Chemical Characterization and Preliminary Evaluation of the Efficacy and Tolerability of a Food Supplement Based on Pomegranate Extract, B Vitamins, and Vitamin C against Prolonged Fatigue in Healthy Consumers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Food Supplement
2.2. Pomegranate Extract Analysis by UHPLC–HRMS
2.3. Evaluation of Efficacy and Tolerability
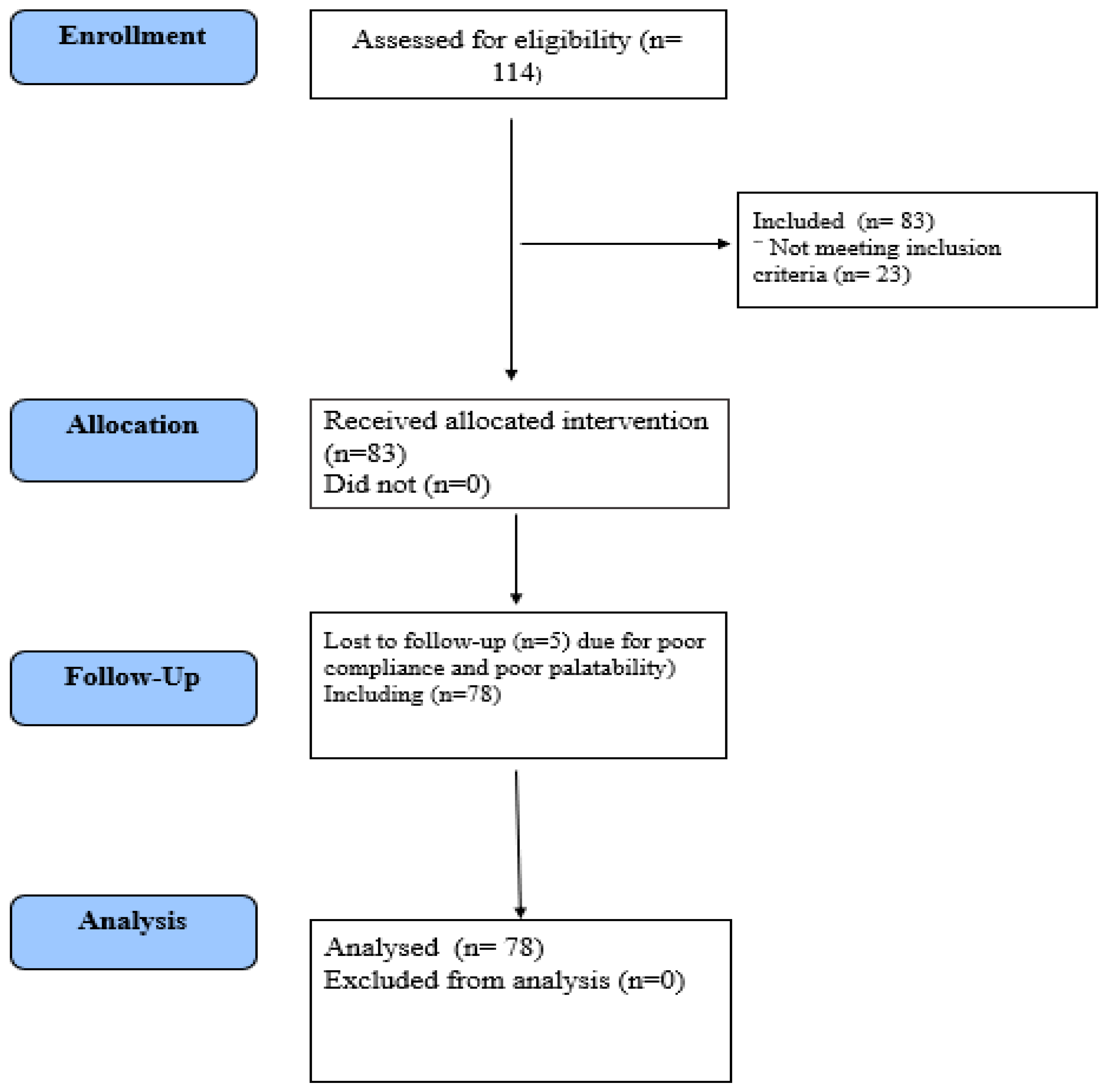
2.3.1. Survey Population
2.3.2. Survey Design
2.3.3. Evaluated Variables
2.4. Statistical Analysis
3. Results
3.1. Pomegranate Extract Chromatographic Analysis
3.2. Survey among Consumers on the Evaluation of Efficacy and Tolerability of the Food Supplement
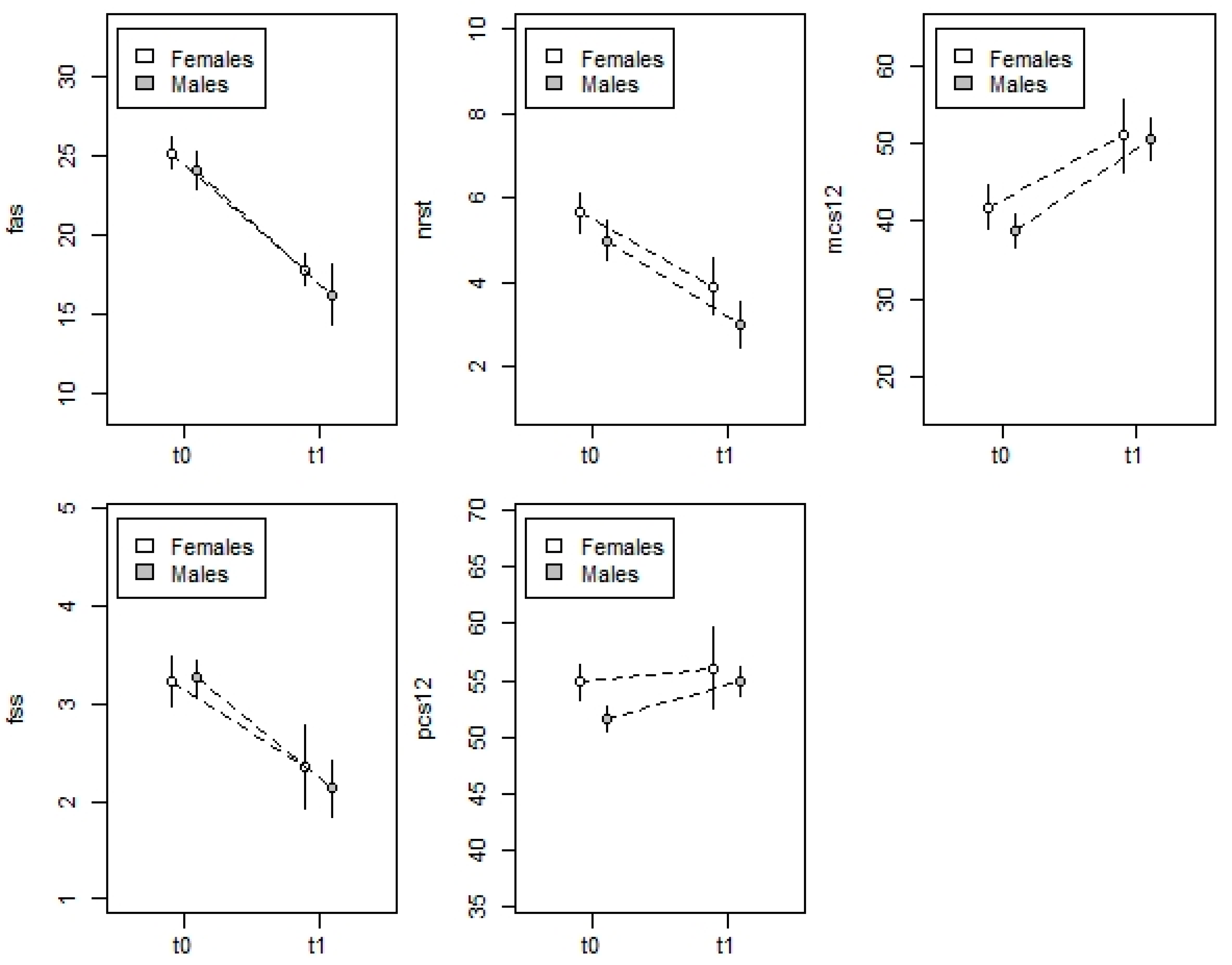
3.3. Primary Outcomes: FAS, FSS, and NRST
3.4. Secondary Endpoint: SF-12 Physical and SF-12 Mental
3.5. Tolerance and Safety Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fukuda, K.; Dobbins, J.G.; Wilson, L.J.; Dunn, R.A.; Wilcox, K.; Smallwood, D. An epidemiologic study of fatigue with relevance for the chronic fatigue syndrome. J. Psychiatr. Res. 1997, 31, 19–29. [Google Scholar] [CrossRef]
- Baek, Y.; Kim, H.; Mun, S.; Lee, S. Three-Component Herbal Tea Alleviates Prolonged Fatigue and Improves Sleep Quality: A Randomized Controlled Pilot Study. EXPLORE 2018, 14, 420–423. [Google Scholar] [CrossRef]
- Zielinski, M.R.; Systrom, D.M.; Rose, N.R. Fatigue, Sleep, and Autoimmune and Related Disorders. Front. Immunol. 2019, 10, 1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jason, L.A.; Evans, M.; Brown, M.; Porter, N. What is Fatigue? Pathological and Nonpathological Fatigue. PM&R 2010, 2, 327–331. [Google Scholar] [CrossRef]
- Klimas, N.G.; Broderick, G.; Fletcher, M.A. Biomarkers for chronic fatigue. Brain Behav. Immun. 2012, 26, 1202–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tardy, A.-L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portero, P.; Gomez-Merino, D. Stanchezza e Motilità. In EMC-Medicina Riabilitativa; Elsevier: Amsterdam, The Netherlands, 2013; Volume 20, pp. 1–12. [Google Scholar] [CrossRef]
