SUMOylation Regulates BmNPV Replication by Moderating PKIP Intracellular Localization
Abstract
1. Introduction
2. Materials and Methods
2.1. Silkworm and Cell Line
2.2. Amplification of BmSUMO by Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.3. Phylogenetic Analysis
2.4. Knockdown of BmSUMO and BmUBC9 in BmN cells
2.5. Virus Infection and Ginkgolic Acid Treatment
2.6. Plasmid Generation
2.7. Pull-Down and Western Blotting Assays
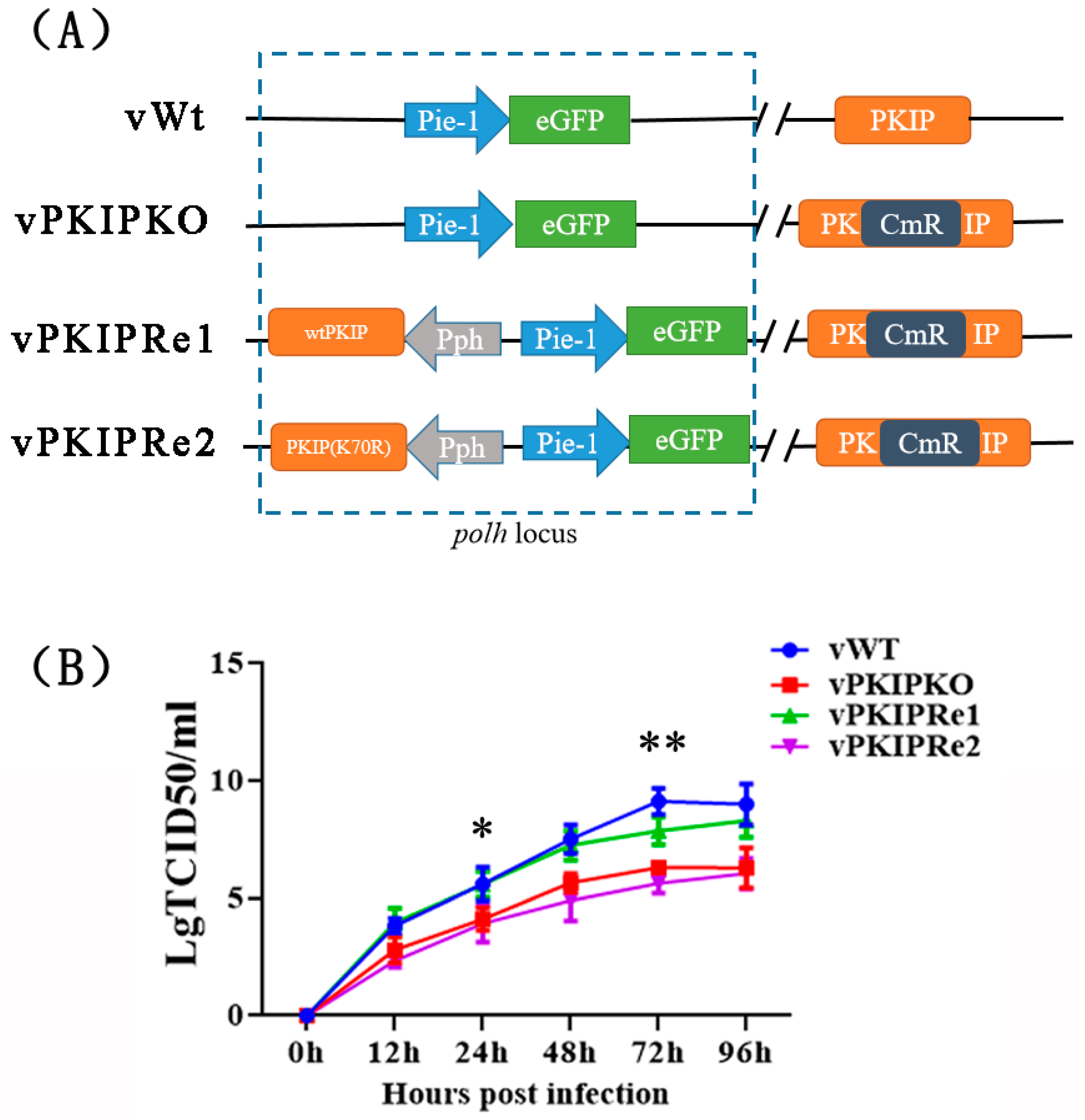
2.8. Construction of Recombinant Viruses
2.9. Virus Growth Curve Analysis
3. Results
3.1. Characterization of the BmSUMO Gene
3.2. Expression of SUMOylation-Related Genes in B. mori after BmNPV Challenge
3.3. Knockdown of BmSUMO and BmUBC9 Inhibited BmNPV Replication
3.4. Ginkgolic Acid Suppressed BmNPV Replication
3.5. PKIP of BmNPV Was SUMOylated at K70
3.6. Localization of PKIP was Related to SUMOylation
3.7. SUMOylation of PKIP Was Essential for BmNPV Replication
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saitoh, H.; Pu, R.; Cavenagh, M.; Dasso, M. RanBP2 associates with Ubc9p and a modified form of RanGAP1. Proc. Natl. Acad. Sci. USA 1997, 94, 3736–3741. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.; Delphin, C.; Guan, T.; Gerace, L.; Melchior, F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 1997, 88, 97–107. [Google Scholar] [CrossRef]
- Baz-Martinez, M.; El Motiam, A.; Ruibal, P.; Condezo, G.N.; de la Cruz-Herrera, C.F.; Lang, V.; Collado, M.; San Martin, C.; Rodriguez, M.S.; Munoz-Fontela, C.; et al. Regulation of Ebola virus VP40 matrix protein by SUMO. Sci. Rep. 2016, 6, 37258. [Google Scholar] [CrossRef] [PubMed]
- Dohmen, R.J. SUMO protein modification. Biochim. Biophys. Acta 2004, 1695, 113–131. [Google Scholar] [CrossRef]
- Su, C.I.; Tseng, C.H.; Yu, C.Y.; Lai, M.M.C. SUMO Modification Stabilizes Dengue Virus Nonstructural Protein 5 To Support Virus Replication. J. Virol. 2016, 90, 4308–4319. [Google Scholar] [CrossRef]
- Hay, R.T. Decoding the SUMO signal. Biochem. Soc. Trans. 2013, 41, 463–473. [Google Scholar] [CrossRef]
- Muller, S.; Dejean, A. Viral immediate-early proteins abrogate the modification by SUMO-1 of PML and Sp100 proteins, correlating with nuclear body disruption. J. Virol. 1999, 73, 5137–5143. [Google Scholar] [CrossRef]
- Schwienhorst, I.; Johnson, E.S.; Dohmen, R.J. SUMO conjugation and deconjugation. Mol. Gen. Genet. 2000, 263, 771–786. [Google Scholar] [CrossRef]
- Song, J.; Durrin, L.K.; Wilkinson, T.A.; Krontiris, T.G.; Chen, Y. Identification of a SUMO-binding motif that recognizes SUMO-modified proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 14373–14378. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Z.; Hu, W.; Chen, Y. Small ubiquitin-like modifier (SUMO) recognition of a SUMO binding motif: A reversal of the bound orientation. J. Biol. Chem. 2005, 280, 40122–40129. [Google Scholar] [CrossRef]
- Mattoscio, D.; Segre, C.V.; Chiocca, S. Viral manipulation of cellular protein conjugation pathways: The SUMO lesson. World. J. Virol. 2013, 2, 79–90. [Google Scholar] [CrossRef] [PubMed]
