1. Introduction
β-mannanase is the common name for mannan endo-1,4-β-mannosidase, endo-1,4-β-mannanase, or 1,4-β-D-mannan mannanohydrolase (E.C. 3.2.1.78). This enzyme catalyzes the random hydrolysis of β-1,4-mannosidic linkages in the main chain of β-mannans and hetero-mannan, which consists of a β-(1-4)-linked backbone of glucose (Glc) and mannose (Man) units [
1], and is valuable in various biotechnological applications, particularly those related to renewable resource utilization. β-mannanases have drawn much interest in degrading mannan-containing polysaccharides because of their important roles. Thus, various applications in the feed, food, pulp/paper, and detergent industries have been reported [
2]. In addition to these industrial applications, mannanases have also shown utility in producing manno-oligosaccharides that benefit human health when used as prebiotics. Biotechnology has increased the discovery of new compounds produced by microorganisms through cultural and genetic engineering technologies.
Hemicelluloses comprise as much as a third of the known lignocellulosic substrates, which consist mainly of xylan and mannan and can be degraded by hemicellulolytic enzymes produced by organisms that degrade plant cell walls. Mannan-degrading enzymes also include several accessory enzymes, such as α-galactosidases (EC 3.2.1.2), β-glucosidases (EC 3.2.1.21), and acetyl mannan esterases [
3], that remove side chains that attach at various points on mannan. The main hydrolysis products obtained by the action of endo β mannanase are mannobiose and mannotriose [
2,
4]. Recently, the importance of mannanases has been recognized for their role in hydrolyzing the hemicellulose fractions in lignocellulosic biomass, which are responsible for the efficient breakdown of complex polysaccharides into simple sugars for bioethanol production [
5].
The mannan-degrading enzyme system occurs in many bacteria and fungi species. Actinomycetes, particularly
Streptomyces, secrete various active enzymes against the main lignocellulose and hemicellulose constituents. Bacterial enzymes should be much easier to produce because most enzyme-producing microorganisms are quickly grown from microorganisms with minimal nutritional requirements and low-cost downstream processing. Additionally, bacterial enzymes offer advantages such as better stability and activity under conditions compatible with industrial applications [
6].
Historically, mannanases from the following origins have been characterized: In fungi such as
Aspergillus awamori K4 [
7],
A. fumigatus IMI 385,708 [
8],
A. niger [
9],
Sclerotium rolfsii [
10], and
Trichoderma reesei [
11]. In bacteria such as
Bacillus subtilis [
12,
13],
Streptomyces sp. S27 [
6],
Geobacillus stearothermophilus [
14],
Thermomonospora fusca [
15],
Thermotoga neopolitana [
16],
B. subtilis WY [
17,
18],
Vibrio sp. strain MA-138 [
19],
B. licheniformis [
20], including
Cellulomonas fimi [
21,
22],
Thermobifida fusca [
23],
T. maritima [
24], and
T. thermarum [
25].
Mannanolytic microbes have developed a unique strategy capable of breaking the natural resistance of mannan and degrading, as well as mineralizing, the polymer. Mannan hydrolysis is carried out by free-living soil microorganisms that include several types of thermophilic bacteria, fungi, and actinomycetes in numerous species of the genus
Streptomyces [
15]. Actinobacteria are a well-known source of bioactive compounds and a promising source of a broad range of industrially important enzymes. However, although endo 1,4-β-mannanases have been isolated from plants, fungi, and bacteria, and many other genes encoding the enzymes have been cloned and sequenced, there have been no reports of β-mannanases derived from
Nonomuraea sp.Screening studies have been conducted to obtain the mannan-degrading enzymes produced by actinomycetes. Following this approach, a novel and more effective recombinant mannanase enzyme produced by rare actinomycete from the
Nonomuraea strain, which belongs to the family
Streptosporangia, may be involved in the spontaneous natural degradation of mannan. This Actinobacterium was recently identified as
Nonomuraea jabiensis ID06-379. Although several structural genes encoding mannanases have been cloned and expressed in bacteria (e.g.,
Escherichia coli) [
26,
27], those genes subcloning
Streptomyces strains are rare. Therefore, we attempted to transform a mannanase gene cloned from the
Nonomuraea strain in
Streptomyces lividans 1326 [
28].
This paper described the cloning and sequence analysis of the gene encoding ManNj6-379, an endo-1,4 β-mannanase secreted by the N. jabiensis ID06-379 strain isolated from soil. The purification of recombinant ManNj6-379 was produced by the heterologous expression in S. lividans 1326, and then we characterized the pattern of manno-oligosaccharide during biomass hydrolysis. We aim to obtain an Indonesian actinomycetes isolate that could produce mannanase for industrial production, particularly from mannan sources such as palm kernel cake (PKC), copra meal, and porang flour, all of which are abundant in Indonesia. Furthermore, we intended to clone the mannan endo-1,4-β-mannanase gene from N. jabiensis ID06-379 to enhance our understanding of this enzyme and its application to the bioconversion of mannan into a commercially usable form of manno-oligosaccharide.
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, Mediums, Cultivation, and Chemicals
Strain
N. jabiensis ID06-379 was provided from Biotechnology Culture Collection (BTCC), Indonesian Institute of Sciences (LIPI), and used throughout this work.
N. jabiensis ID06-379 was grown in an
actinomycetes medium named International
Streptomyces Project 2 (ISP2) at 28 °C for 3–5 days.
Escherichia coli Nova Blue (Invitrogen, Carlsbad, CA, USA) and TOPO Blunt vector (Invitrogen, Carlsbad, CA, USA) was used as the host and vector, respectively, to construct a partial mannanase gene of strain
N. jabiensis ID06-379. For the maintenance and propagation of plasmids, we used pGEM
®-T Easy vector (Promega, Madison, WI, USA),
E. coli Nova Blue (Invitrogen, Carlsbad, CA, USA), and
E. coli JM109 (Takara, Shiga, Japan). For cloning and expression of a mannanase gene, an expression system was used that involved pUC702 (
E. coli-Streptomyces shuttle vector containing
S. cinnamoneus phospholipase D promoter) [
29] and
S. lividans 1326.
E. coli was grown in Luria Bertani (LB) medium at 37 °C for 18–24 h, and
S. lividans 1326 recombinant was grown in Trypticase Soy Broth (TSB) medium supplemented with 1% (
w/
v) tryptone, 3% (
w/
v) glucose and 5 μg/mL of thiostrepton at 28 °C for 72 h with shaking at 180 rpm.
2.2. Molecular Cloning of Endo-1,4-β-Mannanase from N. Jabiensis ID06-379 with Sequence Analysis
Genomic DNA from
N. jabiensis ID06-379 was extracted using a method established by Kieser et al. [
30]. Isolation of recombinant plasmid for DNA sequence analysis using the Qiagen Mini-Prep system (Qiagen, Inc., Chatsworth, Los Angeles, CA, USA). Restriction enzymes were purchased from NEB (New England Biolabs, Tokyo, Japan) and were used according to the manufacturer’s instructions.
A partial fragment of the gene encoding mannanase (250 bp) from the chromosomal DNA of N. jabiensis ID06-379 was cloned using a pair of oligonucleotide primers that were designed using the conserved region of other published sequences from the mannanase genes of actinomycetes isolates listed in the PubMed database. A pair of primers, 6-379_univF: 5′-GTG CAC GAC ACC ACC GGC TAC-3′ and 6-379_univR: 5′-GGA CCA GTC CTG GCC CCA GTT-3′, were used for PCR amplification of the target sequence 250 bp partial gene manNj6-379 using Prime STAR GXL (Takara Bio, Shiga, Japan).
