and the resulting catalyst was used to perform Suzuki coupling reactions, but good yields were obtained only under non-sustainable conditions (DMF as a solvent, 110 °C). Rapid loss of catalytic activity was observed after only 3 runs [38]. Sun and Mohammadnia have designed an efficient Pd catalyst complexed on [2,2′-bipyridin]-4-amine functionalized nanocellulose. The catalytic activity of the nanocatalyst was investigated through one-pot synthesis of biaryl derivatives from the reaction of aryl halides (mainly bromides) with arylboronic acids in DMSO at 70 °C. This simple and mild procedure exhibited excellent recyclability with good yields (55–99%) and short reaction time inferior to 75 min [39].
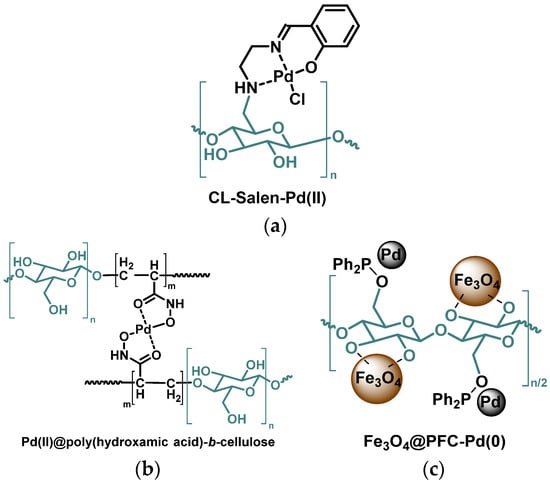
Figure 3.
(a) Cellulose-salen-supported Pd(II) complex [32]; (b) Pd(II) complex supported by poly(hydroxamic acid) derivative of cellulose [34]; (c) Pd NPs supported by magnetic Fe3O4 cellulose [40].
Palladium has also been bonded to microcrystalline cellulose (MCC) via phosphines. One team [41] has synthesized a diphenylphospinite-anchored cellulose while other groups have prepared a cellulose-supported triphenylphosphine [42,43]. Those phosphine-containing celluloses bonded with palladium to form stable Pd NPs that proved to be efficient catalysts in Suzuki coupling reactions. The first catalyst (Cell-OPPh3-Pd) can be reused for 4 runs after recovery by centrifugation and washing but the yield drops from 95% down to 56%, due to Pd aggregation. The other ones (Cell-PPh2-Pd) work with aryliodides and arylbromides; they can be recycled by filtration, washings and drying and used 5 times with almost constant catalytic activity (95–90% yield). In all cases, the optimal conditions are 75–80 °C in water-ethanol with K2CO3 as a base. Cellulose derivatized with a diphenylphosphine on the primary alcohol function was shown to offer a more stable environment than non-derivatized cellulose to fix both Fe3O4 NPs and Pd NPs (Figure 3c). The whole set (Fe3O4@PFC-Pd(0)) has been used as magnetically retrievable nanocatalysts, highly efficient (up to 98% yield) in Suzuki coupling reactions in a deep eutectic solvent (DES, K2CO3/glycerol: 1/5) at 70 °C. Yields almost did not drop after four recyclings [40].
Chemically Modified Cellulose
Pd(II)-complexes have been formed with carboxymethylcellulose (CMC) as the ligand. During a Suzuki coupling reaction, palladium ions first undergo in situ reduction to form Pd(0) NPs supported by the CMC, which further catalyze the cross-coupling. The catalyst was used 6 times to couple iodoanisole and phenylboronic acid, with intermediate filtration, washings, and drying, almost without any loss of catalytic activity [44].
The selective oxidation of the primary hydroxyl groups of cellulose yields carboxyl cellulose (CC), which permits the direct coordination of metal ions [45]. CC has been used as a ligand to complex palladium or nickel ions. PdCC was found to catalyze the cross-coupling of various aryl halides with aryl boronic acids in water/DMF at 30 °C with K2CO3 as a base. After 1 h, several reagent combinations led to quantitative couplings. Bromotoluenes were less reactive (60–79% yields), but 100% yield could be reached at higher temperature (80 °C). Chloroanisole was almost not reactive. Sterically hindered arylbromides reacted poorly (1–22% yields). Suzuki reaction was also found possible with NiCC as a catalyst in toluene at 130 °C, although with only 40% yield.
Pd NPs have been incorporated within a cellulose acetate membrane and this works as dip catalyst in aqueous media for Suzuki reaction [46]. However, minor aggregation of NPs, thermal deformation of the membrane and, more importantly, leaching of Pd(0) lead to a non-linear loss of catalytic activity. The first use has a 100% yield, but after 6 runs it drops to 17% only.
Pd NPs on cellulose sulfate, prepared by reduction of palladium(II) ions, have proved to catalyze the coupling reaction of various aryl halides with phenylboronic acid, with yields up to 99%, within 15 min, in DMF under reflux with NaOH. The catalyst could be reused up to 20 times with a yield still over 80%, if the reaction time was increased from 10 min initially to more than 20 min. Very little Pd leaching was observed between each run [47].
Another team has used a cellulose sponge as a carrier for Pd NPs [48]. The nanoparticles were grown on the surface of cross-linked cellulose nanofibers and then used as an efficient catalyst in Suzuki coupling reaction. The yield remained almost constant (from 99% to 93%) when the same sponge-supported catalyst was recovered by pulling, washed and reused in 6 cycles.
Researchers have partially modified cellulose nanocrystals (CNC) in order to make one face much more hydrophobic than the other (Janus particles) so as to use them as a colloidal surfactant (Pickering emulsion). When loaded with Pd NPs, such amphiphilic CNC can act as an efficient catalyst for Suzuki coupling reaction with a reaction mixture being an emulsion. This proves useful to carry out the coupling hydrophobic organic reagents in water as a green solvent [49].
Dialdehyde nanocellulose was obtained by successive oxidation steps in order to reduce palladium ions. This way Pd NPs were homogeneously deposited onto cellulose nanofibrils and the resulting product successfully applied to Suzuki reaction with 90% yield [50].
Non-Chemically Modified Cellulose
An effective palladium-based catalyst has been developed for the Suzuki coupling reaction of aryl halides (including chlorides) with phenylboronic acid in aqueous conditions at room temperature. Pd(II) ions and neat nanocellulose were added to the reaction mixture and Pd NPs grew in situ within the natural polymer to form the actual heterogeneous nanocatalysts. In addition to the absence of volatile organic solvent, the broad substrate scope and the mild reaction conditions, this method proved to be sustainable and efficient with 90% to 99% product isolated yields and a successful reusability up to 11 cycles [51].
Pd(II) species have been reduced, by solvent or NaBH4, in a cellulose solution in ionic liquid solvent. It led to the formation of Pd(0) cores then microencapsulated in a cellulosic film using anhydrous ethanol as a coagulant [52]. The as-prepared hybrid material proved to be a versatile, robust and highly efficient catalyst for various Suzuki coupling reactions performed in 15–20 min in ethanol under reflux with K2CO3. The catalyst could be retrieved by simple filtration and recycled at least 6 times without important loss of its high catalytic activity (91% to 88 % yields).
Non-chemically modified cellulose has also been used as a support for Pd NPs. Stirring commercially available cellulose with Pd(OAc)2 in ethanol at 25 °C followed by reduction with hydrazine hydrate leads to Pd NPs uniformly distributed on the surface of cellulose [53]. This catalytic system was used in Suzuki coupling of various aryl bromides with phenylboronic acid in water at 100 °C using a phase-transfer agent, reaching excellent yields and it was easily recycled (with 96% yield initially to 94% yield at the fifth run). Cotton fibers are mainly made of cellulose (up to more than 90%). Some researchers have imbedded Pd NPs in nonwoven brown cotton fabric, a cheap cellulose substrate still containing tannins. Those naturally occurring polyphenols reduced palladium ions into Pd NPs which were thus deposited on the fibers. The resulting structure was catalytically active at sub-milliequivalent levels in Suzuki coupling reactions (with yields up to 99%), retained catalytic activity for ten experimental cycles and palladium did not leach into isolated products [54].
A distorted honeycomb-like hollow cage-structured bio-nanocellulose (derived from pomegranate peel by simple microwave technique in water with no other reagent) has been loaded with Pd NPs. That nanocomposite material has been utilized as a heterogeneous catalyst for the C-C coupling of various arylbromides with arylboronic acid derivatives. The Suzuki reactions were carried out in a water-ethanol mixture at room temperature for 20–60 min with 80–98% yields; when heteroaromatic compounds were reacted, the reaction was performed at 60 °C for 24 h and 55–98% yields were reached. The catalyst is reusable up to five catalytic cycles without significant loss of its catalytic activity [55].
Most interestingly, a very efficient and highly recyclable catalytic system was obtained by immobilization of Pd NPs on filter paper (Figure 4) [56]. The loaded paper was then simply dipped in the reacting mixture and pulled for reuse after washing. Excellent yields (up to 100%) were obtained in coupling iodophenol with phenylboronic acid under microwaves with K2CO3 as a base and the catalyst could be used in 5 successive runs with only a small reduction of the catalytic activity (possibly because of a poisoning by iodide ions).
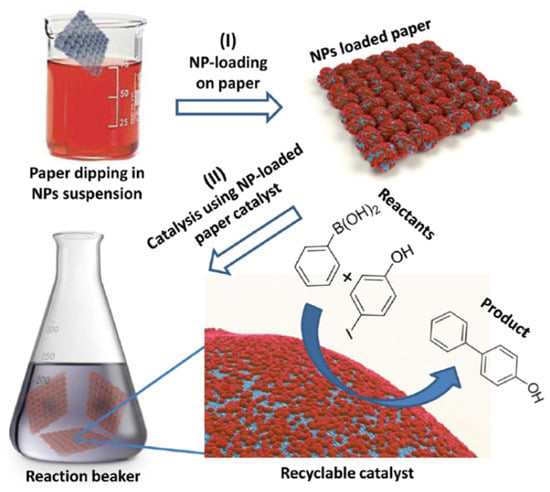
Figure 4.
Schematic representation of the paper-supported dip catalyst preparation (I) and use (II). (I) Assembly of oleylamine-stabilized Pd NPs on cellulose filter paper by dip coating. (II) Immersion of NP-loaded paper strips in a reaction medium to catalyze a cross-coupling reaction [56].
Baruah et al. have synthesized Pd NPs on nanoporous cellulose by reducing palladium(II) ions with a natural wood extract (oxyresveratrol). Those nanoparticles exhibit versatile catalytic activity towards the Suzuki reaction in water under microwave heating with 82–94% yields. The coupling of bromobenzaldehyde with phenylboronic acid was successively catalyzed ten times with almost no leaching of palladium and a yield still over 70% [57]. Similarly, in a green chemistry perspective, Kempasiddaiah et al. have obtained cellulose-supported Pd NPs using waste banana pseudostem both as a source of reducing agent and of cellulose. The dip catalyst obtained (Figure 5) was used in various Suzuki coupling reactions [58]. That of iodobenzene with phenylboronic acid reached a 96% yield under optimized conditions: in water:ethanol (1:1), at room temperature, with Na2CO3 for 30 min. The dip catalyst could be used for 15 cycles with almost no leaching of Pd and a yield still over 88%. A green synthesis of pharmaceutical active principle, Felbinac, has even been successfully performed, by cross-coupling of 4-bromophenylacetic acid with phenylboronic acid, with 94% yield using such dip catalyst.
Figure 5.
Dip catalyst as Pd(0)-loaded cellulose sheets; plausible structure of the Pd NPs-containing cellulose; typical experimental setup using the dip catalyst [58].
Another dip catalyst was prepared as a rectangular sheet by dispersing Pd NPs into the cellulosic pulp of sugarcane bagasse (Figure 6) [59]. Its catalytic activity was demonstrated in the Suzuki cross-coupling reactions of various arylbromides and aryliodides with different arylboronic acids in ethanol–water mixture at room temperature using K2CO3 as a base. Very good yields were reached (up to 98%) and maintained for up to 13 successive runs with the same catalyst sheet simply recovered by pulling with tweezers; at the next cycles, the dip catalyst had been slightly disintegrated on the edges due to abrasion of the stirring bar, which caused a lesser conversion.
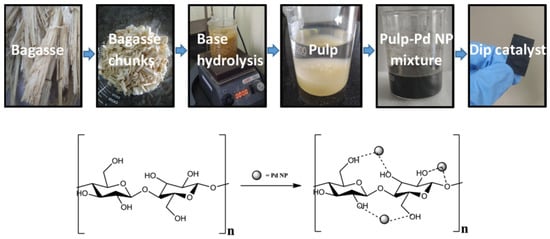
Figure 6.
Preparation steps of the dip catalyst from sugarcane bagasse; representation of cellulose-supported Pd NPs proposed by Kandanthil et al. [59].
The one-pot synthesis of cellulosic–carbon-shielded palladium–magnetic nanoparticle hybrid material (Pd-MNP@SCB) has been described by the same team. Calcined natural sugarcane cellulose was used as a green source of carbon for its large surface area and porosity. The prepared hybrid material proved to have an efficient catalytic activity in Suzuki–Miyaura cross-coupling reaction with excellent functional group tolerance and good recyclability [60].
Cellulose Composites
A hybrid device has been developed with the natural magnetic property from a non-modified mineral. Volcanic pumice magnetic particles (VPMP) have been textured by cellulose to further nest Pd NPs and form a biosourced composite suitable for the catalysis of C-C coupling reactions. Heterogeneity, high stability, and inherent magnetic property enable a remarkable recyclability with 10 times successive use. The actual Pd(0) catalyst was found a bit less efficient when used after prior reduction. Therefore the Pd(II) pre-catalyst was reduced in situ (Scheme 1) in order to obtain a biphenyl with high yield (98%) from 4-nitroiodobenzene and phenyl boronic acid in a short time (10 min) in DMSO at room temperature in the presence of the reductive additives (triphenylphosphine and sodium borohydride) and the base (potassium carbonate) under nitrogen. A series of other aryl halides have been tested with 69–97% coupling yields [61].
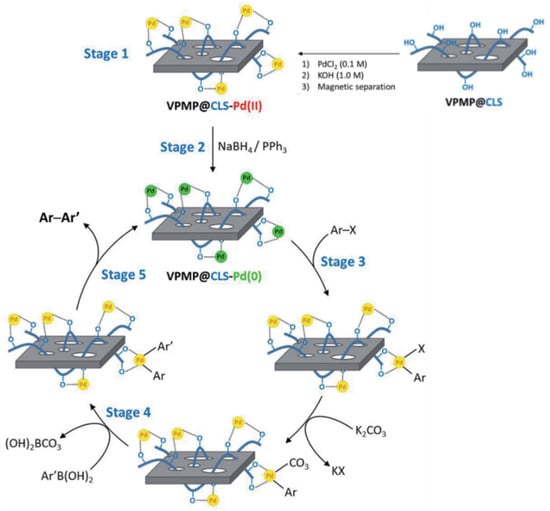
Scheme 1.
Catalytic cycle suggested for VPMP@CLS-Pd(0) in Suzuki coupling reaction [61].
A heterogeneous nanocomposite combining graphene oxide-supported Fe3O4 NPs and cellulose-stabilized Pd NPs (Figure 7a) has been proved an active catalyst in various Suzuki coupling reactions. The optimal reactions were conducted at 70 °C in DES, namely dimethylammonium chloride:glycerol, with K2CO3, and led to 81–99% yields. Aryl iodides reacted much faster (generally 20–30 min) and gave better yields than aryl bromides (40–60 min). Due to the low solubility of catalyst and DES in organic solvents, the separated aqueous phase containing both the catalyst and DES could be readily recovered by evaporating water and reused up to five successive runs with a stable activity. Incidentally, the heterogeneous catalyst could be extracted from the DES by an external magnet [62].
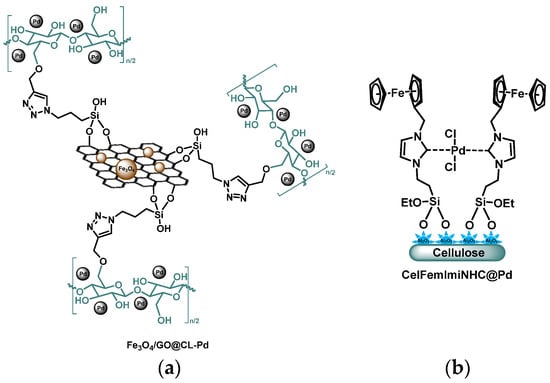
Figure 7.
(a) Palladium supported on cellulose modified Fe3O4-graphene oxide [62]; (b) cellulose–alumina composite supported Fc–NHC Pd(II) complex [63].
Kale et al. have designed a N-heterocyclic carbene-Pd complex anchored on cellulose via Al-O-Si bonds (Figure 7b) [63]. They proved it to be an efficient heterogeneous catalyst in various Suzuki coupling reactions, in ethanol at room temperature using cesium carbonate as a base. Under such conditions, the coupling of phenyl boronic acid with iodobenzene within 3 h led to 78% yield, whereas it grew up to 87% within 30 min when the catalyst was tethered by ferrocene moieties. Indeed, the steric hindrance and the powerful electron donor capacity of the ferrocenyl groups rendered the NHC-Pd complex electron rich. This induced a faster oxidative addition, which is the first and rate-limiting step of the cross-coupling reaction. It is of note that a soluble homogeneous analogue was found more efficient with up to 94% within 30 min, but the heterogeneous catalyst could be recycled for 5 runs with only little loss of catalytic activity. Aminopyridine moieties have been anchored on cellulose-supported alumina with a propylsilyl linker [64]. The nitrogen sites could complex palladium ions thus forming an efficient retrievable hybrid heterogeneous catalyst (Pd(II)-AMP-Cell@Al2O3). The catalyst has been successfully employed in a variety of Suzuki coupling reactions, in DMF at 80 °C for 1 h, using K2CO3 as a base, with up to 94% yield. It could be recycled up to 4 times by filtration, washing, and drying, with almost no Pd leaching and thus minor loss of catalytic activity. Salunkhe and his team have prepared a cellulose–aluminum oxide composite, further silylated with a propyl spacer bearing a primary amine through Al-O-Si bonds [65]. Pd(II) ions were efficiently complexed by the amino groups so that the composite could be used as a highly active heterogeneous catalyst for Suzuki–Miyaura cross-coupling reaction in water and water/DMF mixture at 80 °C (up to 97% yields). Recyclability was limited to 5 successive runs with yield dropping from 97% to 81% and reaction time extending from 20 min to 7 h. Alumina was added in order to supposedly stabilize the hybrid since C-O-Si bonds were said to be easily hydrolyzed. Such instability was however not described by other teams who successfully used C-O-Si bonds in other nanocatalysts [27,28,30,31,66,67].
CMC has been loaded with cerium(IV) hydroxide by successive chelation and precipitation within the biopolymer [68]. The organic/inorganic hybrid was then used to design Pd(II)-complexes similar to those in the previous work, which in the very same way proved to be pre-catalysts for Suzuki–Miyaura reactions. The in situ reduction of palladium occurred in the course of the cross-coupling reaction and the activity of the Pd NPs was enhanced synergistically by the redox properties of cerium and coordination with the carboxyl and hydroxyl groups of CMC (Scheme 2). In DMF/water: 1/1, at 80 °C, using K2CO3 as a base, a variety of biaryls were obtained with up to 97% yield for the coupling of iodoanisole with phenylboronic acid. As commonly reported aryl iodides were more reactive than aryl bromides; aryl chloride only reacted poorly. The catalyst could be easily separated by simple filtration and reused at least five times with yield still over 90%. Interestingly, the results exhibit that this Pd-CMC@Ce(OH)4 catalyst has a much higher activity in Suzuki reaction than Pd@CMC, Pd@CeO2, and Pd@Ce(OH)4.
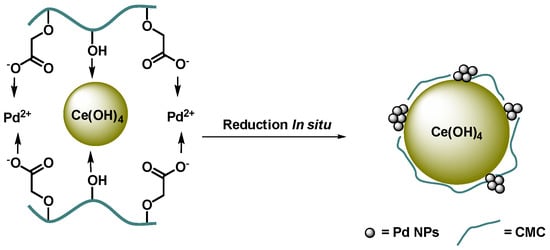
Scheme 2.
Preparation scheme of CMC@Ce(OH)4-supported Pd(II)-complexes and in situ reduction to Pd NPs [68].
Pd NPs have been dispersed onto titanium oxide-cellulose composite by direct reduction [69]. The resulting PdNPs@TiO2-Cell showed an excellent activity as a catalyst in Suzuki–Miyaura reactions, in 95% ethanol at 80 °C with potassium carbonate, with up to 90% yields after 1 h. A wide range of reagents have been used with success, without Pd leaching. The catalyst could be recovered and recycled 4 times with the same yield. A similar catalyst, prepared by the same team with derivatized alumina instead of titanium oxide [65], was more efficient in DMF but less in ethanol. Other Suzuki-type reactions were also performed at room temperature in the presence of K2CO3 using PdNPs@TiO2-Cell as a catalyst to couple aryl boronic acids with: (1) various arenediazonium salts in water with up to 90% yield; (2) a choice of benzoyl chlorides in 95% ethanol with 85–92% yields.
Hyperbranched polystyrene, derivatized so as to exhibit quaternary ammonium groups, have been described by Nishikata et al. as an efficient stabilizer for metallic NPs thanks to the electrostatic and steric properties of its cationic moieties [70]. They further showed that such polystyrene-supported Pd NPs precipitated onto cotton wool or paper cellulose by addition of iodide or bromide ions (Figure 8). The resulting composite proved to catalyze the Suzuki coupling reaction of iodobenzene with phenyl boronic acid in water at 50 °C with K2CO3. After 24 h the reaction was almost quantitative and it remained so after 5 successive runs, with only little Pd leakage over the recycling of the dip catalyst. Interestingly, the same piece of catalyst could be reused four times with different substrate without any cross-contamination or decrease in catalytic activity.
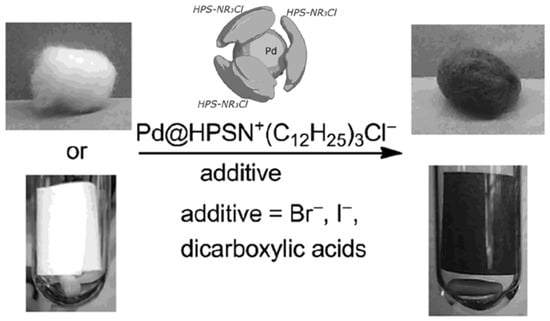
Figure 8.
