Molecular Dynamics Simulation for Structural Evolution of Mixed Ash from Coal and Wheat Straw
Abstract
1. Introduction
2. Methodology
2.1. Ash Samples Preparation
2.2. Simulation Method
2.3. MD Simulation Process
3. Results and Discussion
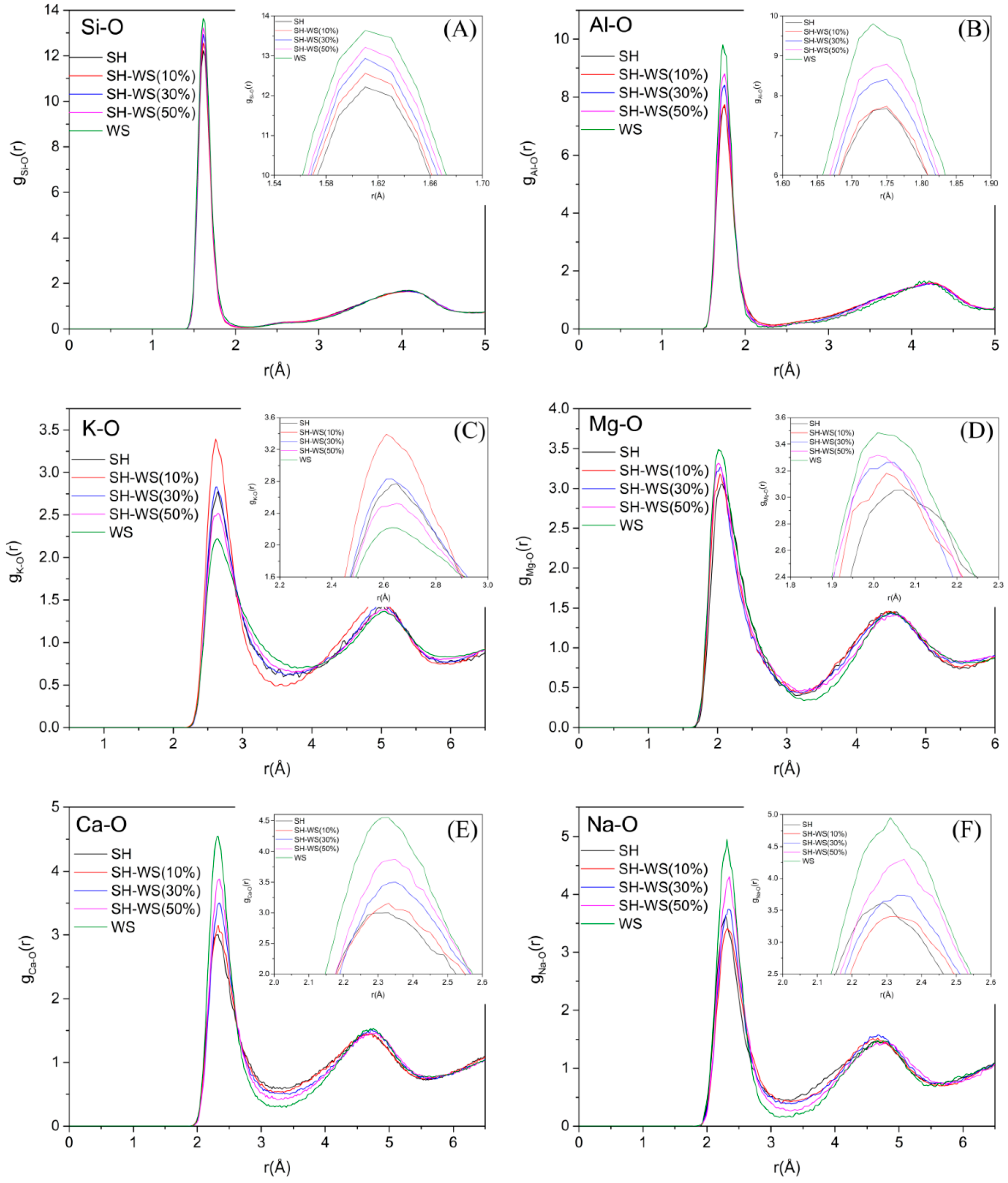
3.1. Radial Distribution Functions (RDFs)
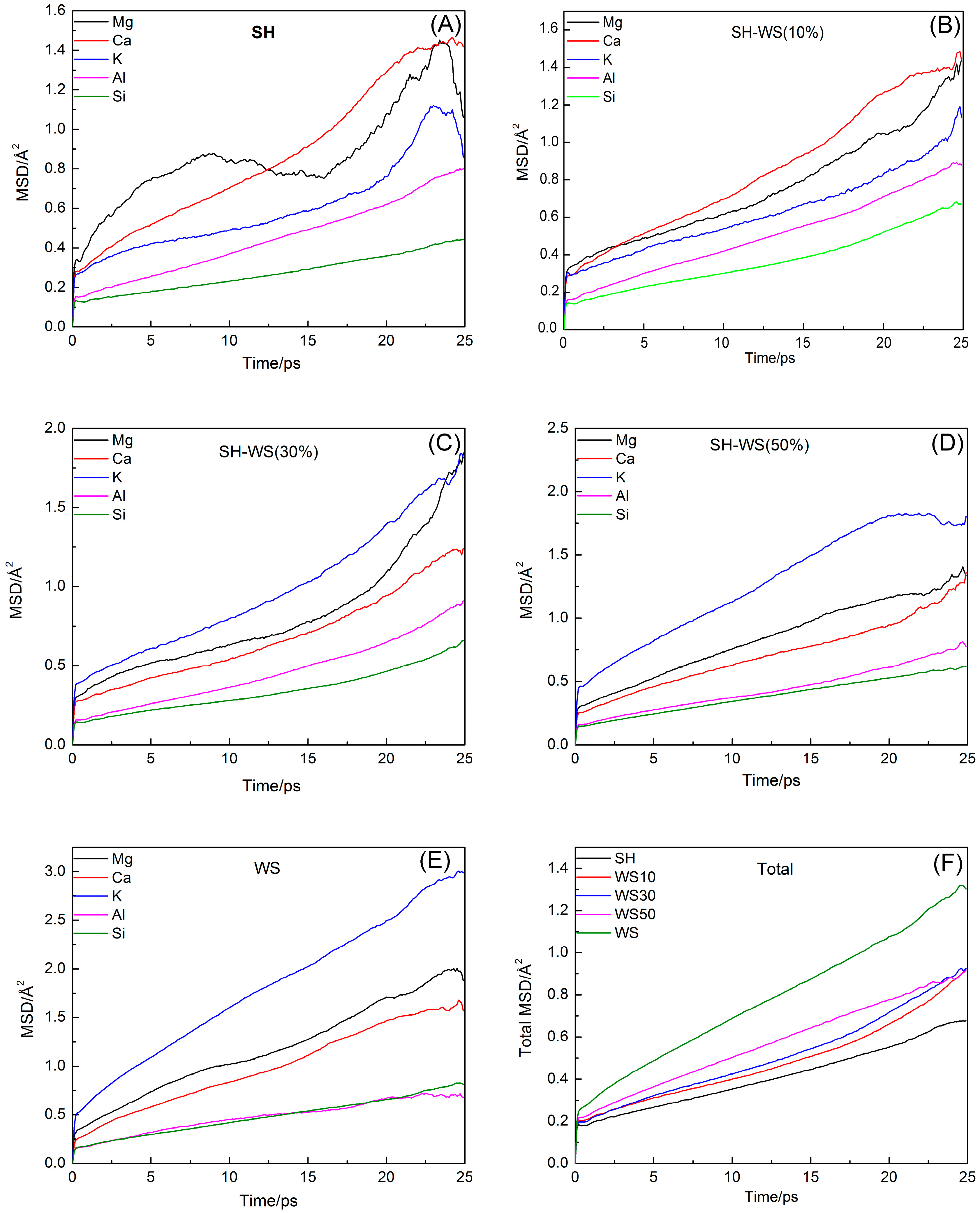