According to the manufacturer’s instruction, the amplified DNA was initially cloned using a Zero Blunt TOPO® vector PCR cloning kit (Invitrogen, Carlsbad, CA, USA) transformed to E. coli Nova Blue and spread on LB agar supplemented with 50 μg/mL of kanamycin. The plates were incubated overnight at 37 °C. The positive transformants grown on plates were picked and reconfirmed by colony PCR. The plasmid harboring the mannanase gene was extracted, and the insert size was confirmed by electrophoresis and verified by DNA sequence analysis.
From the sequence that involved a partial mannanase gene, N. jabiensis ID06-379, we designed a pair of oligonucleotide primers to aid in upstream and downstream identification of the full length of the gene using an LA PCRTM in vitro cloning kit (Takara Bio.Inc, Japan) according to the manufacturer’s instructions.
To clone endo-1,4-β-mannanases into the full-length of the manNj6-379 gene in the expression vector, the endo 1,4-β-mannanase-encoding sequence was amplified from a previously isolated genomic DNA, N. jabiensis ID06-379, with the primers S (S6-379 ORF Primer): 5′-TAAGGATGCAGCATGAGAAGAAGGCTTCTCGCCCTC-3′ that included an SphI site, and AS (AS6-379 HisORF Primer): 5′-AGTCGTCTCAAGATCTTCAGTGGTGGTGGTGGTGGTGGCGGGCGGCGCAGGACGGCGTG-3′ that included a BglII site. The design of these primers was based on the mannanase Open Reading frame (ORF).
The
manNj6-379 gene, fused to a sequence encoding a histidine tag at the C-terminal, was ligated with the PUC702 vector under the control of the
Streptoverticillium cinnamoneum phospholipase D promoter [
29]. The
manNj6-379 was cloned into the
SphI and
BglII sites of the pUC702 vector linearized with the appropriate restriction enzymes (
SphI and
BglII) using an In-Fusion
® HD cloning kit (Takara Bio USA, Inc) according to recommendations from the manufacturer. Proper construction was confirmed by restricting the digestion and DNA sequencing. DNA encoding of a hexa-histidine tag was incorporated into the reverse primers to generate 6 × His-tagged recombinant enzymes for further purification. The constructed plasmid was designated pUC702_
manNj6-379.
2.3. Transformation to S. Lividans 1326
The recombinant plasmid (pUC702_
manNj6-379) was then transformed into protoplast
S. lividans 1326 (NBRC15675). The recombinant plasmid (pUC702_manNj6-379) was then transformed into the prepared protoplast
S. lividans 1326 (NBRC15675) as an expression host according to the method carried out by Kieser et al. [
30], grown at R2YE, and incubated at 28 °C for 2 days. The transformants were selected by overlaying soft agar containing 50 μg/mL of thiostrepton (Merck KGaA, Darmstadt, Germany). After cultivation for 5 days, the transformants were selected and purified.
A loopful of each of the transformants carrying a gene encoding the endo-1,4-β-mannanases gene (manNj6-379) was inoculated in a test tube containing 5 mL of TSB medium supplemented with 5 μg/mL of thiostrepton (Merck KGaA, Darmstadt, Germany), followed by cultivation at 28 °C and 180 rpm for 72 h. Then, the culture supernatant of the transformant was applied to Western Blotting analysis to confirm expression of the manNj6-379 gene.
2.4. Production and Purification of the Recombinant Mannanase ManNj6-379
Streptomyces lividans 1326 harboring the recombinant manNj6-379 gene was inoculated into 5 mL of Tryptic Soy Broth (TSB) medium supplemented with 5 μg/mL of thiostrepton (Merck KGaA, Darmstadt, Germany) incubated at 28 °C for 72 h with shaking at 180 rpm. Next, 1 mL of the 72 h-culture was inoculated into 100 mL of TSB broth containing 5 μg/mL of thiostrepton. The culture was subsequently incubated with vigorous shaking (180 rpm) at 28 °C for 72 h. The culture was harvested by centrifugation at 13,000 rpm for 10 min at 4 °C to separate the cells and supernatant. The supernatant was filtered using Stericup-GP, 0.22 µm, a polyethersulfone (PES) filter (Millipore, Merck KGaA, Darmstadt, Germany), and was concentrated 30-fold using Vivaspin 20 (Sartorius, Goettingen, Germany) with a 5 kDa molecular-weight cut-off.
Immobilized metal affinity chromatography (IMAC) was used for the purification of 6 × His-tagged recombinant β-mannanase by gravity-flow chromatography using Ni Sepharose excel (GE Healthcare, Uppsala, Sweden) according to the manufacturer’s protocol. First, the concentrated culture supernatant was loaded onto a column and washed using a wash buffer. The enzyme was then eluted by elution buffer containing 500 mM imidazole, and the eluted fraction was changed with 50 mM sodium phosphate buffer, pH 6.0, via three sessions of centrifugation using a Vivaspin 20 (Sartorius) with a 5 kDa molecular-weight cut-off at 4 °C, 4000× g for 15 min to remove the imidazole.
2.5. Expression of Mannanase Recombinant ManNj6-379
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was conducted using a 10% (w/v) acrylamide gel for determination of the molecular mass using Precision Plus Protein™ Dual Color Standards from Bio-Rad Laboratories, Inc, Hercules, California 94547 USA, as a standard marker. The protein samples were briefly heated (5 min) in the SDS PAGE protein loading buffer (1:1 v/v) with a total volume of 20 µL at 98 °C using a PCR machine. Proteins in the gel were visualized by staining with Coomassie Brilliant Blue R250 (Nacalai, Kyoto, Japan).
The culture supernatant of wild-type and recombinant S. lividans 1326, as well as the purified ManNj6-379, was analyzed by zymogram using a 12% SDS polyacrylamide gel electrophoresis with 0.1% (w/v) LBG as substrate, followed by refolding with 2.5% (v/v) Triton X for 2 × 15 min. The active protein band was seen using 0.05% (w/v) Congo Red staining (Merck, Darmstadt, Germany).
The culture supernatant of strains expressing ManNj6-379 was subjected to Western Blot analysis on 10% SDS-PAGE gels blotted onto an Immobilon-P transfer membrane (Merck Millipore, Cork, Ireland) from SDS polyacrylamide gel. The His-tagged β-mannanase was allowed to react with Anti-His-tag HRP-DirecT (KDX, Aichi, Japan). The signals were visualized with horseradish peroxidase (HRP), conjugated goat anti-rabbit antibodies (Sigma-Aldrich, St. Louis, MO, USA), and colorimetric detection using BCIP/NBT substrate (Roche Applied Science, Penzberg, Germany), according to manufacturer instructions [
31].