Preparation of dispersible Pd@HPS-NR3+Cl− and its immobilization on cellulose (cotton wool or filter paper) [70].
Baran et al. have designed a greener nanocomposite of chitosan and cellulose to support either Pd(II) complex [71] or Pd NPs [72]. Both structures exhibited a catalytic activity in the coupling reaction of a wide range of aryl halides with phenyl boronic acid. Under mild conditions (solvent free medium, microwave heating at 50 °C, K2CO3 as a base), various yields were reached within 5 min using aryl iodides or bromides: 22–99% with Pd(II) complex and 60–100% with Pd NPs, according to the substituent on the benzene ring. Yields were only 9–25% with Pd(II) and 55–75% with Pd(0) using aryl chlorides. The chitosan-cellulose-Pd(II) catalyst could be recycled and was still active after 9 cycles (although with a yield dropping from 99% to 43%). Another biosourced composite nanocatalyst, made of Pd NPs dispersed within a cellulose-alginate hydrogel, has been found active to couple three different aryl iodides and three bromo isologues with p-tolylboronic acid at 80 °C in ethanol in the presence of K2CO3 under nitrogen atmosphere [73]. The yields reached 85–99% after 2 h. The recycling of the hydrogel bead catalyst was performed, showing negligible Pd leaching and minimal decrease of the product yield after 5 runs.
An active hydrogel has also been developed by incorporation of Pd NPs stabilized on CNCs into a guar gum-based matrix with a boron-based crosslinking [74]. Over 70 °C, the thermoresponsive hydrogel converted to the sol phase; it then proved to be an efficient catalyst in the Suzuki coupling of aryl bromides with phenyl (or tolyl) boronic acid with K2CO3 at 80 °C in ethanol under N2, with the yield culminating at 86–99% after 2 h. After cooling to 20 °C, the catalyst set to the gel phase; it could then be recovered, rinsed with water and directly reused: the reaction yield was still over 85% after the 5th cycle and the Pd content remained essentially the same.
Pd NPs have been immobilized on a biosourced composite by a one-pot reaction of CMC, agar saccharides, and Pd(II) ions, the latter being spontaneously in situ reduced. The Pd-composite was used as a catalyst to cross-couple various aryl halides with phenyl boronic acid in the presence of K2CO3 at 60 °C under ultrasonic sonication [75]. After 30 min, good to excellent yields were reached (up to 98%). Under conventional heating, the yield was lower (only 75%) even after 5 h reaction. Interestingly three homogeneous Pd(II) catalysts were found less active under the same conditions. Furthermore, the heterogeneous catalyst could be recovered by centrifugation and reactivated by washing with hot ethanol, showing that it could be reused even after 6 runs, although the final yield drastically dropped to less than 50%.
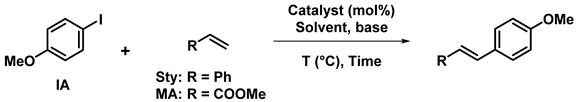
2.1.2. In Mizoroki–Heck coupling
Mizoroki–Heck cross-coupling reaction is useful to create C-C bonds to functionnalize alkenes. It is commonly the reaction between aryl halides and substituted alkenes in the presence of transition metals catalysts.
Cellulose Derivatized with Functionnalized Spacers
Sarkar et al. have synthesized a co-polymer of corn cob cellulose and poly(hydroxamic acid) in order to complex Pd(II) ions between two chains [34,76]. The resulting structure was used as a catalyst for a variety of Heck coupling reactions using different alkenes and whether aryl iodides (89–97% yields), aryl bromides (87–95% yields), heteroaryl halides (72–94% yields), or aryldiazonium tetrafluoroborate salts for a Heck–Matsuda coupling (90–97% yields). All reactions were optimized in non-green conditions: DMF as a solvent, Et3N as a base, at 130 °C (or room temperature for Heck–Matsuda) during 5 h or more. The catalyst was easily recovered by filtration, washed and dried, then reused with very little loss in catalytic activity after up to 7 runs. A pharmaceutical active ingredient, Ozagrel, was successfully prepared under such conditions using such heterogeneous Pd catalyst [76]. In another study, Sarkar’s team has used an analogous Pd(II) complex by replacing poly(hydroxamic acid) with poly(amidoxime) and showed similar catalytic activities: 91–96% (resp. 82–96%) yields for Heck coupling with aryliodides (resp. arylbromides) and 90–97% yields for Heck–Matsuda coupling. That catalyst was also reused with success 7 times with a yield reduced from 95% to 87% [77].
The cellulose-supported Pd(salen)-type catalyst already used in Suzuki reaction has also proved to be efficient to catalyze Heck coupling reactions in the same conditions with 93–99% and good recyclability [32].
A nanomagnetic catalyst has been prepared by chelating palladium(II) ions onto a 5-carboxyoxindole functionalized cellulose already supporting Fe3O4 NPs. Such green composite catalyst was employed for Heck-type arylation of different substituted maleimides with iodoarenes in good to excellent yields, easily magnetically recovered and reused several times with no substantial loss of activity [78].
Pd NPs have been dispersed in different phosphine-containing celluloses. It has already been explained that the resulting stable heterogeneous catalyst was used in Suzuki coupling reactions. The same teams have also proved that both structures could catalyze Heck coupling reactions. Both triphenylphosphine cellulose-supported (Figure 9a) [43] and diphenylphosphinite cellulose-supported nanopalladium [79] are catalytically active in DMF with good yields (ca. 90%) and completely recyclable for 5 cycles.
Another bio-supported catalyst was prepared by uniform distribution of Pd NPs onto ethylenediamine-functionalized cellulose (EDAC). It was successfully used in Heck coupling reactions in water as a green solvent and proved to remain stable when recycled, yields ranging from 99% initially to 94% at the fourth run [80].
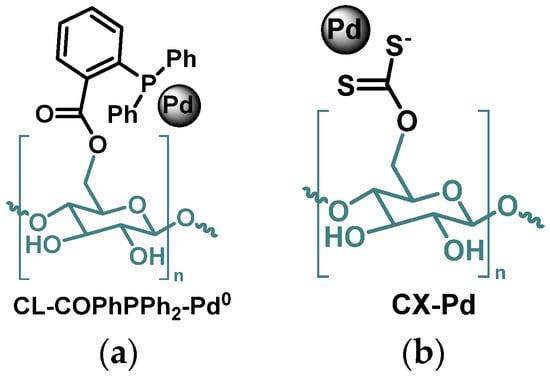
Figure 9.
(a) Triphenylphosphine containing cellulose-supported Pd NPs [43]; (b) cellulose xanthate-supported Pd NPs [81].
Chemically Modified Cellulose
A stable cellulose xanthate-supported palladium(0) has been synthesized using a simple method (Figure 9b) [81]. It was found to be an efficient catalyst for the Heck reaction of acrylic acid or styrene with aryl iodide. Very high yields (up to almost 100%) were reached at 90 °C after about 8 h. The catalyst could be easily recycled and reused although its catalytic activity rapidly dropped down to 55% after 10 runs.
Besides Suzuki coupling reactions, Pd NPs supported by cross-linked cellulose sponge also catalyze Heck coupling reactions and excellent yields (99%) were obtained to couple iodobenzene with three different aryl ethylene. The catalyst is easily recyclable [23].
Non-Chemically Modified Cellulose
Pd NPs microencapsulated in cellulose proved to catalyze Heck coupling reactions of various arylbromides and aryliodides with styrene and acrylic acid in DMF at 130 °C with tributylamine, reaching 66% to 88% yields within 2 to 12 min [52].
Pd(II)-complex on CMC (Figure 10) has proved to catalyze Heck coupling reactions, in addition to Suzuki coupling reaction, after in situ reduction of Pd as nanoparticles [44]. Acrylic acid and styrene were coupled with various aryliodides with good yields (83–90%).
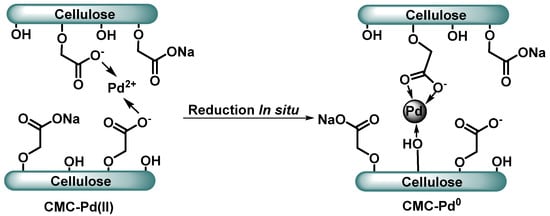
Figure 10.
Pd(II) complex in carboxymethylcellulose and in situ reduction of Pd NPs in CMC [44].
Pd NPs supported by non-chemically modified microcrystalline cellulose, obtained by reduction with hydrazine, already used in Suzuki reaction, has also been shown to act as a catalyst for Heck coupling reactions [36]. Quantitative conversion was observed with various aryliodides and methylacrylate when the reactions were carried out in acetonitrile or DMF with triethylamine as a base. The catalyst could be recycled by filtration, washing and drying, and lost only little catalytic activity after 4 runs, due to a minimal leaching and some aggregation.
Rezayat et al. reduced Pd NPs in cellulose nanocrystals in subcritical and supercritical carbon dioxide as a solvent. The resulting product showed an interesting catalytic activity in two examples of Heck coupling reactions [82]. Pristine cellulose was used by another team to complex Pd(II) ions and the product obtained has been used to catalyze Heck coupling reactions. Using the Pd(II)-cellulose complex, acrylic acid and styrene were coupled with aryliodides in water as a green solvent, in the presence of tributylamine, with yields up to more than 99%. The catalyst could be reused 5 times with yields still over 76% [83]. Pd NPs on cellulose, developed by Baruah’s team, and already mentioned in Suzuki coupling reactions, have shown an interesting catalytic activity in Heck coupling reactions in water under microwave heating with 87–89% yields [57]. A colloidal catalyst for Heck coupling reaction has been developed by reducing Pd NPs onto cellulose nanocrystallites (Figure 11). Using such a catalyst, iodobenzene reacted with styrene in water–acetonitrile at 100 °C with K2CO3 as a base to yield stilbene with 75% conversion [84].
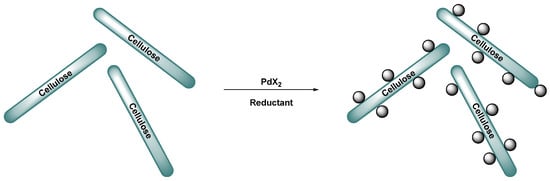
Figure 11.
Reduction of Pd(II) onto cellulose as Pd NPs [84].
Cellulose Composites
Pd NPs supported by an ammonium-derivatized hyperbranched polystyrene have been glued to filter paper and cotton wool to form dip catalysts [70]. Both were utilized to catalyze the quantitative coupling reaction of phenyl iodide with t-butyl acrylate or styrene, at 80 °C in water with K2CO3 for 24 h. The adhesion of the polystyrene-supported NPs to the cellulose was found very strong so that the dip catalyst could be used five times in a row with almost no Pd leaching detected and a yield still over 99%. Interestingly, the same “black filter paper” could be successively recycled to catalyze the couplings of different substrates for each run.
Masteri–Farahani and his team have devised another magnetic nanocomposite, this one made of graphene oxide (GO) and cellulose [85]. Iron ions were precipitated as Fe3O4 NPs in the sites offered by GO. Cellulose was grafted to GO via click-chemistry after adequate derivatization. The abundant OH groups of cellulose highly stabilized Pd NPs uniformly dispersed over it. The resulting nanocomposite served as a heterogeneous catalyst for C-C coupling of iodobenzene with n-butyl acrylate in various deep eutectic solvents (DES). In optimized conditions (at 100 °C in dimethylammonium chloride:glycerol, with K2CO3), 98% yield was reached after 5 h. The Heck reaction was also conducted with different aryl halides and alkenes, generally with yields over 90–95%. The catalyst could easily be retrieved with an external magnet, but its direct recycling was performed without such recovery. The final reaction mixture was quenched with a water/ethyl acetate mixture so that the product was extracted in the separated organic phase with the DES and the catalyst stayed in the aqueous phase and were recovered by water evaporation for further reaction. Eight actual runs were found possible with only small decrease in the final yield.
GO nanosheets have also been combined with chitosan NPs and dialdehyde cellulose nanowhiskers to form a hydrogel support for Pd NPs [86]. The resulting catalyst was utilized to efficiently cross-couple various olefins with phenyl halides in aqueous ethanol under reflux in the presence of K2CO3. Maximum yields were achieved after 30–40 min with iodobenzene (95–99%), after 40–60 min with bromobenzene (93–97%) and after 120–180 min with chlorobenzene (81–87), a shorter carbon-halogen bond resulting in a more difficult metal penetration and a slower cleavage. The reaction rate decreased when an electron-rich substituent (-NH2) was present on the aryl halide. The hydrogel catalyst could be recycled by filtration, water dialysis, and vacuum drying at 80 °C with no important Pd leaching. After 6 uses, the final yield was still 85%.
Heterogeneous nanocatalyst Pd(II)-AMP-Cell@Al2O3, a Pd-complex supported on derivatized hybrid cellulose-alumina, has been shown efficient in various Heck–Mizoroki cross-coupling reactions [64]. For instance, the coupling of iodoanisole with methyl acrylate in DMF at 100 °C with triethylamine for 2 h yielded 92% reaction product and the catalyst could be used 5 times in a row with almost the same activity, due to a high stability of the palladium complex on the amino-pyridine sites.
Pd NPs supported on TiO2-cellulose composite have been used to catalyze Heck–Mizoroki coupling reactions of aryl bromides with various olefins. In optimized conditions, in DMF at 100 °C with K2CO3, yields reached 80–95%. Heck–Matsuda coupling reactions (using diazonium salts instead of bromides) were also possible with that catalyst in water, at room temperature and without a base, with 75–92% yields [69].
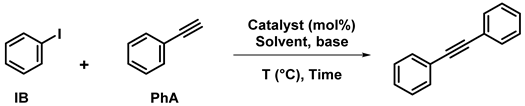
2.1.3. In Sonogashira Coupling
A number of cellulose-supported palladium-based catalysts already used for Heck coupling reactions have also proved to act as catalysts for Sonogashira coupling reactions of arylhalides with phenylacetylene and other alkynes, in similar conditions and with the same ease for recycling. This is the case for EDAC-supported Pd NPs [80] and for cellulose-supported Pd(salen)-type complex [32], using either aryliodides or arylbromides in water in the presence of K2CO3 and CuI, with yields up to 98%. This was also observed with Pd NPs dispersed in phosphine-containing cellulose in DMF with triethylamine and CuI (89–96% yields) [43]. Sonogashira coupling reactions of aryl acetylenes with various aryl halides, performed in deep eutectic solvents at 100 °C, have been catalyzed by Fe3O4@PFC-Pd(0). High yields were obtained (up to 96%) and the catalyst, after magnetic recovery, was reused for five cycles without an appreciable loss of activity [40].
MCC-supported Pd NPs also work as catalysts for Sonogashira reaction, using aryliodides in acetonitrile or DMF with triethylamine as a base but yields were quite contrasted (42–98%), depending on the activation of the aryl group [87]. Honeycomb-structured bio-nanocellulose loaded with Pd NPs has been used to catalyze Sonogashira cross-coupling reactions. The coupling of various alkynes with aryl iodides in water–ethanol at 70 °C or with aryl bromides in DMF at 90 °C were observed with 70–98% yields, using no copper salt but K2CO3 as a base. The reaction was found possible with halo-pyridines and halo-pyrimidines [55].
Another magnetic Pd nanocatalyst has been developed and used for Sonogashira coupling reactions in DES. That nanocomposite of GO-supported Fe3O4 NPs and cellulose-supported Pd NPs has efficiently been used to catalyze the C-C coupling of various aryl halides with phenylacetylene at 120 °C with K2CO3 in a dimethylammonium chloride: glycerol mixture [85]. The products were obtained with 87–96% yields. The nanocatalysts could be recycled by liquid–liquid extraction together with the DES since neither of them were soluble in an organic phase composed of additional ethyl acetate. After 8 runs, the yield decreased from an initial 91% to 80% with negligible leaching. The nanocatalyst could finally be magnetically recovered when necessary.
Jadhav et al. have carried out copper-free Sonogashira coupling reactions of a wide range of arylbromides and aryl alkynes in DMF at 80 °C using K2CO3 as a base and TiO2-cellulose-supported Pd NPs as a catalyst. Yields ranged between 85% and 95% [69].
2.1.4. Other C-C Coupling
Rasouli and Ranjbar claim that they deposited onto microcrystalline cellulose (MCC) Pd(II) ions that spontaneously form stable Pd NPs uniformly distributed within the polymer, without any reducing agent [88]. The product was used as a catalyst for the Ullmann C-C reductive homocoupling of various aryl halides in the presence of zinc, in a water–ethanol mixture, in air. Excellent yields were reached with phenyl bromide (96%) and phenyl iodide (94%) at 25 °C. The reaction was also found possible with various other arenes including aryl chlorides, although with lower yields. The catalyst could be reused but degraded when the reaction temperature was over 75 °C.
The green dip catalyst developed by Kempasiddaiah et al. for Suzuki reaction [58] has also been utilized by the same team to catalyze denitrogenative cross-coupling reactions. They synthesized a broad array of symmetrical and unsymmetrical biaryls using arylhydrazines and aryl halides as coupling partners through C-N bond cleavage in air as green oxidant (Figure 12) [89]. The dip catalyst could be recycled by simple pulling with tweezers from the reaction medium and rinsing; it remained stable and catalytically active (94% initial yield to 85% yields at the sixth run).
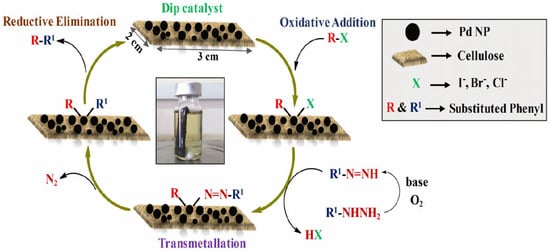
Figure 12.
Mechanism proposed by Kempasiddaiah et al. for denitrogenative cross-coupling reactions [89].
Pastoriza–Santos, Pérez–Juste, and their team have shown that paper-supported Pd nanoparticles efficiently catalyze aqueous aerobic oxidative C-C homocoupling reaction of 4-carboxyphenylboronic acid to form 4,4′-dicarboxybiphenyl in water at 70 °C [56]. The dip catalyst was recovered by simple pulling and washing and could be reused for 10 runs with the same catalytic activity. The cellulose-base Pd(0) dip catalyst prepared by Kandathil et al. has proved to catalyze the 5-arylation reaction of 2-substituted thiophenes. The C-C coupling of various arylbromides and aryliodides with 2-formyl-thiophene and 2-acetyl-thiophene in DMF at 120 °C was found possible by the dip catalyst, in the presence of silver oxide, sodium carbonate and pivalic acid, although with limited yields (52–82%) after 16–18 h [59].
Nishikata et al. have designed “black cotton” and “black filter paper” made of Pd NPs supported by an ammonium-derivative of polystyrene immobilized on cellulose [70]. With those composites, an intramolecular C-C coupling was successfully catalyzed within diaryl iodide compounds to quantitatively yield, after 24 h, the 2H-pyran cycle between the aromatic rings in 9H-10-oxa-phenanthrene-type products. The coupling reaction was carried out at 120 °C in N,N-dimethylacetamide in the presence of potassium acetate. The paper catalyst could be recycled 4 times with the same catalytic activity.
Cotton wool has been treated with chlorosulfuric acid to fabricate a negatively charged derivative of cellulose [90]. Chitosan, a polysaccharide composed of β-(1→4)-linked D-glucosamine-type units, was bound to the cellulose sulfate thanks to its positive charges. This composite served as a support for Pt NPs. The resulting heterogeneous catalyst showed an activity in the reaction of several aromatic aldehydes with 4-hydroxycoumarin to form bis-coumarins. After 5 to 12 min in ethanol under reflux, the reaction was complete with excellent yields (93–98%). Before the crystallization of the product, cellulose sulfate–chitosan-supported platinum was simply recovered by filtration and reused once washed. After 6 successive runs, it retains its catalytic activity, the yield only slightly dropping from 98% to 90%.
2.2. In C-Heteroatom Coupling
A palladium complex has been absorbed under vacuum in porous cellulose. Tsuji Trost coupling reaction of (E)-cinnamyl ethyl carbonate with morpholine was found possible using the resulting compound either as supported aqueous phase catalyst in a mixture of water and benzonitrile or acetonitrile [91] or as heterogeneous catalyst in benzonitrile [92]. However, the reaction rate and yield remained quite poor, except when the amounts of water and surfactant were increased but that then induced an important leaching of palladium, which is polluting and impaired the recyclability of the catalyst. Different C-O couplings (Ullmann condensation or Buchwald–Hartwig etherification) and C-N couplings (Buchwald–Hartwig amination) were shown possible using various examples of cellulose-based Pd catalysts in DMSO at 80 °C with K2CO3 as a base.
Seyednejhad et al. [93] have developed a Pd(II) complex with crystalline nanocellulose 2-(1H-benzo[d]imidazol-2-yl)aniline as a ligand (CNC-BIA-Pd) (Scheme 3). That strong and novel nanocatalyst proved to catalyze the C-O coupling reaction of aryl halides with phenol derivatives with yields up to 98%. Due to its stability, the catalyst could be reused after filtration, washings and drying, for up to 8 runs with a yield still over 90%.
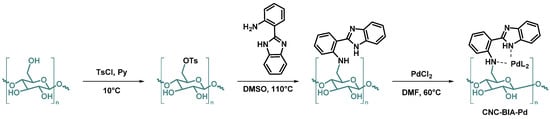
Scheme 3.
Reaction steps for the synthesis of the Pd(II) complex chelated on 2-(1H-Benzo[d]imidazol-2-yl) aniline-derivatized cellulose nanocrystals (CNC-BIA-Pd) [93].
Sun et al. have performed Buchwald–Hartwig-type reactions using recyclable cellulose-supported Pd(salen)-type catalyst [32]. Their work enables the C-N coupling of aniline (resp. the C-O coupling of phenol) with iodobenzene in mild conditions: in DMSO as a solvent, at 80 °C, with K2CO3 as a base, with 78% yield (resp. 93% yield).
Pd(II) chemically anchored on cellulose nanocrystals via a Schiff base and a silyl spacer was applied for C-O couplings using a series of aryl halide and phenol derivatives [30]. It demonstrated high efficiency with 94% reaction yield and indicated good performance after four times recovery and reuse.
