Interaction between Coal and Biomass during Co-Gasification: A Perspective Based on the Separation of Blended Char
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Feedstock
2.2. Preparation of Char Samples
2.3. Characterization Analysis of Char Samples
2.3.1. Content of Elements in Char Samples
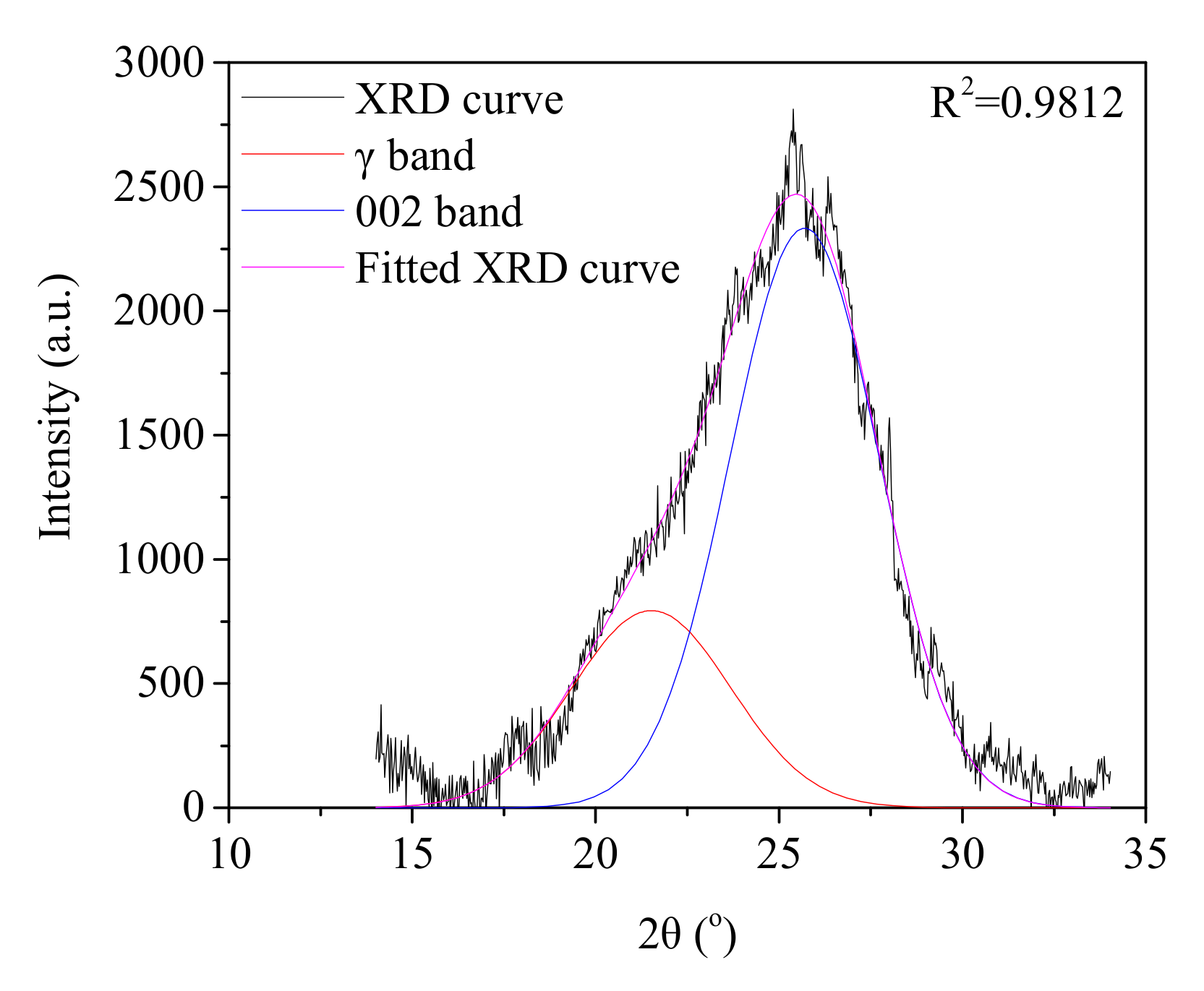
2.3.2. Microcrystalline Structure Analysis of the Char Samples
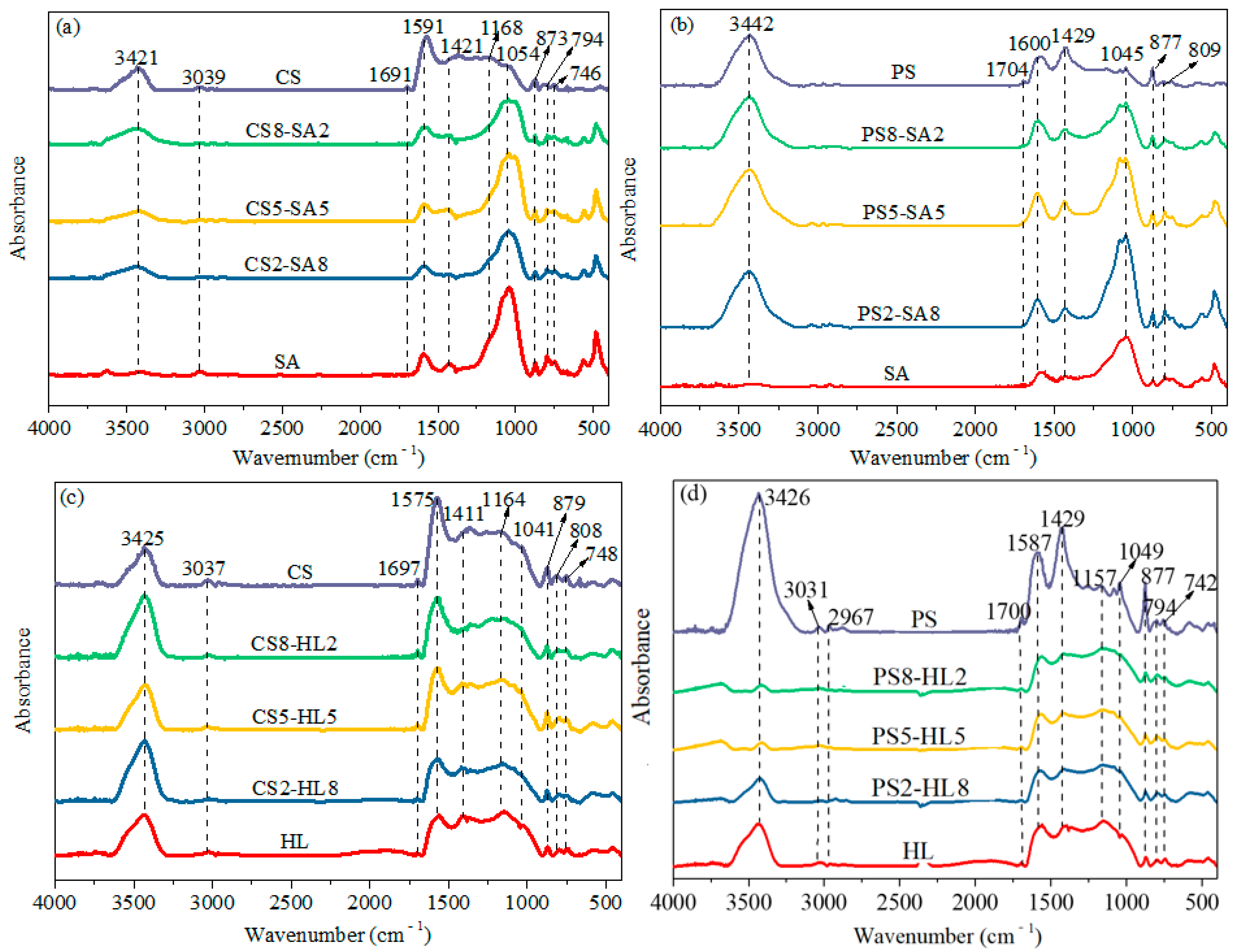