- Yancey, J.R.; Thomas, S.M. Chronic fatigue syndrome: Diagnosis and treatment. Am. Fam. Physician 2012, 86, 741–746. [Google Scholar] [PubMed]
- Bucher, E.; Sandbakk, O.; Donath, L.; Roth, R.; Zahner, L.; Faude, O. Exercise-induced trunk fatigue decreases double poling performance in well-trained cross-country skiers. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 118, 2077–2087. [Google Scholar] [CrossRef]
- Lin, C.F.; Lee, W.C.; Chen, Y.A.; Hsue, B.J. Fatigue-Induced Changes in Movement Pattern and Muscle Activity During Ballet Releve on Demi-Pointe. J. Appl. Biomech. 2016, 32, 350–358. [Google Scholar] [CrossRef]
- Le Mansec, Y.; Pageaux, B.; Nordez, A.; Dorel, S.; Jubeau, M. Mental fatigue alters the speed and the accuracy of the ball in table tennis. J. Sports Sci. 2017, 36, 2751–2759. [Google Scholar] [CrossRef] [PubMed]
- Salva, M.A.Q.; Barbot, F.; Hartley, S.; Sauvagnac, R.; Vaugier, I.; Lofaso, F.; Philip, P. Sleep disorders, sleepiness, and near-miss accidents among long-distance highway drivers in the summertime. Sleep Med. 2014, 15, 23–26. [Google Scholar] [CrossRef]
- Nourbakhsh, B.; Revirajan, N.; Morris, B.; Cordano, C.; Creasman, J.; Manguinao, M.; Krysko, K.; Rutatangwa, A.; Auvray, C.; Aljarallah, S.; et al. Safety and efficacy of amantadine, modafinil, and methylphenidate for fatigue in multiple sclerosis: A randomised, placebo-controlled, crossover, double-blind trial. Lancet Neurol. 2020, 20, 38–48. [Google Scholar] [CrossRef]
- Werbach, M.R. Nutritional strategies for treating chronic fatigue syndrome. Altern. Med. Rev. 2000, 5, 93–108. [Google Scholar]