2.6. Mannanase Assay and Protein Determination
The standard endo-1,4-β-mannanase activity was assayed using the dinitrosalicylic acid (DNS) method previously described by Miller [
32]. The substrate, 0.5% locust bean gum (Sigma-Aldrich, St. Louis, MO, USA), was dissolved in 50 mM sodium phosphate buffer, pH 6.0, via homogenization at 80 °C, and then it was heated to the boiling point, cooled, and stored overnight with continuous stirring. After that, the insoluble elements were removed by centrifugation, as described by Songsiriritthigul et al. [
33]. The ManN6-379 activity was assayed by mixing 50 μL of a solution of purified recombinant enzyme appropriately diluted with 450 μL of 0.5% locust bean gum into 50 mM MES (2-[
N-morpholino] ethanesulfonic acid) buffer (pH 6.0) at 50 °C for 15 min. The amount of reducing sugars liberated in the enzyme reaction was assayed by mixing 500 μL of the enzyme mixture with 500 μL of 3,5-dinitrosalicylic acid (DNS) solution, heating at 100 °C for 15 min, cooling on ice for 10 min, and measuring the absorbance at 540 nm.
One unit of endo-1,4-β-mannanase activity is defined as the amount of enzyme that will liberate 1 μmol of reducing sugar (using D-mannose as a standard) per min under the given assay conditions. The concentration of soluble proteins was determined using the Pierce
TM BCA protein assay kit (Thermo Scientific, Rockford, Illinois, 61101, USA) with BSA as a standard, as described by Huang et al. [
31].
2.7. Biochemical Characterization
The optimal pH of mannan endo-1,4-β-mannanase activity was measured between pH 4.0–10.0 under standard assay conditions using three buffer systems (each 50 mM): sodium acetate (pH 4.0–5.5), MES-NaOH (pH 5.5–6.5), Tris-HCl (pH 6.5–9.0), and Glycine-NaOH (pH 9.0–10.0). To determine the pH stability of endo-1,4-β-mannanase recombinant, the pH stability was determined by incubating the purified mannanase recombinant in various buffers at pH 4.0–10.0 (using buffers as above) at 50 °C for 60 min [
34]. The residual activity was measured under standard assay conditions.
The optimal temperature of the purified recombinant enzyme was measured by incubating the enzyme samples with the substrate at temperatures ranging from 40–100 °C in 50 mM sodium phosphate buffer, pH 6.0. In addition, the thermostability of the enzymes was monitored by pre-incubation in 50 mM MES-NaOH buffer, pH 6.0, at 50, 60, and 70 °C without a substrate for various periods.
The Km and Vmax values for the purified recombinant enzyme were determined using locust bean gum (LBG) as the substrate. Data were plotted in a Lineweaver–Burk diagram, and kinetic parameters were determined from the equation. The mannanase kinetic parameters, Vmax and Km, were calculated in 50 mM MES-NaOH buffer (pH 6.0) after incubation with purified mannanase at 55 °C for 10 min. Each reported result reflects the average of three independent experiments, and every experiment included three samples.
To investigate the effects of various metal ions and chemical reagents on the purified recombinant enzyme activity, 1 mM each of CoCl2, MnCl2, MgCl2, CaCl2, LiCl, FeCl3, CuSO4, ZnSO4, EDTA, and Triton X was individually added to the reaction system.
2.8. N-terminal Amino Acid Sequencing
Purified β-mannanase protein was run on dodecyl sulfate sodium polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a PVDF Immobilon-P Transfer membrane (Merck KGaA, Darmstadt, Germany) using transblot SD (Bio-Rad, Hercules, CA, USA). It was dyed with Ponceau S (Nacalai, Kyoto, Japan). The N-terminal amino acid sequence was analyzed by using a peptide sequencer (Procise 492-HT Protein Sequencer, Applied Biosystems, Applera Corporation, Foster City, CA, USA).
2.9. Product Analysis of ManNj6-379 Using Thin-Layer Chromatography
Commercial mannan substrates, such as LBG, ivory nut mannan, Konjac glucomannan, and β-mannan were each hydrolyzed by recombinant ManNj6-379 prepared at a concentration of 0.5% (w/v) in 50 mM MES-NaOH buffer, pH 6.0. After adding the purified ManNj6-379 enzyme (1.6 U for 1-mL reactions), the solution was incubated at 50 °C. Other biomass substrates, such as palm kernel cake (PKC), copra meal, and porang flour (20 mg), were treated with 3.8 U ManNj6-379 enzyme in 50 mM MES-NaOH buffer at pH 6.0 to achieve a final volume of 1 mL Hydrolysis occurred at 50 °C with constant agitation for up to 24 h. Aliquots were removed from each sample at various times, and the reaction was stopped by heating for 15 min in a 100 °C heat-block furnace.
Hydrolysis products were separated on 60 F254 thin layer chromatography (TLC) silica plates (Merck, Darmstadt, Germany) using a solvent system consisting of
n-butanol-acetic acid-water (2:1:1,
v/
v) as a mobile phase. The sugar products were detected by spraying with a prepared mixture (dimethylamine: aniline: phosphoric acid, 2:1:1
v/
v) followed by heating at 120 °C for 10 min. Manno-oligosaccharides (mannose, M1; mannobiose, M2; mannotriose, M3; mannotetraose, M4; mannopentaose, M5; and mannohexaose, M6 from Megazyme, Wicklow, Ireland) were used as standards, as described by Rahmani et al. [
35].
2.10. Nucleotide Sequence Accession Numbers
The nucleotide sequences for N. jabiensis ID06-379 16S rDNA and manNj6-379 were deposited in the GenBank database under the accession number DDBJ LC012036.
