Isolation and Purification of Mustard Glucosinolates by Macroporous Anion-Exchange Resin: Process Optimization and Kinetics’ Modelling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Ion Exchange Resins
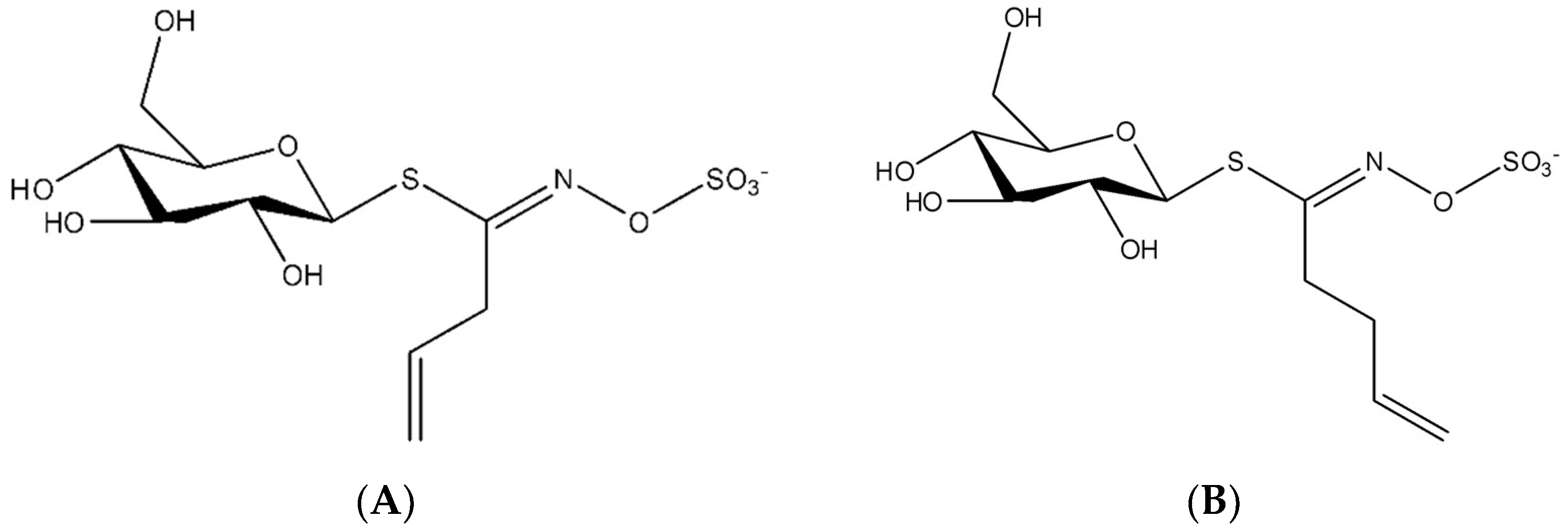
2.3. Glucosinolates Extraction
2.4. Glucosinolates Purification
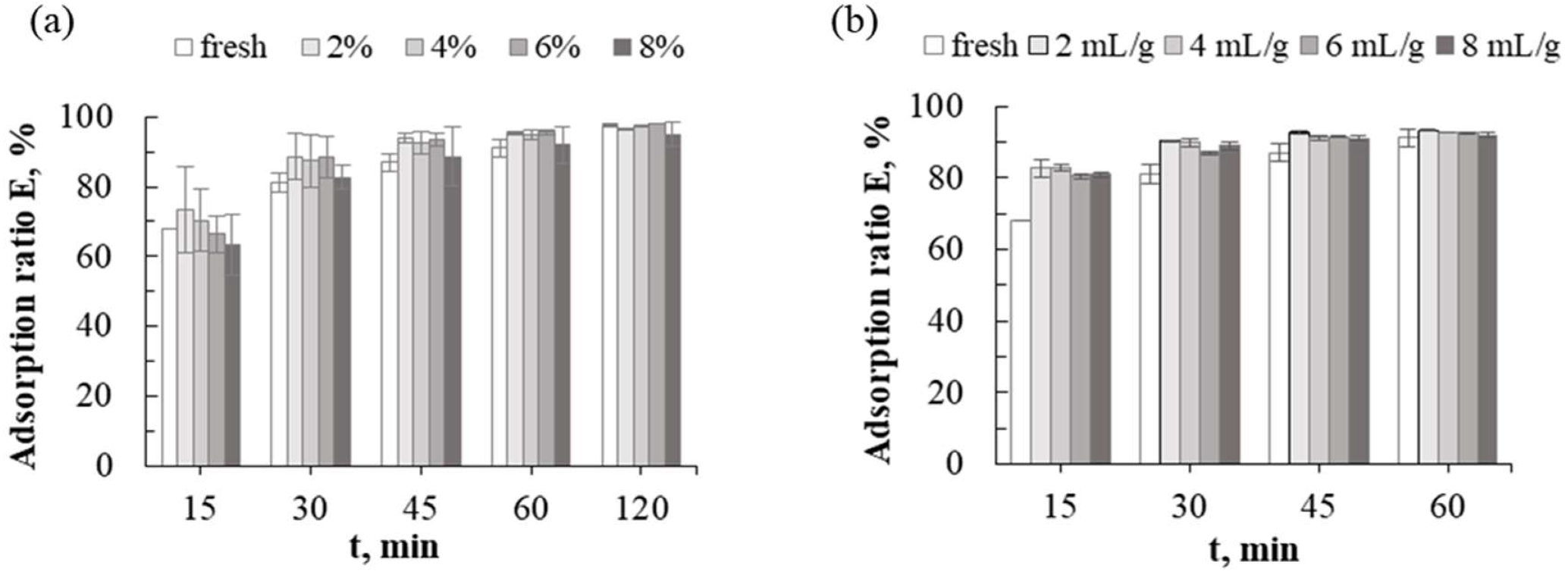
2.4.1. Static (Batch) Purification
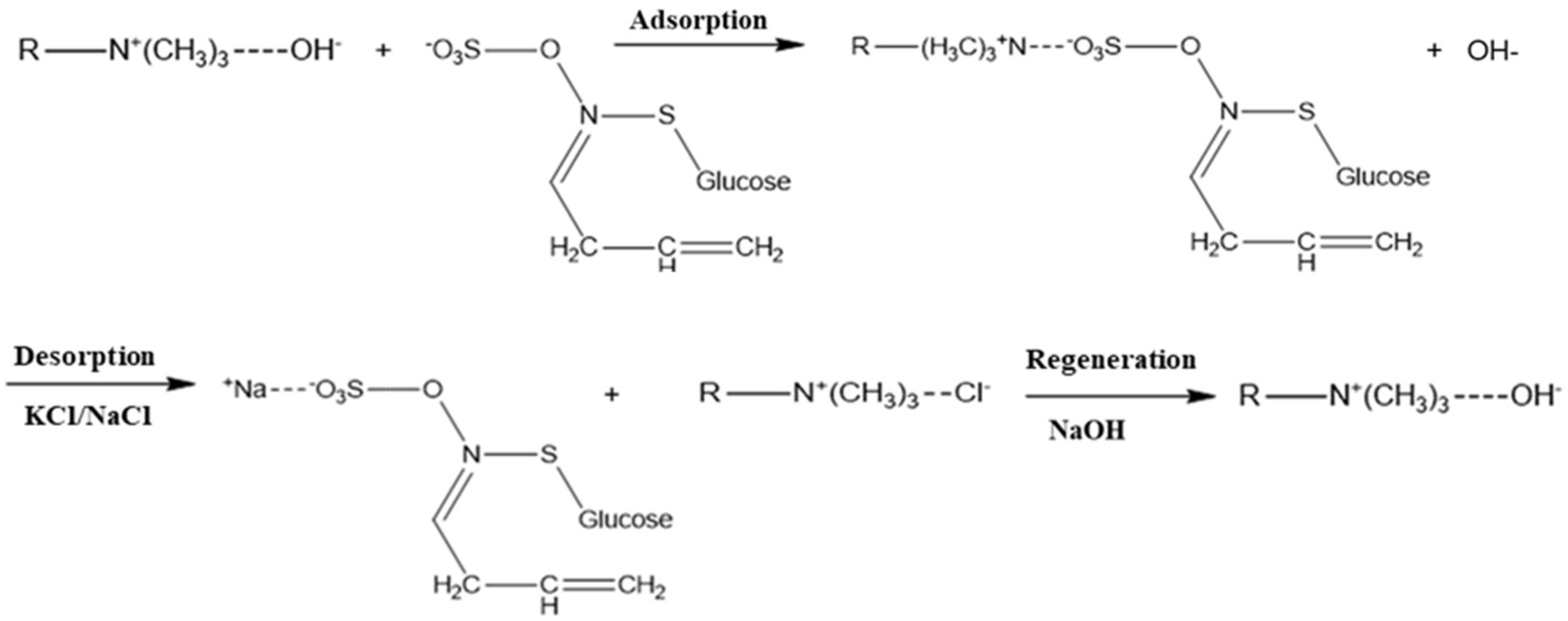
Adsorption and Desorption
Modelling of the Adsorption Process
- Isotherms of adsorption
- Adsorption kinetics
Resins Regeneration
2.4.2. Dynamic (Continuous) Purification
2.5. Analysis
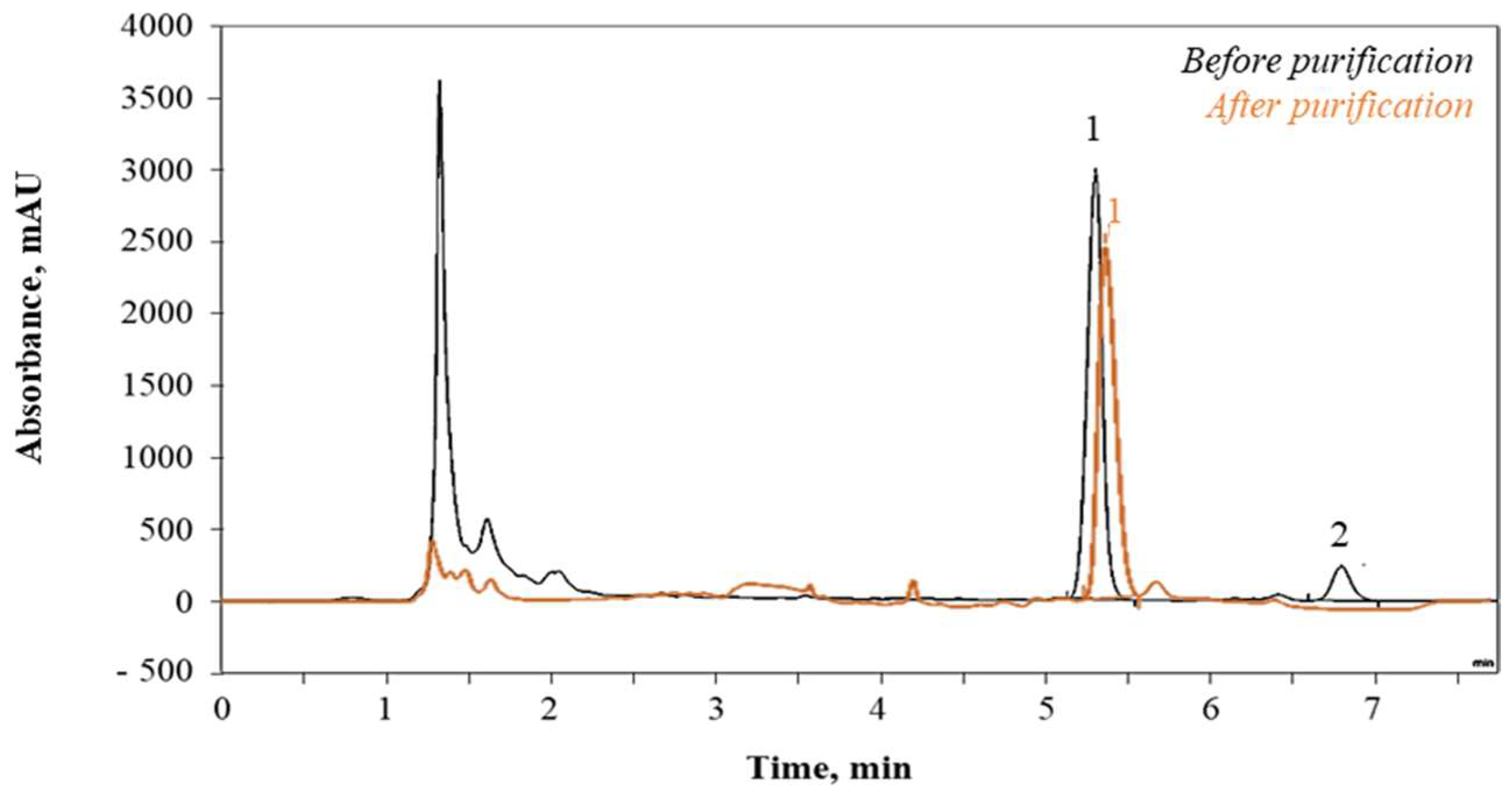
2.5.1. Juice Purity
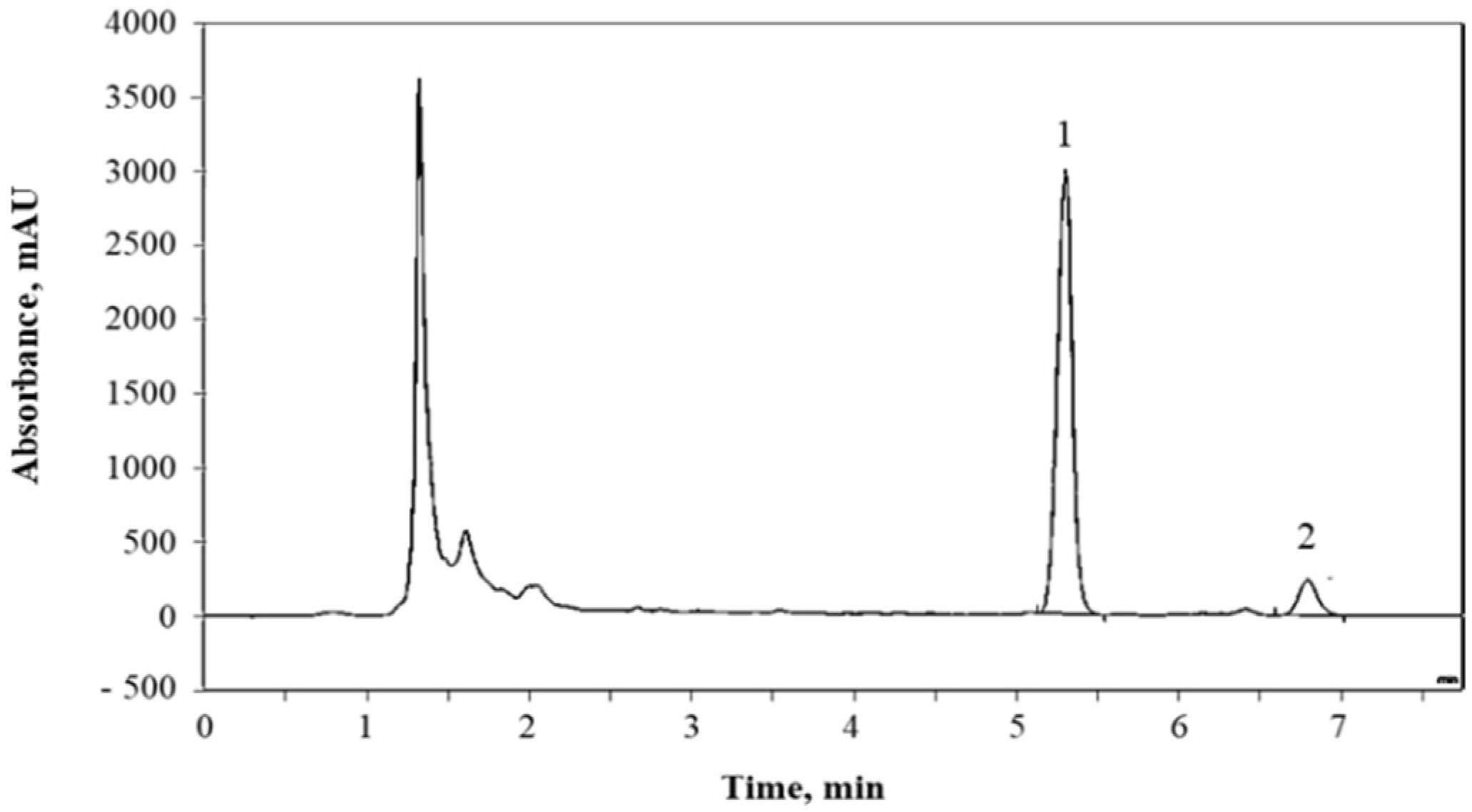
2.5.2. Glucosinolates Quantification
2.5.3. Proteins Quantification
2.5.4. Infrared Characterization (FTIR)
3. Results and Discussion
3.1. Optimization of the Adsorption/Desorption in a Static (Batch) Mode
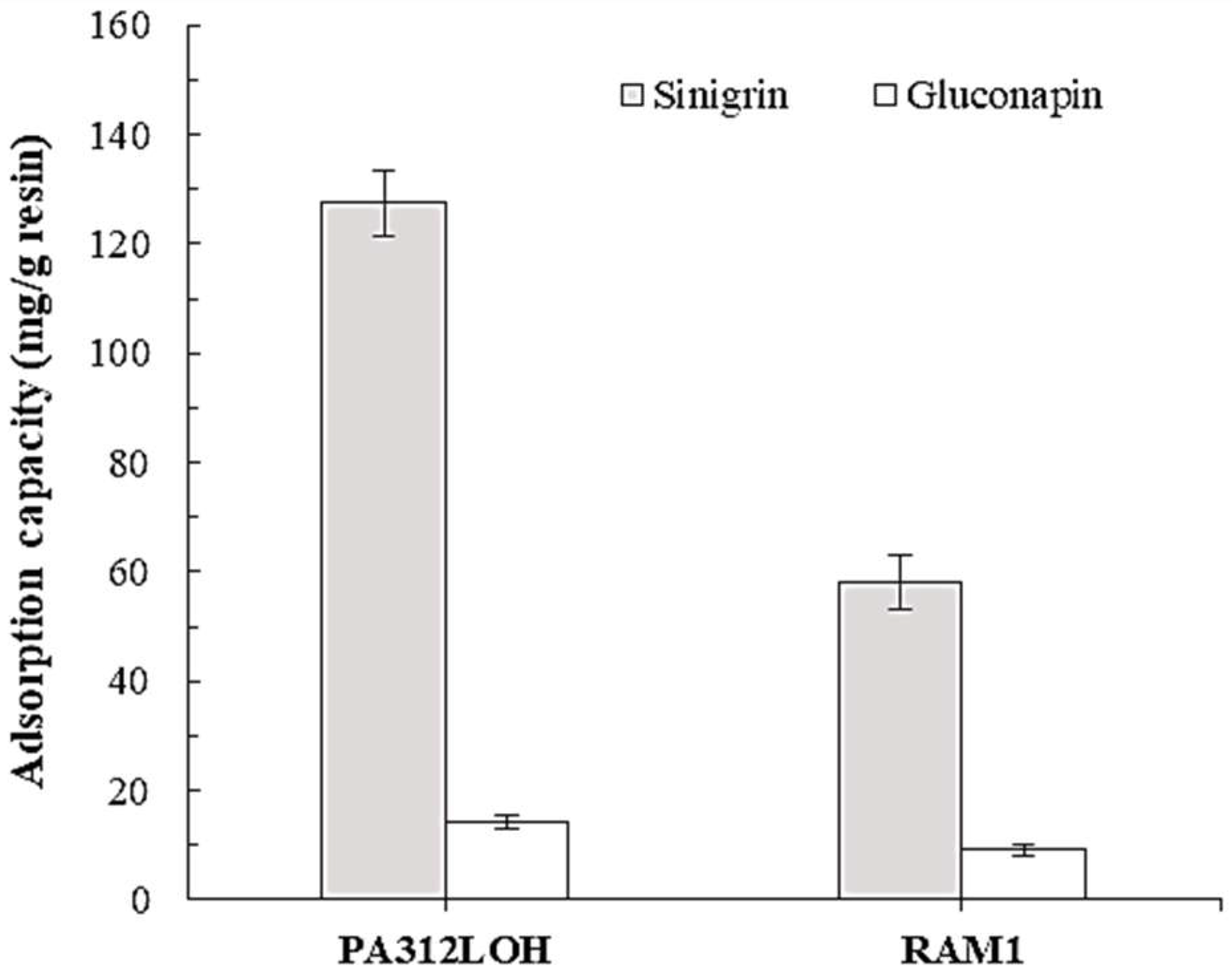
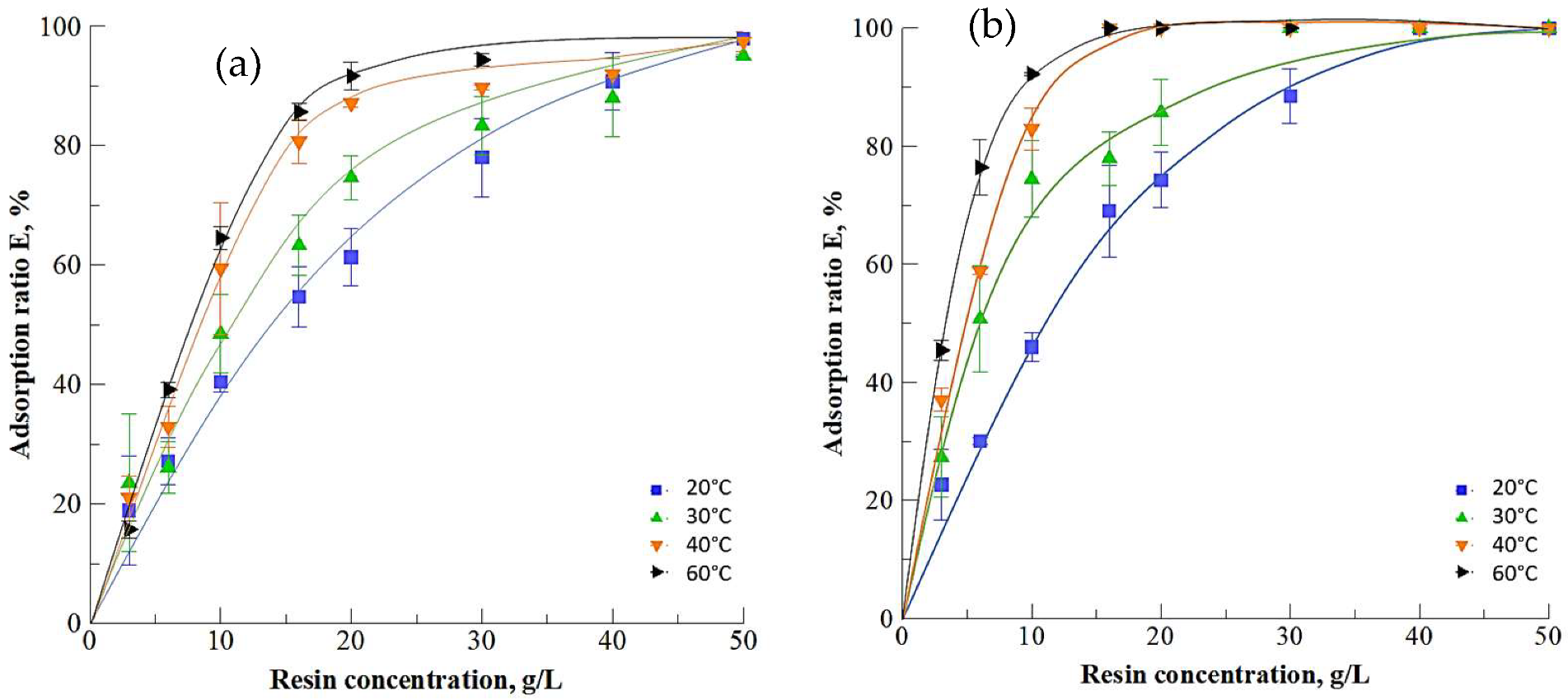
3.1.1. Resin Selection
3.1.2. Impact of Temperature
3.1.3. Adsorption Mechanism
3.1.4. Glucosinolates Desorption Optimization
Influence of the Salt Concentration
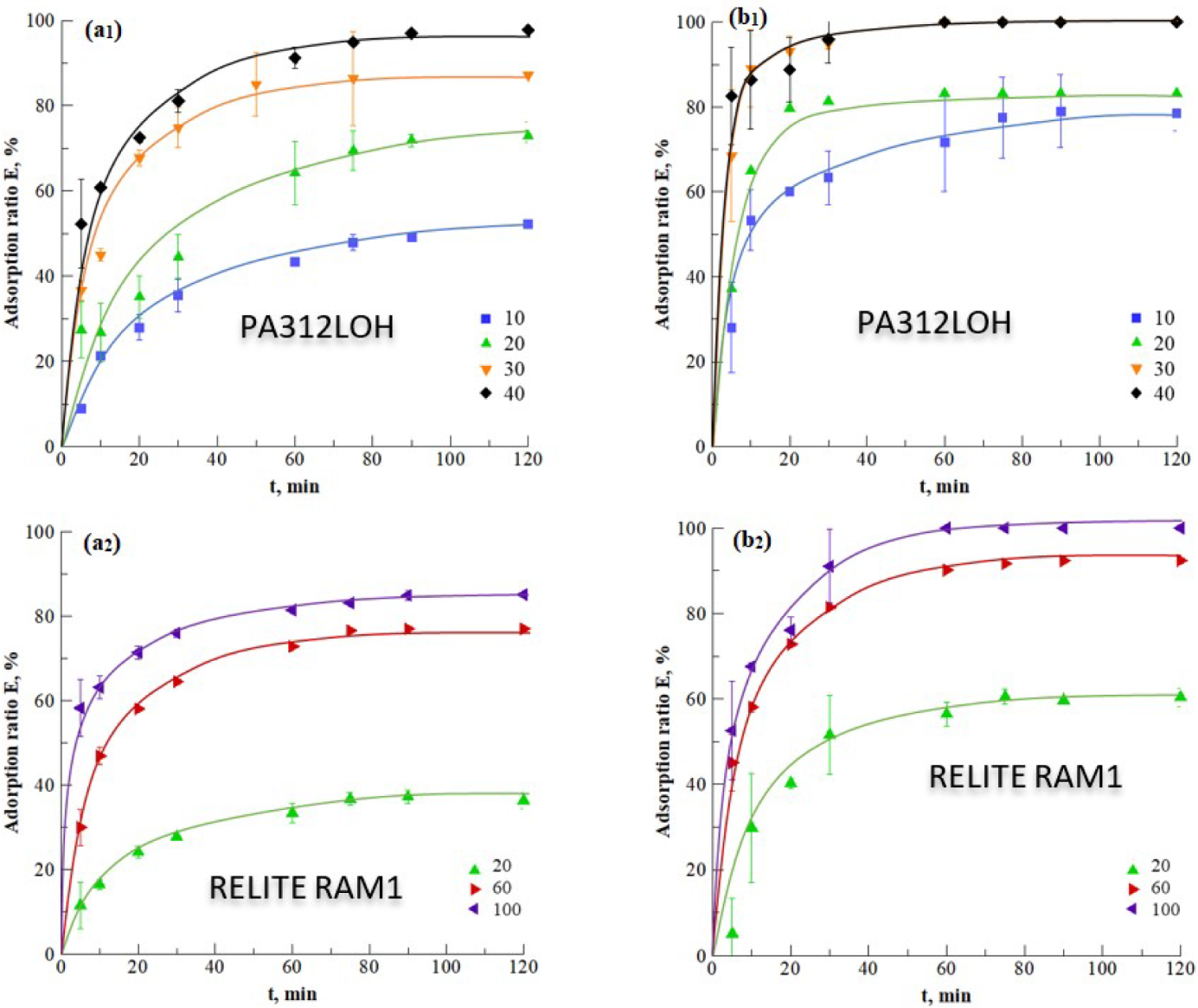
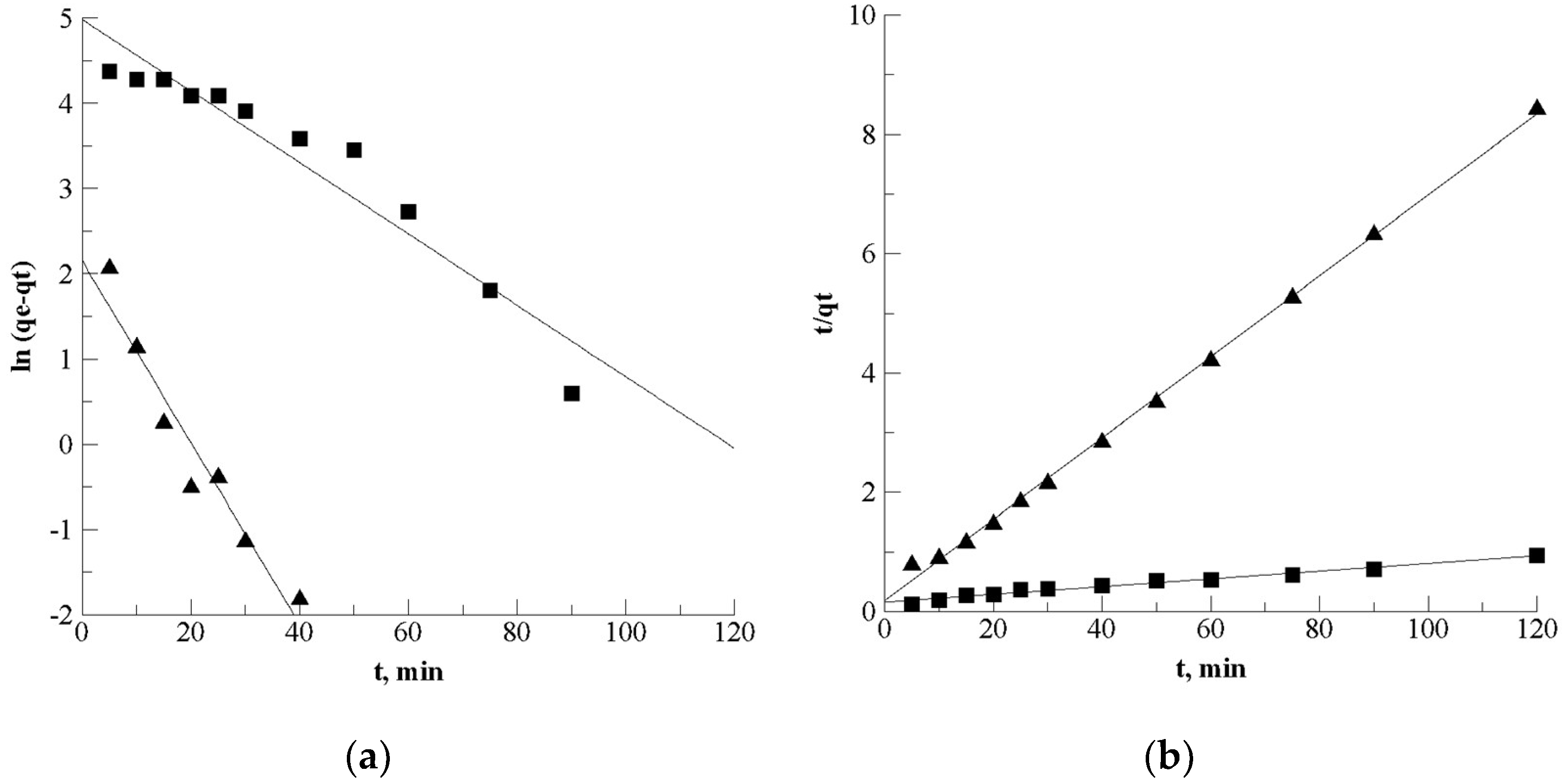
Kinetics of Desorption
3.1.5. Resin Regeneration