- El Motiam, A.; Vidal, S.; Seoane, R.; Bouzaher, Y.H.; Gonzalez-Santamaria, J.; Rivas, C. SUMO and Cytoplasmic RNA Viruses: From Enemies to Best Friends. Adv. Exp. Med. Biol. 2020, 1233, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Mattoscio, D.; Medda, A.; Chiocca, S. Recent Highlights: Onco Viral Exploitation of the SUMO System. Curr. Issues Mol. Biol. 2020, 35, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, Z. Cross-talking between baculoviruses and host insects towards a successful infection. Philos. Trans. R. Soc. Lond B. Biol. Sci. 2019, 374, 20180324. [Google Scholar] [CrossRef]
- Huang, H.; Wu, P.; Zhang, S.; Shang, Q.; Yin, H.; Hou, Q.; Zhong, J.; Guo, X. DNA methylomes and transcriptomes analysis reveal implication of host DNA methylation machinery in BmNPV proliferation in Bombyx mori. BMC. Genomics 2019, 20, 736. [Google Scholar] [CrossRef]
- Mao, F.; Lei, J.; Enoch, O.; Wei, M.; Zhao, C.; Quan, Y.; Yu, W. Quantitative proteomics of Bombyx mori after BmNPV challenge. J. Proteomics 2018, 181, 142–151. [Google Scholar] [CrossRef]
- Xue, J.; Qiao, N.; Zhang, W.; Cheng, R.L.; Zhang, X.Q.; Bao, Y.Y.; Xu, Y.P.; Gu, L.Z.; Han, J.D.; Zhang, C.X. Dynamic interactions between Bombyx mori nucleopolyhedrovirus and its host cells revealed by transcriptome analysis. J. Virol. 2012, 86, 7345–7359. [Google Scholar] [CrossRef]
- Langereis, M.A.; Rosas-Acosta, G.; Mulder, K.; Wilson, V.G. Production of sumoylated proteins using a baculovirus expression system. J. Virol. Methods. 2007, 139, 189–194. [Google Scholar] [CrossRef]
- Liu, L.; Spurrier, J.; Butt, T.R.; Strickler, J.E. Enhanced protein expression in the baculovirus/insect cell system using engineered SUMO fusions. Protein. Expr. Purif. 2008, 62, 21–28. [Google Scholar] [CrossRef]
- Tang, X.; Fu, X.; Hao, B.; Zhu, F.; Xiao, S.; Xu, L.; Shen, Z. Identification of sumoylated proteins in the silkworm Bombyx mori. Int. J. Mol. Sci. 2014, 15, 22011–22027. [Google Scholar] [CrossRef]
- Wang, X.Y.; Shao, Z.M.; Chen, Q.Y.; Xu, J.P.; Sun, X.; Xu, Z.P.; Li, M.W.; Wu, Y.C. Knockdown of BmTCP-1beta Delays BmNPV Infection in vitro. Front. Microbiol. 2019, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Toufeeq, S.; Wang, J.; Zhang, S.Z.; Li, B.; Hu, P.; Zhu, L.B.; You, L.L.; Xu, J.P. Bmserpin2 Is Involved in BmNPV Infection by Suppressing Melanization in Bombyx mori. Insects 2019, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.D.; Xu, Y.P.; Yu, L.L.; Lang, G.J.; Tian, C.H.; Zhao, J.F.; Zhang, C.X. Characterization of a Bombyx mori nucleopolyhedrovirus with Bmvp80 disruption. Virus Res. 2008, 138, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, H.; Liu, H.; Wu, X. Bombyx mori nucleopolyhedrovirus F-like protein Bm14 is a cofactor for GP64-Mediated efficient infection via forming a complex on the envelope of budded virus. Virology 2020, 539, 61–68. [Google Scholar] [CrossRef]
