Influence of Nano-Silica/Chitosan Film Coating on the Quality of ‘Tommy Atkins’ Mango
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Material
2.2. Chemicals
2.3. Preparation of Edible Coatings Film
2.4. Treatment and Coating Application
| T1 | 2% Chitosan |
| T2 | 1% Chitosan nanoparticles |
| T3 | 1% Nano-silicon dioxide |
| T4 | 2% Chitosan + 1% Nano-silicon dioxide |
| T5 | Control (dipping fruits with distilled water) |
2.5. Storage Conditions
2.6. Fruit Quality Attributes
2.6.1. Weight Loss Percentage
2.6.2. Decay Percentage
2.6.3. Hue Angle Skin Color (h°)
2.6.4. Fruit Firmness (lb inch−2)
2.6.5. Ethylene Production
2.6.6. Respiration Rate
2.6.7. Total Soluble Solid (TSS) %
2.6.8. Titratable Acidity (TA) %
2.6.9. Vitamin C Content (mg 100 g−1 FW)
2.6.10. Total Sugar (%)
2.6.11. Total Phenolic Content (mg g−1 FW)
2.6.12. Antioxidant % (DPPH Radical Assay of Fruit Peel)
2.6.13. Sensory Evaluation/Organoleptic Test of Fruits
2.7. Statistical Analysis
3. Results
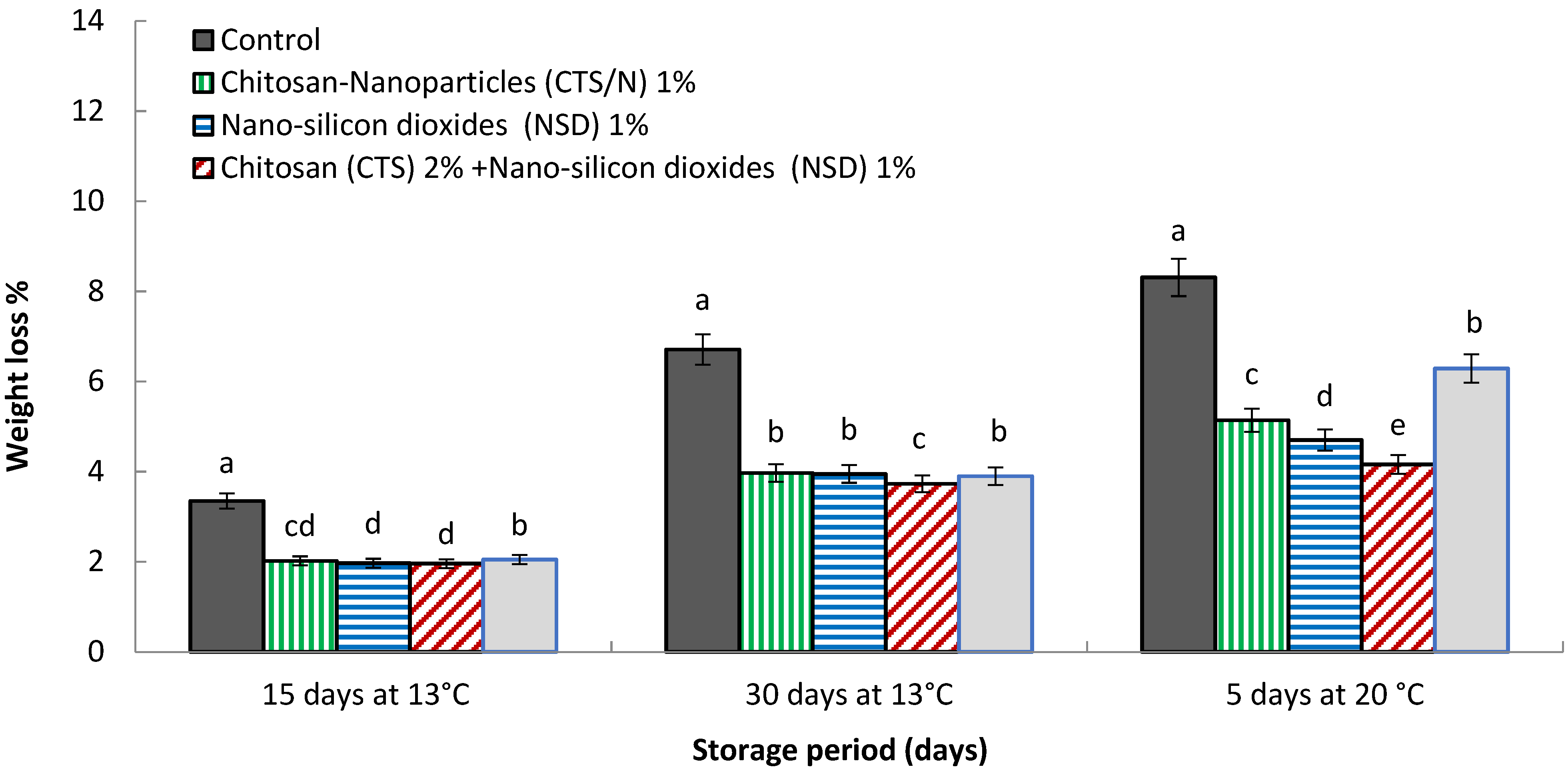
3.1. Weight Loss %
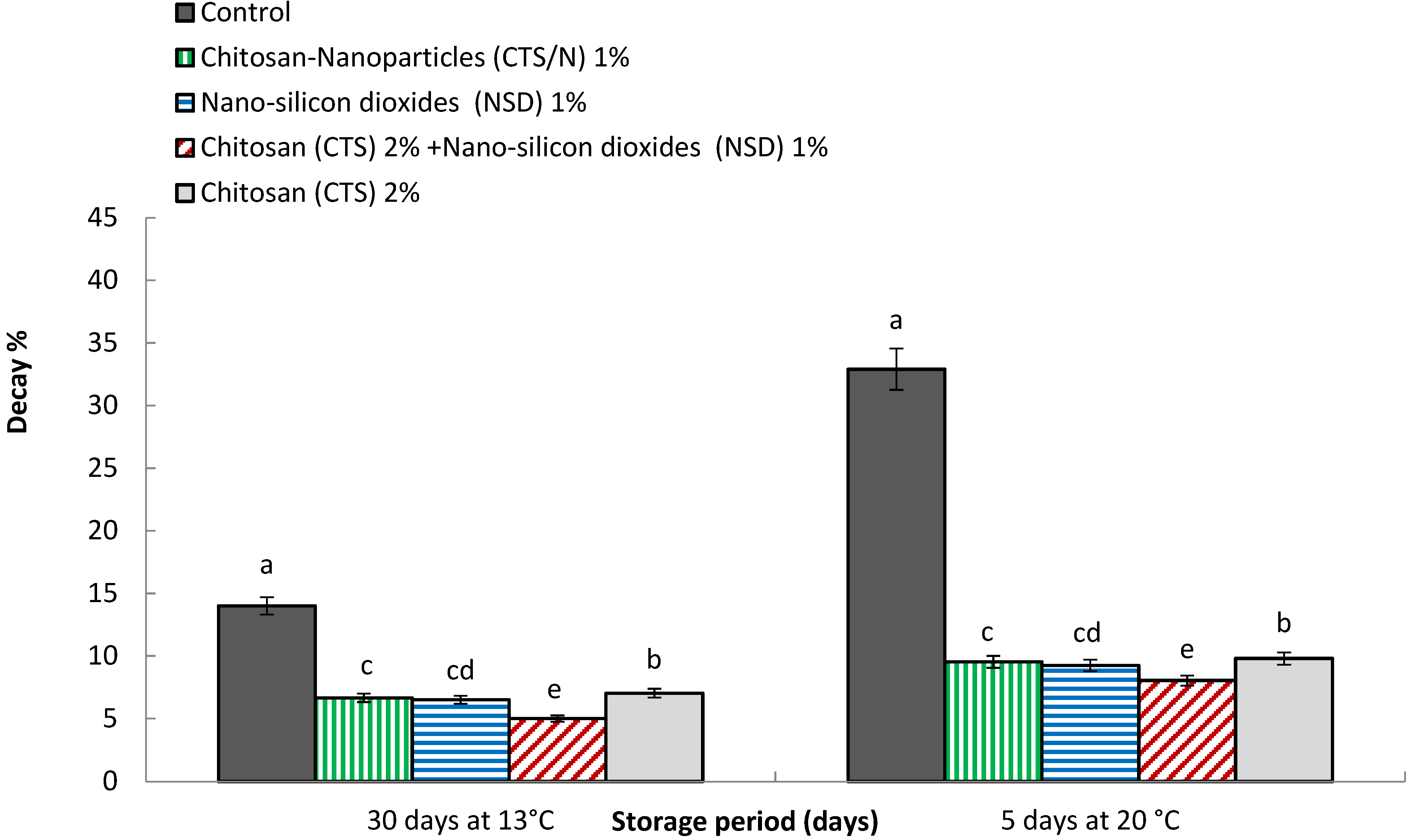
3.2. Decay Percentage
3.3. Skin Hue Color (h°)
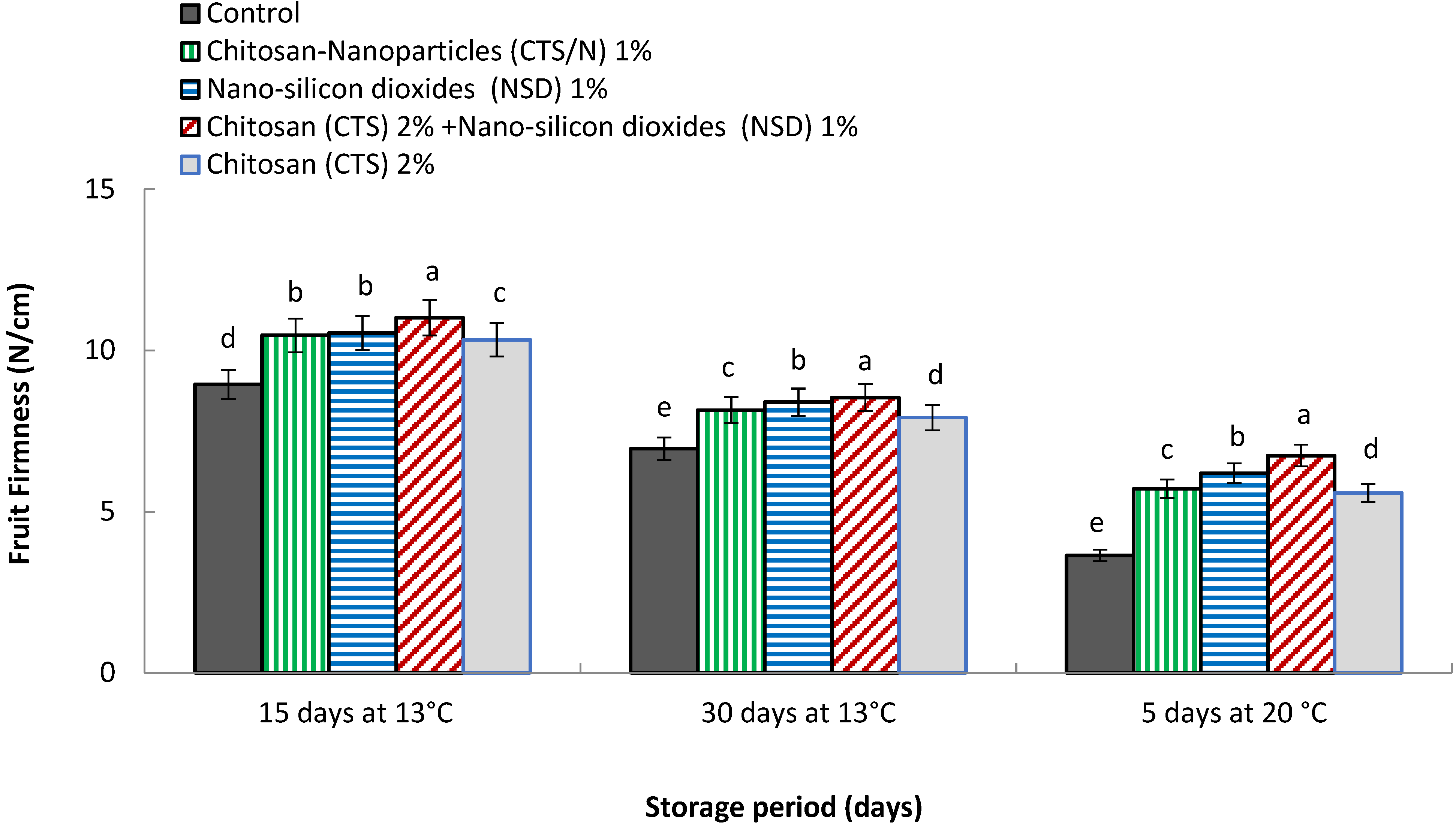
3.4. Fruit Firmness (N/cm)
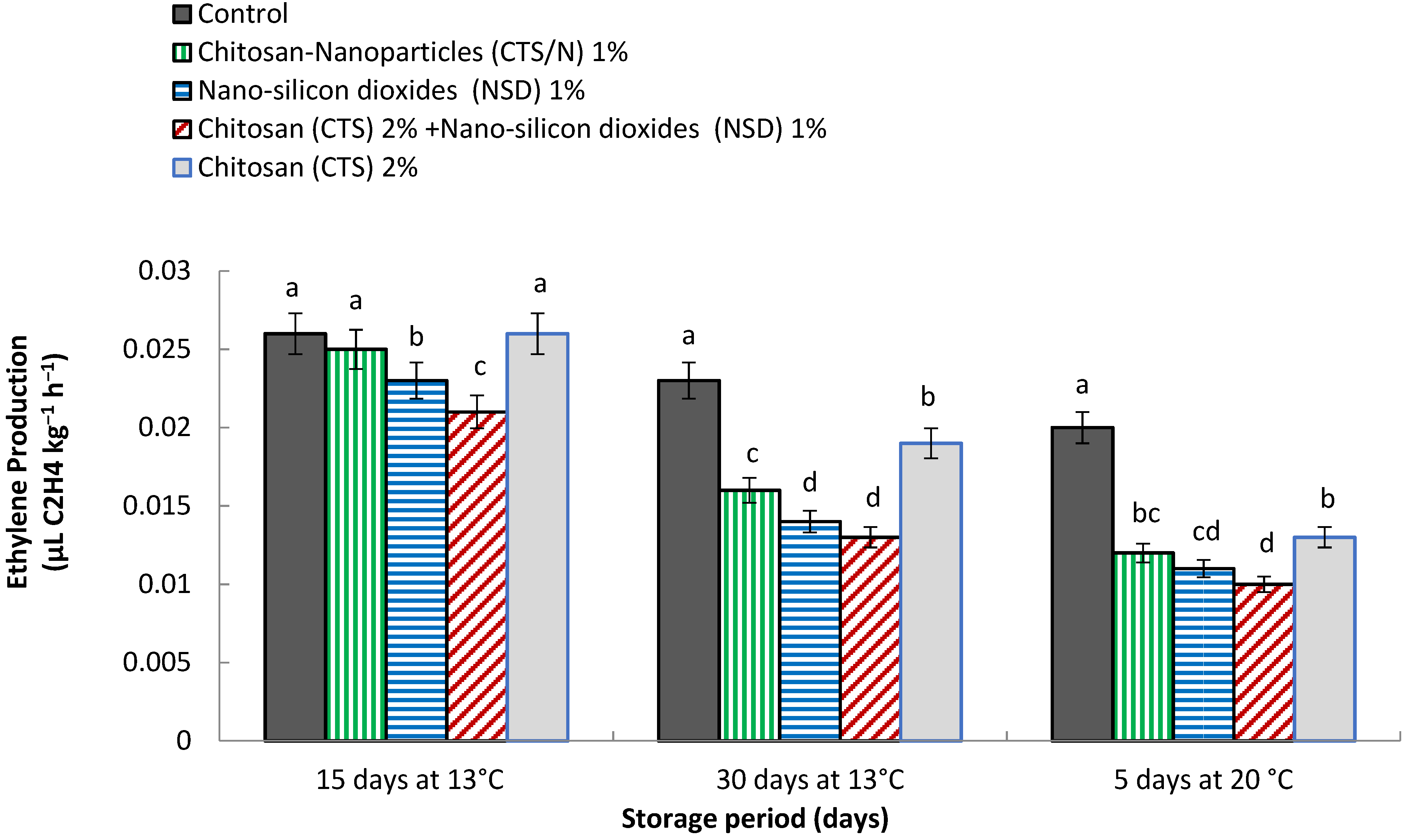
3.5. Ethylene Production (µL C2H4 kg−1 h−1)
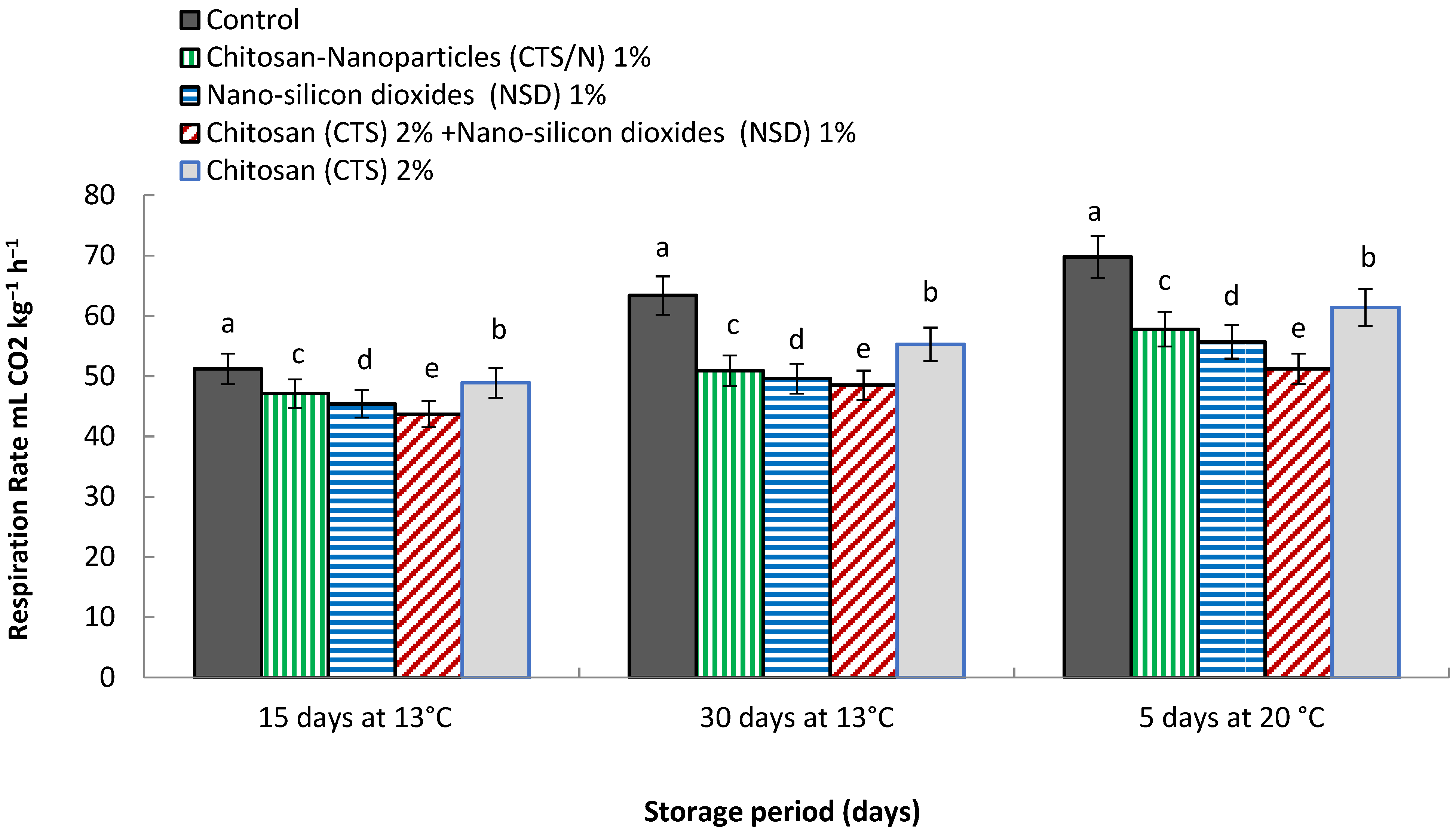
3.6. Respiration Rate mL CO2 kg−1 h−1
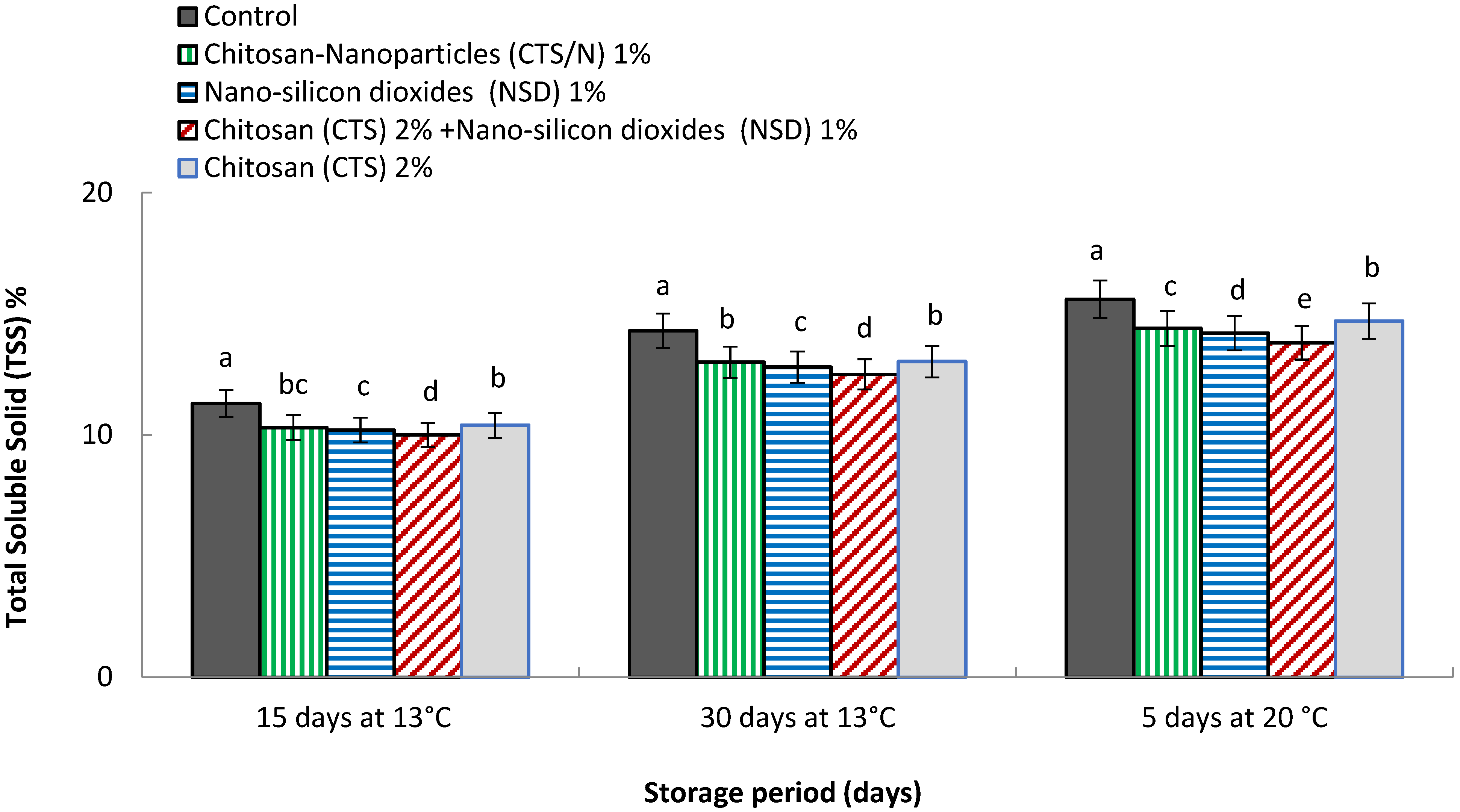
3.7. Total Soluble Solid (TSS %)
3.8. Titratable Acidity (TA)
3.9. Vitamin C (mg 100 g−1 FW)
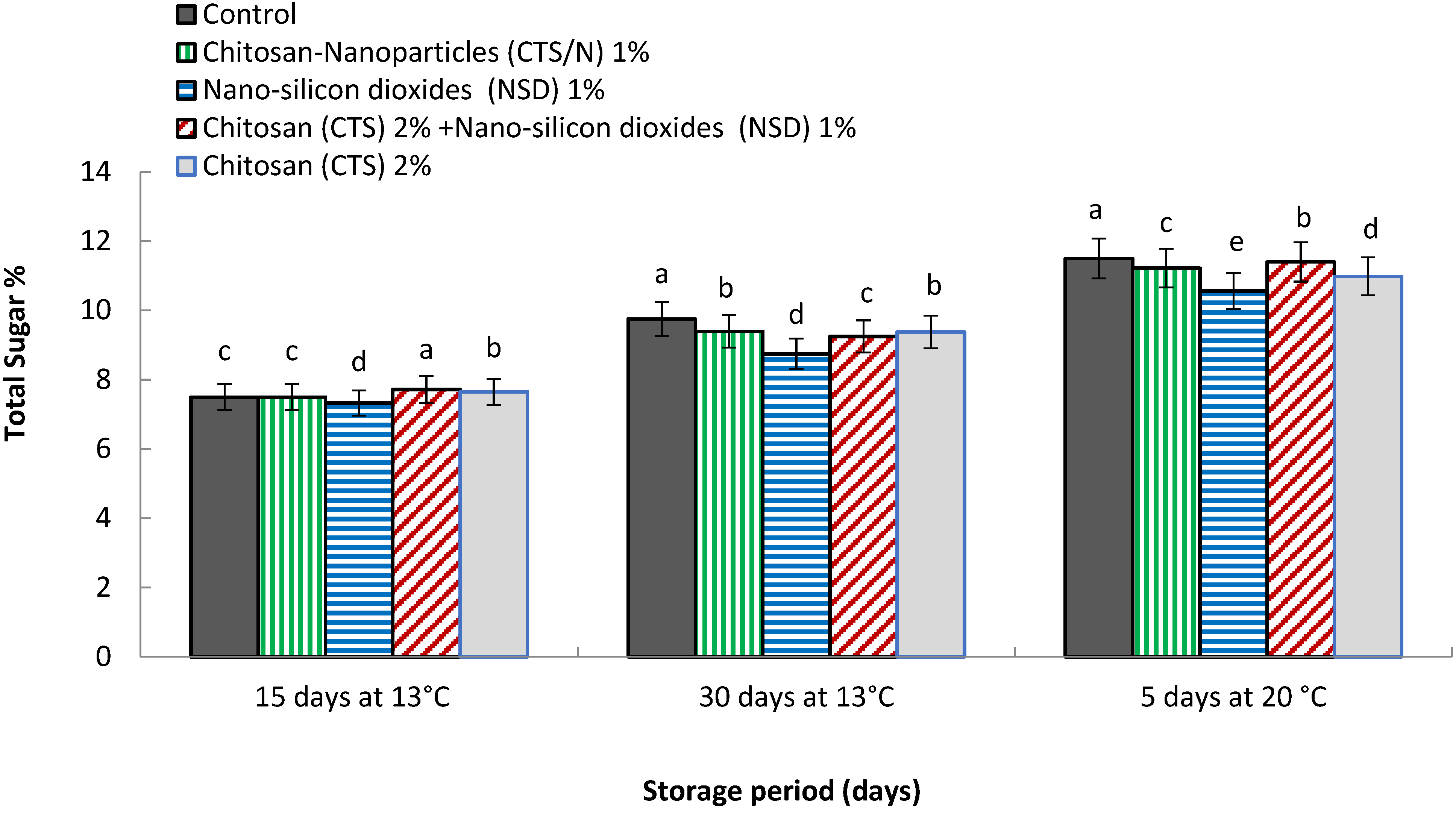
3.10. Total Sugar (mg 100 g−1 FW)
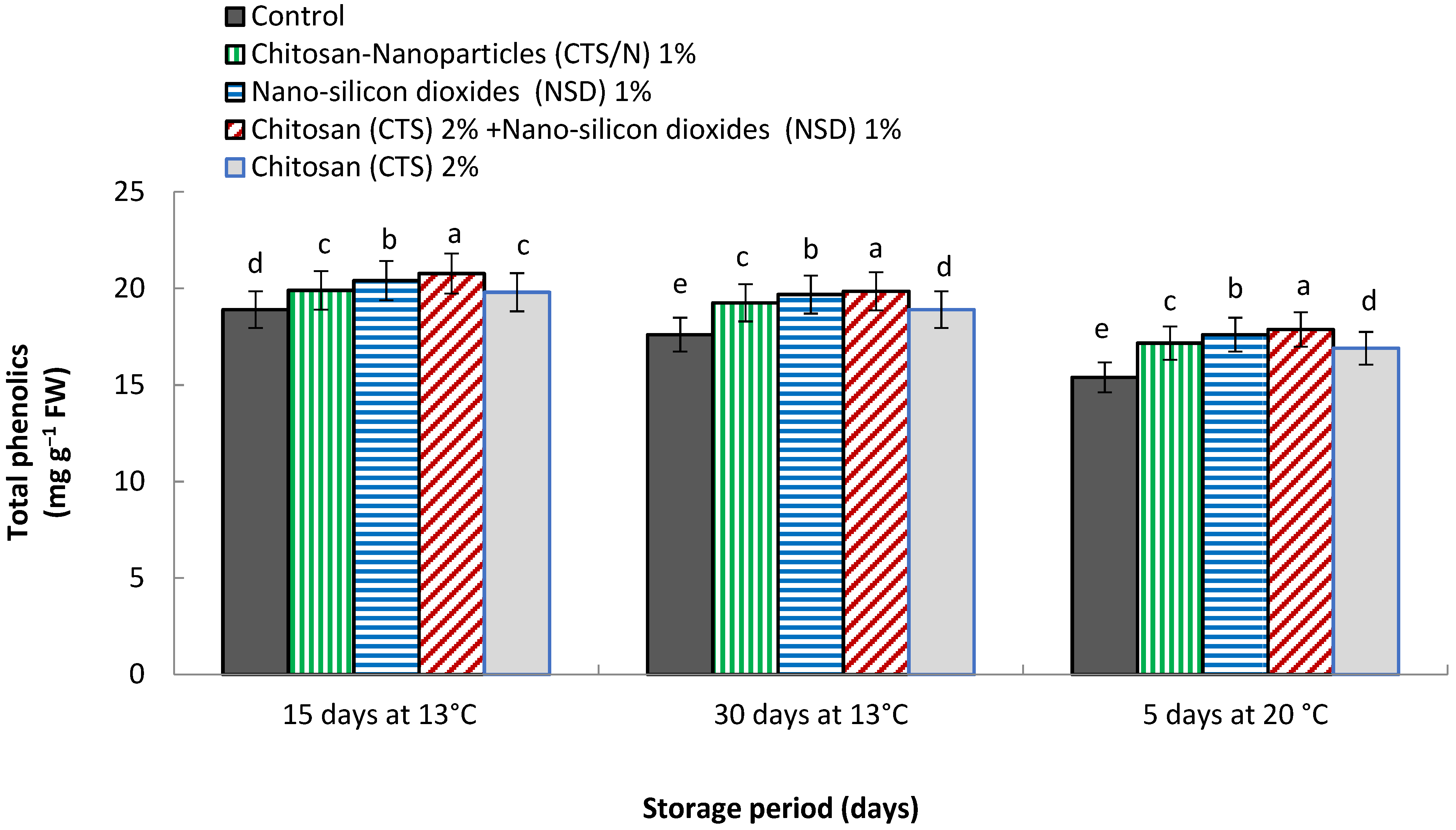
3.11. Total Phenolic Contents (mg 100 g−1 FW)
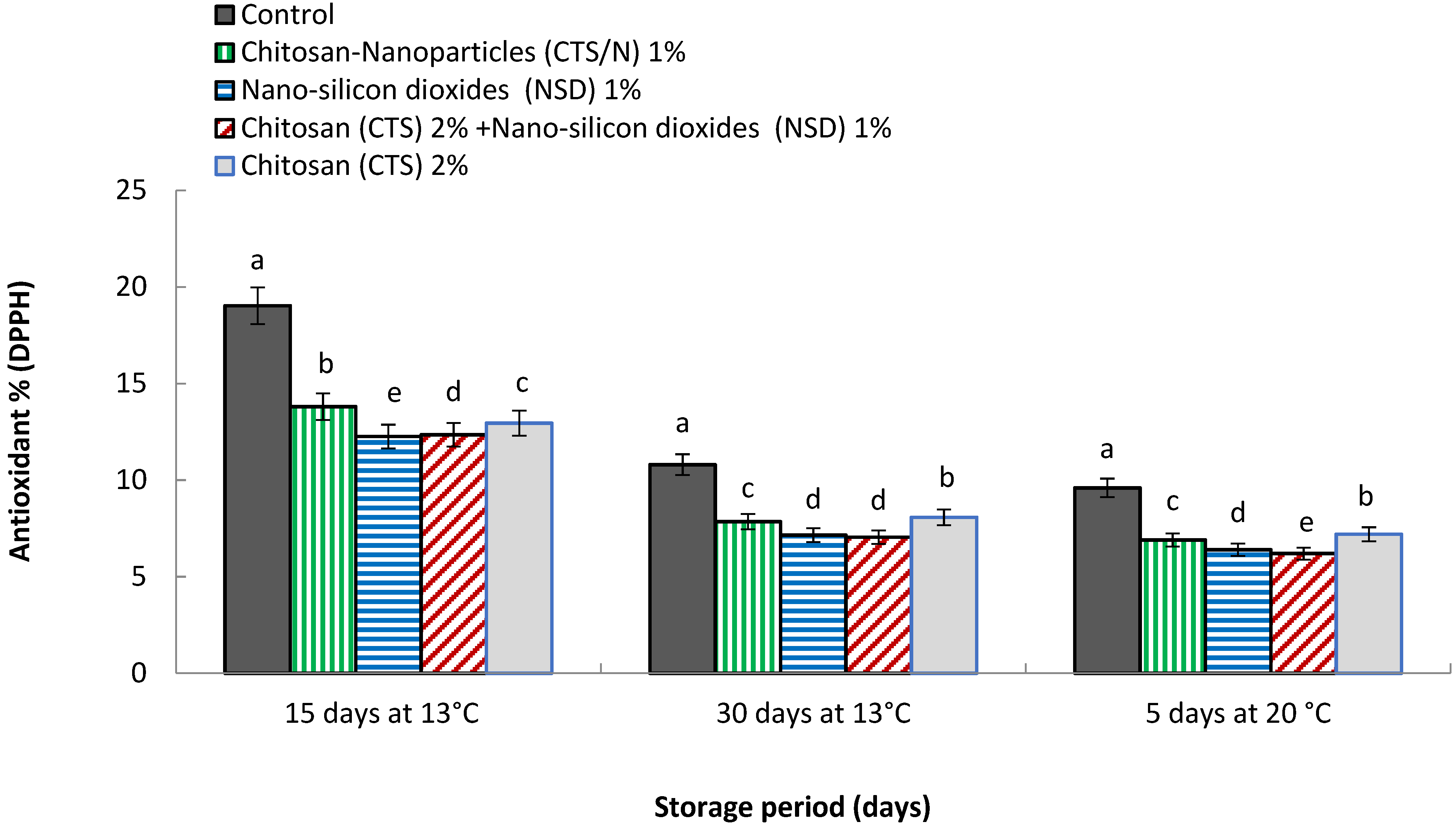
3.12. Antioxidant % (DPPH Radical Scavenging Assay)
3.13. Sensory Evaluation/Organoleptic Test (Taste, Aroma, Texture, Flavor)
4. Discussion
4.1. Weight Loss %
4.2. Decay Percentage
4.3. Skin Hue Color (h°)
4.4. Fruit Firmness (lb inch−2)
4.5. Ethylene Production (µL C2H4 kg−1 h−1)
4.6. Respiration Rate mL CO2 kg−1 h−1
4.7. Total Soluble Solid (TSS %)
4.8. Titratable Acidity (TA)
4.9. Vitamin C (mg 100 g−1 FW)
4.10. Total Sugar (mg 100 g−1 FW)
4.11. Total Phenolic Contents (mg 100 g−1 FW)
4.12. Antioxidant % (DPPH Radical Scavenging Assay)
4.13. Sensory Evaluation/Organoleptic Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akurugu, G.K.; Olympio, N.S.; Ninfaa, D.A.; Karbo, E.Y. Evaluation of Post Harvest Handling and Marketing of Mango (Mangifera indica) in Ghana (a Case Study in Northern Region). J. Res. Agric. Anim. Sci. 2016, 4, 4–9. [Google Scholar]