Hydroxypropyl methylcellulose (HPMC) is a food additive used as an emulsifier [94]. Pd NPs have been formed in situ and trapped in hydrophobic pockets appearing in HPMC when the polymer folds in aqueous media (Figure 13). Such cellulose-supported Pd catalyst was tested on a very wide range of C-N coupling reactions using various NH-bearing compounds (anilines, primary and secondary alkylamines, lactams, amides, ureas, and carbamates) with different aryl bromides. In most of the cases, the Buchwald–Hartwig amination was very fast (3 to 15 min) with good to excellent yields (76–97%). It is of note that the catalyst was applied multigram-scale reactions (up to 10 g).
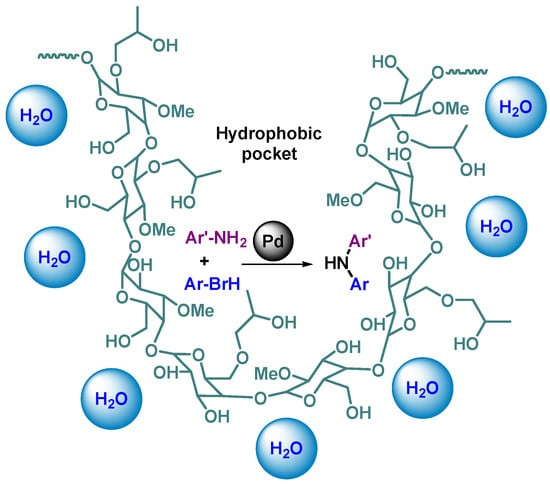
Figure 13.
Pd NP trapped in a hydrophobic pocket of HPMC, catalytic center of C-N coupling [94].
Cellulose deposited over Fe3O4 NPs has been charred at high temperature under inert atmosphere to yield magnetic carbon black suitable to support Pd NPs. The resulting layered composite was found to efficiently catalyze heterogeneously C–H sulfenylation of indoles for the preparation of 3-sulfenylindoles (Scheme 4). The recoverable catalyst was separated from the reaction mixture using an outside magnetic field and could be recycled five times [95].
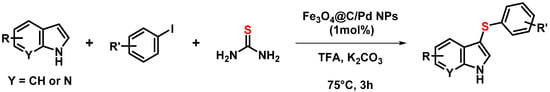
Scheme 4.
C-H sulfenylation of indoles with thiourea and substituted iodobenzenes [95].
2.3. Redox Reactions
2.3.1. Reduction of Nitro Compounds
An efficient catalyst, obtained via modification of cellulose with N-doped graphene quantum dots and Pd nanoparticles, was introduced for the green reduction of a wide range of nitroaromatics [96]. The reaction was carried out using sodium borohydride at room temperature in water or water–ethanol. Aromatic amines were obtained after 2 h with high yields (up to 95%) and the nanocatalyst was recyclable for six times almost without any decrease in activity.
Perfluorohexyl modified cellulose was utilized as support for Pd NPs. The obtained catalyst showed unexpected high activity and selectivity to the selective reduction of nitrobenzene to N-phenylhydroxylamine (PHA) with NaBH4 at room temperature in water. Modified cellulose with a more hydrophobic surface would favor the adsorption of nitrobenzene over PHA thus prevent full hydrogenation to aniline. The catalyst is recyclable the selectivity remaining excellent while the yield decreases from 98% to 80% after 5 runs [97].
Wu et al. report the one-step synthesis of Pd NPs supported in CNCs with no other reagents. Cellulose played a dual role as a supporting matrix and a reductant [98]. The obtained hybrid material exhibits high catalytic activity in the reduction of methylene blue with hydrazine and of 4-nitrophenol with sodium borohydride; reactions were performed at room temperature with stirring in water. CNC have also been coated with melamine-formaldehyde so as to immobilize in situ-reduced Pd NPs with a controlled size and a uniform distribution over the support [99]. The catalytic activity of the nanocatalyst was observed by the effective reduction of 4-nitrophenol to 4-aminophenol with NaBH4 in water at room temperature. The conversion was complete within 13 min and the catalyst could be filtered and recovered for 10 cycles with almost the same activity. To counter the self-agglomeration of CNC in non-polar media, another research team silylated that cellulose with a propyl spacer bearing a primary amine (CNC-APTES) prior to grafting Pd NPs. The resulting catalyst was efficient for the reduction by sodium borohydride of 4-nitrophenol and derivatives to the respective amino compounds at room temperature, with a good pseudo first order rate constant (kapp = 3.40 × 10−3 s−1) and 98% yield. The catalyst could be recycled up to 4 times [66]. Another example of CNC-supported Pd NPs was used to carry out the hydrogenation reaction of nitrobenzene to aniline in water at room temperature under hydrogen gas. The specially designed amphiphilic particles acted both as colloidal surfactant and catalyst [49]. Full conversion of nitrobenzene could be achieved in 1 h at room temperature, which is much efficient compared with other supported Pd catalysts. The hydrophobic group on CNCs surface played an important role in accelerating the catalytic reaction in the interface between organic phase and water. The recyclability of the catalyst was verified: after 8 repetitive hydrogenation reactions, it maintained high conversion; minor Pd leaching was detected in the reaction solutions after each run and a small aggregation of Pd NPs was observed, which can explain the slight deactivation.
Li et al. fabricated a hydrogel using cellulose derivatized with ionic liquid moiety and reduced Pd(II) ions with NaBH4 within it. The Pd NPs-containing hydrogel composite thus created had high catalytic activity and excellent reusability for the reduction of 4-nitrophenol to 4-aminophenol in water [100]. Yu et al. fabricated 3D polyethylene imine-grafted cellulose nanofibrils (CNF-PEI) aerogels [101]. Those porous materials served as supports for Pt NPs to treat 4-nitrophenol from wastewater. Catalytic reduction of aqueous 4-nitrophenol to 4-aminophenol with NaBH4 was observed in the presence of such a Pt-loaded CNF aerogel. Quantitative conversion could be reached within 30 min with a reaction temperature between 25 and 50 °C. The aerogel catalyst was easily recovered and kept the same activity after 5 recycling loops.
Heterogeneous nickel-based catalysts have also been developed to reduce 4-nitrophenol into 4-aminophenol using NaBH4 in water at room temperature [102]. On this purpose, nickel(II) chloride was reduced onto microcrystalline cellulose (MCC). When hydrazine hydrate was used as a reducing agent for nickel, then the catalytic system was highly crystalline and had strong magnetic properties. Ni NPs obtained by reduction with sodium borohydride were amorphous and much less magnetic. However, both catalysts were able to quantitatively reduce 4-nitrophenol within a few to 30 min. Furthermore, both could be recycled and reused for at least 10 successive reductions without significant loss of catalytic activity; the crystalline MCC-supported Ni NPs were retrieved by an external magnet while the amorphous ones were recovered by centrifugation.
Kamal et al. designed a dip catalyst for pollutants degradation [103]. They coated cellulose filter paper with a thin chitosan layer, then adsorbed nickel(II) ions onto it and reduced them to Ni NPs immobilized all over the biopolymeric support. The nanocomposite was found to catalyze reduction reactions. Reduction of 4- and 2-nitrophenol with NaBH4 occurred in the presence of a strip of cellulose-chitosan-supported Ni NPs. The dip catalyst could be recovered by a simple pulling out of the reaction medium and reused 3 times with more than 90% yield, although the reaction rate decreased after each recycling (70% yield being reached after 10 min initially to 16 min at the third run). A team of researchers have developed paper-supported Pd NPs and showed that such dip catalyst could catalyze the reduction of 4-nitrophenol into 4-aminophenol using NaBH4 in water at room temperature to get excellent yields within 15 min. Catalytic conversion efficiency was almost constant over 90% for 11 consecutive reaction cycles using the same paper [56]. Pt NPs have been deposited by simple contact onto a sheet of wipe paper [104]. The macroporous cellulose fibers offered a very stable support for a steady immobilization of reduced platinum. This was used as a dip catalyst for the reduction of 4-nitrophenol to 4-aminophenol with hydrogen gas. More than 93% yield was reached after 8 min.
A magnetic carbon-supported Pd catalyst has been developed by successive deposition of renewable cellulose and Pd NPs over nanoferrites, followed by calcination. The catalyst could be used for quantitative reduction of various nitroarenes in isopropanol at room temperature under atmospheric hydrogen atmosphere and recycled by magnetic retrieval with almost no activity loss after 3 successive runs [105].
A highly effective, stable, and reusable flow microreactor was developed by filling a tube with environmentally sustainable porous monolithic cellulose. The obtained microreactor could be applied to efficiently and continuously catalyzing the model reduction reaction of 4-nitrophenol with sodium borohydride in water without any post-treatment or regeneration of catalysts (Figure 14). This microreactor exhibited extremely high catalytic efficiency (turnover frequency, TOF = 4660 h−1, almost 4 times higher than that of cellulose nanocrystals supported catalyst) and long-term stability (over 90% reaction efficiency after 16 h of continuous flow at the speed of 1 mL/min) [106].
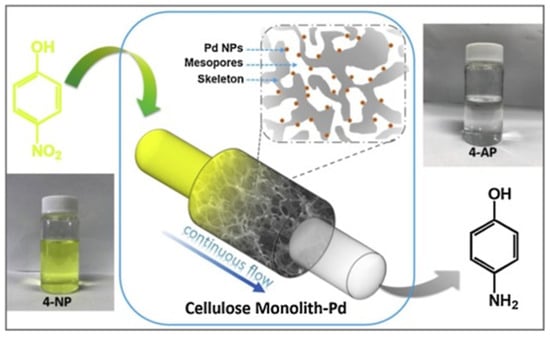
Figure 14.
Reduction of yellow 4-nitrophenol (4-NP) to colorless 4-aminophenol (4-AP) in a continuous flow reactor filled with cellulose monolith-supported Pd NPs [106].
2.3.2. Dyes Depollution
Porous microspheres cellulose-supported Pd NPs have been synthesized according to a simple and environmentally friendly method (direct reduction of palladium(II) with cellulose solution through microwave heating) [107]. Small Pd NPs have been immobilized and reduced on highly porous cellulose nanofibrils that could self-assemble into water-activated aerogels [108]. Both the Pd NPs-containing microspheres and aerogels were found to catalyze the discoloration of methylene blue (Figure 15a) and Congo red (Figure 15b) by sodium borohydride within 5 to 30 min, achieving 99% catalytic efficiency and still higher than 90% after multiple cycles [108].
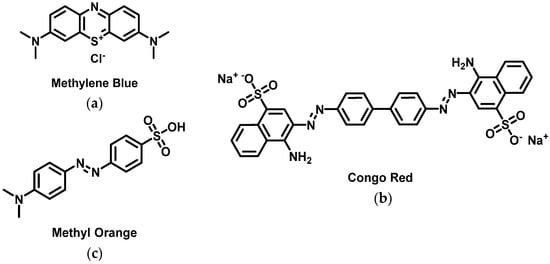
Figure 15.
Structure of methylene blue (a), Congo red (b) and methyl orange (c).
A hydrogel based on biosourced polymers, cellulose and alginate, has been built as a matrix for Pd NPs [73]. To this end, the cellulose was first made cationic by partial oxidation and grafting of quaternary ammonium groups. The gel set by interactions of calcium ions with cationic cellulose and anionic alginate. The hydrogel-supported Pd NPs proved to catalyze the reduction of a methylene blue with NaBH4 at room temperature. The reductive bleaching was also performed with success in a continuous set-up, the catalytic hydrogel being packed inside a column through which the reaction mixture flowed.
Cellulose fibers have been coated by polypyrrole formed by in situ polymerization [109]. The composite then served as a support for metallic nanocrystals obtained by spontaneous reduction of palladium salts. Polypyrrole-coated cellulose fibers with reduced Pd have been burnt under microwave heating to form PdO/PdO2-embedded carbon. Both Pd@py-cellulose and PdO/PdO2@carbon have been used to reduce model water pollutants. Up to 87% of methylene blue could be reduced to leuco-methylene blue at room temperature in water by the Pd-containing catalyst in a concentration of 6.60 mg·mL−1 over 120 min. The PdO/PdO2 nanocomposite was much more slowly active to reduce the dye but could remove up to 72% of octylphenol, 79% of triclosan, and 17% of atrazine over 100 min from a contaminated water.
Islam et al. have designed a dip catalyst by direct impregnation of Pt NPs on wipe paper [110]. They proved that it enables the reduction of an azo-dye, methyl orange (Figure 15c), in aqueous solutions. With dihydrogen bubbling, it afforded 95% reduction of methyl orange after 6 min and 99% after 10 min. Catalytic oxidation of formic acid followed by reduction of methyl orange was quicker and led to 99% removal after 6 min. The same reductions were slightly slower in fresh drinking water as a solvent, but quantitative bleaching was effective after 10 min in both cases. The paper-supported catalyst was easily recovered after each reaction and could be used 5 times with only little lowering of the catalytic activity. It is of note that the paper-supported Pt NPs were still found active after a 10-days rest.
In the presence of sodium borohydride, the discoloration of azo-dyes is due to a reversible reduction of the N=N bond [111]. CMC-stabilized Pd NPs showed an excellent catalytic activity by non-reversible reducing degradation of such azo-dyes, such as p-aminoazobenzene, acid red 66, or acid orange 7 in the presence of sodium borohydride, due to the actual rupture of the N=N bond (Figure 16).
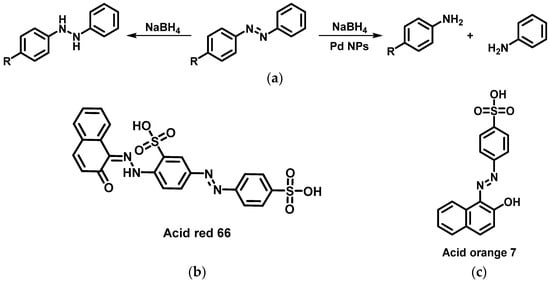
Figure 16.
(a) Degradation of azo-dyes with and without Pd NPs and structure of acid red 66 (b) and acid orange 7 (c) [111].
Degradation reduction of an azo-dye has also been achieved thanks to the catalytic activity of Ni NPs dispersed in a chitosan layer deposited over cellulose filter paper [103]. In contact with such dip catalyst, a complete reduction of methyl orange was observed within a few minutes in a dilute solution with addition of NaBH4 as a reducing agent.
2.3.3. Hydrogenation
Researchers have grafted fluorinated moieties onto cellulose via a silylated spacer. Pd NPs were then straightforwardly deposited onto the resulting amphiphilic support to yield a catalyst exhibiting excellent catalytic activity and selectivity in hydrodeoxygenation of vanillin (a typical model compound of lignin) to 2-methoxy-4-methylphenol under atmospheric hydrogen pressure in neat water without any other additives at 50 °C. The catalyst was reusable for four cycles with only little loss in its activity [67]. A very similar nanocatalyst was constructed with metallic NPs immobilized on amphiphilic cellulose-based polymers [112]. Hydrophilic CNCs have been grafted with more or less numerous hydrophobic C16 carbon chains to produce such amphiphilic nano-support for Pd-Ni alloy NPs. Under hydrogen, in water, at 70 °C, that catalyst displayed an interesting activity in the selective hydrodeoxygenation of vanillin to 2-methoxy-4-methylphenol, reaction in which vanillin alcohol is a common byproduct. An adequate hydrophilic-lipophilic balance was found in the synthesis of the C16-CNC support so that a 100% conversion could be obtained with a 100% selectivity. The selectivity was induced by the chiral cellulosic support and the catalytic activity by the palladium, since supported NPs made of pure Ni were found inactive. The proportion though could vary from PdNi4 to PdNi8 (at least) with no difference in the results. Supported Pd NPs alone proved to be less efficient. Other common metals (Fe, Co, Cu) could not replace Ni without an important loss in yield and selectivity.
CNC supporting monodispersed Pd NPs has been found an active colloidal catalyst for the hydrogenation reaction of phenol to cyclohexanone in water as a solvent, at room temperature, under 4 bars of dihydrogen [84]. Even-dispersed Pd NPs have been confined within a carboxylic cellulose nanofiber (CNF) matrix, appearing as soft hydrophilic foam. The ultrafine Pd NPs (ca. 6 nm) were in situ grown by reduction on the mesoporous matrix with a dense spatial distribution (9.6 wt%) thus generating nano-confinement catalytic effects on the reactants. An enhanced catalytic activity was indeed observed on the model reaction of 4-nitrophenol hydrogenation with sodium borohydride at room temperature in water, with quantitative yields for 6 consecutive cycles. Interestingly, 100% chemoselective reduction of 3-nitrostyrene with NaBH4 at room temperature was made possible using CNF-Pd, according to the choice of solvent: The reaction yielded 50% of 2-ethyl-nitrobenzene in ethanol after 3 h, 98% of 3-aminostyrene in water/methanol: 1/1 after 1 h, and 100% of 2-ethylaniline in pure water or pure methanol after 3 h [113].
A dip catalyst was developed by reducing palladium salt with formic acid onto jute plant stem getting a uniform distribution Pd NPs on the cellulose matrix. The catalyst was successfully applied to the chemoselective transfer hydrogenation of a series of styrenyl, substituted olefins, quinoline and other N-heteroarenes using tetrahydroxydiboron as reductant. Quantitative hydrogenation of various alkenes and quinolines was shown possible in water at 60–70 °C in 1–2 h. This system is stable in water and displays excellent recyclability; it could be used for 32 consecutive cycles, without losing its original crystallinity or requiring replenishment [114]. A variety of alkenes have successfully been hydrogenated using as a catalyst Pd NPs coated with hyperbranched cationic polystyrene and then immobilized on cellulose (on a piece of paper or of cotton) [70]. Quantitative hydrogenations were observed after 5 h in ethyl acetate at room temperature under hydrogen (2 atm). The catalyst could be recycled for 5 runs with only little Pd leaching and an activity remaining constant. Pd@HPS-NR3+Cl− proved to be a robust recyclable catalyst thanks to a strong adhesion of the resinous ammonium-derivative of polystyrene to cellulose due to multiple electrostatic interactions.
Cellulose-immobilized Pd NPs were prepared by reduction of palladium acetate solution drop-cast over paper sheets under dihydrogen flow for mere 90 s. Most interestingly, that technology was developed for use in a continuous flow reactor by simply lining the walls of the reaction tube of a vortex fluidic device with such sheets of paper-supported Pd NPs (Figure 17). The embedded Pd(0) proved efficient in hydrogenation of alkenes, nitroarenes, ketones, and enamides, with products formed in high yield, under ambient pressure and temperature. The system is also effective for transfer hydrogenation using ammonium methanoate as an alternative hydrogen source. High catalyst stability and re-usability is demonstrated along with the chemoselective and scalable synthesis of industrially important fine chemicals, including the bio-based molecule cyrene [115].
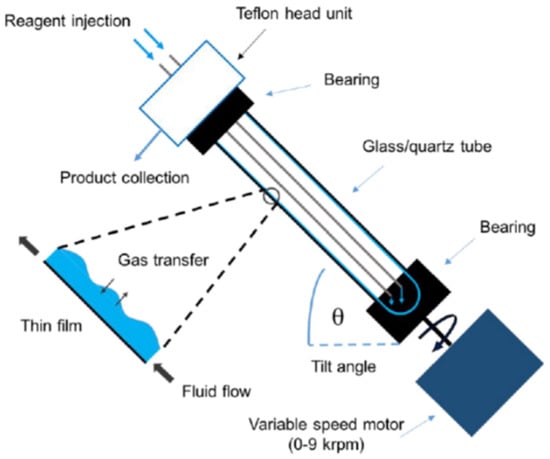
Figure 17.
Scheme of the vortex fluidic device (VFD), a tubular rotating continuous flow reactor, lined with the catalyst-containing paper sheet [115].
The C-supported Pd catalyst developed by Baig and Varma, using cellulose as a source of carbon, was found efficient in the quantitative hydrogenation of various alkenes and of diphenylacetylene in isopropanol at room temperature under atmospheric hydrogen pressure. The catalyst, being magnetically retrievable, could be used at least three times with almost no change in activity and no Pd leaching [105].
Suarez’s team developed metal complexes using carboxyl cellulose (CC) as a ligand. PdCC and NiCC proved to have a catalytic activity in the alkene hydrogenation of the unsaturated fatty chains of a biodiesel [116]. When stirred with a biodiesel obtained from soybean oil, in ethyl acetate at 80 °C under pressured hydrogen, PdCC displayed, after 6 h, a reduction of 60 to 90% of the double bonds when the pressure rose from 10 to 100 bars. Under the same conditions, 55% of the double bonds were reduced in the presence of NiCC catalyst under 100 bars of hydrogen after 24 h. Both catalysts could be recovered and used in 7 consecutive cycles with no change of catalytic activity. Interestingly, in the course of the reaction, the polyunsaturated fatty esters were firstly hydrogenated to monounsaturated compounds, even under low hydrogen pressure.
Moores’ team has devised Pd NPs deposited as patches at the surface of CNCs and used them to catalyze enantioselective hydrogenation of prochiral ketones in water at room temperature under 4 bars of dihydrogen. Cellulose was the sole source of chirality thus induced enantioselectivity with enantiomeric excess as high as 65% with 100% conversions. Recycling test showed that the heterogeneous catalyst could be used up to 5 times with no loss of enantioselectivity while the activity was already reduced from the fourth run onwards [117].
Pd NPs have been formed by reduction with hydrazine and immobilized on cellulose particles (CLP) on the one hand and on a cellulose monolith (CLM) on the other hand. Those composites were applied as hydrogenation catalysts and both were found active (at 1 mol%) to readily reduce a variety of aklynes, alkenes, nitro, and azido compounds in methanol at room temperature under hydrogen at atmospheric pressure with 87–100% yields. Although both catalysts catalyzed the deprotection of benzyloxycarbonyl-protected aromatic amines (Ar-N-Cbz) and aromatic benzyl esters (Ar-CO2Bn), only CLM-supported Pd NPs could accomplish the hydrogenolysis of aliphatic-N-Cbz and aliphatic-CO2Bn protective groups; such protective groups were unchanged when CLP-supported Pd NPs were used. The difference in the physical structure of the cellulose supports induced unique chemoselectivity. Aromatic ketones could be partially hydrogenated in the presence of both catalysts to give the corresponding secondary alcohols as chemoselectively reduced products. On the other hand, both aromatic and aliphatic benzyl ethers were stable under both Pd/CLP and Pd/CLM-catalyzed hydrogenation conditions. Both catalysts could be reused after simple filtration for at least three times [118].