- Haß, U.; Herpich, C.; Norman, K. Anti-Inflammatory Diets and Fatigue. Nutrients 2019, 11, 2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Zhang, Q.; Hou, H.; Liu, Z.; Wang, L.; Rasekhmagham, R.; Kord-Varkaneh, H.; Santos, H.O.; Yao, G. The effects of pomegranate supplementation on biomarkers of inflammation and endothelial dysfunction: A meta-analysis and systematic review. Complement. Ther. Med. 2020, 49, 102358. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Bailey, S.; Chtourou, H.; Trabelsi, K.; Turki, M.; Hökelmann, A.; Souissi, N. Effects of pomegranate supplementation on exercise performance and post-exercise recovery in healthy adults: A systematic review. Br. J. Nutr. 2018, 120, 1201–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbaniak, A.; Skarpańska-Stejnborn, A. Effect of pomegranate fruit supplementation on performance and various markers in athletes and active subjects: A systematic review. Int. J. Vitam. Nutr. Res. 2021, 91, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Drent, M.; Lower, E.E.; De Vries, J. Sarcoidosis-associated fatigue. Eur. Respir. J. 2012, 40, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Kleijn, W.P.; De Vries, J.; Wijnen, P.A.; Drent, M. Minimal (clinically) important differences for the Fatigue Assessment Scale in sarcoidosis. Respir. Med. 2011, 105, 1388–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vries, J.; Michielsen, H.; Van Heck, G.L.; Drent, M. Measuring fatigue in sarcoidosis: The Fatigue Assessment Scale (FAS). Br. J. Health Psychol. 2004, 9, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Krupp, L.B.; LaRocca, N.G.; Muir-Nash, J.; Steinberg, A.D. The Fatigue Severity Scale: Application to Patients With Multiple Sclerosis and Systemic Lupus Erythematosus. Arch. Neurol. 1989, 46, 1121–1123. [Google Scholar] [CrossRef]