2.3.3. Chemical Structure Analysis of the Char Samples
2.4. Isothermal Gasification Experiments of Char Samples
3. Results and Discussion
3.1. Effect of Biomass Type and Blend Ratio on Char Characteristic
3.1.1. Effect of Biomass Type and Blend Ratio on the Content of Elements in Char Samples
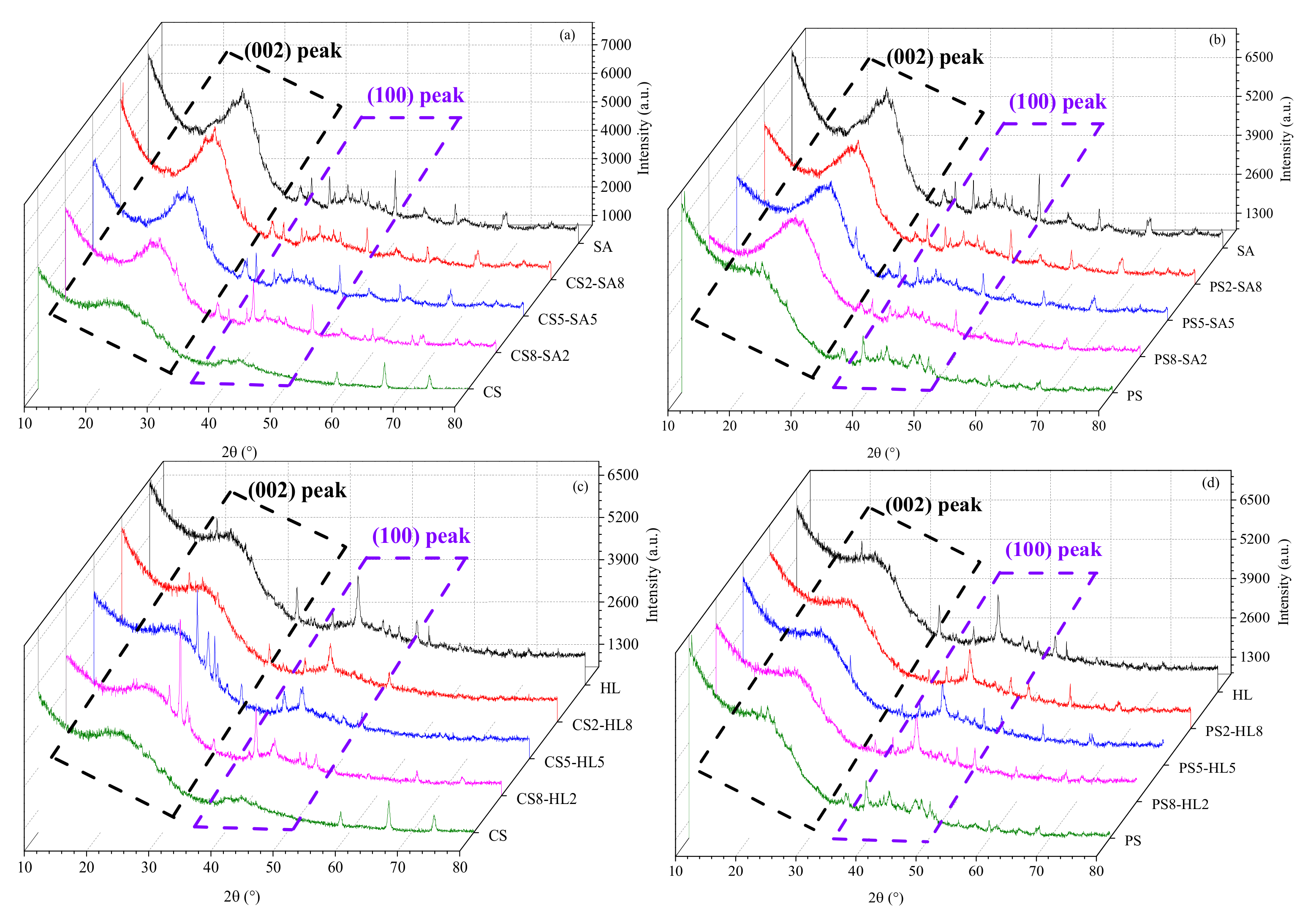
3.1.2. Effect of Biomass Type and Blend Ratio on the Microcrystalline Structure
3.1.3. Effect of Biomass Type and Blend Ratio on the Molecular Structure
3.2. Gasification Characteristics of Char Samples
4. Conclusions
- (1)
- The contents of AAEM species in the coal char sample were determined by the AAEM species and the contents of AAEMs in biomass during co-pyrolysis. Higher K content in CS determined that the increasing degree of K content in coal char caused by the CS addition was higher than that caused by PS addition.