3.2. Dynamic (Continuous) Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. A critical review of the bioavailability of glucosinolates and related compounds. Nat. Prod. Rep. 2004, 21, 425–447. [Google Scholar] [CrossRef]
- Grubb, C.D.; Abel, S. Glucosinolate metabolism and its control. Trends Plant Sci. 2006, 11, 89–100. [Google Scholar] [CrossRef]
- Guerrero-Diaz, M.M.; Lacasa-Martínez, C.M.; Hernández-Piñera, A.; Martínez-Alarcón, V.; Lacasa-Plasencia, A. Evaluation of repeated bio disinfestation using Brassica carinata pellets to control Meloidogyne incognita in protected pepper crops. Span. J. Agric. Res. 2013, 11, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Sotelo, T.; Lema, M.; Soengas, P.; Cartea, M.E.; Velasco, P. In vitro activity of Glucosinolates and their degradation products against Brassica-pathogenic bacteria and fungi. Appl. Environ. Microbiol. 2015, 81, 432–440. [Google Scholar] [CrossRef] [Green Version]
- Karyn, L.B. Glucosinolates. In Nutraceuticals: Efficacy, Safety and Toxicity, 2nd ed.; Ramesh, C.G., Rajiv, L., Ajay, S., Eds.; Elsevier: Boston, MA, USA, 2021; pp. 903–909. [Google Scholar]
- Sarwar, M.; Kirkegaard, J.A.; Wong, P.T.W.; Desmarchelier, J.M. Biofumigation potential of brassicas. Plant Soil. 1998, 201, 103–112. [Google Scholar] [CrossRef]
