Effect of Biochar and Microbial Inoculation on P, Fe, and Zn Bioavailability in a Calcareous Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling
2.2. Preparation of Pruning Waste Biochar of Apple and Grape Trees
2.3. Greenhouse Experiment
2.3.1. Rhizobox Experiment
2.3.2. Soil and Plant Analysis
2.3.3. Statistical Analysis
3. Results and Discussion
3.1. Soil pH, EC, and Organic Carbon
3.2. P, Fe, and Zn Bioavailability in Soil
3.3. P, Fe, and Zn Uptake of Plants
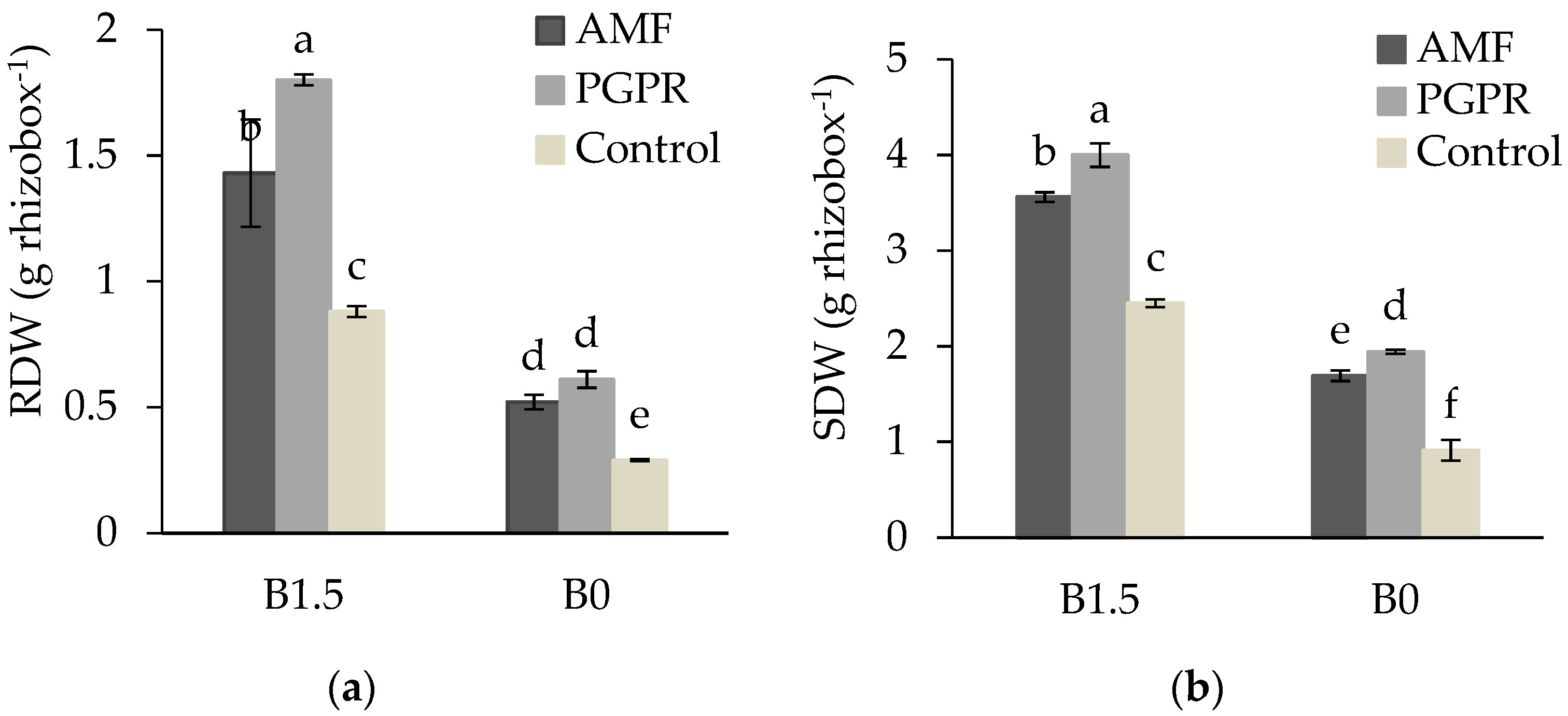
3.4. SDW and RDW
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadeghizadeh, V.; Jalali, V. Improving chemical and hydro-physical properties of semi-arid soils using different magnitudes of crumb rubber. Int. J. Recycl. Org. Waste Agric. 2017, 6, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Abelson, P.H. A potential phosphate crisis. Science 1999, 283, 2015. [Google Scholar] [CrossRef] [PubMed]
- Neset, T.S.; Cordell, D. Global phosphorus scarcity: Identifying synergies for a sustainable future. J. Sci. Food Agric. 2012, 92, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, Z.; Rouached, H.; Rakha, A. Combating mineral malnutrition through iron and zinc biofortification of cereals. Compr. Rev. Food Sci. Food Saf. 2014, 13, 329–346. [Google Scholar] [CrossRef]
- Arora, N.K.; Verma, M. Modified microplate method for rapid and efficient estimation of siderophore produced by bacteria. 3 Biotech 2017, 7, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Singh, D.; Gupta, A.D.; Pandey, K.D.; Singh, K.P.; Kumar, A. Plant Growth Promoting Rhizobacteria. In PGPR Amelioration in Sustainable Agriculture; Elsevier: Amsterdam, The Netherlands, 2019; pp. 41–66. [Google Scholar] [CrossRef]
- Xie, X.; Hu, W.; Fan, X.; Chen, H.; Tang, M. Interactions between Phosphorus, Zinc, and Iron Homeostasis in Nonmycorrhizal and Mycorrhizal Plants. Front. Plant Sci. 2019, 10, 1172. [Google Scholar] [CrossRef]
- Welch, R.; Graham, R. Breeding for micronutrients in staple food crops from a human nutrition perspective. J. Exp. Bot. 2004, 55, 353–364. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I.; Pfeiffer, W.; Mcclafferty, B. Biofortification of durum wheat with zinc and iron. Cereal Chem. 2010, 87, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Dosman, C.; Witmans, M.; Zwaigenbaum, L. Iron’s role in paediatric restless legs syndrome—A review. Paediatr Child Health 2012, 17, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.L. Iron deficiency anemia: A common and curable disease. Cold Spring Harb. Perspect. Med. 2013, 3, a011866. [Google Scholar] [CrossRef] [Green Version]