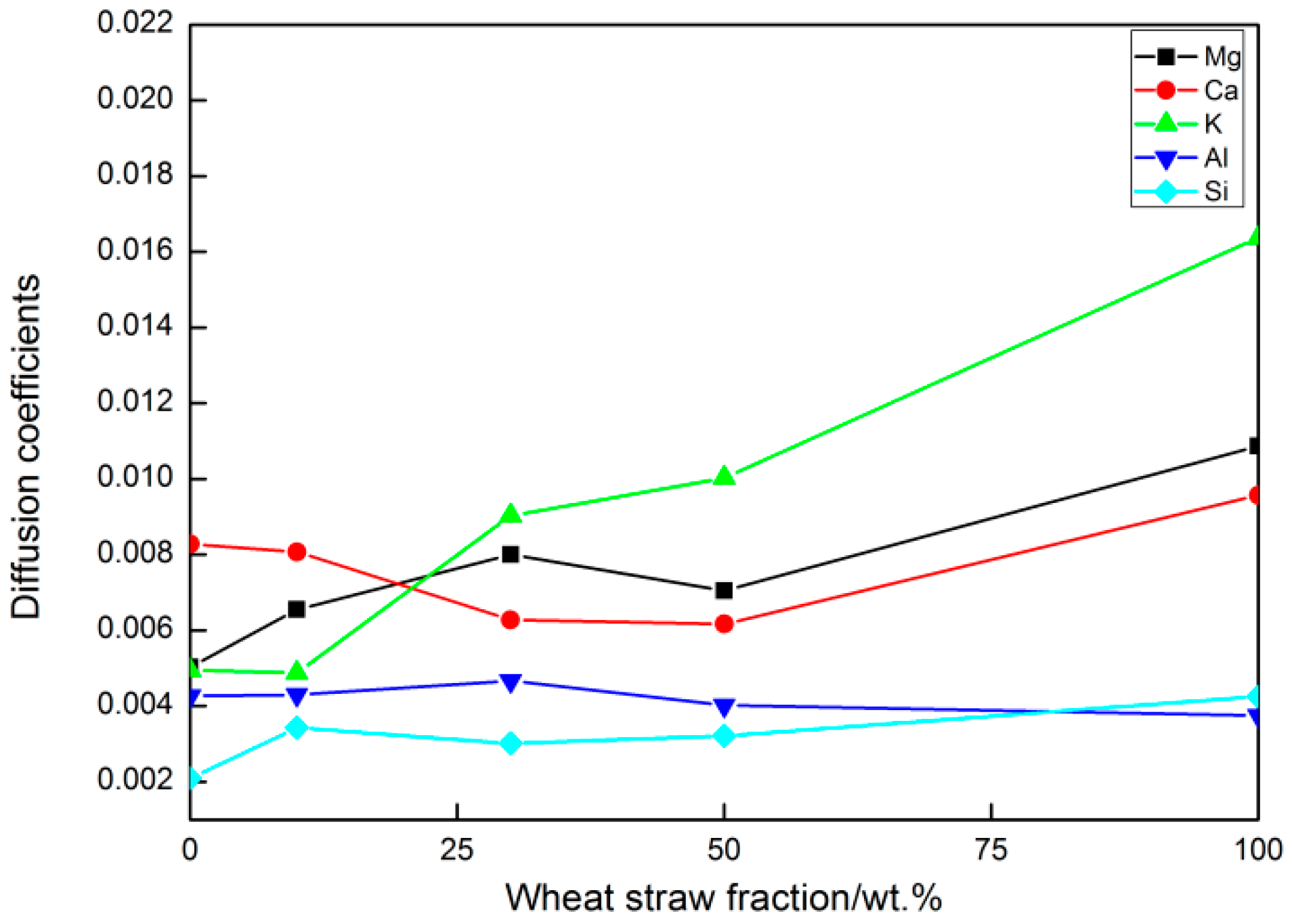
3.2. Mean Square Displacement
3.3. Coordination Numbers
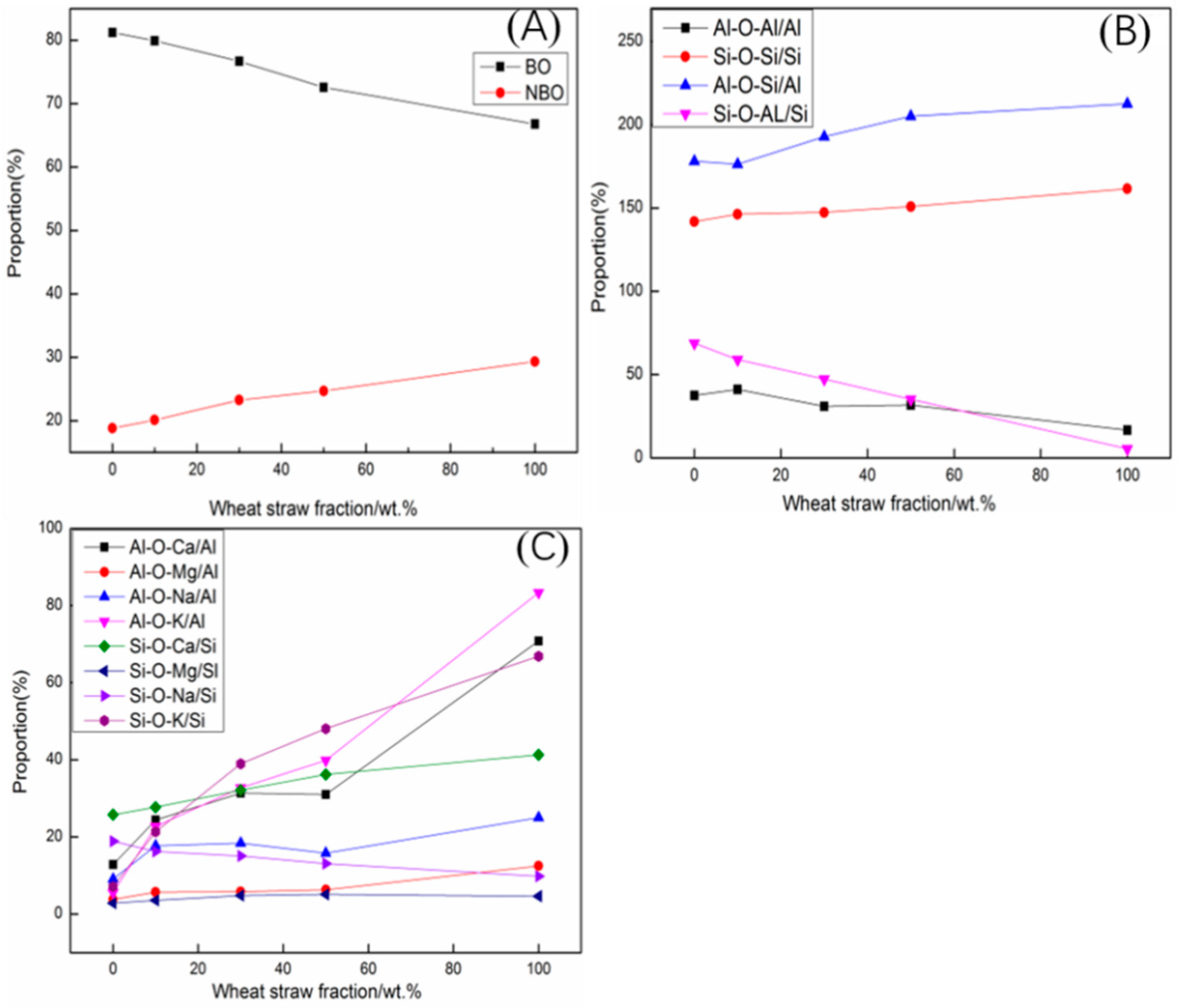
3.4. Distribution of Oxygen Types
3.5. Distribution of Bond Angles
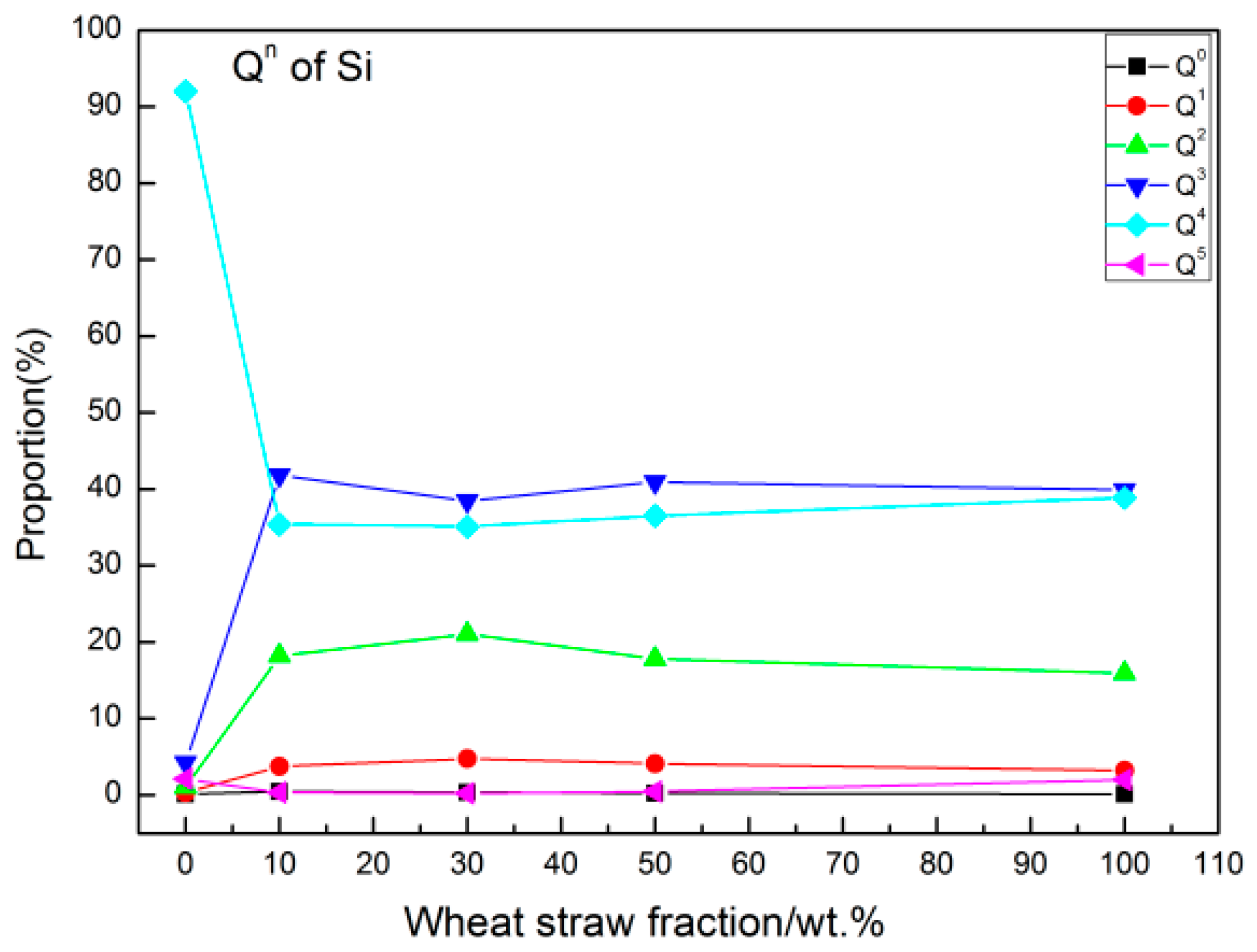
3.6. Distribution of Qn
4. Conclusions
- (1)
- By analyzing the RDFs of the ion–oxygen pair, we found that the WS had no obvious influence on the bond length of Si–O. The widths of the first peaks in other ion–oxygen pairs were larger than that of Si–O, indicating a weaker bond strength than Si–O. The coordination number of Al was influenced by the addition of WS, demonstrating that WS influences the stability of the network structure. Moreover, the [AlO4] tetrahedron was found to be less stable than the [SiO4] tetrahedron.
- (2)
- The concentration of BO decreased and that of NBO increased with increasing WS content. This indicates that the network structure can be destroyed by metal ions, resulting in the conversion of BO to NBO.
- (3)
- The O–Si–O bond angle was constant at approximately 107.5°. The O–Al–O bond angles were wide and varied with increasing WS content.
- (4)
- With increasing WS content, the diffusion coefficients of Si4+ varied only slightly with no obvious trend. The sum of all diffusion coefficients increased with WS content, indicating an increased diffusion capacity of the system.