- Hebert, M.; Mhemdi, H.; Vorobiev, E. Dead-end ultrafiltration of rich glucosinolates juice extracted from mustard defatted meal: Effects of operating conditions on permeate quality and membrane fouling. Food Bioprod. Process. 2021, 128, 133–142. [Google Scholar] [CrossRef]
- Hebert, M. Nouvelles Approches Pour la Valorisation des Graines de Moutarde Riches en Glucosinolates dans un Concept de Bioraffinerie. Ph.D. Thesis, UTC, Compiègne, France, 2020. [Google Scholar]
- Bones, A.M.; Rossiter, J.T. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol. Plant. 1996, 97, 194–208. [Google Scholar] [CrossRef]
- Karcher, A.; Melouk, H.A.; El Rassi, Z. High-performance liquid phase separation of glycosides. 5. Determination of individual glucosinolates in cabbage and rapeseed by laser-induced fluorescence capillary electrophoresis via the enzymatically released isothiocyanate aglycon. J. Agric. Food Chem. 1999, 47, 4267–4274. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Qui, A.; Hu, J. Separation and purification of the main glucosinolates from rapeseeds. Chin. J. Chromatogr. 2005, 23, 411–414. [Google Scholar]
- Song, L.; Thornalley, P.J.; Iori, R. Purification of major glucosinolates from Brassicaceae seeds and preparation of isothiocyanates and amine metabolites. J. Sci. Food Agric. 2006, 86, 1271–1280. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, R.; Wu, Y.; Yang, L.; Wang, Z.; Li, Y. An efficient method for separation and purification of glucosinolates stereoisomeres from radix isatidis. J. Liq. Chrom. Relat. Technol. 2012, 35, 153–161. [Google Scholar] [CrossRef]
- Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Chou, F.E. Separation and purification of glucosinolates from crude plant homogenates by high-speed counter-current chromatography. J. Chromatogr. A 2003, 996, 85–93. [Google Scholar] [CrossRef]
- Rochfort, S.; Caridi, D.; Stinton, M.; Trenerry, V.C.; Jones, R. The isolation and purification of glucoraphanin from broccoli seeds by solid phase extraction and preparative high performance liquid chromatography. J. Chromatogr. A 2006, 1120, 205–210. [Google Scholar] [CrossRef]
- Lee, I.; Boyce, M.C. Extraction and Purification of Glucoraphanin by Preparative High-Performance Liquid Chromatography. J. Chem. Educ. 2011, 88, 832–834. [Google Scholar] [CrossRef]
- Toribio, A.; Nuzillard, J.M.; Renault, J.H. Strong ion-exchange centrifugal partition chromatography as an efficient method for the large-scale purification of glucosinolates. J. Chromatogr. A 2007, 1170, 44–51. [Google Scholar] [CrossRef]
- Hamzaoui, M.; Hubert, J.; Reynaud, R.; Marchal, L.; Foucault, A.; Renault, J.H. Strong ion exchange in centrifugal partition extraction (SIX-CPE): Effect of partition cell design and dimensions on purification process efficiency. J. Chromatogr. A 2012, 1247, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Sun, M. Ion exchange removal and resin regeneration to treat per- and polyfluoroalkyl ether acids and other emerging PFAS in drinking water. Water Res. 2021, 207, 117781. [Google Scholar] [CrossRef]
- Susanto, H.; Roihatin, A.; Widiasa, I.N. Production of colorless liquid sugar by ultrafiltration coupled with ion exchange. Food Bioprod. Process. 2016, 98, 11–20. [Google Scholar] [CrossRef]
- Kiefer, R.; Höll, W.H. Sorption of Heavy Metals onto Selective Ion-Exchange Resins with Aminophosphonate Functional Groups. Ind. Eng. Chem. Res. 2001, 40, 4570–4576. [Google Scholar] [CrossRef]
- Delmousse, G.; Poupinel, J.C. Purification and Stabilization of Fruit Juices by Ion Exchange Treatment. U.S. Patent US2667417A, 19 October 1951. [Google Scholar]
- Vera, E.; Dornier, M.; Ruales, J.; Vaillant, F.; Reynes, R.M. Comparison between different ion exchange resins for the deacidification of passion fruit juice. J. Food Eng. 2003, 57, 199–207. [Google Scholar] [CrossRef]
- Reynaud, E.; Duvat, C.; Baudouin, S.; Meurisse, J. Deacidified Cranberry Juice and the Process for Preparing the Same. U.S. Patent US20190216112A1, 14 June 2018. [Google Scholar]
- Elder, D.P. Pharmaceutical Applications of Ion-Exchange Resins. J. Chem. Educ. 2005, 82, 575. [Google Scholar] [CrossRef]