- Malik, K.A.; Maqbool, A. Transgenic Crops for Biofortification. Front. Sustain. Food Syst. 2020, 4, 571402. [Google Scholar] [CrossRef]
- Bisht, N.; Tiwari, S.; Singh, P.C.; Niranjan, A.; Chauhan, P.S. A multifaceted rhizobacterium Paenibacillus lentimorbus alleviates nutrient deficiency-induced stress in Cicer arietinum L. Microbiol. Res. 2019, 223, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Dixit, V.K.; Misra, S.; Mishra, S.K.; Tewari, S.K.; Joshi, N.; Chauhan, P.S. Characterization of plant growth-promoting alkalotolerant Alcaligenes and Bacillus strains for mitigating the alkaline stress in Zea mays. Antonie Leeuwenhoek 2020, 113, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Msimbira, L.A.; Smith, D.L. The Roles of Plant Growth Promoting Microbes in Enhancing Plant Tolerance to Acidity and Alkalinity Stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Ahmed, W.; Jing, H.; Kaillou, L.; Qaswar, M.; Khan, M.N.; Jin, C.; Geng, S.; Qinghai, H.; Yiren, L.; Guangrong, L.; et al. Changes in phosphorus fractions associated with soil chemical properties under long-term organic and inorganic fertilization in paddy soils of southern China. PLoS ONE 2019, 14, e0216881. [Google Scholar] [CrossRef]
- Pan, S.Y.; Dong, C.D.; Su, J.F.; Wang, P.Y.; Chen, C.W.; Chang, J.S.; Kim, H.; Huang, C.P.; Hung, C.M. The Role of Biochar in Regulating the Carbon, Phosphorus, and Nitrogen Cycles Exemplified by Soil Systems. Sustainability 2021, 13, 5612. [Google Scholar] [CrossRef]
- Huang, P.M.; Violante, A. Influence of organic acids on crystallization and surface properties of precipitation products of aluminum. In Interactions of Soil Minerals with Natural Organics and Microbes; Huang, P.M., Schnitzer, M., Eds.; Soil Science Society of America: Madison, WI, USA, 1986; pp. 159–221. [Google Scholar]
- Teutscherova, N.; Vazquez, E.; Santana, D.; Navas, M.; Masaguer, A.; Benito, M. Influence of pruning waste compost maturity and biochar on c dynamics in acid soil: Incubation study. Eur. J. Soil Biol. 2017, 78, 66–74. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar] [CrossRef]
- Lehmann, J.D.; Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Malo, D.D.; Julson, J.L. Effect of biochars on chemical properties of acidic soil. Arch. Agron. Soil Sci. 2013, 60, 393–404. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Nehls, T.; de Macêdo, J.L.; Blum, W.E.H.; Zech, W. Long term effects of manure, charcoal, and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 2007, 291, 275–290. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Inal, A.; Gunes, A.; Sahin, O.; Taskin, M.B.; Kaya, E.C. Impacts of biochar and processed poultry manure, applied to a calcareous soil, on the growth of bean and maize. Soil Use Manag. 2015, 31, 106–113. [Google Scholar] [CrossRef]