- FAO. Major Tropical Fruits—Statistical Compendium 2018; FAO: Rome, Italy, 2019. [Google Scholar]
- Sivakumar, D.; Jiang, Y.; Yahia, E.M. Maintaining mango (Mangifera indica L.) fruit quality during the export chain. Food Res. Int. 2011, 44, 1254–1263. [Google Scholar] [CrossRef]
- Jahurul, M.; Zaidul, I.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.-L.; Norulaini, N.; Sahena, F.; Omar, A.M. Mango (Mangifera indica L.) by-products and their valuable components: A review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Mango Production and Exports in Egypt; FAOSTAT, FAO: Rome, Italy, 2018. [Google Scholar]
- Cárdenas-Coronel, W.G.; Velez-de la Rocha, R.; Siller-Cepeda, J.H.; Osuna-Enciso, T.; Muy-Rangel, M.D.; Sañudo-Barajas, J.A. Changes in the composition of starch, pectins and hemicelluloses during the ripening stage of mango (Mangifera indica cv. Kent). Rev. Chapingo Ser. Hortic. 2012, 18, 05–19. [Google Scholar]
- Narayana, C.; Pal, R.; Roy, S. Effect of pre-storage treatments and temperature regimes on shelf-life and respiratory behaviour of ripe Baneshan mango. J. Food Sci. Technol. Mysore 1996, 33, 79–82. [Google Scholar]
- Ali, S.; Anjum, M.A.; Khan, A.S.; Nawaz, A.; Ejaz, S.; Khaliq, G.; Iqbal, S.; Ullah, S.; Rehman, R.N.U.; Ali, M.M. Carboxymethyl Cellulose Coating Delays Ripening of Harvested Mango Fruits by Regulating Softening Enzymes Activities. Food Chem. 2021, 168, 131804. [Google Scholar] [CrossRef]
- Lalel, H.J.; Singh, Z.; Tan, S.C. Aroma volatiles production during fruit ripening of ‘Kensington Pride’ mango. Postharvest Biol. Technol. 2003, 27, 323–336. [Google Scholar] [CrossRef]
- Zheng, X.; Ye, L.; Jiang, T.; Jing, G.; Li, J. Limiting the deterioration of mango fruit during storage at room temperature by oxalate treatment. Food Chem. 2012, 130, 279–285. [Google Scholar] [CrossRef]
- Carrillo-Lopez, A.; Ramirez-Bustamante, F.; Valdez-Torres, J.; Rojas-Villegas, R.; Yahia, E. Ripening and quality changes in mango fruit as affected by coating with an edible film. J. Food Qual. 2000, 23, 479–486. [Google Scholar] [CrossRef]
- Maina, B.; Ambuko, J.; Hutchinson, M.J.; Owino, W.O. The Effect of Waxing Options on Shelf Life and Postharvest Quality of “ngowe” Mango Fruits under Different Storage Conditions. Adv. Agric. 2019, 2019, 5085636. [Google Scholar] [CrossRef] [Green Version]