- (2)
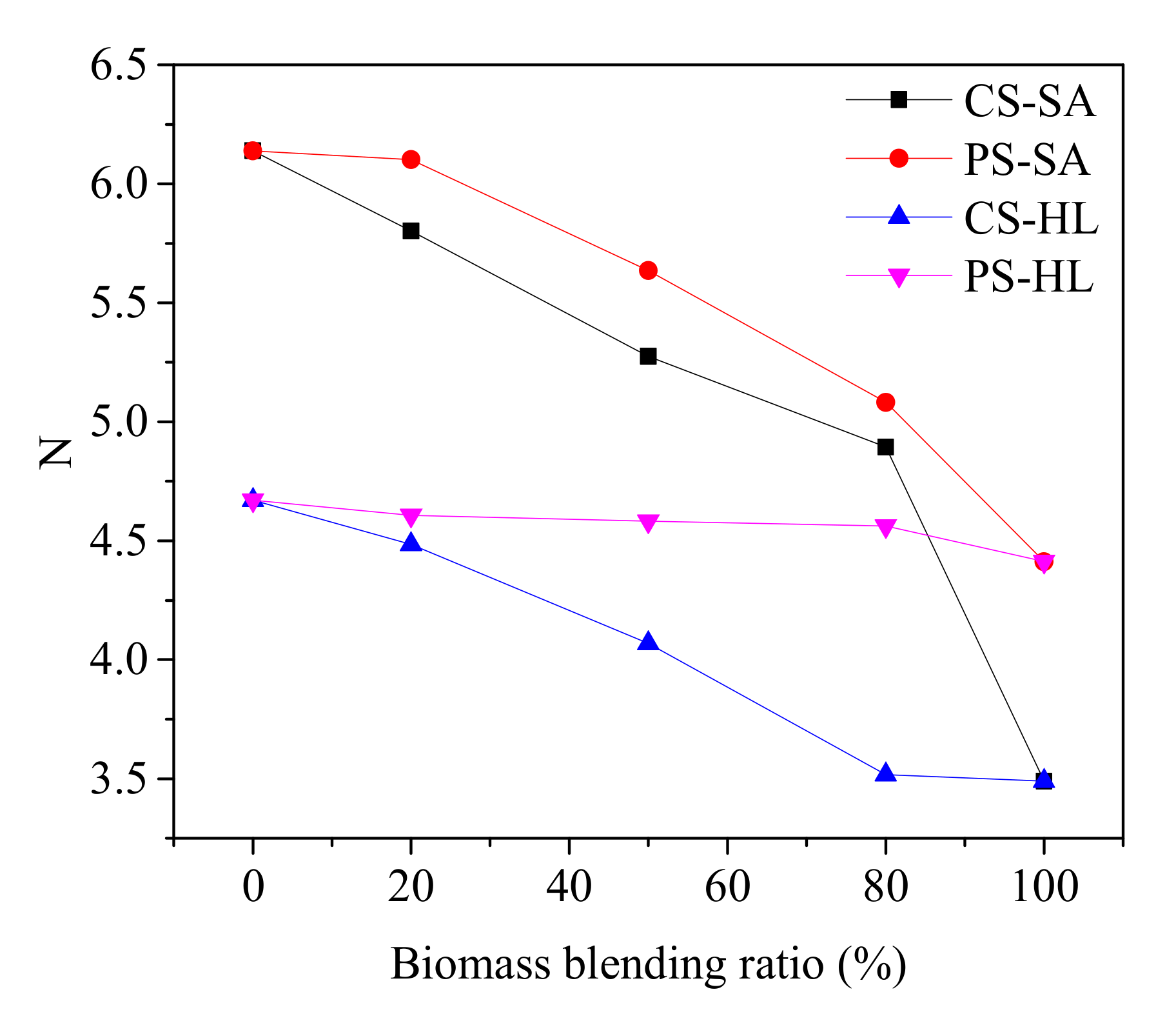
- The graphitization process of coal char was inhibited by the addition of CS and PS during co-pyrolysis, and the inhibition effect of CS and PS on graphitization process was promoted with increasing blend ratio. The reason was that, with the increase in biomass ratio, more AAEM contents in coal char were transferred from biomass. Additionally, the inhibition effect of CS was higher than that of PS at the same blend ratio as a result of the higher AI value as well as more lignin and hemicellulose in CS.
