Recent Advances on Waste Plastic Thermal Pyrolysis: A Critical Overview
Abstract
1. Introduction
1.1. Thermal Pyrolysis Process
1.2. Co-Pyrolysis and Mixed Waste Plastics
2. Common Influential Factors of Waste Plastic Thermal Pyrolysis
2.1. Chemical Composition of the Feedstock
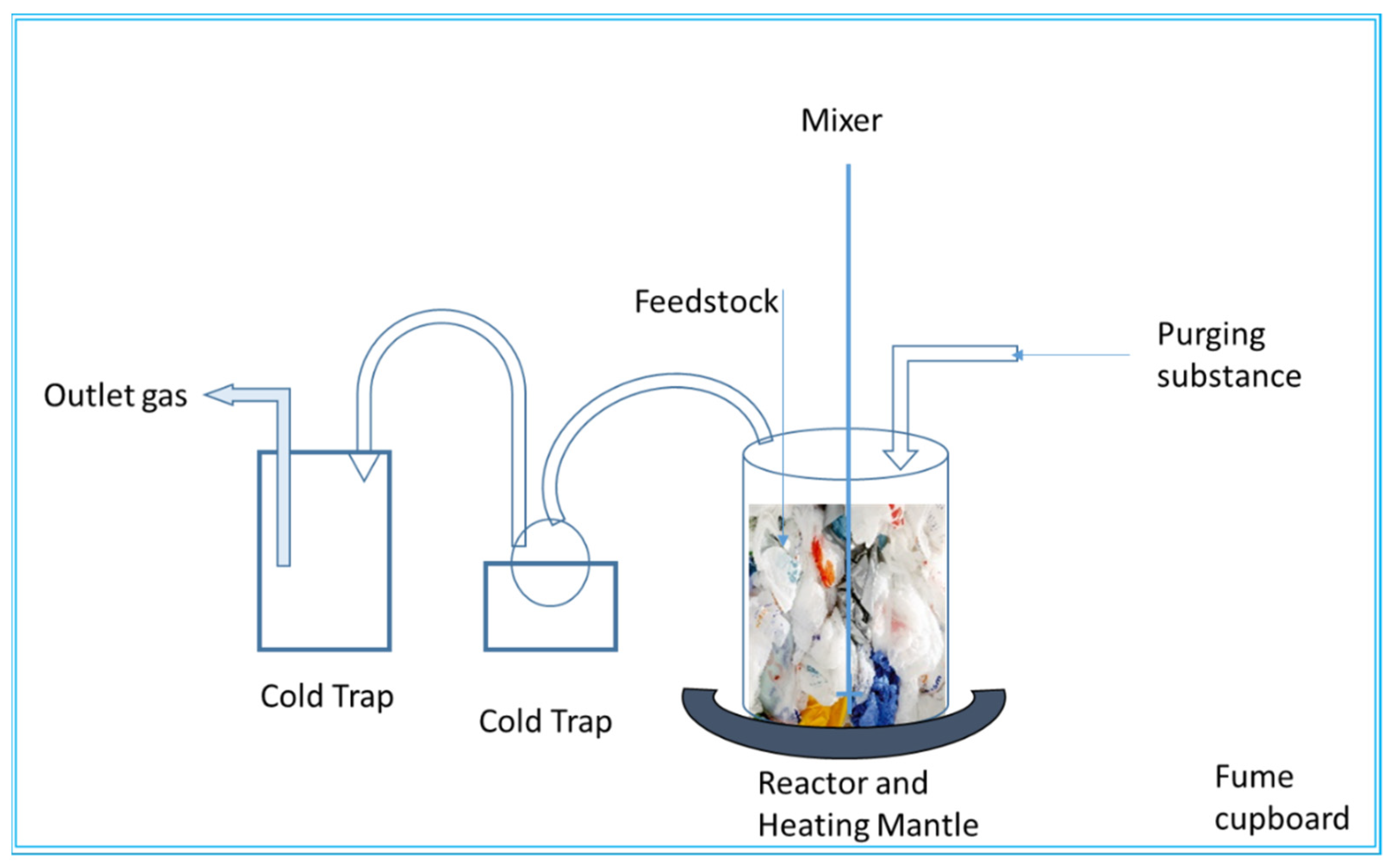
2.2. Reactor Type
- Fusion of the waste plastics.
- Pyrolysis of the fused waste plastics.