3. Results and Discussion
3.1. Cloning and Sequence Analysis of the Endo-1,4-β-Mannanase Gene (manNj6-379) from N. Jabiensis ID06-379
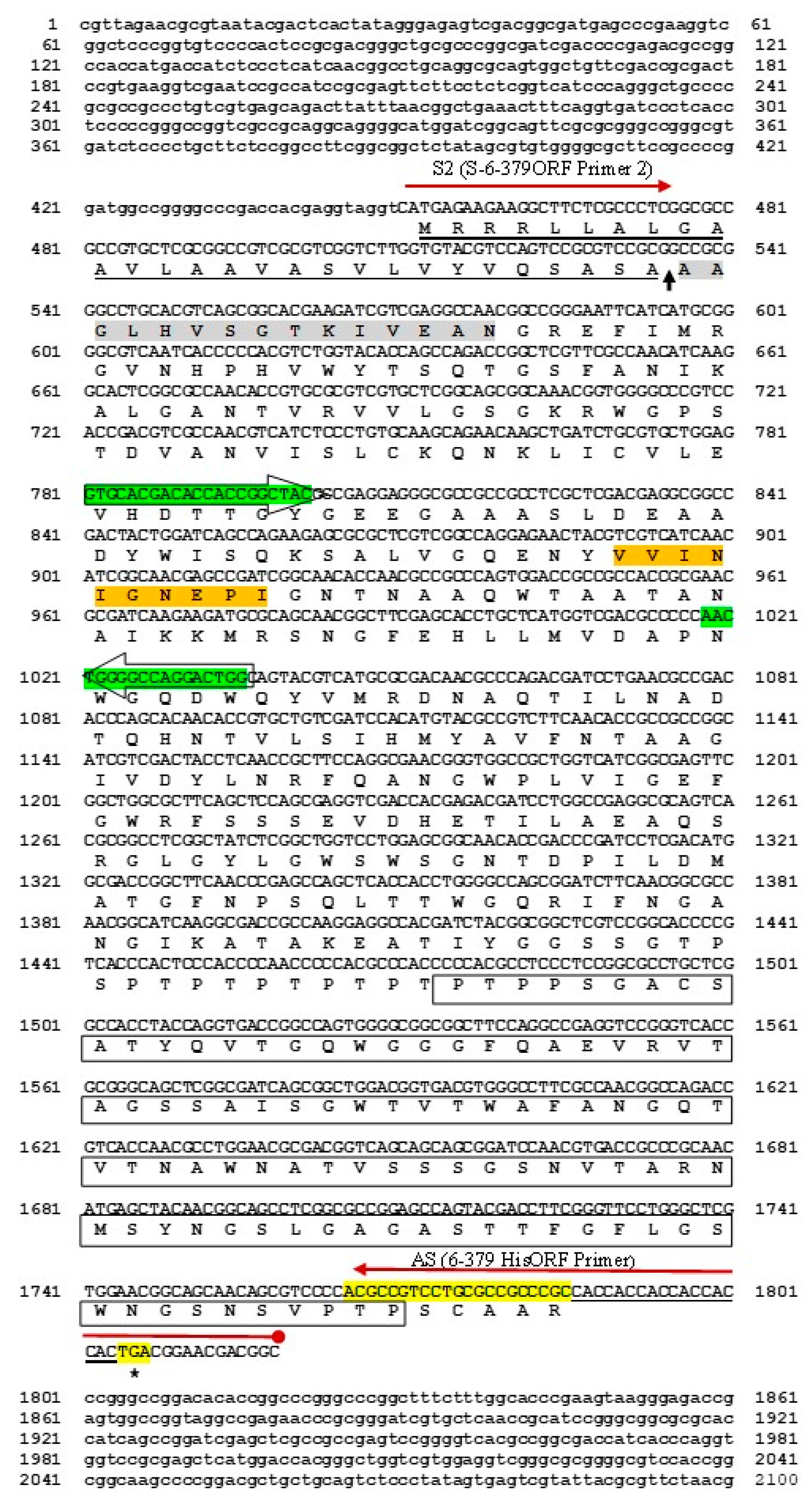
A partial sequence of a 250-bp fragment from the genomic DNA of the strain N. jabiensis ID06-379 with a pair-designed primer PCR indicated that the fragment was most closely related to endo-1,4-β-mannanase from Actinosynnema mirum DSM 43,827 (accession number CP001630.1), with an 89% shared identity. The full-length mannanase gene manNj6-379 (2100 bp) was obtained via the use of an LA PCRTM in vitro cloning kit (Takara Bio Inc., Japan) with a primer design based on the 250 bp sequence. The complete open reading frame (ORF) of manNj6-379 was 1335 bp in length with a G + C content of 71.1%, which started with ATG and was terminated with a TGA stop codon.
Sequence analysis showed that the complete DNA sequence of manNj6-379 consists of 1335 bp and encodes a 445-amino acid polypeptide. The open reading frame corresponding to the manNj6-379 gene is 1251 bp in length and encodes a polypeptide of 417 amino acids that contains a 28-residue signal peptide. The N-terminal sequence of 15 amino acids proved to be AAGLHVSGTKIVEAN, which perfectly agrees with the amino acid sequence at positions 29 to 43 deduced from the DNA sequence. This agreed precisely with the N-terminal amino acid sequence of purified mannanase determined from the culture of the N. jabiensis ID06-379 strain.
This indicates that the N-terminal amino acid sequence of ManNj6-379, which exhibits the typical features of a signal peptide, functions appropriately as a signal peptide in S. lividans. Agreement with the deduced amino acid sequence suggested that the peptide from Met1 to Ala28 would be a signal peptide for the secretion of the enzyme by the N. jabiensis ID06-379 strain. The signal peptidase cleavage site would reside in Ala26-Ser27-Ala28 and Ala29-Ala30, as proven by the N-terminal amino acid sequences of mature ManNj6-379 enzymes.
The deduced amino acid sequence of ManNj6-379 with sequences in the GenBank databases showed that ManNj6-379 consists of a single carbohydrate-binding module (CBM) of the family 2 bacterial type. Furthermore, the deduced ManNj6-379 suggested that the possession of a CBM 2 at residues 360–440, which involved two tryptophan residues (shown in boxes in
Figure 1) conserved in ManNj6-379 and in other
actinomycetes such as GH5 endo-1,4-β-mannanase.
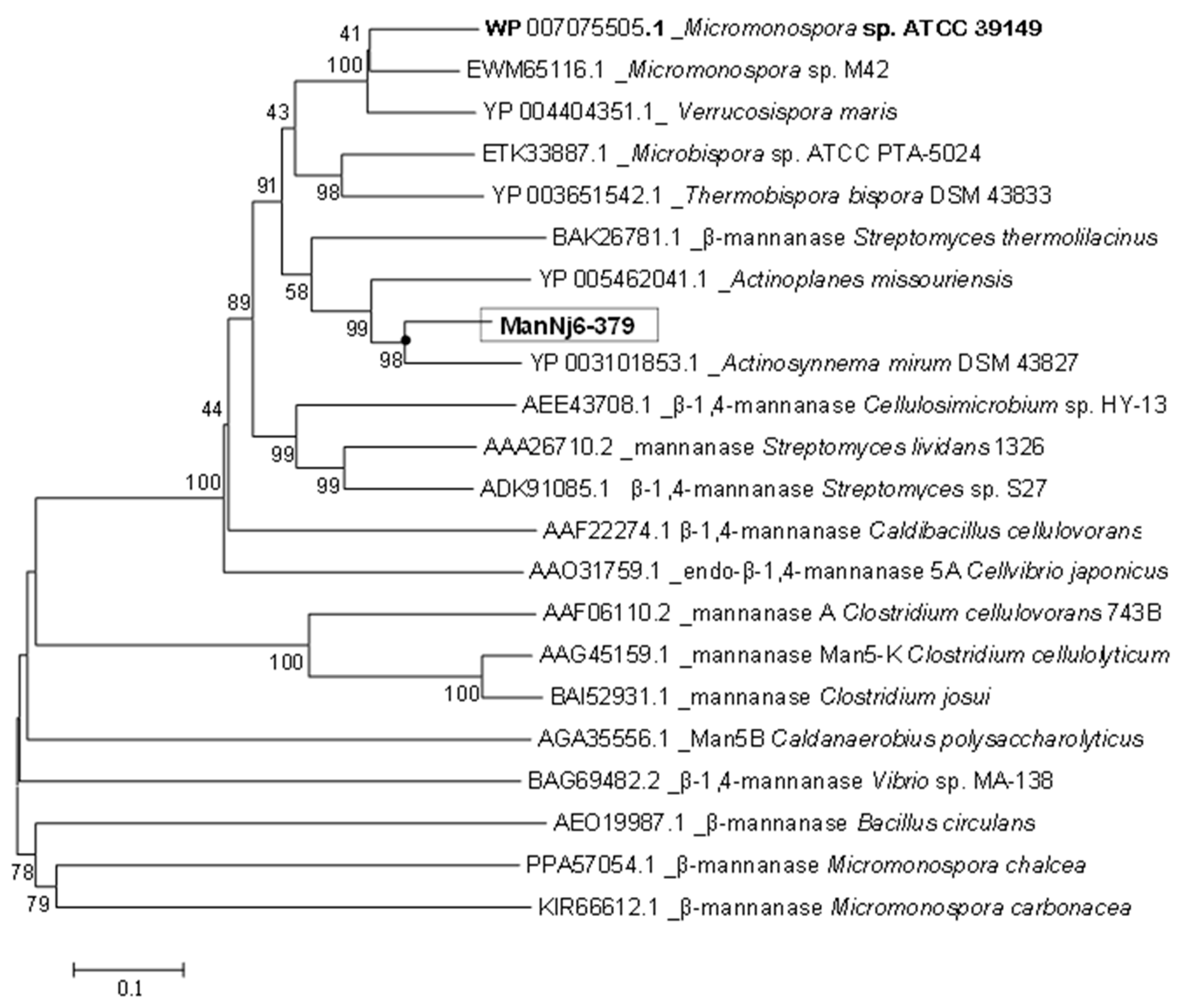
Computer-based homology analyses using an NCBI BLAST algorithm to compare GenBank databases indicated that ManNj6-379 shows many similarities to the endo-β-1,4-mannanase gene in several strains of actinomycetes grouped into family 5 of the GHs (
Figure 2). For example, endo-1,4-β-mannanase from
N. jabiensis ID06-379 belongs to the glycoside hydrolase family 5 (GH5). The amino sequence was more similar to the mannanase sequences of other
Nonomuraea species; however, these highly similar mannanase genes were submitted in the form of sequenced genomes, and none had been previously cloned or expressed.