- (3)
- The catalytic activity of inorganic mineral played a much more important role in predicting gasification reactivity than the graphitization degree. Moreover, the relationship R0.5 = 0.07164AI − 0.9432N + 5.7514 was established to better predict the reactivity of coal char in the process of co-gasification between coal and biomass.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature/Abbreviations
| d002 | Interplanar spacing, Å |
| Lc | Stacking height, Å |
| m0 | Initial mass of char sample, mg |
| mf | Final mass of char sample, mg |
| mt | Instantaneous mass of char sample at the reaction time t, mg |
| N | Stacking layer number |
| r | Reaction rate of char sample, %/h |
| R0.5 | Reactivity index, h−1 |
| R2 | Correlation coefficients |
| t | Time, h |
| Xc | Carbon conversion of char sample, % |
| τ0.5 | Time when the carbon conversion reaches 50%, h |
| AAEMs | Alkali and alkaline earth metals |
| AI | Alkali index |
| CI | Catalytic index |
| CS | Corn straw |
| CS2-HL8 | Separated HL char sample, and the weight ratio of CS was 20% in the blend of CS and HL |
| CS5-HL5 | Separated HL char sample, and the weight ratio of CS was 50% in the blend of CS and HL |
| CS8-HL2 | Separated HL char sample, and the weight ratio of CS was 80% in the blend of CS and HL |
| CS2-SA8 | Separated SA char sample, and the weight ratio of CS was 20% in the blend of CS and SA |
| CS5-SA5 | Separated SA char sample, and the weight ratio of CS was 50% in the blend of CS and SA |
| CS8-SA2 | Separated SA char sample, and the weight ratio of CS was 80% in the blend of CS and SA |
| FTIR | Fourier transform infrared spectrometry |
| HL | Hami lignite |
| ICP-OES | Inductively coupled plasma-optical emission spectrometry |
| PS | Poplar sawdust |
| PS2-HL8 | Separated HL char sample, and the weight ratio of PS was 20% in the blend of PS and HL |
| PS5-HL5 | Separated HL char sample, and the weight ratio of PS was 50% in the blend of PS and HL |
| PS8-HL2 | Separated HL char sample, and the weight ratio of PS was 80% in the blend of PS and HL |
| PS2-SA8 | Separated SA char sample, and the weight ratio of PS was 20% in the blend of PS and SA |
| PS5-SA5 | Separated SA char sample, and the weight ratio of PS was 50% in the blend of PS and SA |
| PS8-SA2 | Separated SA char sample, and the weight ratio of PS was 80% in the blend of PS and SA |
| SA | Shanxi anthracite |
| TGA | Thermogravimetric analyzer |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
References
- Roncancio, R.; Gore, J.P. CO2 char gasification: A systematic review from 2014 to 2020. Energy Convers. Manag. X 2020, 10, 100060. [Google Scholar] [CrossRef]
- Wei, J.; Gong, Y.; Guo, Q.; Chen, X.; Ding, L.; Yu, G. A mechanism investigation of synergy behaviour variations during blended char co-gasification of biomass and different rank coals. Renew. Energy 2019, 131, 597–605. [Google Scholar] [CrossRef]
- Xu, C.; Hu, S.; Xiang, J.; Zhang, L.; Sun, L.; Shuai, C.; Chen, Q.; He, L.; Edreis, E.M.A. Interaction and kinetic analysis for coal and biomass co-gasification by TG-FTIR. Bioresour. Technol. 2014, 154, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, W.; Li, Y.; Zhang, B.; Yang, B. On-line analysis on the interaction between organic compounds from co-pyrolysis of microalgae and low-rank coal: Thermal behavior and kinetic characteristics. Bioresour. Technol. 2018, 268, 672–676. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Liu, A.; Wang, L.; Yu, G. Co-pyrolysis characteristic of biomass and bituminous coal. Bioresour. Technol. 2015, 179, 414–420. [Google Scholar] [CrossRef]