2.3. Decomposition Temperature and Heating Rate
2.4. Residence Time
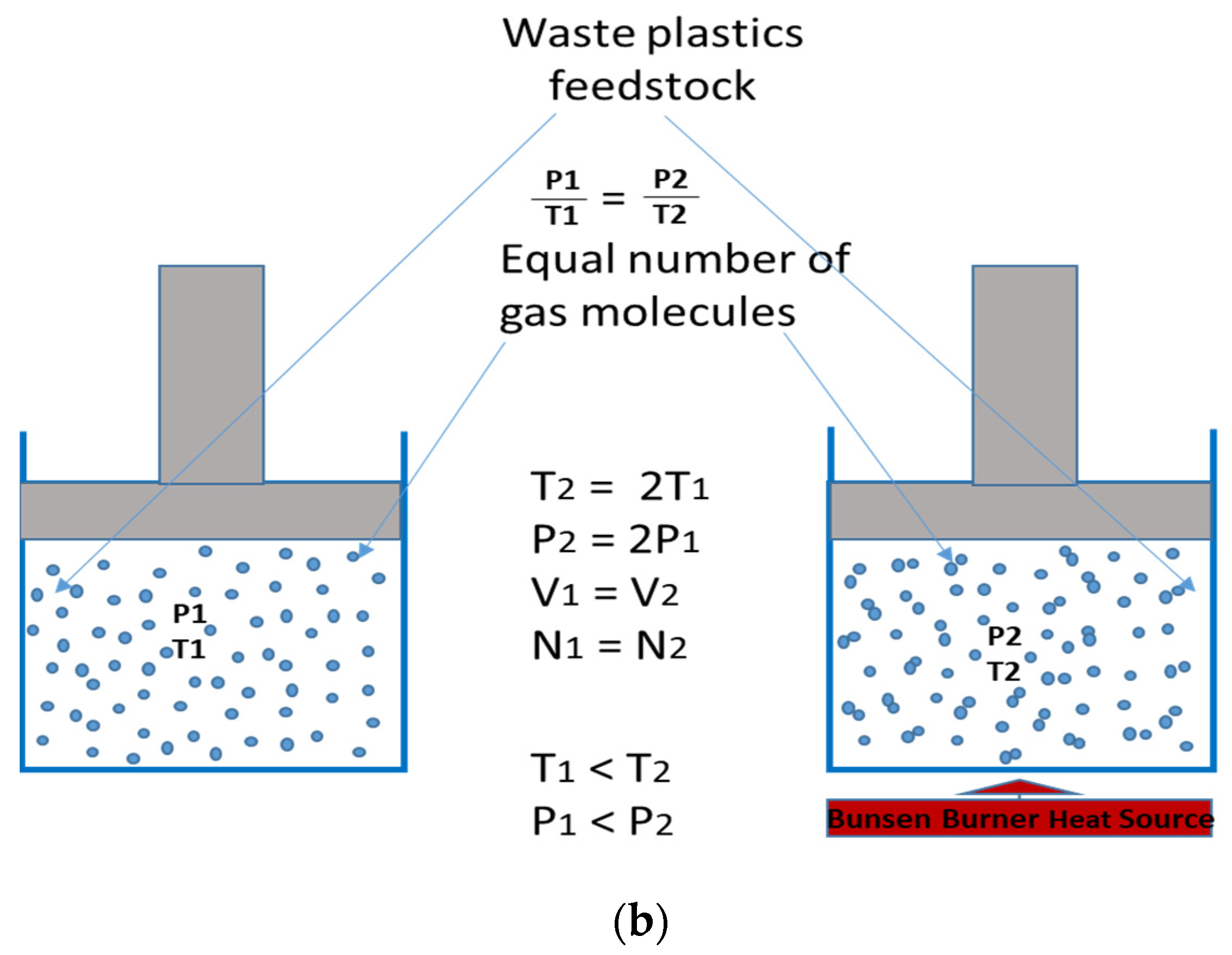
2.5. Pressure
3. Quality Guarantee of Waste Plastic Pyrolysis and Petroleum Products with Emphasis on Diesel: A Brief Comparative Analysis
| Waste Plastics | C (%) | H (%) | O (%) | N (%) | S (%) | HHV (MJ/kg) | References |
|---|---|---|---|---|---|---|---|
| PE | 80.50–85.40 | 14.30–15.50 | 0.03–3.90 | 0.00–0.30 | 0.00–0.30 | 46.1 | [89,94,95] |
| PS | 86.40–92.70 | 7.40–8.50 | 0.00–1.30 | 0.00–6.10 | 0.00–0.10 | 39.00–42.10 | [89,95,96] |
| PP | 85.1–86.50 | 12.90–14.40 | 0.00–0.20 | 0 | 0.00–0.5 | 37.60–46.40 | [89,95] |
| Gasolinea y | 82.68 | 15.13 | 2.09 | 0.0016 | 0.0006 | 45.8 | Summer gasoline in the Republic of Korea. |
| Diesela y | 86.58 | 13.41 | 0.01 | 0.0005 | 0.0005 | 45.96 | [8]; summer diesel in the Republic of Korea. |
4. Active Commercial Plastic Pyrolysis Processes and Technologies
4.1. The Impacts of Feedstock
4.2. Plastic Pyrolysis Technology
4.2.1. Feeding System
4.2.2. Pyrolysis Reactor
4.2.3. Separation and Collection System
4.3. Practical Implications of This Literature
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- PlasticsEurope. Plastics—The Facts 2014/2015. An analysis of European Plastics Production, Demand and Waste Data. PlasticsEurope, Association of Plastics Manufacturers. 2014. Available online: https://plasticseurope.org/wp-content/uploads/2021/10/2014-Plastics-the-facts.pdf (accessed on 9 September 2019).