- McHugh, T.; Senesi, E. Apple wraps: A novel method to improve the quality and extend the shelf life of fresh-cut apples. J. Food Sci. 2000, 65, 480–485. [Google Scholar] [CrossRef]
- Rastegar, S.; Atrash, S. Effect of alginate coating incorporated with Spirulina, Aloe vera and guar gum on physicochemical, respiration rate and color changes of mango fruits during cold storage. J. Food Meas. Charact. 2021, 15, 265–275. [Google Scholar] [CrossRef]
- Sorrentino, A.; Gorrasi, G.; Vittoria, V. Potential perspectives of bio-nanocomposites for food packaging applications. Trends Food Sci. Technol. 2007, 18, 84–95. [Google Scholar] [CrossRef]
- Kumar, N.; Petkoska, A.T.; AL-Hilifi, S.A.; Fawole, O.A. Effect of Chitosan–Pullulan Composite Edible Coating Functionalized with Pomegranate Peel Extract on the Shelf Life of Mango (Mangifera indica). Coatings 2021, 11, 764. [Google Scholar] [CrossRef]
- Tripathi, P.; Dubey, N. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol. Technol. 2004, 32, 235–245. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Hernández-López, M.; Bosquez-Molina, E.; Wilson, C. Effects of chitosan and plant extracts on growth of Colletotrichum gloeosporioides, anthracnose levels and quality of papaya fruit. Crop Prot. 2003, 22, 1087–1092. [Google Scholar] [CrossRef]
- Sousa, F.F.; Junior, J.S.P.; Oliveira, K.T.; Rodrigues, E.C.; Andrade, J.P.; Mattiuz, B.-H. Conservation of ‘palmer’ mango with an edible coating of hydroxypropyl methylcellulose and beeswax. Food Chem. 2021, 346, 128925. [Google Scholar] [CrossRef] [PubMed]
- Eshghi, S.; Hashemi, M.; Mohammadi, A.; Badii, F.; Mohammadhoseini, Z.; Ahmadi, K. Effect of nanochitosan-based coating with and without copper loaded on physicochemical and bioactive components of fresh strawberry fruit (Fragaria x ananassa Duchesne) during storage. Food Bioprocess Technol. 2014, 7, 2397–2409. [Google Scholar] [CrossRef]
- Nasr, F.; Pateiro, M.; Rabiei, V.; Razavi, F.; Formaneck, S.; Gohari, G.; Lorenzo, J.M. Chitosan-Phenylalanine Nanoparticles (Cs-Phe Nps) Extend the Postharvest Life of Persimmon (Diospyros kaki) Fruits under Chilling Stress. Coatings 2021, 11, 819. [Google Scholar] [CrossRef]
- Lustriane, C.; Dwivany, F.M.; Suendo, V.; Reza, M. Effect of chitosan and chitosan-nanoparticles on post harvest quality of banana fruits. J. Plant Biotechnol. 2018, 45, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Resende, N.; Gonçalves, G.; Reis, K.; Tonoli, G.; Boas, E. Chitosan/cellulose nanofibril nanocomposite and its effect on quality of coated strawberries. J. Food Qual. 2018, 2018, 1727426. [Google Scholar] [CrossRef] [Green Version]