- Krerkkaiwan, S.; Fushimi, C.; Tsutsumi, A.; Kuchonthara, P. Synergetic effect during co-pyrolysis/gasification of biomass and sub-bituminous coal. Fuel Processing Technol. 2013, 115, 11–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Yang, M.; Song, Y. Effect of fuel origin on synergy during co-gasification of biomass and coal in CO2. Bioresour. Technol. 2016, 200, 789–794. [Google Scholar] [CrossRef]
- Yang, X.; Liu, X.; Li, R.; Liu, C.; Qing, T.; Yue, X.; Zhang, S. Co-gasification of thermally pretreated wheat straw with Shengli lignite for hydrogen production. Renew. Energy 2018, 117, 501–508. [Google Scholar] [CrossRef]
- Saw, W.L.; Pang, S. Co-gasification of blended lignite and wood pellets in a 100 kW dual fluidised bed steam gasifier: The influence of lignite ratio on producer gas composition and tar content. Fuel 2013, 112, 117–124. [Google Scholar] [CrossRef]
- Cabuk, B.; Duman, G.; Yanik, J.; Olgun, H. Effect of fuel blend composition on hydrogen yield in co-gasification of coal and non-woody biomass. Int. J. Hydrog. Energy 2020, 45, 3435–3443. [Google Scholar] [CrossRef]
- Weiland, N.T.; Means, N.C.; Morreale, B.D. Product distributions from isothermal co-pyrolysis of coal and biomass. Fuel 2012, 94, 563–570. [Google Scholar] [CrossRef]
- Kastanaki, E.; Vamvuka, D.; Grammelis, P.; Kakaras, E. Thermogravimetric studies of the behavior of lignite–biomass blends during devolatilization. Fuel Processing Technol. 2002, 77, 159–166. [Google Scholar] [CrossRef]
- Jong, W.D.; Andries, J.; Hein, K. Coal/biomass co-gasification in a pressurised fluidised bed reactor. Renew. Energy 2014, 16, 1110–1113. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Q.; Rosén, C. Promoted reactivity of char in co-gasification of biomass and coal: Synergies in the thermochemical process. Fuel 1999, 78, 1189–1194. [Google Scholar]
- Li, S.; Chen, X.; Wang, L.; Liu, A.; Yu, G. Co-pyrolysis behaviors of saw dust and Shenfu coal in drop tube furnace and fixed bed reactor. Bioresour. Technol. 2013, 148, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Ellis, N.; Masnadi, M.S.; Roberts, D.G.; Kochanek, M.A.; Ilyushechkin, A.Y. Mineral matter interactions during co-pyrolysis of coal and biomass and their impact on intrinsic char co-gasification reactivity. Chem. Eng. J. 2015, 279, 402–408. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, S.; Zhang, Q.; Hu, J.; Liu, R.; Huang, Z.; Gao, Y. Synergistic effect of the cotton stalk and high-ash coal on gas production during co-pyrolysis/gasification. Bioresour. Technol. 2021, 336, 125336. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, C.; Jiang, Z.; Luo, Z. Structure evolution and gasification characteristic analysis on co-pyrolysis char from lignocellulosic biomass and two ranks of coal: Effect of wheat straw. Fuel 2019, 239, 180–190. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, S.; Luo, Z.; Chen, L.; Meng, H.; Zhao, J. Physico-chemical properties and gasification reactivity of co-pyrolysis char from different rank of coal blended with lignocellulosic biomass: Effects of the cellulose. Bioresour. Technol. 2017, 235, 256–264. [Google Scholar] [CrossRef]