- Singh, R.K.; Ruj, B. Time and temperature depended fuel gas generation from pyrolysis of real world municipal plastic waste. Fuel 2016, 174, 164–171. [Google Scholar] [CrossRef]
- Sophonrat, N.; Sandström, L.; Johansson, A.-C.; Yang, W. Co-pyrolysis of mixed plastics and cellulose: An interaction study by Py-GC × GC/MS. Energy Fuels 2017, 31, 11078–11090. [Google Scholar] [CrossRef]
- Ma, J.; Shi, L.; Shi, Y.; Luo, S.; Xu, J. Pyrolysis of polymethylsilsesquioxane. J. Appl. Polym. Sci. 2002, 85, 1077–1086. [Google Scholar] [CrossRef]
- Fivga, A.; Dimitriou, I. Pyrolysis of plastic waste for production of heavy fuel substitute: A techno-economic assessment. Energy 2018, 149, 865–874. [Google Scholar] [CrossRef]
- Beti, D.R.; Ring, T.A. Programmed Temperature Pyrolysis: Alterations to the Standard Method; Energy and Geoscience Institute, the University of Utah: Salt Lake City, UT, USA; Department of Chemical Engineering, the University of Utah: Salt Lake City, UT, USA, 2019. [Google Scholar]
- Encinar, J.; González, J.F.G. Pyrolysis of synthetic polymers and plastic wastes. Kinetic study. Fuel Process. Technol. 2008, 89, 678–686. [Google Scholar] [CrossRef]
- Zafar, S.; Pyrolysis of Municipal Wastes. BioEnergy Consult Powering a Greener Future. Available online: https://www.bioenergyconsult.com/pyrolysis-of-municipal-waste/ (accessed on 27 November 2021).
- Themelis, N.J.; Mussche, C. 2014 Energy and Economic Value of Municipal Solid Waste (MSW), Including Non-Recycled Plastics (NRP), Currently Landfilled in the Fifty States. Columbia University—Earth Engineering Centre. Advancing the Goals for Sustainable Waste Managements. Available online: https://www.americanchemistry.com/Policy/Energy/Energy-Recovery/2014-Update-of-Potential-for-Energy-Recovery-from-Municipal-Solid-Waste-and-Non-Recycled-Plastics.pdf (accessed on 16 July 2021).
- Gershman, H.W.; Biofuel Opportunities for Solid Waste Management Systems. Gershman, Brickner & Bratton, Inc. Fairfax, VA USA. 12 November, 2013. GBB–Quality–Value–Ethics–Results. 2013. Available online: http://gbbinc.com/wp-content/uploads/2013/06/GershmanRSB2013.pdf (accessed on 12 December 2020).
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. Available online: https://pubmed.ncbi.nlm.nih.gov/19577459/ (accessed on 29 September 2019). [CrossRef]
- Kaminsky, W.; Predel, M.; Sadiki, A. Feedstock recycling of polymers by pyrolysis in a fluidised bed. Polym. Degrad. Stab. 2004, 85, 1045–1050. [Google Scholar] [CrossRef]
- Williams, E.A.; Williams, P. Analysis of products derived from the fast pyrolysis of plastic waste. J. Anal. Appl. Pyrolysis 1997, 40–41, 347–363. [Google Scholar] [CrossRef]
- Chen, D.; Yin, L.; Wang, H.; He, P. Pyrolysis technologies for municipal solid waste: A review. Waste Manag. 2014, 34, 2466–2486. [Google Scholar] [CrossRef]
- McKay, G. Dioxin characterisation, formation and minimisation during municipal solid waste (MSW) incineration: Review. Chem. Eng. J. 2002, 86, 343–368. [Google Scholar] [CrossRef]
- Qinglan, H.; Chang, W.; Dingqiang, L.; Yao, W.; Dan, L.; Guiju, L. Production of hydrogen-rich gas from plant biomass by catalytic pyrolysis at low temperature. Int. J. Hydrogen Energy 2010, 35, 8884–8890. [Google Scholar] [CrossRef]
- Buekens, A. Introduction to Feedstock Recycling of Plastics. In Feedstock Recycling and Pyrolysis of Waste Plastics; Scheirs, J., Kaminsky, W., Eds.; John Wiley & Sons, Ltd.: Brussels, Belgium, 2006; pp. 3–41. [Google Scholar]
- Figueroa, J.E.J.; Ardila, Y.C.; Hoss Lunelli, B.; Filho, R.M.; Wolf Maciel, M.R. Evaluation-of-pyrolysis-and-steam-gasification-processes-of-sugarcane-bagasse-in-a-fixed-bed. Chem. Eng. Trans. 2013, 32, 925–930. [Google Scholar]
- Williams, P.; Slaney, E. Analysis of products from the pyrolysis and liquefaction of single plastics and waste plastic mixtures. Resour. Conserv. Recycl. 2007, 51, 754–769. [Google Scholar] [CrossRef]