- Meena, M.; Pilania, S.; Pal, A.; Mandhania, S.; Bhushan, B.; Kumar, S.; Gohari, G.; Saharan, V. Cu-chitosan nano-net improves keeping quality of tomato by modulating physio-biochemical responses. Sci. Rep. 2020, 10, 21914. [Google Scholar] [CrossRef] [PubMed]
- Amin, U.; Khan, M.K.I.; Khan, M.U.; Akram, M.E.; Pateiro, M.; Lorenzo, J.M.; Maan, A.A. Improvement of the Performance of Chitosan–Aloe vera Coatings by Adding Beeswax on Postharvest Quality of Mango Fruit. Foods 2021, 10, 2240. [Google Scholar] [CrossRef] [PubMed]
- Sami, R.; Elhakem, A.; Alharbi, M.; Benajiba, N.; Fikry, M.; Helal, M. The combined effect of coating treatments to nisin, nano-silica, and chitosan on oxidation processes of stored button mushrooms at 4 C. Sci. Rep. 2021, 11, 6031. [Google Scholar] [CrossRef]
- Eldib, R. Application of Nano-coating and Chitosan Combination Films on Cantaloupe Preservation. Pak. J. Biol. Sci. PJBS 2020, 23, 1037–1043. [Google Scholar] [CrossRef]
- Li, Y.; Rokayya, S.; Jia, F.; Nie, X.; Xu, J.; Elhakem, A.; Almatrafi, M.; Benajiba, N.; Helal, M. Shelf-life, quality, safety evaluations of blueberry fruits coated with chitosan nano-material films. Sci. Rep. 2021, 11, 55. [Google Scholar] [CrossRef]
- Song, H.; Yuan, W.; Jin, P.; Wang, W.; Wang, X.; Yang, L.; Zhang, Y. Effects of chitosan/nano-silica on postharvest quality and antioxidant capacity of loquat fruit during cold storage. Postharvest Biol. Technol. 2016, 119, 41–48. [Google Scholar] [CrossRef]
- Cardoso-Almeida, F.D.A.; Figueiredo-Neto, A.; Lucena-Cavalcante, Í.H. Physical and mechanical parameters correlated to the ripening of mangoes (Mangifera indica L.) cv. ‘Tommy Atkins’. Acta Agron. 2017, 66, 186–192. [Google Scholar]
- Rokayya, S.; Khojah, E.; Elhakem, A.; Benajiba, N.; Chavali, M.; Vivek, K.; Iqbal, A.; Helal, M. Investigating the nano-films effect on physical, mechanical properties, chemical changes, and microbial load contamination of white button mushrooms during storage. Coatings 2021, 11, 44. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Haghighi, M.; Pessarakli, M. Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage. Sci. Hortic. 2013, 161, 111–117. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, D.; Belwal, T.; Li, L.; Chen, H.; Xu, T.; Luo, Z. Effect of nano-SiOx/chitosan complex coating on the physicochemical characteristics and preservation performance of green Tomato. Molecules 2019, 24, 4552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sami, R.; Khojah, E.; Elhakem, A.; Benajiba, N.; Helal, M.; Alhuthal, N.; Alzahrani, S.A.; Alharbi, M.; Chavali, M. Performance study of nano/SiO2 films and the antimicrobial application on cantaloupe fruit shelf-life. Appl. Sci. 2021, 11, 3879. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Pristijono, P.; Golding, J.B.; Bowyer, M.C. Postharvest UV-C treatment, followed by storage in a continuous low-level ethylene atmosphere, maintains the quality of ‘Kensington pride’ mango fruit stored at 20 C. Horticulturae 2019, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Ranganna, S. Handbook of Analysis and Quality Control for Fruit and Vegetable Products; Tata McGraw-Hill Education: New York, NY, USA, 1986. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005; Volume 16. [Google Scholar]
- James, C.S. Analytical Chemistry of Food; University of Plymouth: Plymouth, UK, 1995; pp. 96–97. [Google Scholar]
- Chun, O.K.; Kim, D.-O.; Lee, C.Y. Superoxide radical scavenging activity of the major polyphenols in fresh plums. J. Agric. Food Chem. 2003, 51, 8067–8072. [Google Scholar] [CrossRef]
- Ao, C.; Li, A.; Elzaawely, A.A.; Xuan, T.D.; Tawata, S. Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. fil. extract. Food Control 2008, 19, 940–948. [Google Scholar] [CrossRef]
- Manasa, B. Evaluation of sensory attributes of mango fruits CV ‘Alphonso’ as influenced by pre-harvest treatments. J. Pharmacogn. Phytochem. 2019, 8, 2475–2480. [Google Scholar]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Gardesh, A.S.K.; Badii, F.; Hashemi, M.; Ardakani, A.Y.; Maftoonazad, N.; Gorji, A.M. Effect of nanochitosan based coating on climacteric behavior and postharvest shelf-life extension of apple cv. Golab Kohanz. LWT 2016, 70, 33–40. [Google Scholar] [CrossRef]
- Zaharah, S.S.; Singh, Z.; Symons, G.M.; Reid, J.B. Role of brassinosteroids, ethylene, abscisic acid, and indole-3-acetic acid in mango fruit ripening. J. Plant Growth Regul. 2012, 31, 363–372. [Google Scholar] [CrossRef]
- Romanazzi, G.; Nigro, F.; Ippolito, A.; Divenere, D.; Salerno, M. Effects of pre-and postharvest chitosan treatments to control storage grey mold of table grapes. J. Food Sci. 2002, 67, 1862–1867. [Google Scholar] [CrossRef]
- Bibi, F.; Baloch, M.K. Postharvest Quality and Shelf Life of Mango (Mangifera indica L.) Fruit as Affected by Various Coatings. J. Food Process. Preserv. 2014, 38, 499–507. [Google Scholar] [CrossRef]