The deduced amino acid sequence of ManNj6-379 was compared with other GH5 endo-1,4-β-mannanase sequences available in GenBank. The highest identity was characterized as 77% for endo-1,4-β-mannanase from
A. mirum DSM 43,827 (GenBank accession noYP_003101853.1), followed by a hypothetical protein from
Actinoplanes globisporus (69% identity; GenBank accession no WP_020515925.1). Other characterizations were as follows:
A. missouriensis 431 at 68%,
Microbispora spp. ATCC PCA-5024 at 65%,
Micromonospora spp. ATCC 39,149 at 60%,
Verrucosispora maris AB-18-032 at 59%,
Thermobispora bispora DSM43833 at 61%, and
Micromonospora sp. M42 at 60% (
Figure 2).
Compared with some actinomycetes, and other examples of bacterial β-mannanase, there is a significant difference according to phylogenetic analyses, and they belong to different monophyletic groups. However, a phylogenetic tree (
Figure 3) based on the similarities of amino acid sequences showed that the closest phylogenetic relationship of this mannanase was shared with the β-mannanase of
A. mirum DSM 43827. This suggests that recombinant enzyme ManNj6-379 β-mannanase may have some unique properties.
3.2. Expression of ManNj6-379 in S. Lividans 1326
ManNj6-379 was expressed as an extracellular protein in
S. lividans 1326. The recombinant enzyme in the supernatant was purified using a one-step purification protocol by affinity chromatography. After transformation, β-mannanase activity was detected in the supernatant of
S. lividans 1326 harboring pUC702-
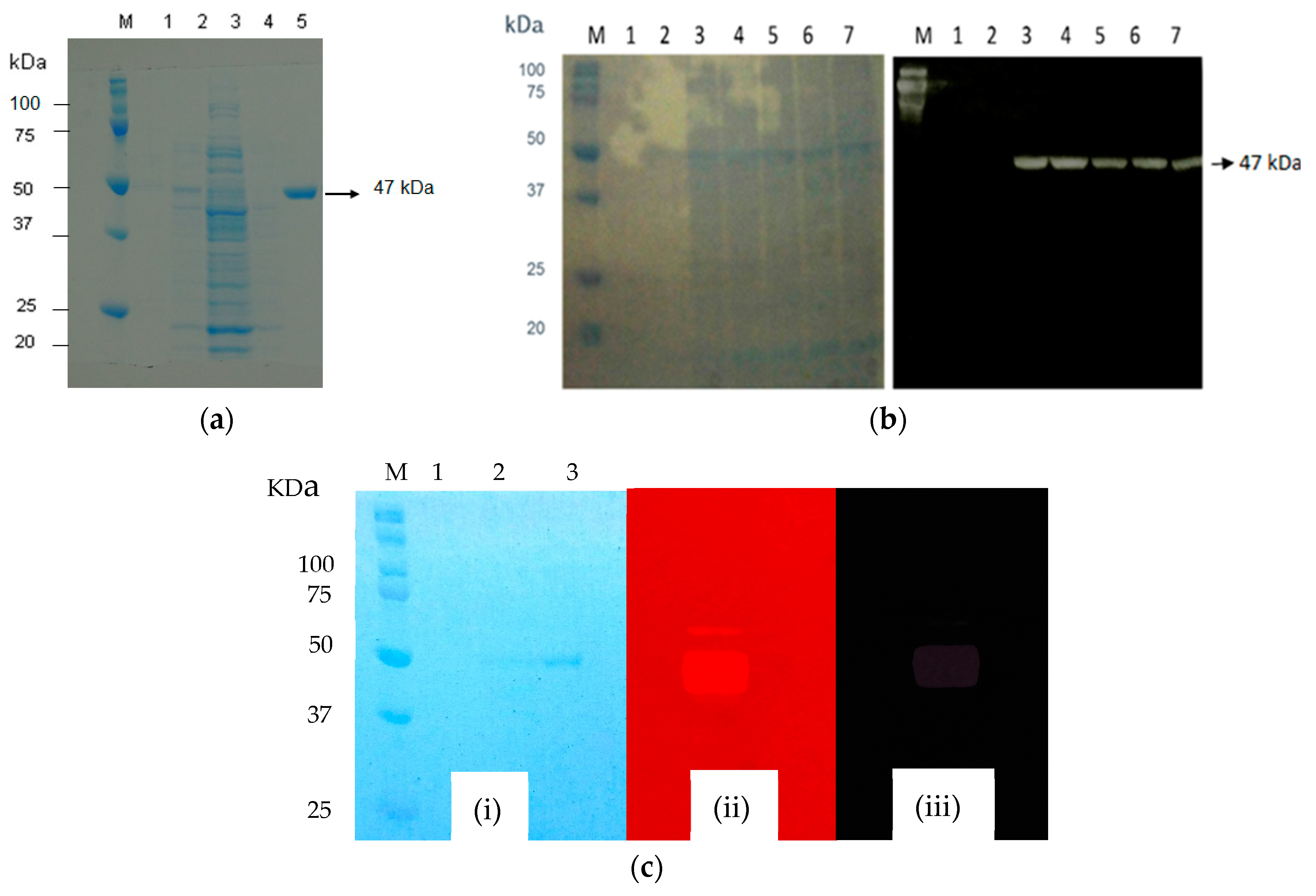
manNj6-379. The secreted recombinant enzyme ManNj6-379 in the culture supernatant was analyzed by SDS-PAGE and Zymogram as well as Western Blotting (
Figure 4a–c).
A single protein band corresponding to recombinant ManNj6-379 was observed in the supernatant, and no protein band was observed in the supernatant of the wild-type
S. lividans 1326 as a control. The apparent molecular weight of the expressed ManNj6-379 protein was about 47.8 kDa (
Figure 4a). Zymogram analysis was also required to determine if the 47 kDa pure protein band is an active mannanase enzyme. Detection of the 47 kDa protein band was performed using locust bean gum as the substrate added to the gel. The results of substrate hydrolysis by the mannanase enzyme showed clear zones with a dark red background from the substrate that was not hydrolyzed after staining with Congo red stain and Congo Red + acetic acid stain (
Figure 4b).