- Miskolczi, N.; Ateş, F.; Borsodi, N. Comparison of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part II: Contaminants, char and pyrolysis oil properties. Bioresour. Technol. 2013, 144, 370–379. [Google Scholar] [CrossRef]
- Sharma, B.K.; Moser, B.R.; Vermillion, K.E.; Doll, K.M.; Rajagopalan, N. Production, characterisation and fuel properties of alternative diesel fuel from pyrolysis of waste plastic grocery bags. Fuel Process. Technol. 2014, 122, 79–90. [Google Scholar] [CrossRef]
- Syamsiro, M.; Saptoadi, H.; Norsujianto, T.; Noviasri, P.; Cheng, S.; Alimuddin, Z.; Yoshikawaa, K. Fuel Oil Production from Municipal Plastic Wastes in Sequential. Energy Procedia 2014, 47, 180–188. [Google Scholar] [CrossRef]
- Muhammad, C.; Onwudili, J.A.; Williams, P.T. Thermal Degradation of Real-World Waste Plastics and Simulated Mixed Plastics in a Two-Stage Pyrolysis–Catalysis Reactor for Fuel Production. Energy Fuels 2015, 29, 2601–2609. [Google Scholar] [CrossRef]
- Fakhrhoseini, S.M.; Dastanian, M. Predicting pyrolysis products of PE, PP, and PET using NRTL activity coefficient model. J. Chem. 2013, 2013, 487676. [Google Scholar] [CrossRef]
- Mastral, F.J.; Esperanza, E.; Garcıa, P.; Juste, M. Pyrolysis of high-density polyethylene in a fluidised bed reactor. Influence of the temperature and residence time. J. Anal. Appl. Pyrolysis 2002, 63, 1–15. [Google Scholar] [CrossRef]
- Butler, E.; Devlin, G.; Meier, D.; McDonnell, K. A review of recent laboratory research and commercial developments in fast pyrolysis and upgrading. Renew. Sustain. Energy Rev. 2011, 15, 4171–4186. [Google Scholar] [CrossRef]
- Helt, J.E.; Agrawal, R.K.; Myles, K.M. Pyrolysis of Municipal Solid Waste. Annual Report, July 1984–June 1985, Argonne National Laboratory, ANL/CNSV-45, 1984. Available online: https://www.osti.gov/biblio/7255421-pyrolysis-municipal-solid-waste-annual-report-july-june (accessed on 5 March 2021).
- Demirbas, A. Pyrolysis of municipal plastic waste for recovery of gasoline range hydrocarbons. J. Anal. Appl. Pyrolysis 2004, 72, 97–102. [Google Scholar] [CrossRef]
- Joo, H.S.; Guin, J.A. Continuous upgrading of a plastics pyrolysis liquid to an environmentally favorable gasoline range product. Fuel Process. Technol. 1998, 57, 25–40. [Google Scholar] [CrossRef]
- Lee, K.-H.; Shin, D.-H. Characteristics of liquid product from the pyrolysis of waste plastic mixture at low and high temperatures: Influence of lapse time of reaction. Waste Manag. 2007, 27, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Marcilla, A.; Beltrán, M.; Navarro, R. Thermal and catalytic pyrolysis of polyethylene over HZSM5 and HUSY zeolites in a batch reactor under dynamic conditions. Appl. Catal. B Environ. 2009, 86, 78–86. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Escola, J.M. Catalytic Upgrading of Plastic Wastes. In Feedstock Recycling and Pyrolysis of Waste Plastics; Scheirs, J., Kaminsky, W., Eds.; John Wiley & Sons, Ltd.: Mostoles, Spain, 2006; pp. 73–110. [Google Scholar]
- Blazso, M. Composition of Liquid Fuels Derived from the Pyrolysis of Plastics. In Feedstock Recycling and Pyrolysis of Waste Plastics; Kaminsky, J.S.A.W., Ed.; John Wiley & Sons, Ltd.: Budapest, Hungary, 2006; pp. 315–344. [Google Scholar]
- Demirbas, A. Effect of initial moisture content on the yields of oily products from pyrolysis of biomass. J. Anal. Appl. Pyrolysis 2004, 71, 803–815. [Google Scholar] [CrossRef]
- McCaffrey, W.C.; Kamal, M.R.; Cooper, D.G. Thermolysis of polyethylene.pdf. Polym. Degrad. Stab. 1995, 47, 133–139. [Google Scholar] [CrossRef]
- Indian Centre for Plastics in the Environment (ICPE) Newsletter. Management of Plastics, Polymer Wastes and Bio-Polymers and Impact of Plastics on the Eco-System. Envis Eco-Echoes. Volume 12, Issue 4, October–December 2011. Available online: http://icpe.in/envis_newsletter/Envis-Eco-Echoes%20Oct_Dec-2011(E%20version).pdf (accessed on 2 January 2020).