- Dautt-Castro, M.; López-Virgen, A.G.; Ochoa-Leyva, A.; Contreras-Vergara, C.A.; Sortillón-Sortillón, A.P.; Martínez-Téllez, M.A.; González-Aguilar, G.A.; Casas-Flores, J.S.; Sañudo-Barajas, A.; Kuhn, D.N. Genome-wide identification of mango (Mangifera indica L.) Polygalacturonases: Expression analysis of family members and Total enzyme activity during fruit ripening. Front. Plant Sci. 2019, 10, 969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jitareerat, P.; Paumchai, S.; Kanlayanarat, S.; Sangchote, S. Effect of chitosan on ripening, enzymatic activity, and disease development in mango (Mangifera indica) fruit. N. Z. J. Crop Hortic. Sci. 2007, 35, 211–218. [Google Scholar] [CrossRef]
- Adiletta, G.; Di Matteo, M.; Petriccione, M. Multifunctional Role of Chitosan Edible Coatings on Antioxidant Systems in Fruit Crops: A Review. Int. J. Mol. Sci. 2021, 22, 2633. [Google Scholar] [CrossRef]
- Mebratie, M.A.; Woldetsadik, K.; Ayalew, A.; Haji, J. Comparative study of different banana ripening methods. Sci. Technol. Arts Res. J. 2015, 4, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Silva, G.M.C.; Silva, W.B.; Medeiros, D.B.; Salvador, A.R.; Cordeiro, M.H.M.; da Silva, N.M.; Santana, D.B.; Mizobutsi, G.P. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 2017, 237, 372–378. [Google Scholar] [CrossRef]
- Woolf, A.B.; Cox, K.A.; White, A.; Ferguson, I.B. Low temperature conditioning treatments reduce external chilling injury of ‘Hass’ avocados. Postharvest Biol. Technol. 2003, 28, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Gol, N.B.; Chaudhari, M.L.; Rao, T.R. Effect of edible coatings on quality and shelf life of carambola (Averrhoa carambola L.) fruit during storage. J. Food Sci. Technol. 2015, 52, 78–91. [Google Scholar] [CrossRef]
- Tefera, A.; Seyoum, T.; Woldetsadik, K. Effects of disinfection, packaging and evaporatively cooled storage on sugar content of mango. Afr. J. Biotechnol. 2008, 7, 65–72. [Google Scholar]
- Zeb, S.; Sajid, M.; Shah, S.T.; Ali, M.; Ali, S.; Nawaz, Z.; Rauf, K.; Khan, A.; Shah, H.; Shah, S.A.A. 34. Influence of post-harvest application of chitosan on physico-chemical changes of apple fruit during storage. Pure Appl. Biol. PAB 2020, 9, 2554–2562. [Google Scholar]
- Zhu, F.; Yun, Z.; Ma, Q.; Gong, Q.; Zeng, Y.; Xu, J.; Cheng, Y.; Deng, X. Effects of exogenous 24-epibrassinolide treatment on postharvest quality and resistance of Satsuma mandarin (Citrus unshiu). Postharvest Biol. Technol. 2015, 100, 8–15. [Google Scholar] [CrossRef]