- Hebert, M.; Mhemdi, H.; Vorobiev, E. Selective and eco-friendly recovery of glucosinolates from mustard seeds (Brassica juncea) using process optimization and innovative pretreatment (high voltage electrical discharges). Food Bioprod. Process. 2020, 124, 11–23. [Google Scholar] [CrossRef]
- Treybal, R.E. Mass Transfer Operation; Tata McGraw Hill: Singapore, 1981. [Google Scholar]
- Kajjumba, G.W.; Emik, S.; Ongen, A.; Ozcan, H.K.; Aydin, S. Modelling of Adsorption Kinetic Processes—Errors, Theory and Application; IntechOpen Limited: London, UK, 2018. [Google Scholar]
- Wang, T.; Liang, H.; Yuan, Q. Separation and Purification of Sinigrin and Gluconapin from Defatted Indian Mustard Seed Meals by Macroporous Anion Exchange Resin and Medium Pressure Liquid Chromatography. Sep. Sci. Technol. 2014, 49, 1838–1847. [Google Scholar] [CrossRef]
- Wang, T.; Liang, H.; Yuan, Q. Separation of sinigrin from Indian mustard (Brassica juncea L.) seed using macroporous ion-exchange resin. Korean J. Chem. Eng. 2012, 29, 396–403. [Google Scholar] [CrossRef]
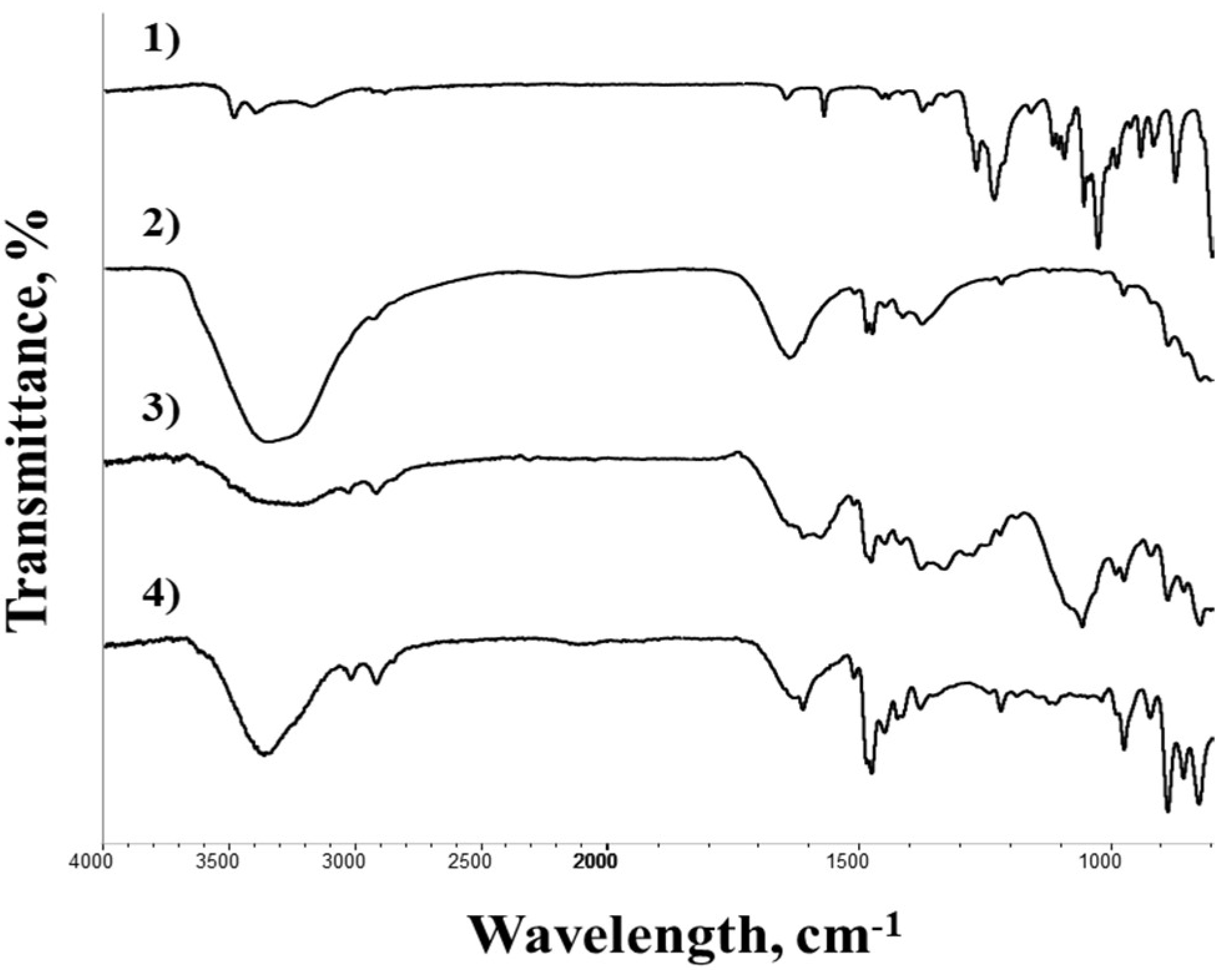







| Parameter | PA312LOH | RELITE RAM1 |
|---|---|---|
| Polymeric matrix | Styrène | Styrène-DVB |
| Functional group | Quaternary amine | Tertiary amine |
| Density (g/mL) | 1.1 | 1.03–1.05 |