- (5)
- For a WS content of 30%, the Q4 and Q5 structural units transformed into Q3, Q2, Q1, and Q0 structural units to the greatest extent, which corresponded to the lowest degree of polymerization and highest ash fluidity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dudley, B. BP Statistical Review of World Energy; Whitehouse Associates: London, UK, 2019. [Google Scholar]
- Jiang, N.; Li, L.; Wang, S.; Li, Q.; Dong, Z.; Duan, S.; Zhang, R.; Li, S. Variation tendency of pollution characterization, sources, and health risks of PM 2.5 -bound polycyclic aromatic hydrocarbons in an emerging megacity in China: Based on three-year data. Atmos. Res. 2019, 217, 81–92. [Google Scholar] [CrossRef]
- Steinberg, M.; Cheng, H.-C. Modern and prospective technologies for hydrogen production from fossil fuels. Int. J. Hydrog. Energy 1989, 14, 797–820. [Google Scholar] [CrossRef]
- Mahgagaokar, U.; Krewinghaus, A.-B. Coal conversion processes (gasification). Encycl. Chem. Technol. 1992, 6, 541–568. [Google Scholar]
- Dai, J.; Cui, H.; Grace, J.-R. Biomass feeding for thermochemical reactors. Prog. Energy Combust. 2012, 38, 716–736. [Google Scholar] [CrossRef]
- Yun, S.; Dai, Z.-H.; Zhou, Z.-J.; Chen, X.-L.; Yu, G.-S.; Wang, F.-C. Rapid co-pyrolysis of rice straw and a bituminous coal in a high-frequency furnace and gasification of the residual char. Bioresour. Technol. 2012, 109, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Ilyushechkin, A.-Y.; Shwe, H.-S.; Chen, X.; Roberts, D.-G. Effect of sodium in brown coal ash transformations and slagging behaviour under gasification conditions. Fuel Process Technol. 2018, 179, 86–98. [Google Scholar] [CrossRef]
- Magdziarz, A.; Gajek, M.; Nowak-Wozny, D.; Wilk, M. Mineral phase transformation of biomass ashes—Experimental and thermochemical calculations. Renew. Energy 2018, 128, 446–459. [Google Scholar] [CrossRef]
- Wei, J.; Gong, Y.; Ding, L.; Yu, J.; Yu, G. Influence of Biomass Ash Additive on Reactivity Characteristics and Structure Evolution of Coal Char–CO2 Gasification. Energy Fuels 2018, 32, 10428–10436. [Google Scholar] [CrossRef]
- Fang, X.; Jia, L. Experimental study on ash fusion characteristics of biomass. Bioresour. Technol. 2012, 104, 769–774. [Google Scholar] [CrossRef]
- Li, K.-J.; Bouhadja, M.; Khanna, R.; Zhang, J.-L.; Liu, Z.-J.; Zhang, Y.-P.; Yang, T.-J.; Sahajwalla, V.; Yang, Y.-D.; Barati, M. Influence of SiO2 reduction on the local structural order and fluidity of molten coke ash in the high temperature zone of a blast furnace. A molecular dynamics simulation investigation. Fuel 2016, 186, 561–570. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Liu, W.; Lv, X.; Bai, C.; Wang, L. Relationship between structure and viscosity of CaO–SiO2–Al2O3–MgO–TiO2 slag. J. Non-Crys. Solids 2014, 402, 214–222. [Google Scholar] [CrossRef]
- Xuan, W.; Wang, H.; Xia, D. Deep structure analysis on coal slags with increasing silicon content and correlation with melt viscosity. Fuel 2019, 242, 362–367. [Google Scholar] [CrossRef]