Huang et al. prepared a silica-supported methylcellulose-L-alanine-palladium complex with the interest of it being chiral [119]. Such highly optically active catalyst enabled the asymmetric hydrogenation of ortho-cresol and meta-cresol to give (S)-2-methylcyclohexanone and (R)-3-methylcyclohexanone, respectively, at 25 °C under an atmospheric hydrogen pressure in 64.6% and 61.3% yields and 91.5% ee and 68.5% ee, respectively, after 12 h. Quantitative reaction was possible after a total of 24 h but the enantioselectivity then drastically dropped. Under optimized conditions, the optical yields were kept over 90% and 60%, respectively, when the catalyst was reused up to 4 times.
CMC has been used to stabilize Pd NPs, thus forming a water-soluble system. Liu et al. [120] have used such CMC-capped Pd NPs to efficiently catalyze the hydrodechlorination of trichloroethylene by hydrogen into biodegradable ethane, in order to depollute soiled waters within minutes. The same research group [121,122] have further immobilized CMC-stabilized Pd NPs onto alumina, a hydrophilic support material, in order to obtain a heterogeneous catalyst. The composite catalyst was able to facilitate rapid and complete hydrodechlorination of trichloroethylene in aqueous media. It offered an activity more than 7 times greater than that of the commercial alumina-supported Pd NPs but much lesser than that of unsupported CMC-stabilized Pd NPs. Recyclability was made possible after pre-calcination treatment in order to limit the leaching of palladium, despite a minor drop in activity.
Palladium and platinum have been simultaneously reduced by hydrazine over non-chemically modified cellulose beads [123]. Two bimetallic nanocatalysts (Pd/Pt 7:1 or 1:7) have been produced this way for the removal of polluting chlorates in water. Aqueous potassium chlorate solutions were efficiently treated for hydrogenation to chloride ions using both catalysts with hydrogen bubbling at 80 °C. The reduction of chlorate ions was observed with a slightly higher reaction rate using the Pt-rich NPs. With a pseudo-first-order kinetics, the rate constants were: with Pd-rich catalyst (74% reduction after 3 h); with Pt-rich catalyst (76% reduction after 3 h). When Fe2O3 was deposited onto the cellulose-supported bimetallic NPs, its promoter effect afforded an increase in the rate constants with much lower contents in precious metals: with Pd-rich catalyst (92% reduction after 3 h); with Pt-rich catalyst (85% reduction after 3 h). Pd-rich bimetallic NPs supported by cellulose beads with addition of Fe2O3 could be recycled 4 times with success despite some metal release. When used in a continuous flow hydrogenation set-up, quantitative removal of chlorate occurred within 160 min.
2.3.4. Oxidation
Cellulose-supported on Pd NPs was found to effectively promote the oxidation of glucose by oxygen into gluconic acid under mild conditions. Glucose was fully converted at room temperature after 3 h by the use of a stoichiometric amount of Na2CO3 to neutralize gluconic acid, and gluconic acid was obtained in a high yield of 91.2%. The catalyst was recovered and reused up to five times without significant loss in its catalytic activity [124]. Pd NPs supported on neat cellulose was found to be highly efficient recyclable heterogeneous catalyst for aerobic oxidation of benzyl alcohols using air as the source of molecular oxygen in acetonitrile. This catalytic system provides benzaldehyde derivatives in high yields (ca. 80%) and selectivity but with a rather modest recyclability [53].
Biosourced microcrystalline cellulose (MCC) can be obtained from cheap and abundant natural cotton [125]. Phtalocyanines of Fe(II) and Ni(II) have been immobilized on MCC via chemical binding. The resulting nanocomposites exhibit a catalytic activity in the selective oxidations of alcohols to aldehydes. Solvent-free reactions were conducted at 80 °C for 2 h with air bubbling, O2 being the oxidant. The iron-based catalyst was systematically more efficient than the nickel-based one with up to 98% yield against up to 77%. Alcohols were converted to aldehydes with 100% selectivity, excepted primary aliphatic alcohols for which the selectivity dropped to 97% with FePcAc@MCC and to 91% with NiPcAc@MCC. Both nanocatalysts were recyclable by simple filtration and the final yield did not significantly decrease after 6 successive runs. It is of note that using crude cellulose instead of MCC as a support led to lower yields. Selective oxidation of ethylbenzene to acetophenone was also observed under the same conditions with no byproduct and 61% yield with the iron-based catalyst and only 33% yield with the nickel analogue.
A copolymeric material has been developed by two successive steps: selective oxidation of the primary hydroxyl groups of a textile cellulose and grafting of a poly(amido amine) dendrimer by amidation of the resulting carboxyl groups [126]. The dendrimer has been loaded beforehand with metallic NPs so as to obtain a hybrid with catalytic activity. In the presence of Pt NPs stabilized in the cellulose-supported dendrimer, formaldehyde gas was absorbed and oxidatively decomposed to CO2 and H2O at room temperature. Within 120 min, up to 65% of the formaldehyde disappeared. Incidentally, an analogue with Ag NPs was found to exhibit an interesting antibacterial activity. Oxidative decomposition of formaldehyde by Pt NPs at room temperature has also been observed when the NPs were supported by cellulose triacetate (CTA) [127]. By comparison to other supported Pt(0) catalysts, the decomposition rate of formaldehyde was 13.4 times and 4.3 times as high in the presence of Pt/CTA as that observed in the presence of MCC-supported Pt NPs and TiO2-supported Pt NPs, respectively. This can be explained by the swift and easy absorption and desorption of formaldehyde, water, and carbon dioxide over CTA while water tends to accumulate on MCC and formaldehyde on TiO2. Incidentally, the catalyst could be shaped as microspheres or as films. Pd NPs have been deposited, together with Fe3O4 NPs, on N-(2-aminoethyl)acetamide-functionalized cellulose for use as a catalyst. It was successfully applied in two oxidative reactions carried out with hydrogen peroxide at 100 °C in aqueous media: the epoxidation of styrene [128] and the oxidation of ethylbenzene [129]. Both reactions proved to be highly selective: only styrene oxide was obtained, without any other oxidized product. The catalyst could be magnetically recovered and reused for 4 runs in a row with the same yield (93% from styrene, 88% from ethylbenzene).
2.4. Others
Nanocellulose has been substituted on carbon number 6 with 2-aminopyrimidine so as to support Pd NPs. That novel bio-compound proved a successful catalyst in Pechmann condensation between various phenols and ethyl acetoacetate to obtain coumarin derivatives in good yields (up to 97%). Interestingly the reaction was optimized under solvent-free conditions, at 130 °C, with no leaching of Pd into the environment making the catalyst recyclable, with only little decrease in the catalytic activity, yields going from 94% initially to 88% at the 4th run [130]. Dong et al. [36] have developed a palladium-based catalyst bonded to diethylenetriamine-derivatized cellulose for Suzuki reaction. The same team also used it as an effective catalyst to synthesize nitroalkenes and 1,3-dinitroalkanes from aromatic aldehydes with nitromethane, via Henry and Mickael reactions. In addition to catalyzing various C-C coupling reactions, cellulose-supported Pd NPs have proved an active catalyst in a tandem C-H activation and annulation to prepare hydroxyisoindolone [43]. The HPMC bearing Pd NPs in its hydrophobic pockets was used to catalyze extremely fast peptide couplings. A wide variety of amines and carboxylic acids were coupled in water at room temperature within one minute thanks to such catalyst (up to 96% yields). Two examples of those reactions were scaled up to 100 g with the same fastness and quantitative yields [94].
Ahmar et al. modified the surface of a glassy carbon electrode with ethylenediamine-cellulose, on which Pd NPs have been immobilized [131]. They used it as an anodic sensor, where they tested the electrochemical oxidation of hydrazine and noted that the overpotential decreased (850 mV) while the current peak increased to four times that of the unmodified carbon electrode. EDAC-supported Pd NPs have been successfully used for an original application. When mounted on a carbon paste electrode, it was an efficient electrocatalyst for the production of hydrogen with high performance and very low palladium load, as compared to other carbon electrodes [132]. A classic cellulose-based carbon (CBC), obtained by basic hydrolysis of MCC at 50 °C for 20 h, has been utilized to support Pd-Ir-M ternary NPs [133]. The catalytic activities of such electrocatalysts were measured in half-cell experiments (chronoamperometry, CO stripping voltammetry, cyclic voltammetry). It was shown that CBC-supported Pd-Ir-Rh, Pd-Ir-Ni, and Pd-Ir-Mo display a better catalytic activity at room temperature towards hydrogen peroxide oxidation as compared to Pd-Ir and Pd catalysts. Amongst all five CBC-supported catalysts developed here, Pd-Ir-Ni/CBC exhibited the best activity in experiments performed on the membraneless sodium perborate fuel cells.
3. Hemicellulose
Hemicellulose is the second most abundant biopolymer after cellulose [134]. It is in fact a heterogeneous family of more or less branched macromolecules constituted of glycosidic monomers, mainly pentoses such as D-xylose and L-arabinose, with some D-hexoses such as glucose, galactose, mannose, rhamnose and glucuronic and galacturonic acids [2]. The exact composition depends on the plant species, the growth location and the part of the plant [135]. Hardwood cells mainly form glucurono xylans and softwood cells galactose glucose mannans. Hemicelluloses have an amorphous structure and a lower degree of polymerization than cellulose, with 50–300 units [136]. Their natural role is to cross-link cellulose microfibrils around the plant cells, with strong hydrogen bonds and Van der Waals force. They also link with lignin, when present, and bridge it to cellulose [137]. Xylan is the most common type of hemicellulose produced industrially. It is made of a β-1,4-glycosidic-bonded polyxylose backbone with some arabinose and glucuronic acid branches (Figure 18).
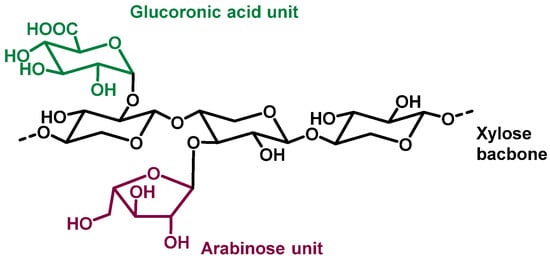
Figure 18.
Xylan-type structure.
3.1. In Suzuki–Miyaura Coupling
Peng’s team and their coworkers reduced Pd NPs into xylan-type hemicellulose to form a versatile heterogeneous nanocatalyst [138]. It showed a catalytic activity in the Suzuki coupling of aryl halides with aryl boronic acids in the presence of potassium carbonate at 50 °C in methanol for 2 h under air atmosphere. Very good to excellent yields were reached with aryl bromides and aryl iodides (over 97% with phenyl boronic acid). The Pd catalyst was successfully recycled for six runs and a final yield still over 93%, almost without palladium leaching. The same group also derivatized xylan-type hemicellulose in two different manners, integrating each time a nitrogenated moiety: 1,10-phenanthroline-5-amine was grafted to carboxymethylxylan to form PACMX and ethylenediamine to xylan to form EDAX [139]. In each case, the bidentate ligand within the polymer was used as a support for palladium ions further locally reduced to Pd NPs. In the presence of one or the other of those catalysts, different aryl iodides and aryl bromides could be cross-coupled with aryl boronic acids at room temperature in ethanol with K2CO3. Up to 100% yield could be reach within 4 h for some substrates. Both xylan-supported catalysts could be recovered by centrifugation and vacuum drying. The recycling of EDAX-Pd showed a drastic drop of catalytic activity from the second run onwards with a yield of 73% and down to 57% after 5 cycles. PACMX-Pd was better recycled, the yield of the Suzuki reaction remaining over 87% after 5 runs. The researchers have fabricated another derivative of xylan-type hemicellulose: terpyridine moieties were substituted on some of the hydroxyl groups of the xylose motifs [140]. It then served as a tridentate ligand for palladium(II) ions. The heterogeneous Pd(II)-complex was then directly used without any further reduction to catalyze the Suzuki coupling of various aryl halides (iodides and bromides) with aryl boronic acids in methanol with K2CO3 as a base at room temperature for 5 h. The yield range was 78–98%. A good recyclability was observed with a yield only decreasing from 94% to 86% after 6 runs. A xylan-type hemicellulose, extracted from sugarcane bagasse, has been oxidized to dialdehyde xylan (DAX) and further derivatized by reduction amination with polyethyleneimine (PEI). Filter paper was coated with a mixed solution of the DAX-PEI obtained and palladium(II) ions [141]. The polymer served to anchor in situ reduced Pd NPs onto the paper surface, forming a dip catalyst. A good catalytic activity was noted in Suzuki reaction cross-coupling of several substrates (aryl halides) with boronic acids. The yield reached up to 91% and the catalyst showed an excellent recyclability, being used 15 times with a yield still around 90%.
3.2. In Mizoroki–Heck Coupling
Heck cross-coupling reactions have also been catalyzed by Pd NPs supported on hemicellulose derivatives. The xylan-supported Pd NPs designed by Peng’s team was successfully used as a catalyst for Heck reactions [138]. Various aryl bromides and aryl iodides have been coupled with vinyl substrates in acetonitrile and triethylamine under reflux for 8 h under air atmosphere. Yields over 94% were reached with an acrylate as a substrate and 86% with styrene. The nanocatalyst could be recovered and after six consecutive uses the final yield was 90% with almost no Pd release. The same group produced Pd(0) supported by carboxymethyl-hemicellulose, by in situ reduction of palladium ions [142]. The nanocomposite has shown a catalytic activity in the coupling of iodobenzene with ethyl acrylate in DMF at 120 °C in the presence of triethylamine for 6 h to 8 h. Easily recovered by filtration, it could be reused with a yield dropping only slightly from 97% initially to 89% at the fifth reaction. Several other aryl halides and olefins have been cross-coupled this way, with yields over 90% for aryl iodides.
3.3. In Sonogashira Coupling
Aryl bromides and aryl iodides have been cross-coupled with terminal alkynes in acetonitrile and triethylamine in the presence of xylan-supported Pd NPs [138]. The reactions were carried out under reflux for 6 h under air atmosphere. Yields over 82% were reached, with even over 92% with arylethynes. The heterogeneous nanocatalyst was easily retrieved by filtration and reused. It was still catalytically active after six runs, the yield being 87%.
4. Lignin
Lignin is widely present in between the cells of all vascular plants, where it serves to strengthen plant tissues. In herbaceous plants, the lignin content is about 15% to 25%, while in woody plants, it can be as high as 20% to 35% [19]. In nature, lignin always coexists with cellulose and hemicellulose, working together to build the plant skeleton. While cellulose and hemicellulose are polysaccharides, lignin is a polymeric aromatic alcohol [2,143]. Lignin is a renewable biological resource in nature because it is non-toxic, highly polymerizable, and easily degraded by microorganisms.
Lignin is composed of three main elements: carbon, hydrogen, and oxygen, which are connected by C-O-C bonds. It is a network polymer with the composition of phenyl propane units, which is a non-crystalline complex with aromatic compound properties. Lignin has three basic structures: guaiac-based structure, syringyl structure and p-hydroxyphenyl structure (Figure 19) [144]. The chemical structure of lignin is relatively complex, with different active groups, such as -OCH3, -COOH, -O-, C=C, -OH, C=O, and so on [145].
Figure 19.
Lignin structure with guaiac-based moiety (blue), syringyl moiety (green) and p-hydroxyphenyl moiety (red).
Therefore, it can undergo different chemical reactions, such as sulfonation, acylation, oxidation, and condensation reaction, etc. The structural composition of lignin varies depending on the plant species and the isolation method. Traditionally, there are two main ways to use lignin in industry: One is to use the lignin in the pulp “black liquor” directly; the other is to add acid to precipitate the lignin in the black liquor, and then use the extraction or ultrafiltration method to separate the lignin. Then it is chemically modified to produce various lignin derivatives, which are used in various aspects of industrial production [146]. More importantly, lignin and its derivatives can be used to prepare carbon-based materials with high specific surface area, which are appropriate as support for precious metal catalysts [147]. The prepared lignin-based catalyst composites can remove pollutants in industrial wastewater and catalyze various chemical reactions, which have the characteristics of high efficiency, environmental protection, and recyclability.
4.1. In C-C Coupling
4.1.1. In Suzuki–Miyaura Coupling
As an abundant biopolymer, lignin has been extensively used as a source to produce activated carbon or carbon nanofibers to support metallic nanoparticles for various applications in catalysis. In 2009, Rodriguez–Mirasol and Cordero et al. prepared new catalysts based on palladium nanoparticles supported on activated carbon. The mesoporous structures were obtained through impregnation of Kraft lignin with phosphoric acid and heating at 500 °C and the nanoparticles were then deposited onto the surface by reduction of a palladium salt with hydrogen [148]. The supported-particles proved efficient in Suzuki–Miyaura cross-coupling reactions with very low catalyst loadings (0.1 mol%). The catalytic activity was evaluated in the synthesis of three biphenyl molecules. The Pd catalyst was easily separated from the reaction medium through filtration. After drying, it could be reused without any significant loss of efficiency.
Lignin could also be used directly (without conversion to activated carbon) as a support for metallic cations [149] or nanoparticles. In the case of palladium nanoparticles, it can play both the role of the reducing agent for the production of the particles and the stabilizing agent (instead of the use of ligands), which can avoid the use of other potentially toxic or expensive reagents. For example, in 2013, d’Alessandro et al. [150] used Kraft lignin or lignosulfonate to reduce a palladium (II) salt at 80 °C in water (Figure 20). The obtained nanoparticles were characterized by XRD and TEM. They had a size around 11 nm (75% of particles having a diameter comprised between 8 and 14 nm). Their catalytic activity was then evaluated in different C-C cross-coupling reactions. In the case of Suzuki–Miyaura reaction, with 0.23 mol% metal loading in water at 70 °C, quantitative yields were usually achieved starting from various aryl iodides, with more modest results when using bromides and very low conversions of the starting materials for aryl chlorides. Interestingly, with two aryl iodides, the reaction could be performed efficiently at 20 °C with quantitative conversions. The recycling and reuse of the nanoparticles were unfortunately not considered in this article.

Figure 20.
(a) Preparation of palladium NPs by using lignin as reductant; (b) TEM image of Pd NPs on Kraft lignin and their size distribution [150].
More recently, Nasrollahzadeth and Luque developed a new catalyst based on palladium nanoparticles deposited on magnetic calcium lignosulfonate [151]. Before the immobilization of the palladium particles, the support is prepared by activation of the calcium lignosulfonate with potassium periodate to generate more bonding functions, then fixation of magnetite nanoparticles. Afterwards, the palladium metal is deposited on this support by reduction of a palladium (II) solution with an extract of hibiscus leaves, the polyphenols contained in this extract being responsible for the reductive properties of this solution (Figure 21a). The synthesized nanoparticles were found to have a size in the range of 20–25 nm (from TEM results), and were also characterized by XRD and EDS. This catalyst was then applied to the preparation of a range of biphenyl derivatives via a Suzuki cross-coupling reaction. It was used in eco-friendly medium (water/ethanol 1:1) at 90 °C with low catalyst loading (0.75 mol%) and proved to be efficient for the conversion of aryl iodides or bromides substituted either by electron-withdrawing or electron-donating substituents. Application of this catalyst to the activation of aryl chlorides was investigated with success on only one example, with a longer reaction time and a slightly lower yield in this case. The catalyst could easily be recovered from the reaction medium using a magnet and could be reused after washing and drying for at least seven cycles without any significant decrease in yield of targeted biaryles (Figure 21). In 2019, Varma and Nasrollahzadeh used lignosulfonate to stabilize magnetic iron oxide nanoparticles [152]. The Fe3O4 particles were then grafted through a silylated linker with tetrazole derivatives. The tetrazole moieties were then used to chelate palladium (II) cations (Figure 21c). The prepared nanostructures were fully characterized. In particular, XRD analysis proved that crystalline structure of Fe3O4 was preserved in the synthesis and confirmed the presence of palladium. FESEM images showed a spherical morphology for the nanostructures with an average particle size of about 20–27 nm. The activity in catalysis was evaluated in Suzuki cross-coupling reactions starting from phenylboronic acid and a range of substituted aryl halides. Good results were obtained regardless of the electronic effect of the substituents and good yields were attained with iodides, bromides, and even with a chloride reagent (one example, 81%), although the reaction time had to be prolonged significantly from iodides to less reactive aryl halides. The best reaction conditions consisted in using 0.05 g of catalyst per mmol of aryl halide in water at reflux. The quantity of palladium on the nanostructures were not determined, but if all the metal used during the complexation step were deposited on the materials, it would represent a 14 mol% palladium loading (the real metal loading is almost certainly lower but it was not determined or estimated in the article). After recovery with a magnet, the catalyst could be reused at least seven times without any significant loss of activity.
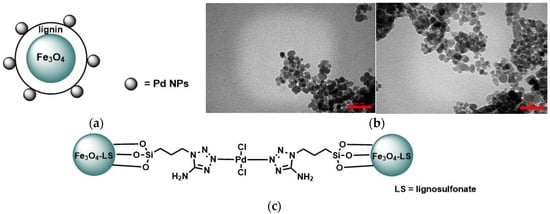
Figure 21.
(a) Structure of the catalyst Pd NPs@Fe3 O4-lignin prepared by Nasrollahzadeh and Luque et al.; (b) TEM images of Pd NPs@Fe3O4-lignin before reaction (left) and after Suzuki-coupling and recycling (right) [151]; (c) structure of the catalyst based on palladium–tetrazole complex supported on Fe3O4-lignosulfonate developed by Nasrollahzadeh and Varma et al. [152].
An efficient Suzuki coupling catalyst was also develop by Baran et al. in 2020 [153]. They grafted palladium nanoparticles on composite materials consisting of Fe3O4, lignin, and chitosan. The iron oxide was loaded onto alkaline lignin by mixing them together at 90 °C for 30 min. Microbeads were then prepared by adding chitosan to the Fe3O4-loaded lignin and cross-linking the obtained gel with glutaraldehyde. The composite microbeads were used as support for the synthesis of palladium nanoparticles: palladium (II) chloride was reduced without the addition of external reducing agents (Figure 22). These nanocatalysts were evaluated in Suzuki C-C couplings under microwave heating with a 0.08 mol% metal loading in a solvent free medium. After 5 min of reaction, excellent to good results (79–96% yields) were observed starting from iodides or bromides, including with ortho-substituted hindered reagents or with less reactive substrates bearing electron-donating groups. Interestingly, this catalyst proved efficient when employing aryl chlorides as starting materials: with the same palladium loading (0.08 mol%) and short reaction time (5min) under microwave irradiation, good yields of the corresponding biaryl products were observed (70–76% yields). The catalyst was recycled and reused several times. It seemed to mostly keep its activity with only a slight decrease in efficiency after seven cycles. These results were explained by the almost negligible metal leaching (<1.5%) as proved by ICP analysis.