| Exchange capacity (eq/L) | 0.9 | 1.5 |
| Particle diameter (mm) | 0.45 | 0.7 |
| Moisture content (%) | 43–55 | 50–58 |
| T (°C) | 20 | 30 | 40 | 60 |
|---|---|---|---|---|
| ∆Q (J/mol) | 32.57 | 41.82 | 45.59 | 55.21 |
| KT (mL/g) | 2.52 | 8.71 | 21.31 | 74.77 |
| R² | 0.931 | 0.990 | 0.930 | 0.932 |
| 20 °C | 30 °C | 40 °C | 60 °C | ||
|---|---|---|---|---|---|
| Sinigrin | Langmuir model | ||||
| R² | 0.929 | 0.903 | 0.953 | 0.997 | |
| q0 (mg/g) | 167.2 | 194.2 | 261.1 | 268.1 | |
| KL | 2.35 | 2.04 | 2.00 | 4.88 | |
| RL | 0.11 | 0.12 | 0.12 | 0.05 | |
| Freundlich model | |||||
| R² | 0.866 | 0.973 | 0.873 | 0.833 | |
| KF | 109.9 | 124.0 | 165.7 | 212.4 | |
| 1/n | 0.23 | 0.48 | 0.38 | 0.28 | |
| Gluconapin | Langmuir model | ||||
| R² | 0.994 | 0.94 | 0.914 | 0.989 | |
| q0 (mg/g) | 20.5 | 44.6 | 71.4 | 84.8 | |
| KL | 22.34 | 11.38 | 10.72 | 25.16 | |
| RL | 0.11 | 0.20 | 0.20 | 0.10 | |
| Freundlich model | |||||
| R² | 0.963 | 0.817 | 0.91 | 0.999 | |
| KF | 27.1 | 66.7 | 89.1 | 125.9 | |
| 1/n | 0.29 | 0.48 | 0.37 | 0.34 | |
| Kinetic Parameters | Sinigrin | Gluconapin |
|---|---|---|
| Pseudo first order | ||
| R² | 0.921 | 0.936 |
| qe (mg/g) | 145.8 | 8.63 |
| K1·10−2 (min−1) | 4.19 | 10.68 |
| Pseudo second order | ||
| R² | 0.985 | 0.998 |
| qe (mg/g) | 148.4 | 14.7 |
| K2·10−2 (g/mg.min) | 0.10 | 2.51 |