- Li, K.; Khanna, R.; Bouhadja, M.; Zhang, J.; Liu, Z.; Su, B.; Yang, T.; Sahajwalla, V.; Singh, C.-V.; Barati, M. A molecular dynamic simulation on the factors influencing the fluidity of molten coke ash during alkalization with K2O and Na2O. Chem. Eng. J. 2017, 313, 1184–1193. [Google Scholar] [CrossRef]
- Wu, T.; Wang, Q.; Yu, C.-F.; He, S.-P. Structural and viscosity properties of CaO-SiO2-Al2O3-FeO slags based on molecular dynamic simulation. J. Non-Crys. Solids 2016, 450, 23–31. [Google Scholar] [CrossRef]
- Jiang, C.; Li, K.; Zhang, J.; Qin, Q.; Liu, Z.; Sun, M.; Wang, Z.; Wang, L. Effect of MgO/Al2O3 ratio on the structure and properties of blast furnace slags: A molecular dynamics simulation. J. Non-Crys. Solids 2018, 502, 76–82. [Google Scholar] [CrossRef]
- PRC National Standard GB/T 212-2008. Methods for Industrial Analysis of Coal; China Quality and Standards Publishing: Beijing, China, 2008. [Google Scholar]
- PRC National Standard GB/T 28731-201. Methods for Industrial Analysis of Solid Biomass Fuel; China Quality and Standards Publishing: Beijing, China, 2012. [Google Scholar]
- ASTM Standard D3174-2012. Standard Test Method for Ash in the Analysis Sample of Coal and Coke from Coal; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Ma, C.; Skoglund, N.; Carlborg, M.; Brostrm, M. Viscosity of molten CaO-K2O-SiO2 woody biomass ash slags in relation to structural characteristics from molecular dynamics simulation. Chem. Eng. Sci. 2020, 215, 115464. [Google Scholar] [CrossRef]
- Dai, X.; Bai, J.; Huang, Q.; Liu, Z.; Bai, X.; Cao, R.; Wen, X.; Li, W.; Du, S. Viscosity temperature properties from molecular dynamics simulation: The role of calcium oxide, sodium oxide and ferrous oxide. Fuel 2019, 237, 163–169. [Google Scholar] [CrossRef]
- Litton, D.-A.; Garofalini, S.-H. Atomistic structure of sodium and calcium silicate intergranular films in alumina. J. Mater. Res. Technol. 1999, 14, 1418–1429. [Google Scholar] [CrossRef][Green Version]
- Zheng, K.; Zhang, Z.; Yang, F.; Sridhar, S. Molecular Dynamics Study of the Structural Properties of Calcium Aluminosilicate Slags with Varying Al2O3/SiO2 Ratios. ISIJ Int. 2012, 52, 342–349. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Z.; Li, R.; Zhu, R.; Xu, J.; Zhao, J.; Wang, C.; Nordlund, K.; Fu, X.; Fang, F. Molecular dynamics simulation of helium ion implantation into silicon and its migration. Nucl. Instrum. Meth. B 2019, 456, 53–59. [Google Scholar] [CrossRef]
- Wang, B.; Cormack, A.-N. Molecular dynamics simulations of Mg-doped beta″-alumina with potential models fitted for accurate structural response to thermal vibrations. Solid State Ionics 2014, 263, 9–14. [Google Scholar] [CrossRef]
- Mongalo, L.; Lopis, A.-S.; Venter, G.-A. Molecular dynamics simulations of the structural properties and electrical conductivities of CaO–MgO–Al2O3–SiO2 melts. J. Non-Crys. Solids 2016, 452, 194–202. [Google Scholar] [CrossRef]




| Sample | Content (wt%) | Gross Calorific Value (cal/g) | |||
|---|---|---|---|---|---|
| Ash | Volatiles | H2O | C-Fix | ||
| SH coal | 8.12 | 28.02 | 9.50 | 54.36 | 6336.8 |