- Li, X.-m.; Zhang, H.; Liu, M.-j.; Zhi, L.-f.; Bai, J.; Bai, Z.-q.; Li, W. Investigation of coal-biomass interaction during co-pyrolysis by char separation and its effect on coal char structure and gasification reactivity with CO2. J. Fuel Chem. Technol. 2020, 48, 897–907. [Google Scholar] [CrossRef]
- Lahijani, P.; Zainal, Z.A.; Mohamed, A.R.; Mohammadi, M. CO2 gasification reactivity of biomass char: Catalytic influence of alkali, alkaline earth and transition metal salts. Bioresour. Technol. 2013, 144, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhiro, S.; Yoshihisa, S.; Yukiaki, H. Influence of Coal Characteristics on CO2 Gasification. Fuel 1982, 61, 717–720. [Google Scholar]
- Liu, M.; Bai, J.; Yu, J.; Kong, L.; Bai, Z.; Li, H.; He, C.; Ge, Z.; Cao, X.; Li, W. Correlation between Char Gasification Characteristics at Different Stages and Microstructure of Char by Combining X-ray Diffraction and Raman Spectroscopy. Energy Fuels 2020, 34, 4162–4172. [Google Scholar] [CrossRef]
- Takarada, T.; Tamai, Y.; Tomita, A. Reactivities of 34 coals under steam gasification. Fuel 1985, 64, 1438–1442. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Wang, X.; Bai, J.; Kong, L.; Bai, Z.; Li, J.; Li, H.; Xing, L.; Li, W. Insights into the effect of particle size on coal char particle gasification by thermogravimetric analyzer and high temperature stage microscope. Fuel 2022, 313, 123010. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, J.; Sheng, C. Effect of pyrolysis temperature on the char micro-structure and reactivity of NO reduction. Korean J. Chem. Eng. 2009, 26, 895–901. [Google Scholar] [CrossRef]
- Hu, J.; Shao, J.; Yang, H.; Lin, G.; Chen, Y.; Wang, X.; Zhang, W.; Chen, H. Co-gasification of coal and biomass: Synergy, characterization and reactivity of the residual char. Bioresour. Technol. 2017, 244, 1–7. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Zhang, L.; Zhao, Y.; Xing, C.; Jiao, Z.; Yang, C.; Qiu, P. Effect of active alkali and alkaline earth metals on physicochemical properties and gasification reactivity of co-pyrolysis char from coal blended with corn stalks. Renew. Energy 2021, 171, 1213–1223. [Google Scholar] [CrossRef]
- Wei, J.; Wang, M.; Wang, F.; Song, X.; Yu, G.; Liu, Y.; Vuthaluru, H.; Xu, J.; Xu, Y.; Zhang, H.; et al. A review on reactivity characteristics and synergy behavior of biomass and coal Co-gasification. Int. J. Hydrog. Energy 2021, 46, 17116–17132. [Google Scholar] [CrossRef]
- He, X.; Liu, X.; Nie, B.; Song, D. FTIR and Raman spectroscopy characterization of functional groups in various rank coals. Fuel 2017, 206, 555–563. [Google Scholar] [CrossRef]
- Yu, J.; Guo, Q.; Ding, L.; Gong, Y.; Yu, G. Studying effects of solid structure evolution on gasification reactivity of coal chars by in-situ Raman spectroscopy. Fuel 2020, 270, 117603. [Google Scholar] [CrossRef]
- Yu, J.; Guo, Q.; Gong, Y.; Ding, L.; Wang, J.; Yu, G. A review of the effects of alkali and alkaline earth metal species on biomass gasification. Fuel Processing Technol. 2021, 214, 106723. [Google Scholar] [CrossRef]
- Li, S.; Song, H.; Hu, J.; Yang, H.; Zou, J.; Zhu, Y.; Tang, Z.; Chen, H. CO2 gasification of straw biomass and its correlation with the feedstock characteristics. Fuel 2021, 297, 120780. [Google Scholar] [CrossRef]