- Jones, D.; Crowe, E.; Stevens, T.A.; Candido, E.P. Functional and phylogenetic analysis of the ubiquitylation system in Caenorhabditis elegans: Ubiquitin-conjugating enzymes, ubiquitin-activating enzymes, and ubiquitin-like proteins. Genome Biol. 2002, 3, research0002.1. [Google Scholar] [CrossRef]
- Su, H.L.; Li, S.S. Molecular features of human ubiquitin-like SUMO genes and their encoded proteins. Gene 2002, 296, 65–73. [Google Scholar] [CrossRef]
- Abed, M.; Bitman-Lotan, E.; Orian, A. The Biology of SUMO-Targeted Ubiquitin Ligases in Drosophila Development, Immunity, and Cancer. J. Dev. Biol. 2018, 6, 2. [Google Scholar] [CrossRef]
- Xu, H.P.; Hao, W.; He, D.; Xu, Y.S. Smt3 is required for the immune response of silkworm, Bombyx mori. Biochimie 2010, 92, 1306–1314. [Google Scholar] [CrossRef]
- Adorisio, S.; Fierabracci, A.; Muscari, I.; Liberati, A.M.; Ayroldi, E.; Migliorati, G.; Thuy, T.T.; Riccardi, C.; Delfino, D.V. SUMO proteins: Guardians of immune system. J. Autoimmun. 2017, 84, 21–28. [Google Scholar] [CrossRef]
- Liu, J.; Qian, C.; Cao, X. Post-Translational Modification Control of Innate Immunity. Immunity 2016, 45, 15–30. [Google Scholar] [CrossRef]
- Schmidt, N.; Domingues, P.; Golebiowski, F.; Patzina, C.; Tatham, M.H.; Hay, R.T.; Hale, B.G. An influenza virus-triggered SUMO switch orchestrates co-opted endogenous retroviruses to stimulate host antiviral immunity. Proc. Natl. Acad. Sci. USA 2019, 116, 17399–17408. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, I.; Ito, A.; Hirai, G.; Nishimura, S.; Kawasaki, H.; Saitoh, H.; Kimura, K.; Sodeoka, M.; Yoshida, M. Ginkgolic acid inhibits protein SUMOylation by blocking formation of the E1-SUMO intermediate. Chem. Biol. 2009, 16, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Xu, L.; Wang, Y.; Luo, W.; Zhu, L.; Yuan, M.; Wu, W.; Yang, K. AcMNPV PKIP is required for hyperexpression of very late genes and involved in the hyperphosphorylation of the viral basic protein P6.9. Virus Res. 2020, 279, 197889. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Zhu, L.; Xu, L.; Yuan, M.; Wu, W.; Yang, K. AcMNPV PKIP is associated with nucleocapsid of budded virions and involved in nucleocapsid assembly. Virus Res. 2019, 268, 27–37. [Google Scholar] [CrossRef]
- Jan Fada, B.; Kaadi, E.; Samrat, S.K.; Zheng, Y.; Gu, H. Effect of SUMO-SIM Interaction on the ICP0-Mediated Degradation of PML Isoform II and Its Associated Proteins in Herpes Simplex Virus 1 Infection. J. Virol. 2020, 94, e00470-20. [Google Scholar] [CrossRef] [PubMed]
- Palacios, S.; Perez, L.H.; Welsch, S.; Schleich, S.; Chmielarska, K.; Melchior, F.; Locker, J.K. Quantitative SUMO-1 modification of a vaccinia virus protein is required for its specific localization and prevents its self-association. Mol. Biol. Cell 2005, 16, 2822–2835. [Google Scholar] [CrossRef]
- Lowrey, A.J.; Cramblet, W.; Bentz, G.L. Viral manipulation of the cellular sumoylation machinery. Cell Commun. Signal. 2017, 15, 27. [Google Scholar] [CrossRef]