- Moses, K. Production and Characterization of Liquid Fuel from Mixed Plastic Wastes Using Catalytic Pyrolysis. Master’s Dissertation, Makerere University, Kampala, Uganda, 2014. [Google Scholar]
- Marcilla, A.; Garcia-Quesada, J.C.; Sanchez, S.; Ruiz, R. Study of the catalytic pyrolysis behaviour of polyethylene polypropylene mixtures. J. Anal. Appl. Pyrol. 2005, 74, 38792. [Google Scholar] [CrossRef]
- Abnisa, F.; Daud, W.M.A.W. A review on co-pyrolysis of biomass: An optional technique to obtain a high-grade pyrolysis oil. Energy Convers. Manag. 2014, 87, 71–85. [Google Scholar] [CrossRef]
- Salaudeen, S.A.; Arku, P.; Dutta, A. Gasification of Plastic Solid Waste and Competitive Technologies. In Plastics to Energy: Fuel, Chemicals, and Sustainability Implications; Plastics Design Library; Elsevier: Amsterdam, The Netherlands, 2019; pp. 269–293. [Google Scholar]
- Wu, S.L.; Kuo, J.H.; Wey, M.Y. Thermaldegradation of waste plastics in a two-stage pyrolysis-catalysis reactor overcore-shell type catalyst. J. Anal. Appl. Pyrolysis 2019, 142, 104641. [Google Scholar] [CrossRef]
- Kim, S. Pyrolysis kinetics of waste PVC pipe. Waste Manag. 2001, 21, 609–616. [Google Scholar] [CrossRef]
- Mansur, D.; Simanungkalit, S.P.; Fitriady, M.A.; Safitri, D. Liquefaction of Plastic for Fuel Production and Application of Volcanic Ash as Catalyst. 2018. Available online: https://aip.scitation.org/doi/pdf/10.1063/1 (accessed on 17 September 2020).
- Liu, Y.; Qian, J.; Wang, J. Pyrolysis of polystyrene waste in a fluidized-bed reactor to obtain styrene monomer and gasoline fraction. Fuel Process. Technol. 2000, 63, 45–55. [Google Scholar] [CrossRef]
- López, A.; de Marco, I.; Caballero, B.; Laresgoiti, M.; Adrados, A. Influence of time and temperature on pyrolysis of plastic wastes in a semi-batch reactor. Chem. Eng. J. 2011, 173, 62–71. [Google Scholar] [CrossRef]
- Dorado, C.; Mullen, C.A.; Boateng, A.A. Origin of carbon in aromatic and olefin products derived from HZSM-5 catalyzed co-pyrolysis of cellulose and plastics via isotopic labeling. Appl. Catal. B Environ 2015, 162, 338–345. [Google Scholar] [CrossRef]
- Ali, M.F.; Siddiqui, M.N. Thermal and catalytic decomposition behaviour of PVC mixed plastic waste with petroleum residue. J. Anal. Appl. Pyrol. 2005, 74, 282–289. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Chen, S.; Wu, J. Catalytic co-pyrolysis of lignocellulosic biomass with polymers: A critical review. Green Chem. 2016, 18, 4145–4169. [Google Scholar] [CrossRef]
- Chen, W.; Shi, S.; Zhang, J.; Chen, M.; Zhou, X. Co-pyrolysis of waste newspaper with high-density polyethylene: Synergistic effect and oil characterization. Energy Convers. Manag. 2016, 112, 41–48. [Google Scholar] [CrossRef]
- Guan, Y.; Ma, Y.; Zhang, K.; Chen, H.; Xu, G.; Liu, W.; Yang, Y. Co-pyrolysis behaviors of energy grass and lignite. Energy Convers. Manag. 2015, 93, 132–140. [Google Scholar] [CrossRef]
- Uzoejinwa, B.B.; He, X.; Wang, S.; Abomohra, A.E.-F.; Hu, Y.; Wang, Q. Co-pyrolysis of biomass and waste plastics as a thermochemical conversion technology for high-grade biofuel production: Recent progress and future directions elsewhere worldwide. Energy Convers. Manag. 2018, 163, 468–492. [Google Scholar] [CrossRef]
- Kositkanawuth, K.; Sattler, M.L.; Dennis, B. Pyrolysis of Macroalgae and Polysytrene: A Review. Curr. Sustain. Energy Rep. 2014, 1, 121–128. [Google Scholar] [CrossRef][Green Version]
- Zhao, Z.-Y.; Yan, H. Assessment of the biomass power generation industry in China. Renew. Energy 2012, 37, 53–60. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review. Renew. Sustain. Energy Rev. 2017, 73, 346–368. [Google Scholar] [CrossRef]
- Yang, J.; Rizkiana, J.; Widayatno, W.B.; Karnjanakom, S.; Kaewpanha, M.; Hao, X.; Abudula, A.; Guan, G. Fast co-pyrolysis of low-density polyethylene and biomass residue for oil production. Energy Convers. Manag. 2016, 120, 422–429. [Google Scholar] [CrossRef]
- Krerkkaiwan, S.; Fushimi, C.; Tsutsumi, A.; Kuchonthara, P. Synergetic effect during co-pyrolysis/gasification of biomass and sub-bituminous coal. Fuel Process. Technol. 2013, 115, 11–18. [Google Scholar] [CrossRef]
- Miranda, R.; Yang, J.; Roy, C.; Vasile, C. Vacuum pyrolysis of PVC I. Kinetic study. Polym. Degrad. Stab. 1999, 64, 127–144. [Google Scholar] [CrossRef]