| Sample | Proximate Analysis (wt./%) | Ultimate Analysis (daf, wt./%) | St,d | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | FCd | C | H | O a | N | ||
| SA | 0.80 | 25.19 | 14.00 | 64.33 | 89.52 | 4.02 | 4.42 | 1.59 | 0.34 |
| HL | 7.46 | 9.93 | 44.18 | 42.33 | 74.36 | 5.46 | 17.58 | 0.92 | 1.51 |
| CS | 4.98 | 5.01 | 80.58 | 18.45 | 48.53 | 5.64 | 45.17 | 0.47 | 0.19 |
| PS | 1.35 | 1.60 | 84.31 | 15.44 | 49.67 | 6.19 | 43.99 | 0.14 | 0.01 |
| Sample | Content wt./% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | TiO2 | K2O | P2O5 | Na2O | |
| SA ash | 49.11 | 29.02 | 12.04 | 3.91 | 0.50 | 2.36 | 1.91 | 0.52 | 0.12 | 0.51 |
| HL ash | 17.08 | 8.22 | 22.26 | 21.76 | 1.11 | 24.82 | 0.22 | 0.28 | 0.17 | 2.77 |
| CS ash | 27.01 | 0.86 | 0.43 | 7.95 | 12.01 | 7.54 | 0.05 | 35.91 | 5.86 | 2.39 |
| PS ash | 53.44 | 3.07 | 0.69 | 15.03 | 7.08 | - | 0.18 | 12.51 | 0.54 | - |
| Sample | Content wt./% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | TiO2 | SO3 | K2O | Na2O | P2O5 | |
| SA | 50.42 | 31.19 | 9.05 | 3.52 | 0.43 | 2.03 | 2.29 | 0.46 | 0.45 | 0.16 |
| CS2-SA8 | 56.62 | 27.41 | 3.69 | 3.59 | 0.94 | 1.24 | 2.21 | 3.2 | 0.81 | 0.29 |
| CS5-SA5 | 54.65 | 25.46 | 3.66 | 3.52 | 1.06 | 1.14 | 2.37 | 7.00 | 0.82 | 0.32 |
| CS8-SA2 | 54.41 | 20.92 | 3.62 | 3.70 | 1.78 | 0.85 | 2.32 | 10.93 | 0.96 | 0.51 |
| CS | 18.07 | 0.74 | 0.64 | 10.17 | 15.14 | 0.03 | 7.02 | 39.73 | 4.59 | 3.87 |
| PS2-SA8 | 54.92 | 27.81 | 4.05 | 4.09 | 0.94 | 1.22 | 1.64 | 1.83 | 0.83 | 0.29 |
| PS5-SA5 | 53.26 | 26.52 | 3.95 | 5.08 | 1.09 | 1.14 | 1.91 | 2.54 | 0.86 | 0.36 |
| PS8-SA2 | 48.53 | 22.79 | 3.59 | 8.27 | 1.53 | 0.99 | 2.45 | 4.64 | 1.12 | 0.60 |
| PS | 52.05 | 2.91 | 0.56 | 14.89 | 6.92 | 0.15 | 4.56 | 12.26 | 1.56 | 0.43 |
| HL | 19.20 | 8.35 | 24.58 | 22.28 | 1.12 | 0.22 | 19.26 | 0.46 | 1.70 | 0.18 |
| CS2-HL8 | 16.01 | 7.36 | 17.64 | 19.31 | 2.25 | 0.20 | 20.23 | 4.87 | 2.72 | 0.42 |
| CS5-HL5 | 15.70 | 5.97 | 13.76 | 16.10 | 4.36 | 0.16 | 16.99 | 13.65 | 2.25 | 0.90 |
| CS8-HL2 | 15.25 | 3.13 | 6.42 | 9.65 | 6.55 | 0.09 | 8.52 | 22.29 | 1.03 | 1.51 |
| PS2-HL8 | 26.61 | 7.48 | 16.89 | 21.97 | 2.01 | 0.19 | 18.69 | 3.36 | 2.43 | 0.37 |
| PS5-HL5 | 29.61 | 6.99 | 12.94 | 18.43 | 3.78 | 0.15 | 14.03 | 11.48 | 1.91 | 0.68 |
| PS8-HL2 | 43.15 | 4.21 | 5.76 | 11.94 | 5.64 | 0.07 | 7.87 | 19.33 | 0.88 | 1.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; He, J.; Liu, M.; Bai, J.; Bai, Z.; Li, W. Interaction between Coal and Biomass during Co-Gasification: A Perspective Based on the Separation of Blended Char. Processes 2022, 10, 286. https://doi.org/10.3390/pr10020286
Li X, He J, Liu M, Bai J, Bai Z, Li W. Interaction between Coal and Biomass during Co-Gasification: A Perspective Based on the Separation of Blended Char. Processes. 2022; 10(2):286. https://doi.org/10.3390/pr10020286
Chicago/Turabian StyleLi, Xiaoming, Jingxia He, Mengjie Liu, Jin Bai, Zongqing Bai, and Wen Li. 2022. "Interaction between Coal and Biomass during Co-Gasification: A Perspective Based on the Separation of Blended Char" Processes 10, no. 2: 286. https://doi.org/10.3390/pr10020286
APA StyleLi, X., He, J., Liu, M., Bai, J., Bai, Z., & Li, W. (2022). Interaction between Coal and Biomass during Co-Gasification: A Perspective Based on the Separation of Blended Char. Processes, 10(2), 286. https://doi.org/10.3390/pr10020286