- Gartler, J.; Robinson, B.; Burton, K.; Clucas, L. Carbonaceous soil amendments to biofortify crop plants with zinc. Sci Total Environ. 2013, 465, 308–313. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota e a review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E. Principles of plant nutrition. Ann Bot. 2004, 93, 479–480. [Google Scholar] [CrossRef] [Green Version]
- Renella, G.; Landi, L.; Ascher, M.T.; Ceccherini, M.T.; Pietramellara, G.; Nannipieri, P. Phosphomonoesterase production and persistence and composition of bacterial communities during plant material decomposition in soils in soil with different pH values. Soil Biol. Biochem. 2006, 38, 795–802. [Google Scholar] [CrossRef]
- Cairney, J.W.; Ashford, A.E. Reducing activity at the root surface in Eucaliptus pilularris-Pisolithus tinctorius ectomycorrhizas. Plant Physiol. 1986, 16, 99–105. [Google Scholar] [CrossRef]
- Domene, X.; Mattana, S.; Hanley, K.; Enders, A.; Lehmann, J. Medium-term effects of corn biochar addition on soil biota activities and functions in a temperate soil cropped to corn. Soil Biol. Biochem. 2014, 72, 152–162. [Google Scholar] [CrossRef] [Green Version]
- Hacisalihoglu, G.; Kochian, L.V. How do some plants tolerate low levels of soil zinc Mechanisms of zinc efficiency in crop plants? New Phytol. 2003, 159, 341–350. [Google Scholar] [CrossRef]
- Wenzel, W.W.; Wieshammer, G.; Fitz, W.J.; Puschenreiter, M. Novel rhizobox design to asses rhizosphere characteristics at high spatial resolution. Plant Soil 2001, 237, 37–45. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. Methods of Soil Analysis: Part 3 Chemical Methods 5.3, 1st ed.; Soil Science Society of America Book Ser. 5; Inc. American Society of Agronomy, Inc.: Madison, WI, USA, 1996; p. 1390. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Camps-Arbestain, M.; Lehmann, J. Biochar: A Guide to Analytical Methods; Csiro Publishing: Melbourne, Australia, 2017; p. 320. [Google Scholar] [CrossRef]
- Vahedi, R.; Rasouli-Sadaghiani, M.H.; Barin, M.; Vetukuri, R.R. Interactions between Biochar and Compost Treatment and Mycorrhizal Fungi to Improve the Qualitative Properties of a Calcareous Soil under Rhizobox Conditions. Agriculture 2021, 11, 993. [Google Scholar] [CrossRef]
- Rorison, I.H. Ecological inferences from laboratory experiments on mineral nutrition. In Ecological Aspects of the Mineral Nutrition of Plants; Rorison, I.H., Ed.; Blackwell Sci. Publ.: Oxford, UK; Edinburgh, UK, 1969; pp. 155–176. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extracting with Sodium Bicarbonate; USDA—Government Printing Office: Washington, DC, USA, 1954; p. 939.