- Morgul, E.; Bener, A.; Atak, M.; Akyel, S.; Aktaş, S.; Bhugra, D.; Ventriglio, A.; Jordan, T.R. COVID-19 pandemic and psychological fatigue in Turkey. Int. J. Soc. Psychiatry 2020, 67, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Hjollund, N.H.; Andersen, J.H.; Bech, P. Assessment of fatigue in chronic disease: A bibliographic study of fatigue measurement scales. Health Qual. Life Outcomes 2007, 5, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladman, D.; Nash, P.; Goto, H.; Birt, J.A.; Lin, C.-Y.; Orbai, A.-M.; Kvien, T.K. Fatigue numeric rating scale validity, discrimination and responder definition in patients with psoriatic arthritis. RMD Open 2020, 6, e000928. [Google Scholar] [CrossRef]
- Gandek, B.; Ware, J.E.; Aaronson, N.K.; Apolone, G.; Bjorner, J.B.; Brazier, J.E.; Bullinger, M.; Kaasa, S.; Leplege, A.; Prieto, L.; et al. Cross-Validation of Item Selection and Scoring for the SF-12 Health Survey in Nine Countries: Results from the IQOLA Project. International Quality of Life Assessment. J. Clin. Epidemiol. 1998, 51, 1171–1178. [Google Scholar] [CrossRef]
- Ware, J.E.; Keller, S.D. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales, 2nd ed.; Health Institute, New England Medical Center: Boston, MA, USA, 1995. [Google Scholar]
- Moga, M.; Dimienescu, O.; Bălan, A.; Dima, L.; Toma, S.; Bîgiu, N.; Blidaru, A. Pharmacological and Therapeutic Properties of Punica granatum Phytochemicals: Possible Roles in Breast Cancer. Molecules 2021, 26, 1054. [Google Scholar] [CrossRef]
- The European Commission. Commission Regulation (EU) No. 432/2012 Establishing a List of Permitted Health Claims Made on Foods, Other Than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health; Official Journal of European Union. 2012. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:136:0001:0040:en:PDF (accessed on 8 December 2021).
- The European Commission. Regulation (EC) No. 1924/2006 of the European Parliament and of the Council on Nutrition and Health Claims Made on Foods; Official Journal of European Union. 2006. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:404:0009:0025:En:PDF (accessed on 8 December 2021).