- Campbell, M.; Izumiya, Y. Post-Translational Modifications of Kaposi’s Sarcoma-Associated Herpesvirus Regulatory Proteins-SUMO and KSHV. Front. Microbiol. 2012, 3, 31. [Google Scholar] [CrossRef]
- Kim, E.T.; Kim, Y.E.; Kim, Y.J.; Lee, M.K.; Hayward, G.S.; Ahn, J.H. Analysis of human cytomegalovirus-encoded SUMO targets and temporal regulation of SUMOylation of the immediate-early proteins IE1 and IE2 during infection. PLoS ONE 2014, 9, e103308. [Google Scholar] [CrossRef]
- Liang, C.; Li, M.; Dai, X.; Zhao, S.; Hou, Y.; Zhang, Y.; Lan, D.; Wang, Y.; Chen, X. Autographa californica multiple nucleopolyhedrovirus PK-1 is essential for nucleocapsid assembly. Virology 2013, 443, 349–357. [Google Scholar] [CrossRef]
- Mishra, G.; Chadha, P.; Das, R.H. Serine/threonine kinase (pk-1) is a component of Autographa californica multiple nucleopolyhedrovirus (AcMNPV) very late gene transcription complex and it phosphorylates a 102 kDa polypeptide of the complex. Virus Res. 2008, 137, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, T.; Zhang, B.; Liu, S.; Song, W.; Qiao, J.; Ruan, H. Types of nuclear localization signals and mechanisms of protein import into the nucleus. Cell Commun. Signal. 2021, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Chang, C.; Li, L.; Klenk, C.; Cheng, J.; Chen, Y.; Xia, N.; Shu, Y.; Chen, Z.; Gabriel, G.; et al. Sumoylation of influenza A virus nucleoprotein is essential for intracellular trafficking and virus growth. J. Virol. 2014, 88, 9379–9390. [Google Scholar] [CrossRef] [PubMed]
- Way, G.; Xiong, Z.; Wang, G.; Dai, H.; Zheng, S.; Garcia-Sastre, A.; Liao, J. A novel SUMOylation site in the influenza a virus NS1 protein identified with a highly sensitive FRET assay. J. Biotechnol. 2020, 323, 121–127. [Google Scholar] [CrossRef]
- Conde, J.N.; Schutt, W.R.; Mladinich, M.; Sohn, S.Y.; Hearing, P.; Mackow, E.R. NS5 Sumoylation Directs Nuclear Responses That Permit Zika Virus To Persistently Infect Human Brain Microvascular Endothelial Cells. J. Virol. 2020, 94, e01086-20. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, R.; Lü, D.; Chen, G.; Liu, M.; Pu, S.; Zhang, Y.; Wang, Q.; Qian, P.; Tang, X. SUMOylation Regulates BmNPV Replication by Moderating PKIP Intracellular Localization. Processes 2022, 10, 261. https://doi.org/10.3390/pr10020261
Shen R, Lü D, Chen G, Liu M, Pu S, Zhang Y, Wang Q, Qian P, Tang X. SUMOylation Regulates BmNPV Replication by Moderating PKIP Intracellular Localization. Processes. 2022; 10(2):261. https://doi.org/10.3390/pr10020261
Chicago/Turabian StyleShen, Rui, Dingding Lü, Guanyu Chen, Mengjin Liu, Shiqi Pu, Yiling Zhang, Qiang Wang, Ping Qian, and Xudong Tang. 2022. "SUMOylation Regulates BmNPV Replication by Moderating PKIP Intracellular Localization" Processes 10, no. 2: 261. https://doi.org/10.3390/pr10020261
APA StyleShen, R., Lü, D., Chen, G., Liu, M., Pu, S., Zhang, Y., Wang, Q., Qian, P., & Tang, X. (2022). SUMOylation Regulates BmNPV Replication by Moderating PKIP Intracellular Localization. Processes, 10(2), 261. https://doi.org/10.3390/pr10020261