- Lindsay, W.L.; Novell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Kookana, R.S.; Sarmah, A.K.; Van Zwieten, L.; Krull, E.; Singh, B. Biochar Application to Soil: Agronomic and Environmental Benefits and Unintended Consequences. Adv. Agron. 2011, 112, 103–143. [Google Scholar] [CrossRef]
- Cheng, C.H.; Lehmann, J. Ageing of black carbon along a temperature gradient. Chemosphere 2009, 75, 1021–1027. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Alkharabsheh, H.M.; Seleiman, M.F.; Battaglia, M.L.; Shami, A.; Jalal, R.S.; Alhammad, B.A.; Almutairi, K.F.; Al-Saif, A.M. Biochar and Its Broad Impacts in Soil Quality and Fertility, Nutrient Leaching and Crop Productivity: A Review. Agronomy 2021, 11, 993. [Google Scholar] [CrossRef]
- Danish, S.; Younis, U.; Akhtar, N.; Ameer, A.; Ijaz, M.; Nasreen, S.; Huma, F.; Sharif, S.; Ehsanullah, M. Phosphorus solubilizing bacteria and rice straw biochar consequence on maize pigments synthesis. Int. J. Biosci. 2015, 5, 31–39. [Google Scholar] [CrossRef]
- Hinsinger, P.; Plassard, C.; Tang, C.; Jaillard, B. Origins of root- mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Pant Soil 2003, 284, 43–59. [Google Scholar] [CrossRef]
- Macias-Benitez, S.; Garcia-Martinez, A.M.; Caballero Jimenez, P.; Gonzalez, J.M.; Tejada Moral, T.; Parrado Rubio, J. Rhizospheric Organic Acids as Biostimulants: Monitoring Feedbacks on Soil Microorganisms and Biochemical Properties. Front. Plant Sci. 2020, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M.; Azcon, R. Symbiotic efficiency and infectivity of an autochthonous arbuscular mycorrhizal Glomus sp. from saline soils and Glomus deserticola under salinity. Mycorrhiza 2000, 10, 137–143. [Google Scholar] [CrossRef]
- Saghafi, D.; Delangiz, N.; Lajayer, B.A.; Ghorbanpour, M. An overview on improvement of crop productivity in saline soils by halotolerant and halophilic PGPRs. 3 Biotech 2019, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Xu, Z. Biochar: Nutrient Properties and Their Enhancement. In Biochar for Environmental Management; Lehmann, J., Joseph, S., Eds.; Science and Technology, Earthscan: London, UK, 2009; pp. 67–84. [Google Scholar] [CrossRef]
- Soumare, M.; Demeyer, A.; Tack, F.M.G.; Verloo, M.G. Chemical Characteristics of Malian and Belgian Solid Waste Composts. Bioresour. Technol. 2002, 81, 97–101. [Google Scholar] [CrossRef]
- Jordan, N.R.; Zhang, J.; Huerd, S. Arbuscular-mycorrhizal fungi: Potential roles in weed management. Weed Res. 2000, 40, 397–410. [Google Scholar] [CrossRef]
- Huang, Q.Y.; Chen, W.L.; Guo, X.J. Sequential fractionation of Cu, Zn and Cd in soils in the absence and presence of rhizobia. In Proceedings of the17th WCSS, Bangkok, Thailand, 14–21 August 2002; p. 1453. [Google Scholar]
- Ippolito, J.A.; Spokas, K.A.; Novak, J.M.; Lentz, R.D.; Cantrell, K.B. Biochar elemental composition and factors influencing nutrient retention. In Biochar for Environmental Management, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Earthscan from Routledge: London, UK, 2015; pp. 139–161. ISBN 978-0-415-70415-1. [Google Scholar]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef]
- Khalil, H.M.A.; Hassan, R.M. Raising the Productivity and Fiber Quality of Both White and Colored Cotton Using Eco-Friendly Fertilizers and Rice Straw. Int. J. Plant Res. 2015, 5, 122–135. [Google Scholar] [CrossRef]
- Lambers, H.; Raven, J.A.; Shaver, G.R.; Smith, S.E. Plant nutrient acquisition strategies change with soil age. Ecol. Evol. 2008, 23, 95–103. [Google Scholar] [CrossRef]
- Leoni, L.; Amborsi, C.; Petrucca, A.; Visca, P. Transcriptional regulation of pseudobactin synthesis in the plant growth promoting pseudomonas B10. FEMS Microbiol. Lett. 2002, 208, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Aliasgharzad, N.; Shirmohamadi, E.; Oustan, S. Siderophore production by mycorrhizal sorghum roots under micronutrient deficient condition. Soil Environ. 2009, 28, 119–123. [Google Scholar]