- Venkatesh, J.; Park, S.W. Role of L-ascorbate in alleviating abiotic stresses in crop plants. Bot. Stud. 2014, 55, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Li, M.; Mitra, S.; Hafiz Muhammad, R.; Debnath, B.; Lu, X.; Jian, H.; Qiu, D. Comparative phytochemical profiles and antioxidant enzyme activity analyses of the southern highbush blueberry (Vaccinium corymbosum) at different developmental stages. Molecules 2018, 23, 2209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, G.B.; Susmitha, P. Silicon uptake, transportation and accumulation in Rice. J. Pharmacogn. Phytochem. 2017, 6, 290–293. [Google Scholar]
- Lattanzio, V.; Kroon, P.A.; Quideau, S.; Treutter, D. Plant phenolics—Secondary metabolites with diverse functions. Recent Adv. Polyphen. Res. 2008, 1, 1–35. [Google Scholar]
- Kondo, S.; Kittikorn, M.; Kanlayanarat, S. Preharvest antioxidant activities of tropical fruit and the effect of low temperature storage on antioxidants and jasmonates. Postharvest Biol. Technol. 2005, 36, 309–318. [Google Scholar] [CrossRef]
- Valenzuela, J.L.; Manzano, S.; Palma, F.; Carvajal, F.; Garrido, D.; Jamilena, M. Oxidative stress associated with chilling injury in immature fruit: Postharvest technological and biotechnological solutions. Int. J. Mol. Sci. 2017, 18, 1467. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, S.; Ren, Y.; Li, H.; Zhang, X.; Di, J. Jujube preservation using chitosan film with nano-silicon dioxide. J. Food Eng. 2012, 113, 408–414. [Google Scholar] [CrossRef]
- Akhtar, S.; Mahmood, S.; Naz, S.; Nasir, M.; Sultan, M. Sensory evaluation of mangoes (Mangifera indica L.) grown in different regions of Pakistan. Pak. J. Bot 2009, 41, 2821–2829. [Google Scholar]
- Engel, K.H.; Tressl, R. Studies on the volatile components of two mango varieties. J. Agric. Food Chem. 1983, 31, 796–801. [Google Scholar] [CrossRef]
- Malundo, T.; Shewfelt, R.; Ware, G.; Baldwin, E. Sugars and acids influence flavor properties of mango (Mangifera indica). J. Am. Soc. Hortic. Sci. 2001, 126, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, N.A.; Iqbal, Z.; Maqbool, M.; Hafiz, I.A. Postharvest quality of mango (Mangifera indica L.) fruit as affected by chitosan coating. Pak. J. Bot 2009, 41, 343–357. [Google Scholar]
- Kaswija, M.; Peter, M.; Leonard, F. Sensory attributes, microbial quality and aroma profiles of off vine ripened mango (Mangifera indica L.) fruit. Afr. J. Biotechnol. 2006, 5, 201–205. [Google Scholar]