- Li, W.; Cheng, C.; He, L.; Liu, M.; Cao, G.; Yang, S.; Ren, N. Effects of feedstock and pyrolysis temperature of biochar on promoting hydrogen production of ethanol-type fermentation. Sci. Total Environ. 2021, 790, 148206. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-W.; Kim, K.; Jeong, T.-U.; Ahn, K.-H. Influence of pyrolysis temperature on characteristics and phosphate adsorption capability of biochar derived from waste-marine macroalgae (Undaria pinnatifida roots). Bioresour. Technol. 2016, 200, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, P.; Yuan, X.; Li, Y.; Han, L. Effect of pyrolysis temperature and correlation analysis on the yield and physicochemical properties of crop residue biochar. Bioresour. Technol. 2019, 296, 122318. [Google Scholar] [CrossRef] [PubMed]
- Banik, C.; Lawrinenko, M.; Bakshi, S.; Laird, D.A. Impact of Pyrolysis Temperature and Feedstock on Surface Charge and Functional Group Chemistry of Biochars. J. Environ. Qual. 2018, 47, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of Feedstock and Pyrolysis Temperature on Biochar Adsorption of Ammonium and Nitrate. PLoS ONE 2014, 9, e113888. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Liu, Y.; Naidu, R.; Parikh, S.J.; Du, J.; Qi, F.; Willett, I.R. Influences of feedstock sources and pyrolysis temperature on the properties of biochar and functionality as adsorbents: A meta-analysis. Sci. Total Environ. 2020, 744, 140714. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Donner, E.; Vasileiadis, S.; Skinner, W.; Smith, E.; Lombi, E. The effect of biochar feedstock, pyrolysis temperature, and application rate on the reduction of ammonia volatilisation from biochar-amended soil. Sci. Total Environ. 2018, 627, 942–950. [Google Scholar] [CrossRef]
- Zhao, X.; Zhan, L.; Xie, B.; Gao, B. Products derived from waste plastics (PC, HIPS, ABS, PP and PA6) via hydrothermal treatment: Characterization and potential applications. J. Chemosphere 2018, 207, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Brandrup, S.; Bittner, M.; Michaeli, W.; Menges, G. Recycling and Recovery of Plastics; Hansa Publishers: New York, NY, USA, 1996. [Google Scholar]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Mastellone, M.L.; Perugini, F.; Ponte, M.; Arena, U. Fluidized bed pyrolysis of a recycled polyethylene. Polym. Degrad. Stab. 2002, 76, 479–487. [Google Scholar] [CrossRef]
- Della Zassa, M.; Favero, M.; Canu, P. Two-steps selective thermal depolymerization of polyethylene. 1: Feasibility and effect of devolatilization heating policy. J. Anal. Appl. Pyrolysis 2010, 87, 248–255. [Google Scholar] [CrossRef]
- Ellens, C.J. Alternative Pyrolyzer Design: Free Fall Reactor. Iowa State University Center for Sustainable Environmental Technologies; 2021. Available online: https://www.cset.iastate.edu/research/current-research/alternative-pyrolyzer-design-free-fall-reactor/ (accessed on 3 January 2020).
- Pandey, U.; Stormyr, J.A.; Hassani, A.; Jaiswal, R.; Haugen, H.H.; Britt, M.E. Pyrolysis of Plastic Waste to Environmentally Friendly Products. In Energy Production and Management in the 21st Century IV; Moldestad University of South-Eastern Norway: Kongsberg, Norway, 2020; p. 61. [Google Scholar] [CrossRef]
- Gao, F. Pyrolysis of Waste Plastics into Fuels. Ph.D. Thesis, University of Canterbury, Christchurch, New Zealand, 2010. [Google Scholar]
- Jung, C.G.; Fontana, A. Production of Gaseous and Liquid Fuels by Pyrolysis and Gasification of Plastics: Technological Approach. Available online: https://onlinelibrary.wiley.com/doi/10.1002/0470021543.ch10 (accessed on 14 September 2020).
- Adrados, A.; de Marco, I.; Caballero, B.; López, A.; Laresgoiti, M.; Torres, A. Pyrolysis of plastic packaging waste: A comparison of plastic residuals from material recovery facilities with simulated plastic waste. Waste Manag. 2012, 32, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, Q.; Dzakpasu, M.; Gao, X.; Yuwen, C.; Wang, X.C. Impacts of different biochar types on hydrogen production promotion during fermentative co-digestion of food wastes and dewatered sewage sludge. Waste Manag. 2018, 80, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Freudenrich, C. How Plastics Work. 2007. Available online: https://science.howstuffworks.com/plastic2.htm (accessed on 7 January 2020).