Western Blot analysis was carried out using His-tagged endo-1,4-β-mannanase from
N. jabiensis ID06-379 strain, which allowed a reaction with Anti-His-tag HRP-DirecT (KDX, Aichi, Japan), as shown in
Figure 4b. Western Blot analysis revealed that the protein was recognized specifically by Anti-His-tag HRP-DirecT (KDX, Aichi, Japan), demonstrating that the expressed heterogeneous protein was recombinant endo-1,4-β-mannanase. The protein recombinant had begun to secrete to the culture supernatant after 48 h of cultivation, and the highest level of expressed protein was reached at 48 to 72 h of incubation (
Figure 4c).
3.3. Purification of Recombinant Endo-1,4-β-Mannanase (ManNj6-379)
The recombinant enzyme ManNj6-379 could be purified in one step by immobilized metal affinity chromatography (IMAC). After purification of the Ni Sepharose excel (GE Healthcare, Uppsala, Sweden) column, as described in the Materials and Methods section, the specific activity of the purified enzyme was obtained at 219.5 U mg
−1. It showed a 12.6-fold increase compared with the crude culture supernatant of
S. lividans 1326 (
Table 1).
After purification, the specific activity of the recombinant enzyme ManNj6-379 was 219.5 U mg
−1. This value was lower than that of ManKs_4-555, as reported by Rahmani et al. (944 U mg-1), for
Kitasatospora sp., β-mannanase expressed in
S. lividans was 1326 U mg
−1 [
35]. There are many previous reports regarding mannanase enzyme activity. For example, the enzyme activity of β-mannanase from the
Cellulosimicrobium sp. strain HY-13 was 14,711 U mg
−1, as reported by Kim et al. [
36].
Bacteroides fragilis enzyme activity was only 2.80 U mg
−1, as reported by Kawaguchi et al. [
18].
Bacillus subtilis MAFIC-S11 β-mannanase showed a high specific activity of 3706 U mg
−1, as written by Lv et al. [
13].
Streptomyces sp. S27 had high specific activity of 2107 U mg
−1, as reported by Shi et al. [
6].
3.4. Characterization of Purified Recombinant ManNj6-379
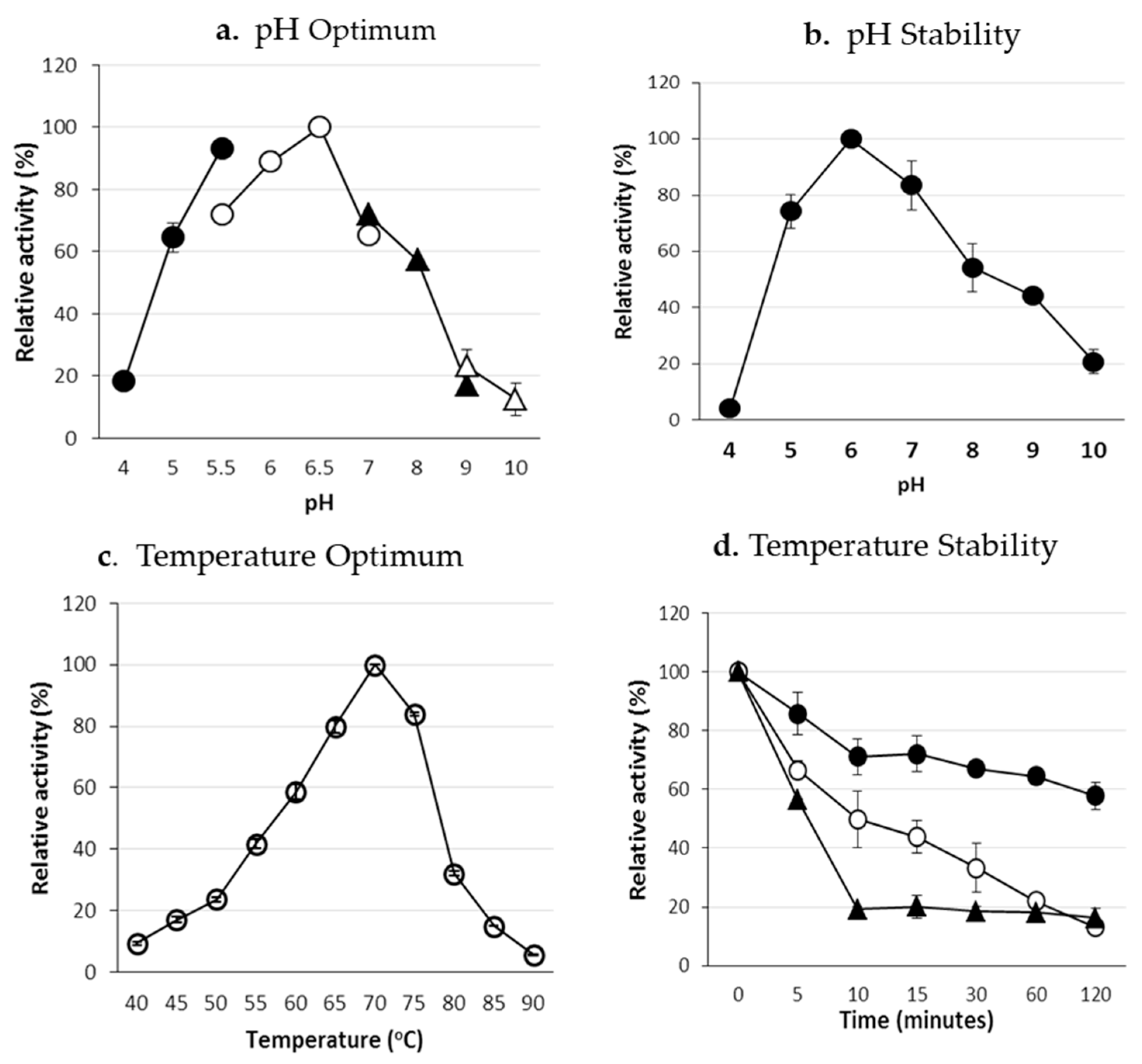
LBG was used as the substrate for enzyme characterization. Purified ManNj6-379 was optimally active at pH 6.5 (at 50 °C). Maximum activity retained more than 50% between pH 5.0–8.0. More than 45% of maximal activity was exhibited at pH 9.0 (
Figure 5a). The enzyme retained more than 44% of the relative activity after incubation in buffers that ranged from pH 5.0 to 9.0 at 50 °C for 60 min (
Figure 5b). As a reference, the optimal activity of β-mannanase from
B. circulans CGMCC 1416 was at pH 7.6, and more than 75% of maximal activity was retained over the pH range 6.8 to 8.0, as reported by Li et al. [
37]. The purified recombinant Man5S27 from
Streptomyces sp. S27 [
38] showed optimal activity at pH 7.0, and more than 70% of maximal activity was retained over pH that ranged from 6.0–9.0. These results confirmed that the enzyme activity of ManNj6-379 was comparable to that of other forms of β-mannanase from other genera.