- Fahmy, H.; Hegazi, N.; El-Shamy, S.; Farag, M.A. Pomegranate juice as a functional food: A comprehensive review of its polyphenols, therapeutic merits, and recent patents. Food Funct. 2020, 11, 5768–5781. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Han, B.; Deng, X.; Deng, S.-Y.; Zhang, Y.-Y.; Shen, P.-X.; Hui, T.; Chen, R.-H.; Li, X.; Zhang, Y. Pomegranate peel extract ameliorates the severity of experimental autoimmune encephalomyelitis via modulation of gut microbiota. Gut Microbes 2020, 12, 1857515. [Google Scholar] [CrossRef] [PubMed]
- Trexler, E.T.; Smith-Ryan, A.E.; Melvin, M.N.; Roelofs, E.J.; Wingfield, H.L. Effects of pomegranate extract on blood flow and running time to exhaustion. Appl. Physiol. Nutr. Metab. 2014, 39, 1038–1042. [Google Scholar] [CrossRef] [Green Version]
- Torregrosa-García, A.; Ávila-Gandía, V.; Luque-Rubia, A.J.; Abellán-Ruiz, M.S.; Querol-Calderón, M.; López-Román, F.J. Pomegranate Extract Improves Maximal Performance of Trained Cyclists after an Exhausting Endurance Trial: A Randomised Controlled Trial. Nutrients 2019, 11, 721. [Google Scholar] [CrossRef] [Green Version]
- Roelofs, E.J.; Smith-Ryan, A.E.; Trexler, E.T.; Hirsch, K.R.; Mock, M.G. Effects of pomegranate extract on blood flow and vessel diameter after high-intensity exercise in young, healthy adults. Eur. J. Sport Sci. 2016, 17, 317–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louati, K.; Berenbaum, F. Fatigue in chronic inflammation—A link to pain pathways. Arthritis Res. Ther. 2015, 17, 254. [Google Scholar] [CrossRef] [Green Version]
- Maes, M.; Twisk, F.; Kubera, M.; Ringel, K. Evidence for inflammation and activation of cell-mediated immunity in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Increased interleukin-1, tumor necrosis factor-α, PMN-elastase, lysozyme and neopterin. J. Affect. Disord. 2012, 136, 933–939. [Google Scholar] [CrossRef]
- Lee, C.-H.; Giuliani, F. The Role of Inflammation in Depression and Fatigue. Front. Immunol. 2019, 10, 1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastrogiovanni, F.; Mukhopadhya, A.; Lacetera, N.; Ryan, M.T.; Romani, A.; Bernini, R.; Sweeney, T. Anti-Inflammatory Effects of Pomegranate Peel Extracts on In Vitro Human Intestinal Caco-2 Cells and Ex Vivo Porcine Colonic Tissue Explants. Nutrients 2019, 11, 548. [Google Scholar] [CrossRef] [Green Version]