- Naresh, S.; Rockwell, J.; Huffman, G.P. Conversion of Waste Plastic to Oil: Direct Liquefaction versus Pyrolysis and Hydro-Processing; CFFLS, 533 S. Limestone St., University of Kentucky: Lexington, KY, USA, 1999. [Google Scholar]
- Aguado, J.; Serrano, D.P.; Vicente, G.; Sánchez, N. Enhanced Production of α-Olefins by Thermal Degradation of High-Density Polyethylene (HDPE) in Decalin Solvent: Effect of the Reaction Time and Temperature. Ind. Eng. Chem. Res. 2007, 46, 3497–3504. [Google Scholar] [CrossRef]
- Ludlow-Palafox, C.; Chase, H.A. Microwave-induced pyrolysis of plastic wastes. Ind. Eng. Chem. Res. 2001, 40, 4749–4756. [Google Scholar] [CrossRef]
- Miller, S.J.; Shah, N.; Huffman, G.P. Conversion of waste plastic to lubricating base oil. Energy Fuels 2005, 19, 1580–1586. [Google Scholar] [CrossRef]
- Kaminsky, W. Pyrolysis with Respect to Recycling of Polymer†. First Published: October 1995. Available online: https://onlinelibrary.wiley.com/doi/10.1002/apmc.1995.052320110 (accessed on 14 September 2020).
- Ayhan, D. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Tsai, W.; Lee, M.; Chang, Y. Fast pyrolysis of rice husk: Product yields and compositions. Bioresour. Technol. 2007, 98, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.K.K.; Sakata, Y. Effect of pressure on thermal degradation of polyethylene. J. Anal. Appl. Pyrolysis 2004, 71, 569–589. [Google Scholar]
- Chem.Fsu.Edu, (n.d). Gas Laws—Gay Lussac’s Law. Available online: https://www.chem.fsu.edu/chemlab/chm1045/gas_laws.html (accessed on 5 July 2021).
- Central Pollution Control Board (CPCB) Report. Material on plastic waste management. Parivesh Bhaham 2004, East Argum Nagar Delhi-110032. Available online: https://www.nswai.org/docs/An%20Overview%20of%20Plastic%20Waste%20Management%20by%20CPCB.pdf (accessed on 14 September 2020).
- Helmenstine, A. Gay-Lussac’s Law—Definition, Formula, Examples. Available online: https://sciencenotes.org/gay-lussacs-law-definition-formula-examples/ (accessed on 5 July 2021).
- Jung, S.-H.; Cho, M.-H.; Kang, B.-S.; Kim, J.-S. Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process. Technol. 2010, 91, 277–284. [Google Scholar] [CrossRef]
- Sophonrat, N.; Sandström, L.; Zaini, I.N.; Yangaa, W. Stepwise Pyrolysis of Mixed Plastics and Paper for Separation of Oxygenatedand Hydrocarbon Condensates; Department of Materials Science and Engineering, KTH Royal Institute of Technology: Stockholm, Sweden, 2018; Available online: www.elsevier.com/locate/apenergy (accessed on 9 May 2021).
- Gerpen, J.V. Diesel Combustion and Fuels. In Diesel Engine Reference Book, 2nd ed.; Challen, B., Baranescu, R., Eds.; Society of Automotive Engineers, Inc.: Warrendale, PA, USA, 1999. [Google Scholar]
- Scheirs, J.; Kaminsky, W. Feedstock Recycling and Pyrolysis of Waste Plastics: Converting waste plastics into diesel and other fuels. In Wiley Series in Polymer Science; Scheirs, J., Ed.; John Wiley & Sons, Ltd.: Milton, Australia, 2006. [Google Scholar]
- Koppolu, L.; Agblevor, F.A.; Clements, L.D. Pyrolysis as a technique for separating heavy metals from hyperaccumulators. Part II: Lab-scale pyrolysis of synthetic hyperaccumulator biomass. Biomass-Bioenergy 2003, 25, 651–663. [Google Scholar] [CrossRef]
- Zannikos, F.; Kalligeros, S.; Anastopoulos, G.; Lois, E. Converting Biomass and Waste Plastic to Solid Fuel Briquettes. J. Renew. Energy 2012, 2013, 360368. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, W. Effect of heat transfer model on the prediction of refuse-derived fuel pyrolysis process. Fuel 2015, 142, 46–57. [Google Scholar] [CrossRef]
- Park, H.J.; Park, Y.K.; Dong, J.I.; Kim, J.S.; Jeon, J.K.; Kim, S.S.; Kim, J.; Song, B.; Park, J.; Lee, K.J. Pyrolysis characteristics of oriental white oak: Kinetic study and fast pyrolysis in a fluidized bed with an improved reaction system. Fuel Process. Technol. 2009, 90, 86–195. [Google Scholar] [CrossRef]
- Aboulkas, A.; El harfi, K.; El Bouadili, A. Thermal degradation behaviours of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Sims, B. The Dangers of Polystyrene. Future Centre Trust. Available online: http://gracz-brand.com/en/life/4 (accessed on 14 February 2021).