Figure 22.
FE-SEM images of Fe3O4-lignin-chitosan beads (a) and Pd NPs@Fe3O4-lignin-chitosan (b,c) [153].
4.1.2. In Mizoroki–Heck Coupling
The palladium nanoparticles stabilized by lignin and prepared by d’Alessandro’s team were also evaluated for Heck reactions [150]. In aqueous solution and without the addition of any ligand, good results were observed with 0.23 mol% catalyst loading starting from aryl iodides. Total conversions of the starting halides were observed in most cases and good selectivities were generally obtained (the lower yields for the synthesis of cinnamyl alcohols were the results of oxidation to the corresponding aldehydes). Unfortunately, the conversions were much lower with aryl bromides and the nanoparticles showed no catalytic activity starting from the chlorides’ derivatives.
A similar catalyst was developed in Kumar’s team [154]. They tried two different methods for the preparation of the palladium nanoparticles and their deposition on high molecular weight lignin: either reduction of a palladium (II) chloride solution with hydrazine at room temperature or direct use of lignin as a reducing agent in water at reflux. The palladium particles were fully characterized and their properties were found to be identical, with a spherical morphology and good monodispersity and an average size of 1–5 nm (Figure 23a). The only difference between the nanoparticles obtained via the two different methods was the quantity of palladium deposited on the natural polymer. The use of lignin as reducing agent providing the catalyst the most concentrated in palladium. The catalytic activity was evaluated in the Heck coupling reaction for the preparation of various cinnamic esters starting from aryl halides and n-butyl acrylate. The immobilized nanoparticles proved to be efficient, when using tripropylamine as a base and 0.28 mol% of catalyst, in aerobic conditions without solvent at 140 °C. With aryl iodides substituted with electron-withdrawing or electron-donating groups, excellent yields of the targeted cinnamic esters were achieved (90–94% yield, for 6 examples) in short reaction times (30 min or less). The catalyst was also capable of converting aryl bromides or chlorides but in this case, longer reaction times were necessary and lower yields were obtained (between 55 and 90% for 6 examples). The possibility to recycle and reuse the catalyst was also envisioned. After the initial reaction, the particles supported on lignin were recovered by filtration, then washed and dried and then reuse in another Heck reaction. The catalyst could be recycled five times with a slow decrease of the observed yield at each cycle (from 94% for the first use to 75% yield after the fifth re-use) (Figure 23b).
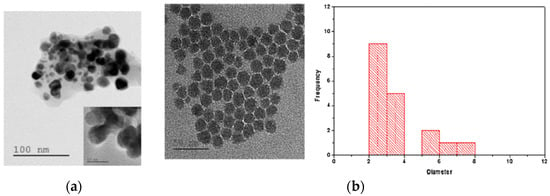
Figure 23.
(a) TEM images of Pd NPs deposited on lignin and prepared by Kumar et al.; (b) TEM images and size distribution of the same catalysts after the sixth run of Heck reaction [154].
More recently, Panchangam’s team designed a new catalyst based on palladium nanoparticles attached to Fe3O4-lignin nanocomposites [155]. The iron oxide nanoparticles were prepared by co-precipitation of iron (III) and (II) salts. These magnetic nanoobjects were then mixed with high molecular weight alkali lignin, and sonicated for 5 h to obtain the nanocomposites, which were isolated after centrifugation and magnetic decantation. Palladium nanoparticles were grafted onto these composites by reduction of a palladium (II) salt. These prepared metallic-loaded nanoparticles contained around 27% of palladium (w/w) as the results of EDX analysis showed. They were tested in Mizoroki–Heck couplings starting from butyle acrylate or styrene as the alkene source. The best conditions were when the reaction was performed without any solvent and using tripropylamine as a base, at 140 °C. With these reaction conditions, the iodoaryl reagents were efficiently converted to the corresponding substituted stilbenes or cinnamic esters (between 75% and 99% yields). From iodides to bromides and to chlorides, the observed yields decreased and the reaction time had to be increased in order to completely convert all the reagents. The nanocatalyst could easily be recovered thanks to its magnetic properties. Its recycling was evaluated through six cycles. It appeared that the catalyst seemed to keep its activity relatively well. After the sixth recycling, a yield about 10% lower than during the first run was observed and slightly longer reaction times were needed.
4.1.3. In Sonogashira Coupling
The catalyst previously described by Kumar’s team for Heck reactions was also used in copper-free Sonogashira reaction [156]. The nanoparticles immobilized on lignin proved to promote good catalytic activity for the synthesis of diarylacetylenes with 0.28 mol% of palladium in DMF at 100 °C starting from either aryl iodides or bromides, although a longer reaction time was necessary for the bromides. Activation of aryl chlorides seems to be possible with this catalyst, but the yields were lower in this case. The catalyst could handily be recycled and reuse at least three times, with only a slight decrease in the yield of the synthesized diarylacetylenes (88% in the third reuse compared to 93% in the first use).
After the evaluation of its catalytic activity in Suzuki and Heck reactions, the catalyst designed by d’Alessandro et al. [150] was also tested in Sonogashira couplings. Unfortunately, in copper-free conditions, the lignin-supported nanoparticles were found to be much less efficient than in other coupling reactions. Only 30% conversion of the starting materials was observed when using iodobenzene or 2-bromothiophene with phenylacetylene at 50 °C in aqueous medium. In the same conditions, no catalytic activity was found when using bromo or chlorobenzene as the reagent.
4.1.4. In Other C-C Coupling
Heterogeneous palladium catalysts supported on lignin have also been described for other catalytic reactions involving the creation of C-C bonds. The d’Alessandro’s catalyst was evaluated in Stille coupling reactions [150]. Disappointingly, the nanoparticles supported on lignin provided less encouraging results in this case than in Suzuki or Sonogashira reactions, with a maximum conversion of 45% even when using the more reactive iodobenzene.
The catalyst consisting of palladium nanoparticles grafted onto Fe3O4-lignin-chitosan microbeads composites, described by Baran et al. for Suzuki coupling applications, were also employed in catalytic cyanation of aryl halides [153]. Using potassium hexacyanoferrate as a cyanating agent and a 0.3 mol% metal loading, good to excellent yields of diversely substituted benzonitriles were from aryl iodides or bromides (82–97% yields). However, more modest results were observed strating from the aryl chlorides. As it was the case in Suzuki reactions, the catalyst could be reused at least seven times after magnetic separation from the medium with a good conservation of its catalytic activity.
4.2. In C-N Coupling
Catalysts supported on lignin and its derivatives can also be employed in the formation of C-N bonds. Indeed, Orooji, Nasrollahzadeh et al. used Fe3O4-lignosulfonate nanocomposites to prepare palladium (II) complexes [157]. The lignosulfonate was activated through reaction with sodium periodate, then mixed with the iron oxide nanoparticles to obtain the nanocomposites. These nanoparticles were then functionalized by reaction with (3-chloropropyl)trimethoxysilane on the free hydroxyl sites and a tetranitrogenated ligand was grafted using the chloride introduced just before. The palladium complex was obtained after adding palladium (II) chloride to the potential tetradentate ligand and separating the magnetic nanoparticles with a magnet (Figure 24a). These nanocatalysts were then evaluated in the C-N coupling of aryl halides and para-toluenesulfonamide. By using around 1.7 mol% of palladium contained in the nanocomposites, the best results were observed with toluene as a solvent and potassium carbonate as a base, with yields ranging from 75–95% when starting from iodides and 45–65% from bromides. The results were more modest when chlorides were used as reagents (Figure 24b). Due to its magnetic properties, the catalyst could easily be recovered after the reaction and reused in other catalytic reactions. It was found that the catalyst kept more of 90% of its activity after five recycling tests.
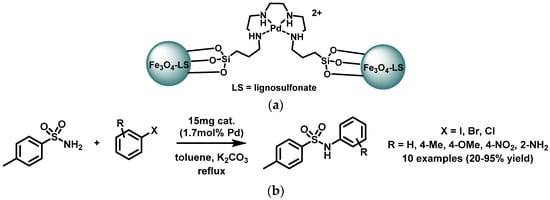
Figure 24.
(a) Possible structure for the palladium complex supported on magnetic lignosulfonate prepared by Orooji, Nasrollahzadeh et al.; (b) N-arylation of 4-methylbenzenesulfonamide catalyzed by this palladium complex [157].
4.3. Redox Reactions
4.3.1. Reduction of Nitro Compounds, Dyes or Cr(VI) for Depollution
Lignin was also used as biosourced support for catalysts in oxydo-reduction reactions either for synthesis purposes or for depolluting applications (for a recent review on catalysts supported on lignin for oxidation reaction see: [158]). The catalytic system consisting of supported metallic nanoparticles obtained by direct reduction of palladium cations by lignin, designed by d’Alessandro’s team and evaluated in various C-C couplings [150], was also applied to redox reactions. Following the same procedure, they prepared palladium and platinum nanoparticles supported on different water-soluble lignins as well as on fulvic acid. All these nanocatalysts were then evaluated in the reduction of 4-nitrophenol [159], a chemical frequently found in the water waste of drugs or textiles industries. The reaction took place at room temperature with about 0.5 mol% of metal in the presence of sodium borohydride as the reducing agent. Although all the different catalysts tested showed some catalytic activity, the platinum nanoparticles supported on ammonium lignosulfonates were the most efficient converting all the nitro starting materials to 4-aminophenol in 30 min. Unfortunately, the possibility to recycle and reuse the particles were not evaluated.
More recently, Jaleh, Nasrollahzadeh, Varma et al. described palladium nanoparticles prepared by the method of laser ablation in liquid [160]. The produced nanoparticles were stabilized by lignosulfonate (Figure 25) and either deposited on a stainless-steel mesh to make an electrode for evaluation of the hydrogen storage potential or tested in various redox reactions for depollution and environmental remediation [161]. Reduction of 4-nitrophenol was performed using the supported palladium nanoparticles and 50 equivalents of sodium borohydride at ambient temperature in less than two minutes. After filtration, washing with water and ethanol and drying, the catalyst could be reused several times with only a slight decrease in catalytic activity (only 3% decrease in yield after the eighth consecutive runs was observed). The same catalyst was also employed efficiently for the reduction of methylene blue with sodium borohydride as reducing agent and the conversion of Cr(VI) to Cr(III) in the presence of formic acid as the hydrogen source. In both cases, the depollution process was very fast, with complete conversion occurring within minutes in the optimized conditions.
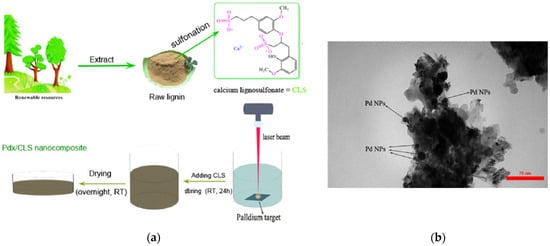
Figure 25.
(a) Preparation of the lignosulfonate-supported palladium nanoparticles by laser ablation; (b) TEM image of the prepared catalyst used for depollution [161].
Performances in depollution by reduction of Cr(VI) and pigments were also evaluated with a nanocomposite catalyst consisting of palladium nanoparticles deposited on lignin-based phenolic resin nanospheres [162]. The nano-objects were prepared by mixing lignin and phenol with a formaldehyde solution and heating at 65 °C for 1 h and 90 °C for 30 min (it corresponds to 40% replacement of phenol by lignin in the phenolic resin). By acting as a surfactant at the surface of the phenolic resin spheres, lignin reduces the surface tension and plays an important role in determining the size of the objects (Figure 26a). Following collection by centrifugation and washing, the resin nanospheres served as the stabilizing support and the reducing agent for the preparation of the palladium nanoparticles. Characterization by SEM and TEM images showed that the obtained nanospheres had a size of 200–500 nm with a rough surface due to some protuberances which are responsible for an increase in the specific surface area compared to spheres prepared without lignin. Dispersion of the palladium nanoparticles was found to be rather uniform, and their average size was around 30 nm (Figure 26b). They were very efficient in the catalysis of the reduction of Cr(VI) with formic acid, enabling the reaction with one of the best TOF (2.045 mol Cr(VI) per mol Pd cat per min) of the previous literature. The catalyst was recycled after centrifugation and used for up to ten cycles without any significant loss of activity. The catalytic ability of these nanospheres were also tested in the degradation of two hazardous organic pigments: anionic methyl orange and cationic Rhodamine B. The reactions were performed with sodium borohydride and followed by UV-visible spectrophotometry. In both cases, the decoloration confirming the degradation of the dyes occurred rapidly compared to previously reported Pd-catalysts.
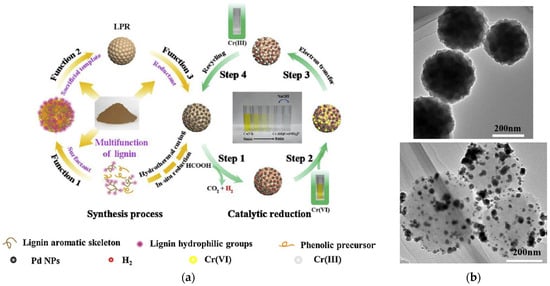
Figure 26.
(a) Preparation of the catalyst supported on lignin-based phenolic resin (LPR) and reaction mechanism for the reduction of Cr(VI); (b) TEM images of LPR nanospheres (top) and palladium nanoparticles on LPR nanospheres (bottom) prepared by Wang, Si et al. [162].
In 2020, the palladium catalyst developed by Luque and Nasrollahzadeh [153], which was efficient in Suzuki coupling, was also investigated in the Cr(VI) reduction for depollution purposes. The best result was obtained using 0.88 mol% of palladium and in this case, Cr(VI) was entirely reduced to less toxic Cr(III) species in 24 min with formic acid as the stoichiometric reducing agent.
4.3.2. Hydrogenation
Platinum and palladium supported on lignin and its derivatives can also be used as efficient hydrogenation catalysts. For example, the catalyst synthesized by Rodriguez–Mirasol, Cordero et al. [148], which consists of palladium nanoparticles deposited on mesoporous activated carbon derived from lignin, proved efficient in the hydrogenation of different alkenes under 35 psi H2 at room temperature in an eco-friendly medium (water/ethanol mixture). The reaction occurred in high yields even starting from trisubstituted or conjugated alkenes with a low catalyst loading (0.05 mol%) in 2 h. This catalyst was recycled by filtration and reused five times without any observed decrease in its catalytic activity.
In the same way, in 2015, d’Alessandro and Tonucci’s team explored the catalytic activity of their system, previously used in C-C couplings [150] or depollution [159], in hydrogenation reactions. Following their efficient procedure, they prepared palladium or platinum nanoparticles by using six different water-soluble lignins both as reducing agent for the metal salts and as stabilizing support [163]. TEM characterization of the obtained metallic particles showed that the palladium ones were spherical whereas the platinum ones prepared by the same method were mostly irregular in shape. They were both studied in the hydrogenation of various allylic alcohols at 20 °C in water solution under 1 atm H2. Interestingly, the results obtained from the two metals were quite different. Indeed, platinum nanoparticles produced much lower conversion of the starting materials and the reaction seemed to stop after a few hours. On the contrary, palladium nanoparticles were much more active. The activity was the same when, after the reaction was completed, fresh amounts of allylic alcohol were added to the reactor. TEM images of the catalysts after the reaction provided an explanation to this difference in catalytic activity: The reaction conditions seem to provoke the aggregation of the platinum nanoparticles, but for the palladium ones, the opposite was observed. Extensive disaggregation occurred and the palladium particles size were much smaller after the reaction. After the hydrogenation process, a mixture of different products was obtained: saturated alcohol (from the alkene hydrogenation), saturated aldehyde (from isomerization of the allylic alcohol), and alkenes (from deoxygenation). Except for the hydrogenation of 2-propen-1-ol with palladium which mostly provided the aldehyde, the saturated alcohol was generally the major products obtained but often with not so good selectivities (Figure 27).
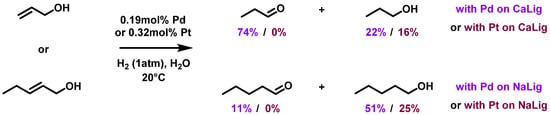
Figure 27.
Examples of results obtained with palladium or platinum nanoparticles deposited on sodium or calcium lignosulfonate in the hydrogenation of allylic alcohols by d’Alessandro and Tonucci et al. [163].
More recently, palladium nanocatalysts prepared by Kumar et al. [154] and previously described in this review for Heck and Sonogashira couplings were employed in the reduction of carbonyl compounds by hydride transfer using ammonium formate as the source of hydrogen [164]. With a 0.28 mol% catalyst loading at room temperature, various aromatic aldehydes or methyl ketones were successfully reduced to the corresponding benzylic alcohols in 4–5 h, with good to excellent yields (68–99%). The process tolerated electron-donating and electron-withdrawing substituents on the aromatic rings with no evident impact on the obtained yield. Only in the case of heteroaromatic rings (pyridine or thiophene) as substituents of the carbonyl groups were the results more modest (between 35 and 40% yield). Recycling of this supported catalyst was easy and it could be reused at least five times after the first reaction with only a slight decrease in the yield of product.
In 2020, Yuan and Xie et al. [165] prepared platinum nanoparticles by reducing the platinum salt (H2PtCl6) at 80 °C for 3 h with sodium lignosulfonate as a reducing agent. Through 2D HSQC NMR spectroscopy, it was proven that the reduction of the metallic cation was performed by the alcohol functions situated on the side chain of the lignin structure with the concomitant formation of carbonyl groups. The lignin not only served as the reducer but also as the stabilizer for the metallic nanoparticles. The catalytic activity was evaluated in the hydrogenation of alpha-pinene. The catalyst showed good activity (with an 84% conversion after 1.5 h) and selectivity (94% of cis-pinane versus trans-pinane) in relatively mild conditions: with 0.25 mol% metal loading, with 1 MPa of H2 and heating at 70 °C. After a five-run recycling test, the efficiency of the platinum nanoparticles in this reaction was almost unchanged (Figure 28).

Figure 28.
Application of platinum nanoparticles deposited sodium lignosulfonate to the hydrogenation of alpha-pinene [165].
4.3.3. Oxidation
The catalytic system, consisting of palladium nanoparticles supported on mesoporous activated carbon derived from lignin, which was designed by Rodriguez–Mirasol and Cordero et al. and previously evaluated in C-C couplings and hydrogenation [148], was also successfully used in the complete oxidation of aromatic compounds to CO2 [166] for depollution purposes. The reaction was performed in a fixed bed microreactor using a catalytic bed of supported metal nanoparticles (containing 0.5 wt% Pd plus the carbon support) diluted in silicon carbide. The oxidation process was studied in an air flow at atmospheric pressure and at high temperatures (between 150 and 425 °C). The best catalytic activity was observed on the oxidation of xylene (mixture of isomers) and the efficiency of the process decreased when applied to toluene. Benzene was the most difficult aromatic to oxidize, requiring higher temperatures.
In 2012, the d’Alessandro’s nanocatalyts, prepared from the direct reduction of palladium or platinum salts with the reductive power of lignin, were used in the oxidation of alcohols using oxygen as the oxidant [159]. Oxidation of 2-cyclohexenol to 2-cyclohexenone was performed at 80 °C in water during 24 h under aerobic conditions. The expected ketone was obtained in 85% yield in this case with a platinum catalyst (0.83 mol%) supported on ammonium lignosulfonate. The prepared palladium nanoparticles were totally inactive in this reaction. Unfortunately, non-allylic alcohols were much more difficult to oxidize with this method. For example, only 20% yield of butan-2-one (from butan-2-ol) or 22% yield of cyclohexanone (from cyclohexanol) were obtained.
Other examples of platinum catalysts supported on lignin and its derivatives include their use for electrochemical applications. Indeed, platinum nanoparticles were used to prepare anode catalysts in electro-oxidation of alcohols, for potential applications in fuel cells. In 2017, Rodriguez–Mirasol’s team developed new electrocatalysts based on metallic nanoparticles supported on carbon fibers [167]. The catalysts were obtained by electrospinning of a solution containing lignin, ethanol, phosphoric acid, and platinum acetylacetonate to form lignin fibers, which were then carbonized at 900 °C in nitrogen atmosphere to transform the lignin fibers into carbon fibers. The fibers prepared by this method had a size between 600 nm and 3 μm and a high porosity. The platinum nanoparticles, with the size around 2 nm, were very well-dispersed on the fibers. They were evaluated in the electro-oxidation of ethanol and methanol and showed remarkable performances regarding the current density, with relatively low onset potential and high specific oxidation currents. In 2019, Li et al. [168] prepared metallic nanoparticles by reduction of a platinum (II) salt by NaBH4. The particles were immobilized on carbon dots (average size 4.7 nm) synthesized by carbonization of lignin at elevated temperatures (500–800 °C). The obtained Pt nanocatalysts were then evaluated in the electro-oxidation of methanol in acidic solutions. They showed better stability and activity than commercial catalysts such as Pt/C (40% Pt) for example.
Even if nickel is less frequently employed than platinum and palladium as a catalyst in redox reactions, Jiang, Chen, and Liu et al. designed a catalyst consisting of nickel nanoparticles for the oxidation of benzylic alcohols [169]. The nickel salts was mixed with lignin in aqueous solution to obtain the metal–lignin complex. Then, this complex was homogeneously grinded with dicyandiamide and carbonized at 1000 °C during 1 h to obtain the catalyst supported on nitrogen-doped carbon. Other catalysts with different metals were also synthesized (Fe, Co, Cu) and evaluated. Unfortunately, the nickel catalyst showed a very poor activity in the oxidative esterification of primary benzylic or allylic alcohols. Only 6.4% of conversion of the starting alcohol was observed, whereas in the same conditions, the reaction was almost completed with the cobalt catalyst.
4.4. Others
In 2013, Bedia’s Team used activated carbon synthesized from chemical activation (with Lewis or Brönsted acids or bases) of lignin, followed by thermal treatment at high temperature for supporting palladium nanoparticles. The metal was deposited by impregnation method, then reduced under a dihydrogen flow. These supported catalysts were successfully evaluated in the hydrodechlorination of chloroform. The best results were obtained starting from the catalyst prepared from lignin activated with potassium hydroxide, with selectivities to the formation of ethane and propane of more than 80% in this case [170].