| qe experimental (mg/g) | 127.5 | 14.2 |
| Intra-particle diffusion | ||
| R1² | 0.967 | 0.926 |
| Ki1 (mg/g.min−0.5) | 11.60 | 3.29 |
| C1 | 16.03 | 0.27 |
| R2² | 0.821 | 0.665 |
| Ki2 (mg/g.min−0.5) | 2.48 | 0.104 |
| C2 | 79.86 | 13.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hebert, M.; Serra, E.; Vorobiev, E.; Mhemdi, H. Isolation and Purification of Mustard Glucosinolates by Macroporous Anion-Exchange Resin: Process Optimization and Kinetics’ Modelling. Processes 2022, 10, 191. https://doi.org/10.3390/pr10020191
Hebert M, Serra E, Vorobiev E, Mhemdi H. Isolation and Purification of Mustard Glucosinolates by Macroporous Anion-Exchange Resin: Process Optimization and Kinetics’ Modelling. Processes. 2022; 10(2):191. https://doi.org/10.3390/pr10020191
Chicago/Turabian StyleHebert, Mathieu, Emmanuel Serra, Eugène Vorobiev, and Houcine Mhemdi. 2022. "Isolation and Purification of Mustard Glucosinolates by Macroporous Anion-Exchange Resin: Process Optimization and Kinetics’ Modelling" Processes 10, no. 2: 191. https://doi.org/10.3390/pr10020191
APA StyleHebert, M., Serra, E., Vorobiev, E., & Mhemdi, H. (2022). Isolation and Purification of Mustard Glucosinolates by Macroporous Anion-Exchange Resin: Process Optimization and Kinetics’ Modelling. Processes, 10(2), 191. https://doi.org/10.3390/pr10020191