- Boldaji, R.B.; Akhlaghi, M.; Sagheb, M.M.; Esmaeilinezhad, Z. Pomegranate juice improves cardiometabolic risk factors, biomarkers of oxidative stress and inflammation in hemodialysis patients: A randomized crossover trial. J. Sci. Food Agric. 2019, 100, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Shishehbor, F.; Shahi, M.M.; Zarei, M.; Saki, A.; Zakerkish, M.; Shirani, F.; Zare, M. Effects of Concentrated Pomegranate Juice on Subclinical Inflammation and Cardiometabolic Risk Factors for Type 2 Diabetes: A Quasi-Experimental Study. Int. J. Endocrinol. Metab. 2016, 14, e33835. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, B.; Saedisomeolia, A.; Wood, L.G.; Yaseri, M.; Tavasoli, S. Effects of pomegranate extract supplementation on inflammation in overweight and obese individuals: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2016, 22, 44–50. [Google Scholar] [CrossRef]
- Frémont, M.; Coomans, D.; Massart, S.; De Meirleir, K. High-throughput 16S rRNA gene sequencing reveals alterations of intestinal microbiota in myalgic encephalomyelitis/chronic fatigue syndrome patients. Anaerobe 2013, 22, 50–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logan, A.C.; Rao, A.V.; Irani, D. Chronic fatigue syndrome: Lactic acid bacteria may be of therapeutic value. Med. Hypotheses 2003, 60, 915–923. [Google Scholar] [CrossRef]
- Sheedy, J.R.; Wettenhall, R.E.H.; Scanlon, D.; Gooley, P.R.; Lewis, D.P.; McGregor, N.; Stapleton, D.I.; Butt, H.L.; De Meirleir, K.L. Increased d-lactic Acid intestinal bacteria in patients with chronic fatigue syndrome. In Vivo 2009, 23, 621–628. [Google Scholar] [PubMed]
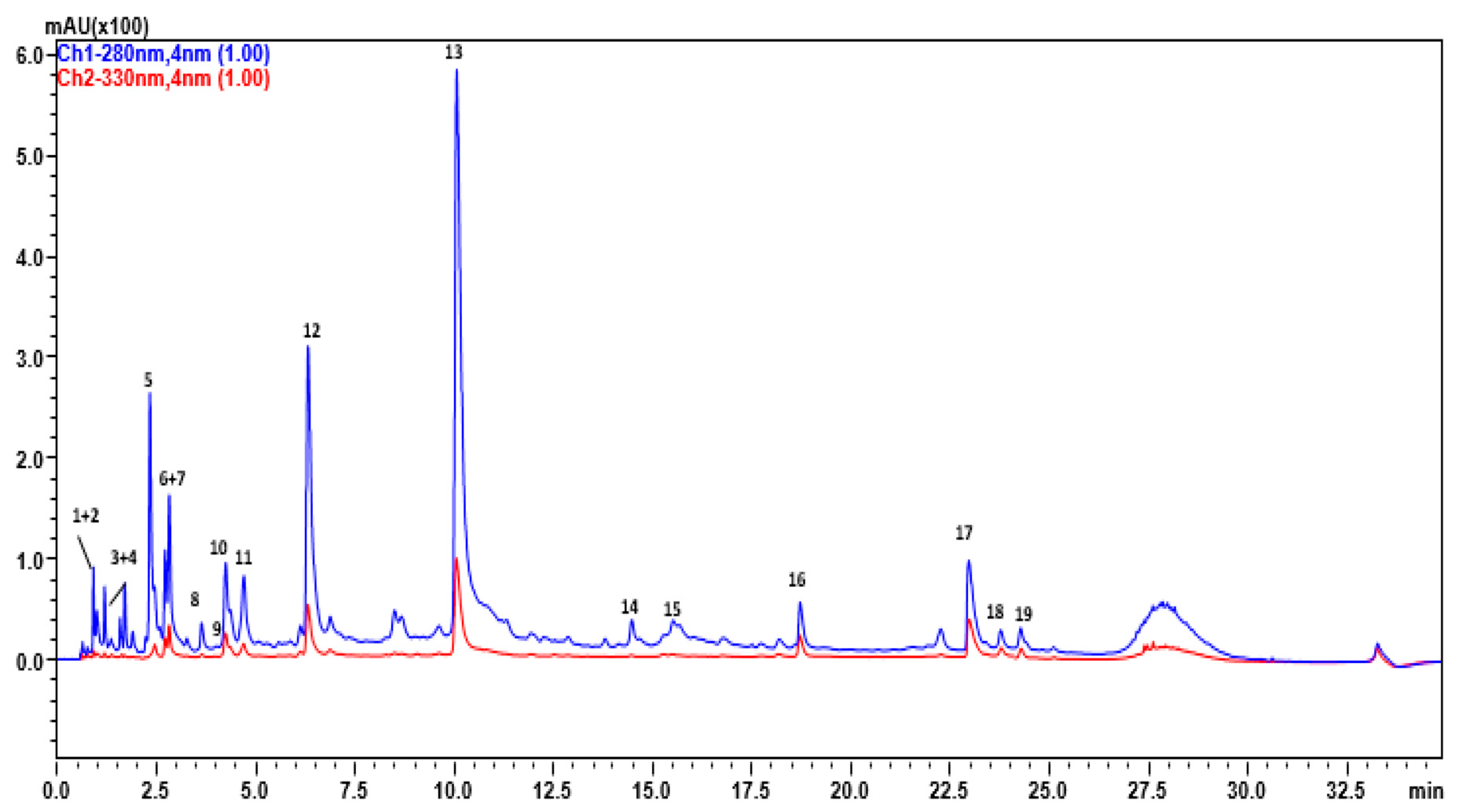
| Peak | Tr | Compound | Mol.formula | [M-H]-/[M-2H]2- | [MS/MS] | Error (ppm) |
|---|---|---|---|---|---|---|
| 1 | 1,25 | HHDP-hexose | C20H18O14 | 481.0693 | 300, 275 | 6.9 |
| 2 | 1,51 | Citric acid | C6H8O7 | 191.0194 | 111, 173 | 0.85 |
| 3 | 1,6 | Galloyl-hexoside | C13H15O10 | 331.0577 | 211, 169 | 1.54 |
| 4 | 1,74 | Gallic acid | C7H6O5 | 169.0158 | 125, 107 | 0 |
| 5 | 2,6 | Galloyl-hexoside # | C13H15O10 | 331.0577 | 211, 169 | 1.54 |
| 6 | 2,85 | Punicalin β | C34H22O22 | 781.0593 | 721, 601, 575, 392, 298, 273, | 0 |