Interestingly, catalysts supported on carbon derived from lignin could be used for depolymerization of lignin itself. In 2019, Wang et al. prepared a nickel catalyst by impregnation of lignin with a nickel (II) nitrate solution, then carbonization of the lignin at 800 °C for 4 h under nitrogen flow [171]. To activate the catalyst by exposing the Ni-site at its surface, it was heated at 400 °C for 2 h in air. The depolymerization of lignin was conducted in 1,4-dioxane by heating the lignin suspension at 260 °C for 10 h under 10 bars of dihydrogen pressure. In these conditions, 87% lignin conversion was observed, proving a catalytic activity higher than Raney nickel (80% conversion observed in this case). Along with oligomers, 23% of converted lignin mass were identified as monomers with the main ones being 2-methoxy-4-propenylphenol and 4-allyl-2,6-dimethoxyphenol (10% and 24%, respectively). More recently, Wang and Zhou’s team used nickel catalyst supported on carbon nanofibers for the same process of lignin depolymerization [172]. The carbon nanofibers were prepared from lignin by electrospinning of a mixture of lignin, polyethylene glycol, and nickel (II) nitrate to obtain lignin-based fibers then carbonization of these precursors (Figure 29). Depolymerization of lignin with this catalyst was performed in a mixture of ethanol/water at 300 °C during 5 h under 10 bars of hydrogen. In these conditions, an excellent conversion of lignin was observed (91%) and a mixture of phenolic compounds was obtained in 7% yield (in addition to large amounts of light and heavy oligomers). The nickel catalyst could be retrieved and reused over three catalytic cycles with unchanged activity and selectivity.

Figure 29.
SEM images of the catalyst (based on nickel deposited on lignin-based carbon nanofiber) used by Wang and Zhou et al. for lignin depolymerization [172].
5. Wood as Future
From a chemical point of view, wood is mainly composed of three biopolymers: cellulose (40–50%), hemicelluloses (25–35%), and lignin (20–30%). They all contribute to the rigidity of plants. Cellulose is woven in the plant cell walls. Hemicelluloses form junctions between the cellulose chains, and lignin takes its place in the most solid tissues in between the other fibers. Raw wood has also been used as a support for metallic nanoparticles without any chemical treatment or separation of lignin, cellulose, and hemicelluloses.
Pd NPs have been embedded into carbonized cherry wood by controlled in situ reduction under microwave irradiation [173]. The resulting heterogeneous composite exhibited a catalytic activity in C-C cross-coupling reactions under air atmosphere. The Suzuki reaction of phenyl iodide with phenyl boronic acid in water with K2CO3 under reflux for 20 h yielded 84–97% biaryl product and the catalyst could be recycled 5 times with a drop in the activity after the second run. The Heck reaction of phenyl iodide with n-butyl acrylate in toluene with triethylamine at 85 °C for 20h yielded butyl cinnamate in 57%; the catalyst was recovered and reused 4 times although the yield rapidly decreased. The copper-free Sonogashira reaction of phenyl iodide with phenylacetylene in ethanol with potassium carbonate at 50 °C for 24 h yielded the product in 75%; upon recycling of the catalyst, the conversion significantly decreased to low levels at the third and fourth runs. In all cases the Pd NPs deactivated probably by clogging of the pores in the wood matrix but no metal leaching was observed.
Lin et al. experimented it with cypress timber ground to ultrafine wood flour [174]. The hydroxyl groups of the wood nanofibers proved to efficiently reduce platinum(II) ions to Pt(0) nanoparticles. Wood nanomaterial-supported Pt NPs were successfully used to reduce p-nitrophenol to p-aminophenol. The different sizes and shapes of the Pt NPs had repercussions on the catalytic activity: It decreased with increase in the particle size; the reaction was quicker with spherical Pt NPs than with spherical nanoclusters, than with cubic Pt NPs. The nanocatalyst could easily be retrieved by centrifugation and reused for at least three successive reduction reactions.
6. Summary
In order to give an overview of the catalyst performances, some results have been gathered in the following tables. The turnover number (TON) is one of the best indicator of the catalytic activity, as it reflects the number of catalytic cycles performed by each catalytic site. The turnover frequency (TOF) (which corresponds to the TON divided by the reaction time) could often not be calculated for the articles of this review, because the reaction time were almost always not optimized. Therefore, only TON was chosen to compare the catalytic systems described in this review. In the following tables, TON sometimes needed to be roughly evaluated because the quantity of metal contained in the catalytic system was not always determined. In these cases, the quantity of metal present in the support was estimated to correspond to the total quantity of metal used for the preparation of the supported-catalyst. The real metal contain of these catalysts are surely overestimated, but it is the only way the comparison can be effectively made. It further proves the importance of the full characterization of the prepared catalysts, and in particular of the determination of the metal content. Without these data, it is very difficult to precisely discuss and compare the catalytic efficiency.
The first Table shows some examples of catalysts involved in a Suzuki–Miyaura cross coupling reaction between 4-bromoanisole and phenylboronic acid. In Table 1, entries 1–24 correspond to cellulose-supported catalysts, 25–27 to hemicellulose-supported catalysts, and 28–31 to lignin-supported catalysts. In most cases, catalysts were used in green solvents, water, ethanol, or a mixture of both. Two experiments (entries 11 and 22) were run in deep-eutectic solvents and three others (entries 4, 23, and 31) in solvent-free conditions. Several of these catalysts are recyclable. Their recovery is particularly easy when they contain magnetic Fe3O4 or are immobilized on macroscale supports (dip catalyst). Only PdNPs@NC (entry 18) was a homogeneous catalyst, therefore not recyclable. The catalytic loading was inferior or equal to 1 mol% and, generally, very high yields were reached within less than 1 h (up to maximum 4 h). The two thirds of the yields were even over 90%, and no difference was noticeable between Pd(II) complexes and Pd(0) NPs. The best catalytic performances were observed with Cell-Sc-Pd(II), CL-gly-Pt, or Pd and Pd@dip catalyst with TON of 56,875, 44–47,000 and 20,250, respectively. It should be noticed that CL-gly-Pt or Pd were used under microwave irradiations, without solvent and the reaction times are very short (7 min). Interestingly, the dip catalyst made of paper (entry 19), despite a lower yield, was able to work at room temperature with a very low catalytic load and a very good recyclability.

Table 1.
Suzuki–Miyaura cross-coupling reaction between 4-methoxyphenyl bromide (BrA) and phenylboronic acid (B). Comparison of the reaction conditions and results from different articles.
The catalysts presented in Table 2 were used in Mizoroki–Heck cross coupling reaction between 4-iodoanisole and styrene or methalacrylate. They were mostly used in DMF or acetonitrile at high temperature (often under reflux) and the base was generally a tertiary amine. Hemicellulose-supported catalysts (entries 9–12) were more loaded than most of the cellulose-supported ones (entries 1–8) and needed longer reaction times (6–8 h, against 2–6 h). The best catalytic performance (TON = 7600) was found with the lignin-based material (entry 13-PdNPs@Fe3O4/L/Chi). In this case, 4-methoxystilbene is produced with 95% yield within 25 min under solvent-free conditions. When styrene was used as an alkene substrate, the yield was over 85% and over 90% with methyl acrylate. Entry 1 shows interesting green conditions (ethanol as a solvent, slightly lower reaction temperature, potassium carbonate as a base) with a very good yield.

Table 2.
Mizoroki–Heck cross-coupling reaction between 4-methoxyphenyl iodide and styrene (Sty) or methylacrylate (MA). Comparison of the reaction conditions and results from different articles.
The last table compares the catalityc activities with regard to Sonogashira reaction (phenylacetylene and iodobenzene). The catalysts collated in Table 3 were used in a variety of solvents at high temperature with excellent yields (over 90%), with one noticeable exception (entry 6: 30% for PdNPs@L). Promising green conditions are exemplified by entries 1 and 2, with reaction-time down to 1–3 h and 96–98% yields. Interestingly, in the case of entry 2, the Sonogashira cross couping was performed in copper-free conditions in a Deep Eutectic Solvent (DES).

Table 3.
Sonogashira cross-coupling reaction between iodobenzene (IB) and phenyl acetylene (PhA). Comparison of the reaction conditions and results from different articles.
From all those data, it appears clearly that the catalyst support e.g., cellulose, hemicellulose or lignin has no direct impact on the catalytic activity. The most important parameters are certainly the geometric structure of the support around the metal core and therefore its accessibility. The chemical nature of the ligand, its morphology and its porosity are at stake to design the catalyst. Some examples of direct impreganation of metal nanoparticles on the biopolymeric support without prior chemical modification exhibit good results in cross coupling reaction and question the need for more complicated ligands.
7. Conclusions
The main role of catalysts is to reduce the activation energy of chemical reactions and accelerate the reaction rate, so they are widely used in oil refining, chemical, pharmaceutical, and environmental protection industries. Technological advances in catalysts are one of the most effective drivers for the development of these industries. The introduction of a new catalytic material or a new catalytic process often triggers a revolutionary industrial change, accompanied by huge social and economic benefits. It is required to establish and develop an eco-industry with full recycling of materials and to achieve clean production to application. The realization of these goals is closely related to catalysts and catalytic technology. Therefore, we should pay great attention to the development of new catalysts and catalytic technology, and make catalyst technology a priority development in the new century. With the development of catalytic technology, noble metal nanoparticles have been widely used in biochemistry, nanomaterials, renewable energy, environmental protection, and biomedicine. Especially, the important application value of catalysts in organic chemical catalysis and electrochemical catalysis is more prominent. Therefore, the research on catalyst carriers needs to be further strengthened. The development of precious metal catalysts with high loading on their support and strong catalytic performances remains an important challenge.
Biopolymer-loaded metal catalysts have become a hot research topic in recent years due to the advantages of good catalytic activity, high stability, easy separation from the products after the reaction and recyclability. The biopolymers in wood, such as cellulose, hemicellulose, and lignin, are abundant natural polymers in nature. They are stable in most organic solvents and have a large specific surface area and many hydroxyl groups in their structure. Therefore, they are excellent carrier candidates for precious metal catalysts. Catalysts prepared from non-toxic, degradable, and renewable biopolymers as carriers have a wide range of applications in organic reactions. The conversion of homogeneous catalysts into non-homogeneous catalysts can overcome the problems of difficult separation of catalytic products from catalysts, low stability of metal complexes, difficult recycling of catalysts, and residual precious metals. Compared with inorganic carriers and synthetic organic polymer carriers, natural biopolymer supports have obvious advantages. C-C coupling reactions, catalyzed by group 10 transition metals and more particularly palladium, can efficiently synthesize a series of biphenyls, aryl olefins and other interesting compounds, which have important potential applications in fine chemicals and organic synthesis. Other reactions, in which nickel, palladium and platinum supported on wood or biopolymers extracted from it have proved useful, also include a wide variety of redox reactions both for depollution (reduction of pigments or chemical waste, total oxidation of organic compound to carbon dioxide) and synthetic purposes (oxidation of alcohols to carbonyls, hydrogenation of alkenes).
Some of the supported-catalytic systems described in this review seem to provide answers to some of very important challenges hampering the expansion of catalysis in industrial processes. Indeed, some of the catalysts showed excellent catalytic activities with low metal loadings needed to perform the reaction, combined with very good recyclability (both in terms of easiness in recycling and conservation of the activity after recovery). Moreover, other principles of green chemistry were often taken into account both for the preparation of the catalysts and their use in catalyzed reactions: use of green solvents such as water, or alternative activation methods such as microwave irradiation, for example. These group 10 transition metal catalysts supported on wood-sourced polymers will no doubt find many more and more applications in the near future. However, at this time, all the catalysts of this review were only evaluated in some model reactions in batch and on small scales. To fully explore the potential of this family of catalysts, researchers working on this field need to start to apply their catalytic systems to less reactive reagents and to the synthesis of more complex molecules with known industrial applications. The use of these supported-catalysts in larger scale reactions as well as in continuous flow should also be considered to ensure that they could be employed in industrial processes.
Author Contributions
Conceptualization, V.J. and V.T.; bibliographical research, M.N., Z.Z. and C.F.; writing—original draft preparation, M.N., Z.Z., V.J. and V.T.; writing—review and editing, A.R., E.G., V.J. and V.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by a grant from SFR Condorcet—FR CNRS 3417 (project CelluMetaCat, 10/2019/SFR/CC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
Authors acknowledge the financial support of Algeria government for Mekki Negui’s thesis fellowship (Excellence program) and of China Scholarship Council for Zhao Zhang’s thesis fellowship. We would also like to acknowledge Marie Bresson for the help in English revising of this manuscript. At last, we also acknowledge the support of Ecole Supérieure de Chimie Organique et Minérale (ESCOM) and Université de Technologie de Compiègne (UTC).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Choplin, A.; Quignard, F. From Supported Homogeneous Catalysts to Heterogeneous Molecular Catalysts. Coord. Chem. Rev. 1998, 178–180, 1679–1702. [Google Scholar] [CrossRef]
- Petterson, R.C. The Chemistry of Solid Wood—The Chemical Composition of Wood; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1984; Volume 207, ISBN 978-0-8412-0796-7. [Google Scholar]
- Kumbhar, A.; Salunkhe, R. Recent Advances in Biopolymer Supported Palladium in Organic Synthesis. Curr. Org. Chem. 2015, 19, 2075–2121. [Google Scholar] [CrossRef]
- Munakata, N.; Reinhard, M. Palladium Catalysis for the Treatment of Contaminated Waters: A Review. In Physicochemical Groundwater Remediation; Smith, J.A., Burns, S.E., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2002; pp. 45–71. ISBN 978-0-306-46569-7. [Google Scholar]
- Hooshmand, S.E.; Heidari, B.; Sedghi, R.; Varma, R.S. Recent Advances in the Suzuki–Miyaura Cross-Coupling Reaction Using Efficient Catalysts in Eco-Friendly Media. Green Chem. 2019, 21, 381–405. [Google Scholar] [CrossRef]
- Yamaguchi, J.; Muto, K.; Itami, K. Recent Progress in Nickel-Catalyzed Biaryl Coupling: Recent Progress in Nickel-Catalyzed Biaryl Coupling. Eur. J. Org. Chem. 2013, 2013, 19–30. [Google Scholar] [CrossRef]
- Kadu, B.S. Suzuki–Miyaura Cross Coupling Reaction: Recent Advancements in Catalysis and Organic Synthesis. Catal. Sci. Technol. 2021, 11, 1186–1221. [Google Scholar] [CrossRef]
- Mondal, S.; Ballav, T.; Biswas, K.; Ghosh, S.; Ganesh, V. Exploiting the Versatility of Palladium Catalysis: A Modern Toolbox for Cascade Reactions. Eur. J. Org. Chem. 2021, 2021, 4566–4602. [Google Scholar] [CrossRef]
- Mohajer, F.; Heravi, M.M.; Zadsirjan, V.; Poormohammad, N. Copper-Free Sonogashira Cross-Coupling Reactions: An Overview. RSC Adv. 2021, 11, 6885–6925. [Google Scholar] [CrossRef]
- Zhou, T.; Szostak, M. Palladium-Catalyzed Cross-Couplings by C–O Bond Activation. Catal. Sci. Technol. 2020, 10, 5702–5739. [Google Scholar] [CrossRef]
- Kanwal, I.; Mujahid, A.; Rasool, N.; Rizwan, K.; Malik, A.; Ahmad, G.; Shah, S.A.A.; Rashid, U.; Nasir, N.M. Palladium and Copper Catalyzed Sonogashira Cross Coupling an Excellent Methodology for C-C Bond Formation over 17 Years: A Review. Catalysts 2020, 10, 443. [Google Scholar] [CrossRef]
- Ntainjua, N.E.; Taylor, S.H. The Catalytic Total Oxidation of Polycyclic Aromatic Hydrocarbons. Top. Catal. 2009, 52, 528–541. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Shokouhimehr, M.; Varma, R.S. Recent Developments in Palladium (Nano)Catalysts Supported on Polymers for Selective and Sustainable Oxidation Processes. Coord. Chem. Rev. 2019, 397, 54–75. [Google Scholar] [CrossRef]
- Chen, Q.-A.; Ye, Z.-S.; Duan, Y.; Zhou, Y.-G. Homogeneous Palladium-Catalyzed Asymmetric Hydrogenation. Chem. Soc. Rev. 2013, 42, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, J. Selective Hydrogenation of Phenol for Cyclohexanone: A Review. J. Ind. Eng. Chem. 2021, 94, 78–91. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, M.; Wang, A.; Zhang, T. Selective Hydrogenation over Supported Metal Catalysts: From Nanoparticles to Single Atoms. Chem. Rev. 2020, 120, 683–733. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Ha, S.K.; Verma, K.; Tiwari, S.K. Recent Progress in Selected Bio-Nanomaterials and Their Engineering Applications: An Overview. J. Sci. Adv. Mater. Devices 2018, 3, 263–288. [Google Scholar] [CrossRef]
- He, X.; Lu, W.; Sun, C.; Khalesi, H.; Mata, A.; Andaleeb, R.; Fang, Y. Cellulose and Cellulose Derivatives: Different Colloidal States and Food-Related Applications. Carbohydr. Polym. 2021, 255, 117334. [Google Scholar] [CrossRef] [PubMed]
- Ververis, C.; Georghiou, K.; Christodoulakis, N.; Santas, P.; Santas, R. Fiber Dimensions, Lignin and Cellulose Content of Various Plant Materials and Their Suitability for Paper Production. Ind. Crops Prod. 2004, 19, 245–254. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Hui Chuin, C.T.; Sabar, S.; Fazita, M.R.N.; Taiwo, O.F.A.; Hassan, T.M.; Haafiz, M.K.M. Microcrystalline Cellulose: Isolation, Characterization and Bio-Composites Application—A Review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and Modification of Nanofibrillated Cellulose Using Various Mechanical Processes: A Review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef]