3.5. Optimum Temperature and Thermostability of Recombinant β-Mannanase (ManNj6-379) Activity
The optimal temperature for enzyme activity was 70 °C at pH 6.0. The enzyme retained 40–60% of the maximum activity when assayed at 55–60 °C and more than 80% of the maximum activity at temperatures of 65 and 75 °C (
Figure 5c). After incubation at 50 °C for 2 h, the enzyme retained more than 50% of its initial activity. Above 60 °C, the enzyme’s stability decreased rapidly (
Figure 5d). The optimal temperature of ManNj6-379 for enzyme activity was 70 °C. The protein was active over a broad temperature range.
Optimal temperatures for different β-mannanases have previously been reported; that of the β-mannanase from
S. lividans 66 was 58 °C [
39]. The optimal temperature for MANB48 activity from
Bacillus circulans was 58 °C [
37]. For β-mannanase produced from
Streptomyces sp. S27 [
6], the optimal temperature for Man5S27 was 65 °C. In the thermostability assay, Man5S27 remained stable at 50 °C after incubation at pH 7.0 for 1 h. Man5S27 lost almost all of its activity after incubation at 60 °C for 1 h. Two forms of endo-1,4-β-mannanase from the thermotolerant fungus
A. fumigatus IMI 385,708 showed the highest activity at 60 °C (at pH 4.5) [
8].
The enzyme properties varied for β-mannanase from various sources. In the present study, the optimal temperature of ManNj6-379 was determined to be 70 °C (
Figure 5a), and within 50–70 °C, the enzyme maintained >50% of its maximal activity. However, the thermal resistance of ManNj6-379 was weak. The enzyme activities decreased dramatically after 10 min of incubation at 60 and 70 °C, and activity of 20% was observed after 120 min at these two temperatures. From several published reports, the recombinant
B. circulans mannan endo-1,4-β-mannanase retained 90% of its activity after incubation at 50 °C for 60 min, but no activity remained after incubation at 60 °C for 20 min, as described by Li et al. [
37].
3.6. Effect of Various Metal Ions and Chemical Reagents on the Activity of ManNj6-379
An increase (approximately 1.15-fold of its original activity) in ManNj6-379 activity was observed in the presence of divalent cations (1 mM) in Co
2+. The activity of these β-mannanases was also reported by Rahmani et al. [
35] and Kim et al. [
36] to be enhanced with Co
2+.
The recombinant ManNj6-379 activity was not inhibited by metal ions (1 mM) such as Mn
2+, Mg
2+, Ca
2+, and Li
+, but inhibited by Fe
3+, Cu
2+, Zn
2+, and chemicals: 5 mM EDTA and 5 mM Triton X (
Table 2). Several β-mannanases from actinomycetes such as
S. tendea [
40],
Streptomyces sp. CS147 [
41], and
Kitasatospora sp. [
35] had similar trends to strongly inhibit ManNj6-379 activity with Zn
2+ and EDTA.
3.7. Product Analyses
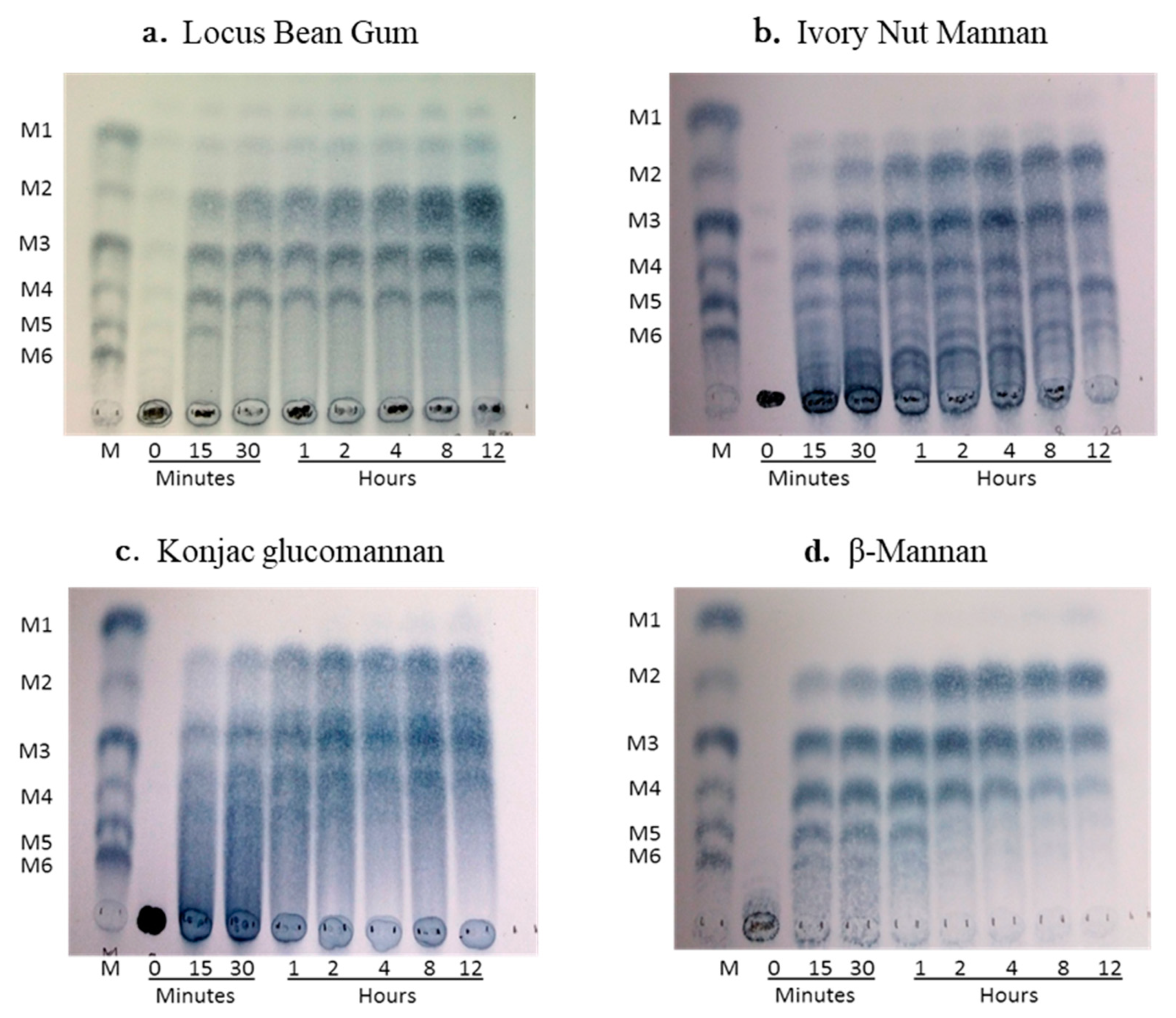
Analysis of oligosaccharide products obtained during enzymatic hydrolysis of commercial substrates using thin-layer chromatography revealed that the recombinant endo-1,4-β-mannanase (ManNj6-379) yields mannobiose (M2), mannotriose (M3), and mannotetraose (M4) as its main products. No trace of mannose could be detected in the hydrolysis experiments of LBG, ivory nut mannan, konjac glucomannan, or β-mannan hydrolysates generated by purified ManNj6-379 mannanase when analyzed by thin-layer chromatography (
Figure 6a–d).