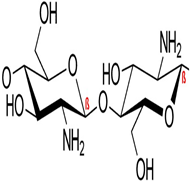 | 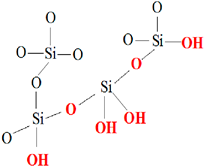 |
| (a) CAS Registry Number: 9012-76-4 Mol weight: 50,000–190,000 Da (based on viscosity) Viscosity: 20–300 cP, 1 wt.% in 1% acetic acid (25 °C, Brookfield)(lit.) Solubility: Dilute aqueous acid: soluble | (b) CAS Registry Number: 7631-86-9 Chemical formula: SiO2 Molar mass: 60.08 g/mol Density: 2.648 (α-quartz), 2.196 (amorphous) g cm−3 Melting point: 1.713 °C (Amp) Thermal conductivity: 12 (|| c-axis), 6.8 (⊥axis), 1.4 (am.) W/(m·K) |
| Scale | Peel Color and Appearance | Pulp Color and Appearance | Pulp Texture | Pulp Taste and Flavor | Overall Acceptability |
|---|---|---|---|---|---|
| 1 | Poor | Poor | Poor | Poor | Poor |
| 3 | Fair | Fair | Fair | Fair | Fair |
| 5 | Good | Good | Good | Good | Good |
| 7 | Very good | Very good | Very good | Very good | Very good |
| 9 | Excellent | Excellent | Excellent | Excellent | Excellent |
| Treatments | Peel Color and Appearance | Pulp Color and Appearance | Pulp Texture | Pulp Taste and Flavor | Overall Acceptability |
|---|---|---|---|---|---|
| 2% Chitosan | 5.40 bc | 5.71 bc | 5.87 bc | 5.89 bc | 5.72 bc |
| 1% Chitosan nanoparticles | 6.40 b | 6.54 b | 6.77 b | 6.51 b | 6.55 b |
| 1% Nano-silicon dioxide | 6.74 b | 6.84 b | 6.92 b | 6.44 b | 6.73 b |
| 2% Chitosan + 1% Nano-silicon dioxide | 7.80 a | 8.20 a | 8.55 a | 8.35 a | 8.22 a |
| Control (dipped fruits with distilled water) | 4.43 c | 4.86 c | 4.57 c | 4.33 c | 4.55 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassem, H.S.; Tarabih, M.E.; Ismail, H.; Eleryan, E.E. Influence of Nano-Silica/Chitosan Film Coating on the Quality of ‘Tommy Atkins’ Mango. Processes 2022, 10, 279. https://doi.org/10.3390/pr10020279
Kassem HS, Tarabih ME, Ismail H, Eleryan EE. Influence of Nano-Silica/Chitosan Film Coating on the Quality of ‘Tommy Atkins’ Mango. Processes. 2022; 10(2):279. https://doi.org/10.3390/pr10020279
Chicago/Turabian StyleKassem, Hazem S., Mohamed E. Tarabih, Hamed Ismail, and Eman E. Eleryan. 2022. "Influence of Nano-Silica/Chitosan Film Coating on the Quality of ‘Tommy Atkins’ Mango" Processes 10, no. 2: 279. https://doi.org/10.3390/pr10020279
APA StyleKassem, H. S., Tarabih, M. E., Ismail, H., & Eleryan, E. E. (2022). Influence of Nano-Silica/Chitosan Film Coating on the Quality of ‘Tommy Atkins’ Mango. Processes, 10(2), 279. https://doi.org/10.3390/pr10020279