- Islam, M.T.; Alam, M.M.; Patrucco, A.; Montarsolo, A.; Zoccola, M. Preparation of Nanocellulose: A Review. AATCC J. Res. 2014, 1, 17–23. [Google Scholar] [CrossRef]
- Wang, X.; Hu, P.; Xue, F.; Wei, Y. Cellulose-Supported N-Heterocyclic Carbene-Palladium Catalyst: Synthesis and Its Applications in the Suzuki Cross-Coupling Reaction. Carbohydr. Polym. 2014, 114, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wu, X.; Chen, X.; Wei, Y. N-Methylimidazole Functionalized Carboxymethycellulose-Supported Pd Catalyst and Its Applications in Suzuki Cross-Coupling Reaction. Carbohydr. Polym. 2017, 160, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Bi, J.; Zhang, S.; Zhu, D.; Meng, D.; Ming, S.; Qin, K.; Liu, Q.; Guo, L.; Li, T. Palladium Supported on N-Heterocyclic Carbene Functionalized Hydroxyethyl Cellulose as a Novel and Efficient Catalyst for the Suzuki Reaction in Aqueous Media. Appl. Surf. Sci. 2020, 531, 147392. [Google Scholar] [CrossRef]
- Yılmaz Baran, N.; Baran, T.; Menteş, A. Fabrication and Application of Cellulose Schiff Base Supported Pd(II) Catalyst for Fast and Simple Synthesis of Biaryls via Suzuki Coupling Reaction. Appl. Catal. A Gen. 2017, 531, 36–44. [Google Scholar] [CrossRef]
- Dong, Y.; Bi, J.; Zhu, D.; Meng, D.; Ming, S.; Guo, W.; Chen, Z.; Liu, Q.; Guo, L.; Li, T. Functionalized Cellulose with Multiple Binding Sites for a Palladium Complex Catalyst: Synthesis and Catalyst Evaluation in Suzuki–Miyaura Reactions. Cellulose 2019, 26, 7355–7370. [Google Scholar] [CrossRef]
- Baran, T.; Yılmaz Baran, N.; Menteş, A. Preparation, Structural Characterization, and Catalytic Performance of Pd(II) and Pt(II) Complexes Derived from Cellulose Schiff Base. J. Mol. Struct. 2018, 1160, 154–160. [Google Scholar] [CrossRef]
- Seyednejhad, S.; Khalilzadeh, M.A.; Sadeghifar, H.; Zareyee, D. Cellulose Nanocrystals-Palladium, a Novel Recyclable Catalyst for Coupling Reaction. Eurasian Chem. Commun. 2020, 2, 349–361. [Google Scholar] [CrossRef][Green Version]
- Pharande, P.S.; Rashinkar, G.S.; Pore, D.M. Cellulose Schiff Base-Supported Pd(II): An Efficient Heterogeneous Catalyst for Suzuki Miyaura Cross-Coupling. Res. Chem. Intermed. 2021, 47, 4457–4476. [Google Scholar] [CrossRef]
- Sun, P.; Yang, J.; Chen, C.; Xie, K.; Peng, J. Synthesis of a Cellulosic Pd(Salen)-Type Catalytic Complex as a Green and Recyclable Catalyst for Cross-Coupling Reactions. Catal. Lett. 2020, 150, 2900–2910. [Google Scholar] [CrossRef]
- Sultana, T.; Mandal, B.H.; Rahman, M.L.; Sarkar, S.M. Bio-Waste Corn-Cob Cellulose Supported Poly(Amidoxime) Palladium Nanoparticles for Suzuki-Miyaura Cross-Coupling Reactions. ChemistrySelect 2016, 1, 4108–4112. [Google Scholar] [CrossRef]
- Sarkar, S.M.; Rashid, S.S.; Karim, K.M.R.; Mustapha, S.N.H.; Lian, Y.M.; Zamri, N.; Khan, M.M.R.; O’Reilly, E.J.; Rahman, M.L. Cellulose Supported Pd(II) Complex Catalyzed Carbon–Carbon Bonds Formation. J. Nanosci. Nanotechnol. 2019, 19, 2856–2861. [Google Scholar] [CrossRef] [PubMed]
- Sabaqian, S.; Nemati, F.; Nahzomi, H.T.; Heravi, M.M. Palladium Acetate Supported on Amidoxime-Functionalized Magnetic Cellulose: Synthesis, DFT Study and Application in Suzuki Reaction. Carbohydr. Polym. 2017, 177, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Lai, Y.; Wang, X.; Gao, M.; Xue, F.; Chen, X.; Ma, Y.; Wei, Y. Design and Synthesis of Amine-Functionalized Cellulose with Multiple Binding Sites and Their Application in C C Bond Forming Reactions. Int. J. Biol. Macromol. 2019, 130, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Dong, Y.; Wu, X.; Wei, Y. 2-Aminopyridine Functionalized Cellulose Based Pd Nanoparticles: An Efficient and Ecofriendly Catalyst for the Suzuki Cross-Coupling Reaction. Front. Chem. Sci. Eng. 2016, 10, 389–395. [Google Scholar] [CrossRef]
- Jebali, Z.; Granados, A.; Nabili, A.; Boufi, S.; do Rego, A.M.B.; Majdoub, H.; Vallribera, A. Cationic Cellulose Nanofibrils as a Green Support of Palladium Nanoparticles: Catalyst Evaluation in Suzuki Reactions. Cellulose 2018, 25, 6963–6975. [Google Scholar] [CrossRef]
- Sun, Y.; Mohammadnia, M. Synthesis and Characterization of Pd Based on [2,2′-Bipyridin]-4-Amine Functionalized Nano Cellulose as a Novel and Recyclable Nano Catalyst for Suzuki Reaction. Inorg. Chem. Commun. 2020, 118, 107993. [Google Scholar] [CrossRef]
- Salamatmanesh, A.; Heydari, A.; Nahzomi, H.T. Stabilizing Pd on Magnetic Phosphine-Functionalized Cellulose: DFT Study and Catalytic Performance under Deep Eutectic Solvent Assisted Conditions. Carbohydr. Polym. 2020, 235, 115947. [Google Scholar] [CrossRef]
- Du, Q.; Li, Y. Air-Stable, Recyclable, and Time-Efficient Diphenylphosphinite Cellulose-Supported Palladium Nanoparticles as a Catalyst for Suzuki–Miyaura Reactions. Beilstein J. Org. Chem. 2011, 7, 378–385. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Wang, F.; Wei, Y. Functionalized Cellulose-Supported Triphenylphosphine and Its Application in Suzuki Cross-Coupling Reactions. J. Appl. Polym. Sci. 2015, 132, 41427. [Google Scholar] [CrossRef]
- Lu, Z.; Jasinski, J.B.; Handa, S.; Hammond, G.B. Recyclable Cellulose-Palladium Nanoparticles for Clean Cross-Coupling Chemistry. Org. Biomol. Chem. 2018, 16, 2748–2752. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Lu, Z.; Li, Y. Carboxymethylcellulose-Supported Palladium Nanoparticles Generated in Situ from Palladium(II) Carboxymethylcellulose: An Efficient and Reusable Catalyst for Suzuki–Miyaura and Mizoroki–Heck Reactions. Ind. Eng. Chem. Res. 2015, 54, 790–797. [Google Scholar] [CrossRef]
- Martins, G.; dos Santos, M.; Rodrigues, M.; Sucupira, R.; Meneghetti, L.; Monteiro, A.; Suarez, P. Cellulose Oxidation and the Use of Carboxyl Cellulose Metal Complexes in Heterogeneous Catalytic Systems to Promote Suzuki-Miyaura Coupling and C−O Bond Formation Reaction. J. Braz. Chem. Soc. 2017, 34, 21–22. [Google Scholar] [CrossRef]
- Faria, V.W.; Oliveira, D.G.M.; Kurz, M.H.S.; Gonçalves, F.F.; Scheeren, C.W.; Rosa, G.R. Palladium Nanoparticles Supported in a Polymeric Membrane: An Efficient Phosphine-Free “Green” Catalyst for Suzuki–Miyaura Reactions in Water. RSC Adv. 2014, 4, 13446–13452. [Google Scholar] [CrossRef]
- Jokar, M.; Naeimi, H.; Nabi Bidhendi, G. Preparation and Characterization of Cellulose Sulfate/Pd Nanocatalsysts with Remarkable Efficiency for Suzuki–Miyaura Reaction. Appl. Organomet. Chem. 2021, 35, e6266. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Xu, B.; Mao, Z.; Xu, H.; Zhong, Y.; Zhang, L.; Wang, B.; Sui, X. Cellulose Sponge Supported Palladium Nanoparticles as Recyclable Cross-Coupling Catalysts. ACS Appl. Mater. Interfaces 2017, 9, 17155–17162. [Google Scholar] [CrossRef]
- Li, D.; Jiang, J.; Cai, C. Palladium Nanoparticles Anchored on Amphiphilic Janus-Type Cellulose Nanocrystals for Pickering Interfacial Catalysis. Chem. Commun. 2020, 56, 9396–9399. [Google Scholar] [CrossRef]
- Zhang, K.; Shen, M.; Liu, H.; Shang, S.; Wang, D.; Liimatainen, H. Facile Synthesis of Palladium and Gold Nanoparticles by Using Dialdehyde Nanocellulose as Template and Reducing Agent. Carbohydr. Polym. 2018, 186, 132–139. [Google Scholar] [CrossRef]
- Aabaka, S.R.; Mao, J.; Lavanya, M.; Venkateswarlu, K.; Huang, Z.; Mao, J.; Yang, X.; Lin, C. Nanocellulose Supported PdNPs as in Situ Formed Nano Catalyst for the Suzuki Coupling Reaction in Aqueous Media: A Green Approach and Waste to Wealth. J. Organomet. Chem. 2021, 937, 121719. [Google Scholar] [CrossRef]
- Chen, F.; Huang, M.; Li, Y. Synthesis of a Novel Cellulose Microencapsulated Palladium Nanoparticle and Its Catalytic Activities in Suzuki–Miyaura and Mizoroki–Heck Reactions. Ind. Eng. Chem. Res. 2014, 53, 8339–8345. [Google Scholar] [CrossRef]
- Jamwal, N.; Sodhi, R.K.; Gupta, P.; Paul, S. Nano Pd(0) Supported on Cellulose: A Highly Efficient and Recyclable Heterogeneous Catalyst for the Suzuki Coupling and Aerobic Oxidation of Benzyl Alcohols under Liquid Phase Catalysis. Int. J. Biol. Macromol. 2011, 49, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Easson, M.W.; Jordan, J.H.; Bland, J.M.; Hinchliffe, D.J.; Condon, B.D. Application of Brown Cotton-Supported Palladium Nanoparticles in Suzuki–Miyaura Cross-Coupling Reactions. ACS Appl. Nano Mater. 2020, 3, 6304–6309. [Google Scholar] [CrossRef]
- Dewan, A.; Sarmah, M.; Bharali, P.; Thakur, A.J.; Boruah, P.K.; Das, M.R.; Bora, U. Pd Nanoparticles-Loaded Honeycomb-Structured Bio-Nanocellulose as a Heterogeneous Catalyst for Heteroaryl Cross-Coupling Reaction. ACS Sustain. Chem. Eng. 2021, 9, 954–966. [Google Scholar] [CrossRef]
- Zheng, G.; Kaefer, K.; Mourdikoudis, S.; Polavarapu, L.; Vaz, B.; Cartmell, S.E.; Bouleghlimat, A.; Buurma, N.J.; Yate, L.; de Lera, Á.R.; et al. Palladium Nanoparticle-Loaded Cellulose Paper: A Highly Efficient, Robust, and Recyclable Self-Assembled Composite Catalytic System. J. Phys. Chem. Lett. 2015, 6, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Baruah, D.; Das, R.N.; Hazarika, S.; Konwar, D. Biogenic Synthesis of Cellulose Supported Pd(0) Nanoparticles Using Hearth Wood Extract of Artocarpus Lakoocha Roxb—A Green, Efficient and Versatile Catalyst for Suzuki and Heck Coupling in Water under Microwave Heating. Catal. Commun. 2015, 72, 73–80. [Google Scholar] [CrossRef]
- Kempasiddaiah, M.; Kandathil, V.; Dateer, R.B.; Sasidhar, B.S.; Patil, S.A. Immobilizing Biogenically Synthesized Palladium Nanoparticles on Cellulose Support as a Green and Sustainable Dip Catalyst for Cross-Coupling Reaction. Cellulose 2020, 27, 3335–3357. [Google Scholar] [CrossRef]
- Kandathil, V.; Kempasiddaiah, M.; Sasidhar, B.S.; Patil, S.A. From Agriculture Residue to Catalyst Support; A Green and Sustainable Cellulose-Based Dip Catalyst for C C Coupling and Direct Arylation. Carbohydr. Polym. 2019, 223, 115060. [Google Scholar] [CrossRef]
- Kandathil, V.; Veetil, A.K.; Patra, A.; Moolakkil, A.; Kempasiddaiah, M.; Somappa, S.B.; Rout, C.S.; Patil, S.A. A Green and Sustainable Cellulosic-Carbon-Shielded Pd–MNP Hybrid Material for Catalysis and Energy Storage Applications. J. Nanostruct. Chem. 2021, 11, 395–407. [Google Scholar] [CrossRef]
- Soltani, S.S.; Taheri-Ledari, R.; Farnia, S.M.F.; Maleki, A.; Foroumadi, A. Synthesis and Characterization of a Supported Pd Complex on Volcanic Pumice Laminates Textured by Cellulose for Facilitating Suzuki–Miyaura Cross-Coupling Reactions. RSC Adv. 2020, 10, 23359–23371. [Google Scholar] [CrossRef]
- Niakan, M.; Masteri-Farahani, M.; Karimi, S.; Shekaari, H. Hydrophilic Role of Deep Eutectic Solvents for Clean Synthesis of Biphenyls over a Magnetically Separable Pd-Catalyzed Suzuki-Miyaura Coupling Reaction. J. Mol. Liq. 2021, 324, 115078. [Google Scholar] [CrossRef]
- Kale, D.; Rashinkar, G.; Kumbhar, A.; Salunkhe, R. Facile Suzuki-Miyaura Cross Coupling Using Ferrocene Tethered N-Heterocyclic Carbene-Pd Complex Anchored on Cellulose. React. Funct. Polym. 2017, 116, 9–16. [Google Scholar] [CrossRef]
- Mhaldar, P.; Vibhute, S.; Rashinkar, G.; Pore, D. Highly Effective Cellulose Supported 2-Aminopyridine Palladium Complex (Pd(II)-AMP-Cell@Al2O3) for Suzuki-Miyaura and Mizoroki–Heck Cross-Coupling. React. Funct. Polym. 2020, 152, 104586. [Google Scholar] [CrossRef]
- Kumbhar, A.; Jadhav, S.; Kamble, S.; Rashinkar, G.; Salunkhe, R. Palladium Supported Hybrid Cellulose–Aluminum Oxide Composite for Suzuki–Miyaura Cross Coupling Reaction. Tetrahedron Lett. 2013, 54, 1331–1337. [Google Scholar] [CrossRef]
- Karami, K.; Saadatzadeh, H.; Ramezanpour, A. Synthesis and Characterization of Palladium Nanoparticles Immobilized on Modified Cellulose Nanocrystals as Heterogeneous Catalyst for Reduction of Nitroaromatic Compounds. ChemistrySelect 2021, 6, 2746–2759. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Cai, C. Pd Nanoparticles Supported on Cellulose as a Catalyst for Vanillin Conversion in Aqueous Media. J. Org. Chem. 2018, 83, 7534–7538. [Google Scholar] [CrossRef]
- Lin, B.; Liu, X.; Zhang, Z.; Chen, Y.; Liao, X.; Li, Y. Pd(0)–CMC@Ce(OH) 4 Organic/Inorganic Hybrid as Highly Active Catalyst for the Suzuki–Miyaura Reaction. J. Colloid Interface Sci. 2017, 497, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.; Jagdale, A.; Kamble, S.; Kumbhar, A.; Salunkhe, R. Palladium Nanoparticles Supported on a Titanium Dioxide Cellulose Composite (PdNPs@TiO2–Cell) for Ligand-Free Carbon–Carbon Cross Coupling Reactions. RSC Adv. 2016, 6, 3406–3420. [Google Scholar] [CrossRef]
- Nishikata, T.; Tsutsumi, H.; Gao, L.; Kojima, K.; Chikama, K.; Nagashima, H. Adhesive Catalyst Immobilization of Palladium Nanoparticles on Cotton and Filter Paper: Applications to Reusable Catalysts for Sequential Catalytic Reactions. Adv. Synth. Catal. 2014, 356, 951–960. [Google Scholar] [CrossRef]
- Baran, T.; Sargin, I.; Kaya, M.; Menteş, A. Green Heterogeneous Pd(II) Catalyst Produced from Chitosan-Cellulose Micro Beads for Green Synthesis of Biaryls. Carbohydr. Polym. 2016, 152, 181–188. [Google Scholar] [CrossRef]
- Yılmaz Baran, N.; Baran, T.; Menteş, A. Production of Novel Palladium Nanocatalyst Stabilized with Sustainable Chitosan/Cellulose Composite and Its Catalytic Performance in Suzuki-Miyaura Coupling Reactions. Carbohydr. Polym. 2018, 181, 596–604. [Google Scholar] [CrossRef]
- Wang, B.; Ran, M.; Fang, G.; Wu, T.; Tian, Q.; Zheng, L.; Romero-Zerón, L.; Ni, Y. Palladium Nano-Catalyst Supported on Cationic Nanocellulose–Alginate Hydrogel for Effective Catalytic Reactions. Cellulose 2020, 27, 6995–7008. [Google Scholar] [CrossRef]
- Wang, B.; Dai, L.; Yang, G.; Bendrich, G.; Ni, Y.; Fang, G. A Highly Efficient Thermo Responsive Palladium Nanoparticles Incorporated Guar Gum Hydrogel for Effective Catalytic Reactions. Carbohydr. Polym. 2019, 226, 115289. [Google Scholar] [CrossRef] [PubMed]
- Baran, T. Ultrasound-Accelerated Synthesis of Biphenyl Compounds Using Novel Pd(0) Nanoparticles Immobilized on Bio-Composite. Ultrason. Sonochem. 2018, 45, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.M.; Rahman, M.L.; Chong, K.F.; Yusoff, M.M. Poly(Hydroxamic Acid) Palladium Catalyst for Heck Reactions and Its Application in the Synthesis of Ozagrel. J. Catal. 2017, 350, 103–110. [Google Scholar] [CrossRef]
- Islam, M.S.; Rahman, M.L.; Yusoff, M.M.; Sarkar, S.M. Highly Active Bio-Waste Cellulose Supported Poly(Amidoxime) Palladium(II) Complex for Heck Reactions. J. Clean. Prod. 2017, 149, 1045–1050. [Google Scholar] [CrossRef]
- Mohammadnia, M.; Poormirzaei, N. Preparation and Characterization of Pd Supported on 5-Carboxyoxindole Functionalized Cell@Fe 3 O 4 Nanoparticles as a Novel Magnetic Catalyst for the Heck Reaction. Nanoscale Adv. 2021, 3, 1917–1926. [Google Scholar] [CrossRef]
- Du, Q.; Li, Y. Application of an Air-and-Moisture-Stable Diphenylphosphinite Cellulose-Supported Nanopalladium Catalyst for a Heck Reaction. Res. Chem. Intermed. 2012, 38, 1807–1817. [Google Scholar] [CrossRef]
- Keshipour, S.; Shojaei, S.; Shaabani, A. Palladium Nano-Particles Supported on Ethylenediamine-Functionalized Cellulose as a Novel and Efficient Catalyst for the Heck and Sonogashira Couplings in Water. Cellulose 2013, 20, 973–980. [Google Scholar] [CrossRef]
- Xu, Y.; Xue, M.; Li, J.; Zhang, L.; Cui, Y. Synthesis of a Cellulose Xanthate Supported Palladium(0) Complex and Its Catalytic Behavior in the Heck Reaction. React. Kinet. Mech. Catal. 2010, 497, 134–143. [Google Scholar] [CrossRef]
- Rezayat, M.; Blundell, R.K.; Camp, J.E.; Walsh, D.A.; Thielemans, W. Green One-Step Synthesis of Catalytically Active Palladium Nanoparticles Supported on Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2014, 2, 1241–1250. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Cui, Y. Catalytic Performance of Cellulose Supported Palladium Complex for Heck Reaction in Water. J. Appl. Polym. Sci. 2008, 110, 2996–3000. [Google Scholar] [CrossRef]
- Cirtiu, C.M.; Dunlop-Brière, A.F.; Moores, A. Cellulose Nanocrystallites as an Efficient Support for Nanoparticles of Palladium: Application for Catalytichydrogenation and Heck Coupling under Mild Conditions. Green Chem. 2011, 13, 288–291. [Google Scholar] [CrossRef]
- Niakan, M.; Masteri-Farahani, M.; Shekaari, H.; Karimi, S. Pd Supported on Clicked Cellulose-Modified Magnetite-Graphene Oxide Nanocomposite for C-C Coupling Reactions in Deep Eutectic Solvent. Carbohydr. Polym. 2021, 251, 117109. [Google Scholar] [CrossRef] [PubMed]
- Ashiri, S.; Mehdipour, E. Preparation of a Novel Palladium Catalytic Hydrogel Based on Graphene Oxide/Chitosan NPs and Cellulose Nanowhiskers. RSC Adv. 2018, 8, 32877–32885. [Google Scholar] [CrossRef]
- Rajender Reddy, K.; Kumar, N.S.; Surendra Reddy, P.; Sreedhar, B.; Lakshmi Kantam, M. Cellulose Supported Palladium(0) Catalyst for Heck and Sonogashira Coupling Reactions. J. Mol. Catal. A Chem. 2006, 252, 12–16. [Google Scholar] [CrossRef]
- Rasouli, M.A.; Ranjbar, P.R. Reductive Ullmann Coupling of Aryl Halides by Palladium Nanoparticles Supported on Cellulose, a Recoverable Heterogeneous Catalyst. Z. Für Nat. B 2013, 68, 946–950. [Google Scholar] [CrossRef]
- Kempasiddaiah, M.; Kandathil, V.; Dateer, R.B.; Sasidhar, B.S.; Patil, S.A.; Patil, S.A. Palladium-Catalyzed Denitrogenative Cross-Coupling of Aryl Halides with Arylhydrazines under Mild Reaction Conditions. Transit. Met. Chem. 2021, 46, 273–281. [Google Scholar] [CrossRef]
- Jokar, M.; Naeimi, H.; Nabi Bidhendi, G. Design and Preparation of Platinum Anchored on Cellulose as Heterogeneous Nanocatalyst for Synthesis of Bis-Coumarin Derivatives. Polycycl. Aromat. Compd. 2021, 68, 1–12. [Google Scholar] [CrossRef]
- Buisson, P.; Quignard, F. Polysaccharides: Natural Polymeric Supports for Aqueous Phase Catalysts in the Allylic Substitution Reaction. Aust. J. Chem. 2002, 55, 73. [Google Scholar] [CrossRef]
- Quignard, F.; Choplin, A. Cellulose: A New Bio-Support for Aqueous Phase Catalysts. Chem. Commun. 2001, 34, 21–22. [Google Scholar] [CrossRef]
- Seyednejhad, S.; Khalilzadeh, M.A.; Zareyee, D.; Sadeghifar, H.; Venditti, R. Cellulose Nanocrystal Supported Palladium as a Novel Recyclable Catalyst for Ullmann Coupling Reactions. Cellulose 2019, 26, 5015–5031. [Google Scholar] [CrossRef]
- Petkova, D.; Borlinghaus, N.; Sharma, S.; Kaschel, J.; Lindner, T.; Klee, J.; Jolit, A.; Haller, V.; Heitz, S.; Britze, K.; et al. Hydrophobic Pockets of HPMC Enable Extremely Short Reaction Times in Water. ACS Sustain. Chem. Eng. 2020, 8, 12612–12617. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Jin, J.; Tong, J.; Shen, C. Recyclable Cellulose-Derived Fe3O4@Pd NPs for Highly Selective C–S Formation by Heterogeneously C–H Sulfenylation of Indoles. Catal. Lett. 2020, 150, 2409–2414. [Google Scholar] [CrossRef]
- Keshipour, S.; Adak, K. Pd(0) Supported on N-Doped Graphene Quantum Dot Modified Cellulose as an Efficient Catalyst for the Green Reduction of Nitroaromatics. RSC Adv. 2016, 6, 89407–89412. [Google Scholar] [CrossRef]
- Li, D.; Lu, G.; Cai, C. Modified Cellulose with Tunable Surface Hydrophilicity/Hydrophobicity as a Novel Catalyst Support for Selective Reduction of Nitrobenzene. Catal. Commun. 2020, 137, 105949. [Google Scholar] [CrossRef]