- Arena, U.; Mastellone, M.L. Fluidized Bed Pyrolysis of Plastic Wastes. In Feedstock Recycling and Pyrolysis of Waste Plastics; Kaminsky, J.S.a.W., Ed.; John Wiley & Sons, Ltd.: Caserta, Italy, 2006; p. 440. [Google Scholar]
- Zadgaonkar, A. Process and Equipment for Conversions of Waste Plastics into Fuels. In Feedstock Recycling and Pyrolysis of Waste Plastics; Kaminsky, J.S.a.W., Ed.; John Wiley & Sons, Ltd.: Nagpur, India, 2006; pp. 709–728. [Google Scholar]
- Bagri, R.; Williams, P.T. Catalytic of pyrolysis of polyethylene. J. Anal. Appl. Pyrolysis 2002, 63, 29–41. [Google Scholar] [CrossRef]
- Shoaib, A.M.; El-Adly, R.A.; Hassanean, M.H.M.; Youssry, A.; Bhran, A.A. Developing a free-fall reactor for rice straw fast pyrolysis to produce bio-products. Egypt. J. Pet. 2018, 27, 1305–1311. [Google Scholar] [CrossRef]
- Uemichi, Y.; Nakamura, J.; Itoh, T.; Sugioka, M.; Garforth, A.A.; Dwyer, J. Conversion of Polyethylene into Gasoline-Range Fuels by Two-Stage Catalytic Degradation Using Silica−Alumina and HZSM-5 Zeolite. Ind. Eng. Chem. Res. 1999, 38, 385–390. [Google Scholar] [CrossRef]
- Dou, B.; Wang, K.; Jiang, B.; Song, Y.; Zhang, C.; Chen, H.; Xu, Y. Fluidized-bed gasification combined continuous sorption-enhanced steam reforming system to continuous hydrogen production from waste plastic. Int. J. Hydrogen Energy 2016, 41, 3803–3810. Available online: www.sciencedirect.com (accessed on 1 August 2021). [CrossRef]
- Troitsch, J. International Plastics Flammability Handbook; Hanser Publishers: Munich, Germany, 1990. [Google Scholar]
- Okuwaki, A. Feedstock recycling of plastics in Japan. Polym. Degrad. Stab. 2004, 85, 981–988. [Google Scholar] [CrossRef]


| Conditions of Pyrolysis | Cracking Temperature (°C) | Heating Rate | Derived Products |
|---|---|---|---|
| Slow carbonisation | 450–600 | Very low | Charcoal |
| Slow pyrolysis | 450–600 | 10–100 K/min | Gas, oil and char |
| Fast pyrolysis | 550–650 | Up to 1000 K/s | Gas, oil and (char) |
| Flash pyrolysis | 450–900 | Up to 10,000 K/s | Gas, oil and (char) |
| Conditions of Pyrolysis | Residence Time | Derived Products |
|---|---|---|
| Slow carbonisation | Over 24 h | Charcoal |
| Slow pyrolysis | 10–60 min | Gas, oil and char |
| Fast pyrolysis | 0.5–5 s | Gas, oil, (char) |
| Flash pyrolysis | <1 s | Gas, oil, (char) |
| Gas Yield/wt vs. Degeneration Pressure/Mpa | |||||
|---|---|---|---|---|---|
| @ 410 °C | 6.4 vs. 0.1 | 7.0 vs. 0.2 | 9.0 vs. 0.4 | 10.4 vs. 0.6 | 13.0 vs. 0.8 |
| @ 420 °C | 4.4 vs. 0.1 | 5.4 vs. 0.2 | 6.0 vs. 0.4 | 7.3 vs. 0.6 | 8.0 vs. 0.8 |
| @ 430 °C | 4.3 vs. 0.1 | 4.4 vs. 0.2 | 5.2 vs. 0.4 | 6.0 vs. 0.6 | 6.2 vs. 0.8 |
| @ 440 °C | 3.8 vs. 0.1 | 4.0 vs. 0.2 | 4.8 vs. 0.4 | 5.0 vs. 0.6 | 5.8 vs. 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yansaneh, O.Y.; Zein, S.H. Recent Advances on Waste Plastic Thermal Pyrolysis: A Critical Overview. Processes 2022, 10, 332. https://doi.org/10.3390/pr10020332
Yansaneh OY, Zein SH. Recent Advances on Waste Plastic Thermal Pyrolysis: A Critical Overview. Processes. 2022; 10(2):332. https://doi.org/10.3390/pr10020332
Chicago/Turabian StyleYansaneh, Osman Y., and Sharif H. Zein. 2022. "Recent Advances on Waste Plastic Thermal Pyrolysis: A Critical Overview" Processes 10, no. 2: 332. https://doi.org/10.3390/pr10020332
APA StyleYansaneh, O. Y., & Zein, S. H. (2022). Recent Advances on Waste Plastic Thermal Pyrolysis: A Critical Overview. Processes, 10(2), 332. https://doi.org/10.3390/pr10020332