- Jin, H.Y. Characterization of Microbial Life Colonizing Biochar and Biochar-Amended Soils. Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 2010. [Google Scholar]
- Budania, A.K.; Janardan, Y. Effects of PGPR blended biochar and different levels of phosphorus on yield and nutrient uptake by chickpea. Ann. Agri-Bio. Res. 2014, 19, 408–412. [Google Scholar]
- Vanek, S.J.; Lehmann, J. Phosphorus availability to beans via interactions between mycorrhizas and biochar. Plant Soil 2014, 395, 105–123. [Google Scholar] [CrossRef]
- Becker, M.; Asch, F. Iron toxicity in rice—conditions and management concepts. J. Soil Sci. Plant Nutr. 2005, 168, 558–573. [Google Scholar] [CrossRef]
- Singh, T.; Singh, A.P.; Singh, S.K.; Rai, S.; Kumar, D. Impact of Addition of Biochar Along with PGPR on Rice Yield. Availability of Nutrients and their Uptake in Alluvial Soil. J. Pure Appl. Microbiol. 2016, 10, 2181–2188. [Google Scholar]
- Adejumo, S.A.; Owolabi, M.O.; Odesola, I.F. Agro-physiologic effects of compost and biochar produced at different temperatures on growth, photosynthetic pigment and micronutrients uptake of maize crop. Afr. J. Agric. Res. 2016, 11, 661–673. [Google Scholar] [CrossRef] [Green Version]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. J. Biol. Fertil. 2011, 48, 271–284. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Asghar, H.N.; Saleem, M.; Khan, M.Y.; Zahir, Z.A. Synergistic effect of rhizobia and biochar on growth and physiology of maize. Search Results Web Results J. Agron. 2015, 107, 2327–2334. [Google Scholar] [CrossRef]



| Parameters | Unit | Soil |
|---|---|---|
| pH | - | 7.53 |
| EC | dS m−1 | 0.47 |
| OC | % | 0.25 |
| CaCO3 | % | 14.25 |
| N | % | 0.08 |
| P | % | 7.64 |
| K | % | 98 |
| Fe | mg kg−1 | 1.44 |
| Zn | mg kg−1 | 0.62 |
| Characteristics | Unit | Pruning Waste Biochar |
|---|---|---|
| pH (1:10) | - | 7.29 |
| EC | dS m−1 | 0.08 |
| N | % | 0.54 |
| C | % | 67.53 |
| C/N | - | 125 |
| P (Total) | mg kg−1 | 2748.07 |
| Fe | mg kg−1 | 303.45 |
| Zn | mg kg−1 | 40.88 |
| AMF | PGPR | Control | ||||
|---|---|---|---|---|---|---|
| B1.5 | B0 | B1.5 | B0 | B1.5 | B0 | |
| pH | 7.68 b | 7.42 c | 7.65 b | 7.32 d | 7.82 a | 7.64 b |
| EC (dS m−2) | 0.53 b | 0.49 b c | 0.65 a | 0.47 c | 0.53 b | 0.36 d |
| SOM (%) | 1.81 a | 1.67 d e | 1.65 b | 0.76 d | 1.14 c | 0.59 e |
| POlsen (mg kg−1) | 27.81 a | 10.91 d | 24.58 a | 9.60 d | 14.02 c | 6.13 e |
| Fe (mg kg−1) | 4.27 a | 2.52 c | 3.38 b | 2.73 c | 1.97 d | 1.38 e |
| Zn (mg kg−1) | 1.31 a | 1.11 c | 1.32 a | 1.17 b | 1.01 d | 0.93 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vahedi, R.; Rasouli-Sadaghiani, M.H.; Barin, M.; Vetukuri, R.R. Effect of Biochar and Microbial Inoculation on P, Fe, and Zn Bioavailability in a Calcareous Soil. Processes 2022, 10, 343. https://doi.org/10.3390/pr10020343
Vahedi R, Rasouli-Sadaghiani MH, Barin M, Vetukuri RR. Effect of Biochar and Microbial Inoculation on P, Fe, and Zn Bioavailability in a Calcareous Soil. Processes. 2022; 10(2):343. https://doi.org/10.3390/pr10020343
Chicago/Turabian StyleVahedi, Roghayeh, Mir Hassan Rasouli-Sadaghiani, Mohsen Barin, and Ramesh Raju Vetukuri. 2022. "Effect of Biochar and Microbial Inoculation on P, Fe, and Zn Bioavailability in a Calcareous Soil" Processes 10, no. 2: 343. https://doi.org/10.3390/pr10020343
APA StyleVahedi, R., Rasouli-Sadaghiani, M. H., Barin, M., & Vetukuri, R. R. (2022). Effect of Biochar and Microbial Inoculation on P, Fe, and Zn Bioavailability in a Calcareous Soil. Processes, 10(2), 343. https://doi.org/10.3390/pr10020343