- Wu, X.; Lu, C.; Zhang, W.; Yuan, G.; Xiong, R.; Zhang, X. A Novel Reagentless Approach for Synthesizing Cellulose Nanocrystal-Supported Palladium Nanoparticles with Enhanced Catalytic Performance. J. Mater. Chem. A 2013, 1, 8645. [Google Scholar] [CrossRef]
- Wu, X.; Shi, Z.; Fu, S.; Chen, J.; Berry, R.M.; Tam, K.C. Strategy for Synthesizing Porous Cellulose Nanocrystal Supported Metal Nanocatalysts. ACS Sustain. Chem. Eng. 2016, 4, 5929–5935. [Google Scholar] [CrossRef]
- Li, X.; Dong, F.; Zhang, L.; Xu, Q.; Zhu, X.; Liang, S.; Hu, L.; Xie, H. Cellulosic Protic Ionic Liquids Hydrogel: A Green and Efficient Catalyst Carrier for Pd Nanoparticles in Reduction of 4-Nitrophenol in Water. Chem. Eng. J. 2019, 372, 516–525. [Google Scholar] [CrossRef]
- Yu, H.; Oh, S.; Han, Y.; Lee, S.; Jeong, H.S.; Hong, H.-J. Modified Cellulose Nanofibril Aerogel: Tunable Catalyst Support for Treatment of 4-Nitrophenol from Wastewater. Chemosphere 2021, 285, 131448. [Google Scholar] [CrossRef]
- Zeynizadeh, B.; Karami, S. Synthesis of Ni Nanoparticles Anchored on Cellulose Using Different Reducing Agents and Their Applications towards Reduction of 4-Nitrophenol. Polyhedron 2019, 166, 196–202. [Google Scholar] [CrossRef]
- Kamal, T.; Khan, S.B.; Asiri, A.M. Nickel Nanoparticles-Chitosan Composite Coated Cellulose Filter Paper: An Efficient and Easily Recoverable Dip-Catalyst for Pollutants Degradation. Environ. Pollut. 2016, 218, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Sultana, K.A.; Noveron, J.C. Borohydride-Free Catalytic Reduction of Organic Pollutants by Platinum Nanoparticles Supported on Cellulose Fibers. J. Mol. Liq. 2019, 296, 111988. [Google Scholar] [CrossRef]
- Nasir Baig, R.B.; Varma, R.S. Magnetic Carbon-Supported Palladium Nanoparticles: An Efficient and Sustainable Catalyst for Hydrogenation Reactions. ACS Sustain. Chem. Eng. 2014, 2, 2155–2158. [Google Scholar] [CrossRef]
- Xie, Z.-T.; Asoh, T.-A.; Uyama, H. Monolithic Cellulose Supported Metal Nanoparticles as Green Flow Reactor with High Catalytic Efficiency. Carbohydr. Polym. 2019, 214, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-Y.; Zhang, X.-Q.; Yao, X.-H.; Wan, Y.; Song, P.; Liu, Z.-Y.; Fu, Y.-J. Microwave-Assisted Synthesis of PdNPs by Cellulose Solution to Prepare 3D Porous Microspheres Applied on Dyes Discoloration. Carbohydr. Polym. 2020, 247, 116569. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Hu, C.; Zhang, W.; Dichiara, A.B. Reagentless Preparation of Shape Memory Cellulose Nanofibril Aerogels Decorated with Pd Nanoparticles and Their Application in Dye Discoloration. Appl. Catal. B Environ. 2018, 237, 482–490. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Desai, I.; Cruz, C.; Yang, D.J. Novel Pd Based Catalyst for the Removal of Organic and Emerging Contaminants. RSC Adv. 2012, 2, 7540. [Google Scholar] [CrossRef]
- Islam, M.T.; Rosales, J.A.; Saenz-Arana, R.; Ghadimi, S.J.; Noveron, J.C. Rapid Synthesis of Ultrasmall Platinum Nanoparticles Supported on Macroporous Cellulose Fibers for Catalysis. Nanoscale Adv. 2019, 1, 2953–2964. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Wang, Z.; Liu, H. Green Synthesis of Palladium Nanoparticles with Carboxymethyl Cellulose for Degradation of Azo-Dyes. Mater. Chem. Phys. 2017, 187, 133–140. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Jiang, J.; Cai, C. Amphiphilic Cellulose Supported PdNi Alloy Nanoparticles towards Biofuel Upgrade under Mild Conditions. Catal. Commun. 2019, 122, 43–46. [Google Scholar] [CrossRef]
- Meng, J.; Liu, Y.; Shi, X.; Chen, W.; Zhang, X.; Yu, H. Recyclable Nanocellulose-Confined Palladium Nanoparticles with Enhanced Room-Temperature Catalytic Activity and Chemoselectivity. Sci. China Mater. 2021, 64, 621–630. [Google Scholar] [CrossRef]
- Shaikh, M.N. Pd Nanoparticles on Green Support as Dip-Catalyst: A Facile Transfer Hydrogenation of Olefins and N -Heteroarenes in Water. RSC Adv. 2019, 9, 28199–28206. [Google Scholar] [CrossRef]
- Phillips, J.M.; Ahamed, M.; Duan, X.; Lamb, R.N.; Qu, X.; Zheng, K.; Zou, J.; Chalker, J.M.; Raston, C.L. Chemoselective and Continuous Flow Hydrogenations in Thin Films Using a Palladium Nanoparticle Catalyst Embedded in Cellulose Paper. ACS Appl. Bio Mater. 2019, 2, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.R.; Rodrigues, M.V.R.; Santos, A.B.S.; Valerio, M.G.; Martins, G.B.C.; Sucupira, R.R.; Meneghetti, L.; Suarez, P.A.Z. Metal-Cellulose Catalytic Systems for Biodiesel Preparation and Reductive Stabilization. J. Mol. Catal. A Chem. 2016, 422, 131–141. [Google Scholar] [CrossRef]
- Kaushik, M.; Basu, K.; Benoit, C.; Cirtiu, C.M.; Vali, H.; Moores, A. Cellulose Nanocrystals as Chiral Inducers: Enantioselective Catalysis and Transmission Electron Microscopy 3D Characterization. J. Am. Chem. Soc. 2015, 137, 6124–6127. [Google Scholar] [CrossRef]
- Yamada, T.; Teranishi, W.; Park, K.; Jiang, J.; Tachikawa, T.; Furusato, S.; Sajiki, H. Development of Carbon-Neutral Cellulose-Supported Heterogeneous Palladium Catalysts for Chemoselective Hydrogenation. ChemCatChem 2020, 12, 4052–4058. [Google Scholar] [CrossRef]
- Huang, K.; Hu, J.; Huang, M.-Y.; Jiang, Y.-Y. Asymmetric Hydrogenation Ofo-Cresol Andm-Cresol Catalyzed by Silica-Supported Methylcellulose-L-Alanine-Pd Complex. Polym. Adv. Technol. 2001, 12, 711–715. [Google Scholar] [CrossRef]
- Liu, J.; He, F.; Durham, E.; Zhao, D.; Roberts, C.B. Polysugar-Stabilized Pd Nanoparticles Exhibiting High Catalytic Activities for Hydrodechlorination of Environmentally Deleterious Trichloroethylene. Langmuir 2008, 24, 328–336. [Google Scholar] [CrossRef]
- Zhang, M.; Bacik, D.B.; Roberts, C.B.; Zhao, D. Catalytic Hydrodechlorination of Trichloroethylene in Water with Supported CMC-Stabilized Palladium Nanoparticles. Water Res. 2013, 47, 3706–3715. [Google Scholar] [CrossRef]
- Bacik, D.B.; Zhang, M.; Zhao, D.; Roberts, C.B.; Seehra, M.S.; Singh, V.; Shah, N. Synthesis and Characterization of Supported Polysugar-Stabilized Palladium Nanoparticle Catalysts for Enhanced Hydrodechlorination of Trichloroethylene. Nanotechnology 2012, 23, 294004. [Google Scholar] [CrossRef]
- Sikora, E.; Katona, K.K.; Muránszky, G.; Bánhidi, O.; Kristály, F.; Szabó, J.T.; Windisch, M.; Fiser, B.; Vanyorek, L. Cellulose-Based Catalyst Design for Efficient Chlorate Reduction. Arab. J. Chem. 2021, 14, 103202. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, H.; Chi, Q.; Liu, X.; Chen, L. Cellulose-Supported Pd Nanoparticles: Effective for the Selective Oxidation of Glucose into Gluconic Acid. Polym. Bull. 2020, 77, 1003–1014. [Google Scholar] [CrossRef]
- Bahluli, R.; Keshipour, S. Microcrystalline Cellulose Modified with Fe(II)– and Ni(II)–Phthalocyanines: Syntheses, Characterizations, and Catalytic Applications. Polyhedron 2019, 169, 176–182. [Google Scholar] [CrossRef]
- Kebede, M.A.; Imae, T.; Sabrina; Wu, C.-M.; Cheng, K.-B. Cellulose Fibers Functionalized by Metal Nanoparticles Stabilized in Dendrimer for Formaldehyde Decomposition and Antimicrobial Activity. Chem. Eng. J. 2017, 311, 340–347. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Zhao, X.; Wei, T.; Wang, H.; Li, X.; Gu, X.; Yan, N.; Li, L.; Xiao, H. Excellent Low-Temperature Formaldehyde Decomposition Performance over Pt Nanoparticles Directly Loaded on Cellulose Triacetate. Ind. Eng. Chem. Res. 2020, 59, 21720–21728. [Google Scholar] [CrossRef]
- Keshipour, S.; Khaltech, N.K. Pd and Fe3O4 Nanoparticles Supported on N-(2-Aminoethyl)Acetamide Functionalized Cellulose as an Efficient Catalyst for Epoxidation of Styrene. Int. J. Nanosci. Nanotechnol. 2017, 13, 219–226. [Google Scholar]
- Keshipour, S.; Kalam Khalteh, N. Oxidation of Ethylbenzene to Styrene Oxide in the Presence of Cellulose-Supported Pd Magnetic Nanoparticles: Oxidation of Ethylbenzene to Styrene Oxide. Appl. Organomet. Chem. 2016, 30, 653–656. [Google Scholar] [CrossRef]
- Mirosanloo, A.; Zareyee, D.; Khalilzadeh, M.A. Recyclable Cellulose Nanocrystal Supported Palladium Nanoparticles as an Efficient Heterogeneous Catalyst for the Solvent-Free Synthesis of Coumarin Derivatives via von Pechmann Condensation: Heterogeneous Nanocatalyst. Appl. Organomet. Chem. 2018, 32, e4546. [Google Scholar] [CrossRef]
- Ahmar, H.; Keshipour, S.; Hosseini, H.; Fakhari, A.R.; Shaabani, A.; Bagheri, A. Electrocatalytic Oxidation of Hydrazine at Glassy Carbon Electrode Modified with Ethylenediamine Cellulose Immobilized Palladium Nanoparticles. J. Electroanal. Chem. 2013, 690, 96–103. [Google Scholar] [CrossRef]
- Moradpour, A.; Ghaffarinejad, A.; Maleki, A.; Eskandarpour, V.; Motaharian, A. Low Loaded Palladium Nanoparticles on Ethylenediamine-Functionalized Cellulose as an Efficient Catalyst for Electrochemical Hydrogen Production. RSC Adv. 2015, 5, 70668–70674. [Google Scholar] [CrossRef]
- Vijayaramalingam, K.; Karthikeyan, A.; Selvarani, V.; Kiruthika, S.; Muthukumaran, B. Enhanced Electrocatalytic Activity of Pd–Ir–Ni, Pd–Ir–Mo and Pd–Ir–Rh Nanoparticles Supported on Cellulose-Based Carbon (CC) for Membraneless Sodium Perborate Fuel Cells (MLSPBFCs). J. App. Pharm. Sci. 2018, 34, 129–137. [Google Scholar] [CrossRef][Green Version]
- Nechita, P.; Mirela, R.; Ciolacu, F. Xylan Hemicellulose: A Renewable Material with Potential Properties for Food Packaging Applications. Sustainability 2021, 13, 13504. [Google Scholar] [CrossRef]
- Huang, L.-Z.; Ma, M.-G.; Ji, X.-X.; Choi, S.-E.; Si, C. Recent Developments and Applications of Hemicellulose From Wheat Straw: A Review. Front. Bioeng. Biotechnol. 2021, 9, 690773. [Google Scholar] [CrossRef]
- Farhat, W.; Venditti, R.; Ayoub, A.; Prochazka, F.; Fernández-de-Alba, C.; Mignard, N.; Taha, M.; Becquart, F. Towards Thermoplastic Hemicellulose: Chemistry and Characteristics of Poly-(ε-Caprolactone) Grafting onto Hemicellulose Backbones. Mater. Des. 2018, 153, 298–307. [Google Scholar] [CrossRef]
- Pereira, C.S.; Silveira, R.L.; Dupree, P.; Skaf, M.S. Effects of Xylan Side-Chain Substitutions on Xylan–Cellulose Interactions and Implications for Thermal Pretreatment of Cellulosic Biomass. Biomacromolecules 2017, 18, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhong, L.; Peng, X.; Wang, K.; Chen, Z.; Sun, R. Xylan-Type Hemicellulose Supported Palladium Nanoparticles: A Highly Efficient and Reusable Catalyst for the Carbon–Carbon Coupling Reactions. Catal. Sci. Technol. 2014, 4, 1426–1435. [Google Scholar] [CrossRef]
- Du, F.; Zhang, L.; Ma, J.; Peng, X. Hemicelluloses Supported Palladium/Xylan Nanocomposites Containing N and O Ligands: Highly-Performance Heterogeneous Catalysts for Suzuki Reaction. Carbohydr. Polym. 2019, 217, 224–231. [Google Scholar] [CrossRef]
- Chen, W.; Zhong, L.; Peng, X.; Lin, J.; Sun, R. Xylan-Type Hemicelluloses Supported Terpyridine–Palladium(II) Complex as an Efficient and Recyclable Catalyst for Suzuki–Miyaura Reaction. Cellulose 2014, 21, 125–137. [Google Scholar] [CrossRef]
- He, M.; Song, T.; Qi, H.; Xiang, Z. An Environment-Friendly Dip-Catalyst with Xylan-Based Catalytic Paper Coatings. Carbohydr. Polym. 2022, 275, 118707. [Google Scholar] [CrossRef]
- Wu, C.; Peng, X.; Zhong, L.; Li, X.; Sun, R. Green Synthesis of Palladium Nanoparticles via Branched Polymers: A Bio-Based Nanocomposite for C–C Coupling Reactions. RSC Adv. 2016, 6, 32202–32211. [Google Scholar] [CrossRef]
- Hosoya, T.; Kawamoto, H.; Saka, S. Cellulose–Hemicellulose and Cellulose–Lignin Interactions in Wood Pyrolysis at Gasification Temperature. J. Anal. Appl. Pyrolysis 2007, 80, 118–125. [Google Scholar] [CrossRef]
- Zhang, Z.; Terrasson, V.; Guénin, E. Lignin Nanoparticles and Their Nanocomposites. Nanomaterials 2021, 11, 1336. [Google Scholar] [CrossRef] [PubMed]
- El Mansouri, N.-E.; Salvadó, J. Analytical Methods for Determining Functional Groups in Various Technical Lignins. Ind. Crops Prod. 2007, 26, 116–124. [Google Scholar] [CrossRef]
- Fu, K.; Yue, Q.; Gao, B.; Sun, Y.; Zhu, L. Preparation, Characterization and Application of Lignin-Based Activated Carbon from Black Liquor Lignin by Steam Activation. Chem. Eng. J. 2013, 228, 1074–1082. [Google Scholar] [CrossRef]
- Zevallos Torres, L.A.; Lorenci Woiciechowski, A.; de Andrade Tanobe, V.O.; Karp, S.G.; Guimarães Lorenci, L.C.; Faulds, C.; Soccol, C.R. Lignin as a Potential Source of High-Added Value Compounds: A Review. J. Clean. Prod. 2020, 263, 121499. [Google Scholar] [CrossRef]
- Guillén, E.; Rico, R.; López-Romero, J.M.; Bedia, J.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Pd-Activated Carbon Catalysts for Hydrogenation and Suzuki Reactions. Appl. Catal. A Gen. 2009, 368, 113–120. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.; Shan, X. Adsorption of Metal Ions on Lignin. J. Hazard. Mater. 2008, 151, 134–142. [Google Scholar] [CrossRef]
- Coccia, F.; Tonucci, L.; d’Alessandro, N.; D’Ambrosio, P.; Bressan, M. Palladium Nanoparticles, Stabilized by Lignin, as Catalyst for Cross-Coupling Reactions in Water. Inorg. Chim. Acta 2013, 399, 12–18. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Bidgoli, N.S.S.; Issaabadi, Z.; Ghavamifar, Z.; Baran, T.; Luque, R. Hibiscus rosasinensis L. Aqueous Extract-Assisted Valorization of Lignin: Preparation of Magnetically Reusable Pd NPs@Fe3O4-Lignin for Cr(VI) Reduction and Suzuki-Miyaura Reaction in Eco-Friendly Media. Int. J. Biol. Macromol. 2020, 148, 265–275. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Issaabadi, Z.; Varma, R.S. Magnetic Lignosulfonate-Supported Pd Complex: Renewable Resource-Derived Catalyst for Aqueous Suzuki–Miyaura Reaction. ACS Omega 2019, 4, 14234–14241. [Google Scholar] [CrossRef]
- Baran, T.; Sargin, I. Green Synthesis of a Palladium Nanocatalyst Anchored on Magnetic Lignin-Chitosan Beads for Synthesis of Biaryls and Aryl Halide Cyanation. Int. J. Biol. Macromol. 2020, 155, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Marulasiddeshwara, M.B.; Kumar, P.R. Synthesis of Pd(0) Nanocatalyst Using Lignin in Water for the Mizoroki–Heck Reaction under Solvent-Free Conditions. Int. J. Biol. Macromol. 2016, 83, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Madrahalli Bharamanagowda, M.; Panchangam, R.K. Fe3O4-Lignin@Pd-NPs: A Highly Efficient, Magnetically Recoverable and Recyclable Catalyst for Mizoroki-Heck Reaction under Solvent-free Conditions. Appl. Organomet. Chem. 2020, 34, 265–275. [Google Scholar] [CrossRef]
- Marulasiddeshwara, M.B.; Raghavendra Kumar, P. Phosphine and Copper-Free Sonogashira Coupling Reaction Catalyzed by Lignin Supported Palladium Nanoparticles. Mater. Today Proc. 2018, 5, 20811–20818. [Google Scholar] [CrossRef]
- Orooji, Y.; Pakzad, K.; Nasrollahzadeh, M.; Tajbakhsh, M. Novel Magnetic Lignosulfonate-Supported Pd Complex as an Efficient Nanocatalyst for N-Arylation of 4-Methylbenzenesulfonamide. Int. J. Biol. Macromol. 2021, 182, 564–573. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Shafiei, N.; Nezafat, Z.; Bidgoli, N.S.S. Recent Progresses in the Application of Lignin Derived (Nano)Catalysts in Oxidation Reactions. Mol. Catal. 2020, 489, 110942. [Google Scholar] [CrossRef]
- Coccia, F.; Tonucci, L.; Bosco, D.; Bressan, M.; d’Alessandro, N. One-Pot Synthesis of Lignin-Stabilised Platinum and Palladium Nanoparticles and Their Catalytic Behaviour in Oxidation and Reduction Reactions. Green Chem. 2012, 14, 1073. [Google Scholar] [CrossRef]
- Fazio, E.; Gökce, B.; De Giacomo, A.; Meneghetti, M.; Compagnini, G.; Tommasini, M.; Waag, F.; Lucotti, A.; Zanchi, C.G.; Ossi, P.M.; et al. Nanoparticles Engineering by Pulsed Laser Ablation in Liquids: Concepts and Applications. Nanomaterials 2020, 10, 2317. [Google Scholar] [CrossRef]
- Mohazzab, B.F.; Jaleh, B.; Nasrollahzadeh, M.; Khazalpour, S.; Sajjadi, M.; Varma, R.S. Upgraded Valorization of Biowaste: Laser-Assisted Synthesis of Pd/Calcium Lignosulfonate Nanocomposite for Hydrogen Storage and Environmental Remediation. ACS Omega 2020, 5, 5888–5899. [Google Scholar] [CrossRef]
- Chen, S.; Wang, G.; Sui, W.; Parvez, A.M.; Dai, L.; Si, C. Novel Lignin-Based Phenolic Nanosphere Supported Palladium Nanoparticles with Highly Efficient Catalytic Performance and Good Reusability. Ind. Crops Prod. 2020, 145, 112164. [Google Scholar] [CrossRef]
- Di Pietrantonio, K.; Coccia, F.; Tonucci, L.; d’Alessandro, N.; Bressan, M. Hydrogenation of Allyl Alcohols Catalyzed by Aqueous Palladium and Platinum Nanoparticles. RSC Adv. 2015, 5, 68493–68499. [Google Scholar] [CrossRef]
- Marulasiddeshwara, M.B.; Raghavendra Kumar, P. Hydrogenation of Carbonyl Compounds to Alcohols Catalyzed by Lignin Supported Palladium Nanoparticles. Mater. Today Proc. 2019, 9, 295–305. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, B.; Yu, F.; Liu, Y.; Xie, C.; Yu, S. Hydrogenation of α-Pinene over Platinum Nanoparticles Reduced and Stabilized by Sodium Lignosulfonate. ACS Omega 2020, 5, 8902–8911. [Google Scholar] [CrossRef]
- Bedia, J.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Pd Supported on Mesoporous Activated Carbons with High Oxidation Resistance as Catalysts for Toluene Oxidation. Appl. Catal. B Environ. 2010, 94, 8–18. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Cordero-Lanzac, T.; Berenguer, R.; Morallón, E.; Cazorla-Amorós, D.; Rodríguez-Mirasol, J.; Cordero, T. Lignin-Derived Pt Supported Carbon (Submicron)Fiber Electrocatalysts for Alcohol Electro-Oxidation. Appl. Catal. B Environ. 2017, 211, 18–30. [Google Scholar] [CrossRef]
- Li, X.; Lv, Y.; Pan, D. Pt Catalysts Supported on Lignin-Based Carbon Dots for Methanol Electro-Oxidation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 569, 110–118. [Google Scholar] [CrossRef]
- Zhou, H.; Hong, S.; Zhang, H.; Chen, Y.; Xu, H.; Wang, X.; Jiang, Z.; Chen, S.; Liu, Y. Toward Biomass-Based Single-Atom Catalysts and Plastics: Highly Active Single-Atom Co on N-Doped Carbon for Oxidative Esterification of Primary Alcohols. Appl. Catal. B Environ. 2019, 256, 117767. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, C.; Bedia, J.; Bonal, P.; Rodriguez, J.J.; Gómez-Sainero, L.M. Chloroform Conversion into Ethane and Propane by Catalytic Hydrodechlorination with Pd Supported on Activated Carbons from Lignin. Catal. Sci. Technol. 2018, 8, 3926–3935. [Google Scholar] [CrossRef]
- Wang, D.; Li, G.; Zhang, C.; Wang, Z.; Li, X. Nickel Nanoparticles Inlaid in Lignin-Derived Carbon as High Effective Catalyst for Lignin Depolymerization. Bioresour. Technol. 2019, 289, 121629. [Google Scholar] [CrossRef]
- Du, B.; Liu, C.; Wang, X.; Han, Y.; Guo, Y.; Li, H.; Zhou, J. Renewable Lignin-Based Carbon Nanofiber as Ni Catalyst Support for Depolymerization of Lignin to Phenols in Supercritical Ethanol/Water. Renew. Energy 2020, 147, 1331–1339. [Google Scholar] [CrossRef]
- Heinrich, F.; Keßler, M.T.; Dohmen, S.; Singh, M.; Prechtl, M.H.G.; Mathur, S. Molecular Palladium Precursors for Pd0 Nanoparticle Preparation by Microwave Irradiation: Synthesis, Structural Characterization and Catalytic Activity. Eur. J. Inorg. Chem. 2012, 2012, 6027–6033. [Google Scholar] [CrossRef]
- Lin, X.; Wu, M.; Wu, D.; Kuga, S.; Endo, T.; Huang, Y. Platinum Nanoparticles Using Wood Nanomaterials: Eco-Friendly Synthesis, Shape Control and Catalytic Activity for p-Nitrophenol Reduction. Green Chem. 2011, 13, 283–287. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).