Mannobiose (M2) and mannotriose (M3) were the main products of mannan hydrolysis, whereas no mannose was detected among the four specific substrate hydrolysates. The enzyme was active on LBG, ivory nut mannan, konjac glucomannan, and β-mannan (
Figure 6). Hydrolysis from LBG purified enzyme mainly produced M2, M3, and M4 (
Figure 6a). Mannobiose, M3, M4, and M5 were the final products released separately from ivory nut mannan by the purified ManNj6-379 (
Figure 6b). Mannose was not released from either ivory nut mannan or LBG. The konjac glucomannan is a polysaccharide composed of D-glucose and D-mannose backbones with branches through α-1, 6-glucosyl units, and this substrate released M3 and M4 as the final products (
Figure 6c). The β-mannan substrate, a linear mano-oligosaccharide without side groups, released M2 and M3 as the main end products with less M4 produced (
Figure 6d). Some manno-oligosaccharide fragments containing glucosyl groups and galactosyl groups can be produced from polysaccharides other than β-mannan during hydrolysis, which we can see in
Figure 6 as spots with different
Rf ranges from the mobility of standards.
These results indicated that the enzyme ManNJ6-379 has endo β-mannanase activity that can efficiently and randomly cleave higher molecular-weight mannans consisting of more than six mannose monomers. There was no detectable mannose, which indicated that the enzyme had no β-mannosidase activity.
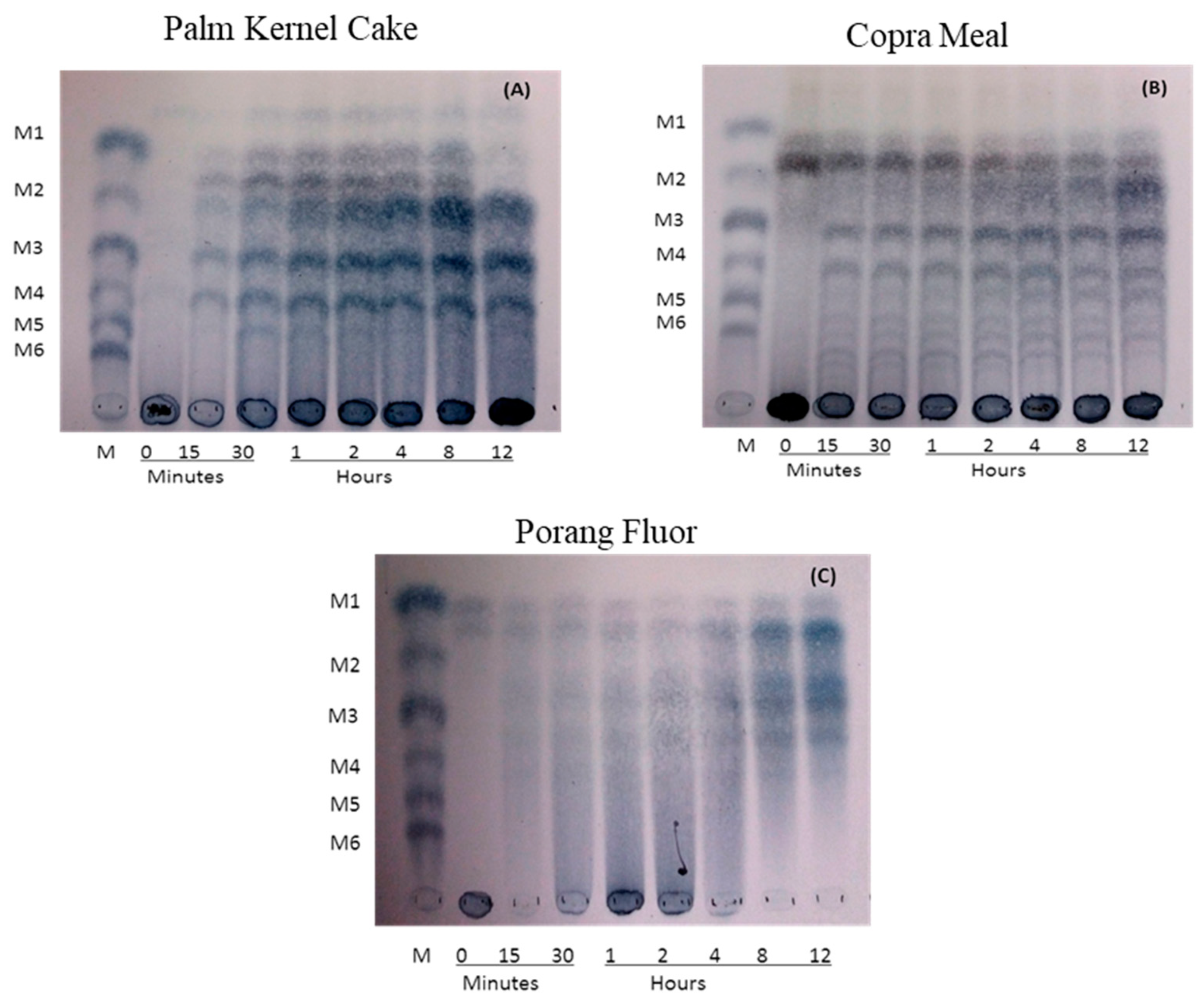
The ability of ManNj6-379 to hydrolyze biomass substrates was examined by using TLC. Manno-oligosaccharide products, including mannose (M1), mannobiose (M2), mannotriose (M3), and mannotetraose (M4), were detected after PKC, copra meal, and porang flour were incubated with the enzyme for times that varied from 15 min to 24 h at 50 °C (
Figure 7). The major hydrolysis products obtained from PKC were M2, M3, and M4 (
Figure 7A), whereas M2 and M3 were the major products of copra meal hydrolysis with smaller amounts of M3 (
Figure 7B). When using ManNj6-379, the main hydrolysis products from porang flour were M2 and M3 (
Figure 7C).
These results showed that ManNj6-379 could efficiently hydrolyze PKC, copra meal, and porang flour to release various shorter-chain manno-oligosaccharides, which can function as an energy source for yeast fermentation in a biorefinery platform. The production of M2 and M3 by the enzyme was in agreement with previous reports by Harnpicharnchai et al. [
42].
The different types of manno-oligosaccharides obtained after hydrolyzing PKC and copra meal yielded mostly M2 and M3, whereas porang flour hydrolysis yielded mostly M2 and M3. This was supposedly due to the different compositions of linear mannan from each biomass.
3.8. Enzyme Kinetics of ManNj6-379
Endo-1,4-β-mannanase recombinant (ManNj6-379) from
N. jabiensis ID06-379 efficiently hydrolyzes galactomannan, glucomannan, and β-1,4-mannan from several substrates. The present study proved that ManNj6-379 actively degrades several structurally unique mannans. The Michaelis–Menten,
Km, and
Vmax parameters, using LBG, ivory nut, and konjac glucomannan, are shown in
Table 3.
The
Km and
Vmax values for β-mannanase ManNj6-379 were calculated based on the Lineweaver–Burk plot. The
Km and
Vmax values for the hydrolysis of LBG were 0.385 mg mL
−1 and 769.23 μmol min
−1 mg
−1. The
Km value of ManNj6-379 for an LBG substrate (0.385 mg mL
−1) was relatively low compared with the
Km of bacterial β-mannanase Man3 (3.4 mg mL
−1) [
43] and with that of mannanase from
S. lividans IAF36 [
39], but it was higher than the
Streptomyces sp. S27 strain (0.16 mg mL
−1) [
38]. These data suggest that ManNj6-379 has better substrate affinity and higher catalytic activity than previously reported.