| WS | 11.5 | 66.4 | 7.95 | 14.15 | 4354.4 |
| Sample | Content (wt%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Na2O | MgO | AI2O3 | SiO2 | P2O5 | SO3 | K2O | CaO | Fe2O3 | |
| SH ash | 1.86 | 2.34 | 19.12 | 58.03 | 0.50 | 5.66 | 1.09 | 8.29 | 3.11 |
| WS ash | 0.66 | 4.28 | 1.19 | 58.76 | 2.39 | 4.74 | 19.39 | 7.54 | 1.04 |
| Sample | Mol% | |||||
|---|---|---|---|---|---|---|
| Na2O | MgO | Al2O3 | K2O | CaO | SiO2 | |
| SH | 2.1 | 4.2 | 13.3 | 0.9 | 10.6 | 68.9 |
| SH-WS(10%) | 1.9 | 4.6 | 11.5 | 2.8 | 10.4 | 68.8 |
| SH-WS(30%) | 1.6 | 5.4 | 8.4 | 14.4 | 10.1 | 68.6 |
| SH-WS(50%) | 1.3 | 6.1 | 5.9 | 8.9 | 9.8 | 68.5 |
| WS | 0.7 | 7.5 | 0.9 | 14.4 | 9.4 | 68.2 |
| Sample | Atomic Number | Density (g/cm3) | Length (Å) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Na | Mg | Al | Si | K | Ca | O | |||
| SH | 55 | 53 | 342 | 884 | 22 | 135 | 2508 | 3.0 | 35.82 |
| SH-WS(10%) | 50 | 60 | 299 | 894 | 72 | 134 | 2491 | 3.0 | 35.93 |
| SH-WS(30%) | 42 | 72 | 223 | 908 | 161 | 134 | 2459 | 3.0 | 36.13 |
| SH-WS(50%) | 34 | 82 | 158 | 922 | 239 | 132 | 2432 | 3.0 | 36.31 |
| WS | 21 | 104 | 24 | 947 | 400 | 130 | 2374 | 3.0 | 36.67 |
| Atoms | Parameter | |||
|---|---|---|---|---|
| i | j | Aij (fJ) | βij (pm) | ρij (pm) |
| Na | Na | 0.2159 | 230 | 29 |
| Na | Al | 0.2178 | 230 | 29 |
| Na | Si | 0.2001 | 230 | 29 |
| Na | O | 0.3195 | 234 | 29 |
| Mg | Mg | 1.0643 | 231.8 | 29 |
| Mg | Al | 0.20846 | 230 | 29 |
| Mg | Si | 0.2216 | 230 | 29 |
| Mg | O | 0.2842 | 234 | 29 |
| Al | Al | 0.0500 | 235 | 29 |
| Al | Si | 0.2523 | 233 | 29 |
| Al | O | 0.2490 | 234 | 29 |
| Si | Si | 0.1877 | 230 | 29 |
| Si | O | 0.2962 | 234 | 29 |
| K | K | 0.99706 | 253.8 | 29 |
| K | Al | 0.4448 | 236.6 | 29 |
| K | Si | 0.4420 | 253.8 | 29 |
| K | O | 0.60802 | 253.8 | 29 |
| Ca | Ca | 0.7000 | 230 | 29 |
| Ca | Al | 0.2178 | 230 | 29 |
| Ca | Si | 0.2215 | 230 | 29 |
| Ca | O | 0.5700 | 234 | 29 |
| O | O | 0.0725 | 234 | 29 |
| Atomtriplet | λij (fJ) | γij (pm) | Rij (pm) | ωjik (deg) |
|---|---|---|---|---|
| Al/Si–O–Al/Si | 0.001 | 200 | 260 | 109.5 |
| O–Al/Si–O | 0.024 | 280 | 300 | 109.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, H.; Li, X.; Zhang, M.; Li, Z.; Zhou, Y.; Ma, X. Molecular Dynamics Simulation for Structural Evolution of Mixed Ash from Coal and Wheat Straw. Processes 2022, 10, 215. https://doi.org/10.3390/pr10020215
Ji H, Li X, Zhang M, Li Z, Zhou Y, Ma X. Molecular Dynamics Simulation for Structural Evolution of Mixed Ash from Coal and Wheat Straw. Processes. 2022; 10(2):215. https://doi.org/10.3390/pr10020215
Chicago/Turabian StyleJi, Hengsong, Xiang Li, Mei Zhang, Zhenqiang Li, Yan Zhou, and Xiang Ma. 2022. "Molecular Dynamics Simulation for Structural Evolution of Mixed Ash from Coal and Wheat Straw" Processes 10, no. 2: 215. https://doi.org/10.3390/pr10020215
APA StyleJi, H., Li, X., Zhang, M., Li, Z., Zhou, Y., & Ma, X. (2022). Molecular Dynamics Simulation for Structural Evolution of Mixed Ash from Coal and Wheat Straw. Processes, 10(2), 215. https://doi.org/10.3390/pr10020215