| 7 | 3,3 | HHDP galloyl hexose | C27H22O18 | 633.0867 | 463, 300, 275, 249, 169, 125 | −1.3 |
| 8 | 3,67 | Citric acid derivative | C17H12O11 | 391.0268 | 270 | −4.35 |
| 9 | 4,03 | Pedunculagin (di-HHDP-hexose) | C34H24O22 | 783.0629 | 481, 300 275, 249 | 0 |
| 10 | 4,29 | Punicalagin * | C48H28O30 | 541.0266 * | 301, 601, 275 | −0.27 |
| 11 | 4,76 | Galloyl-HHDP-gluconate | C27H22O19 | 649.0617 | 301, 497, 626 | −1.3 |
| 12 | 6,35 | Punicalagin *# | C48H28O30 | 541.0266 | 301, 601, 275 | −0.27 |
| 13 | 10,08 | Punicalagin *# | C48H28O30 | 541.0266 | 301, 601, 275 | −0.27 |
| 14 | 14,49 | HHDP galloyl hexose # | C27H22O18 | 633.0867 | 463, 300, 275, 249, 169, 125 | −1.3 |
| 15 | 15,54 | Pedunculagin (di-HHDP-hexose) # | C34H24O22 | 783.0629 | 481, 300 275, 249 | 0 |
| 16 | 18,73 | Ellagic acid-hexoside | C26H32O13 | 551.1749 | 301, 389, 341 | −4.92 |
| 17 | 23 | Ellagic acid | C14H6O8 | 301.0029 | 229, 284, 267 | −4.35 |
| 18 | 23,81 | Ellagic acid-pentoside | C19H14O12 | 433.0413 | 301 | 0 |
| 19 | 24,26 | Kaempferol hexoside | C20H16O12 | 447.0167 | 285, 255 | 5.3 |
| Demographic Characteristics | |
|---|---|
| Man | 21 |
| Woman | 57 |
| Age in Men | 42.5 ± 14.5 |
| Age in Women | 39.8 ± 13.8 |
| Scale | Woman | Man | ||
|---|---|---|---|---|
| t0 | t1 | t0 | t1 | |
| FAS | 25.2 ± 3.7 | 17.8 ± 4.5 | 24.1 ± 2.2 | 16.2 ± 4.4 |
| (10–33) | (11–29) | (22–30) | (9–24) | |
| FSS | 3.2 ± 1 | 2.4 ± 0.7 | 3.3 ± 1.0 | 2.1 ± 0.7 |
| (1.2–4.8) | (1.2–4.6) | (1.6–4.9) | (1–3.8) | |
| NRS | 5.6 ± 1.8 | 3.9 ± 1.8 | 5.0 ± 1.5 | 3.0 ± 1.2 |
| (1–10) | (1–8) | (2–8) | (2–6) | |
| SF12-physical | 54.9 ± 5.8 | 56.0 ± 4.3 | 51.6 ± 8.3 | 54.9 ± 2.9 |
| (35.8–65.8) | (41.2–69.2) | (36–64.6) | (48.5–61.5) | |
| SF12-mental | 41.9 ± 10.6 | 51.1 ± 8.1 | 38.8 ± 11.2 | 50.7 ± 6.2 |
| (15.7–58.8) | (19–64.8) | (19–58.7) | (35.3–58.7) | |
| Model | F | gdl | p |
|---|---|---|---|
| FAS | |||
| Measurement | 122.17 | 1.76 | <0.001 |
| Sex | 2.960 | 1.75 | 0.089 |
| Age | 1.433 | 1.75 | 0.230 |
| Measurement × sex | 0.117 | 1.76 | 0.730 |
| FSS | |||
| Measurement | 85.694 | 1.76 | <0.001 |
| Sex | 0.134 | 1.75 | 0.710 |
| Age | 1.881 | 1.75 | 0.170 |
| Measurement × sex | 1.507 | 1.76 | 0.220 |
| NRS | |||
| Measurement | 39.239 | 1.76 | <0.001 |
| Sex | 6.119 | 1.75 | 0.016 |
| Age | 0.331 | 1.75 | 0.570 |
| Measurement × sex | 0.195 | 1.76 | 0.660 |
| SF12-physical component | |||
| Measurement | 5.909 | 1.76 | 0.017 |
| Sex | 4.361 | 1.75 | 0.040 |
| Age | 0.029 | 1.75 | 0.860 |
| Measurement × sex | 1.323 | 1.76 | 0.250 |
| SF12-mental component | |||
| Measurement | 39.029 | 1.76 | <0.001 |
| Sex | 0.961 | 1.75 | 0.340 |
| Age | 0.188 | 1.75 | 0.670 |
| Measurement × sex | 0.620 | 1.76 | 0.430 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, C.; Santarcangelo, C.; Di Minno, A.; Sacchi, R.; Sommella, E.; De Lellis, L.F.; De Pasquale, M.A.; Montarolo, F.; Campiglia, P.; Baldi, A.; et al. Chemical Characterization and Preliminary Evaluation of the Efficacy and Tolerability of a Food Supplement Based on Pomegranate Extract, B Vitamins, and Vitamin C against Prolonged Fatigue in Healthy Consumers. Processes 2022, 10, 208. https://doi.org/10.3390/pr10020208
Esposito C, Santarcangelo C, Di Minno A, Sacchi R, Sommella E, De Lellis LF, De Pasquale MA, Montarolo F, Campiglia P, Baldi A, et al. Chemical Characterization and Preliminary Evaluation of the Efficacy and Tolerability of a Food Supplement Based on Pomegranate Extract, B Vitamins, and Vitamin C against Prolonged Fatigue in Healthy Consumers. Processes. 2022; 10(2):208. https://doi.org/10.3390/pr10020208
Chicago/Turabian StyleEsposito, Cristina, Cristina Santarcangelo, Alessandro Di Minno, Roberto Sacchi, Eduardo Sommella, Lorenza Francesca De Lellis, Maria Antonietta De Pasquale, Francesca Montarolo, Pietro Campiglia, Alessandra Baldi, and et al. 2022. "Chemical Characterization and Preliminary Evaluation of the Efficacy and Tolerability of a Food Supplement Based on Pomegranate Extract, B Vitamins, and Vitamin C against Prolonged Fatigue in Healthy Consumers" Processes 10, no. 2: 208. https://doi.org/10.3390/pr10020208
APA StyleEsposito, C., Santarcangelo, C., Di Minno, A., Sacchi, R., Sommella, E., De Lellis, L. F., De Pasquale, M. A., Montarolo, F., Campiglia, P., Baldi, A., Riccioni, C., & Daglia, M. (2022). Chemical Characterization and Preliminary Evaluation of the Efficacy and Tolerability of a Food Supplement Based on Pomegranate Extract, B Vitamins, and Vitamin C against Prolonged Fatigue in Healthy Consumers. Processes, 10(2), 208. https://doi.org/10.3390/pr10020208










