Physicochemical Properties of Starch Binary Mixtures with Cordia and Ziziphus Gums
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chickpea Starch Isolation
2.3. Wheat Starch Isolation
2.4. Isolation of Gums
2.5. Gums Modification
2.6. Determination of Degree of Acetylation
2.7. Preparation of Gum Starch Blends (Binary Composites)
2.8. Fourier Transform Infrared (FTIR) Analysis of Native and Acetylated Gums
2.9. Differential Scanning Calorimetry (DSC)
2.10. Rapid Visco Analyzer Measurements (RVA)
2.11. Freeze Thaw Stability of Starch Gels
2.12. Gel Texture
2.13. Rheological Measurements and Temperature Dependence
2.14. Statistical Analysis
3. Results and Discussion
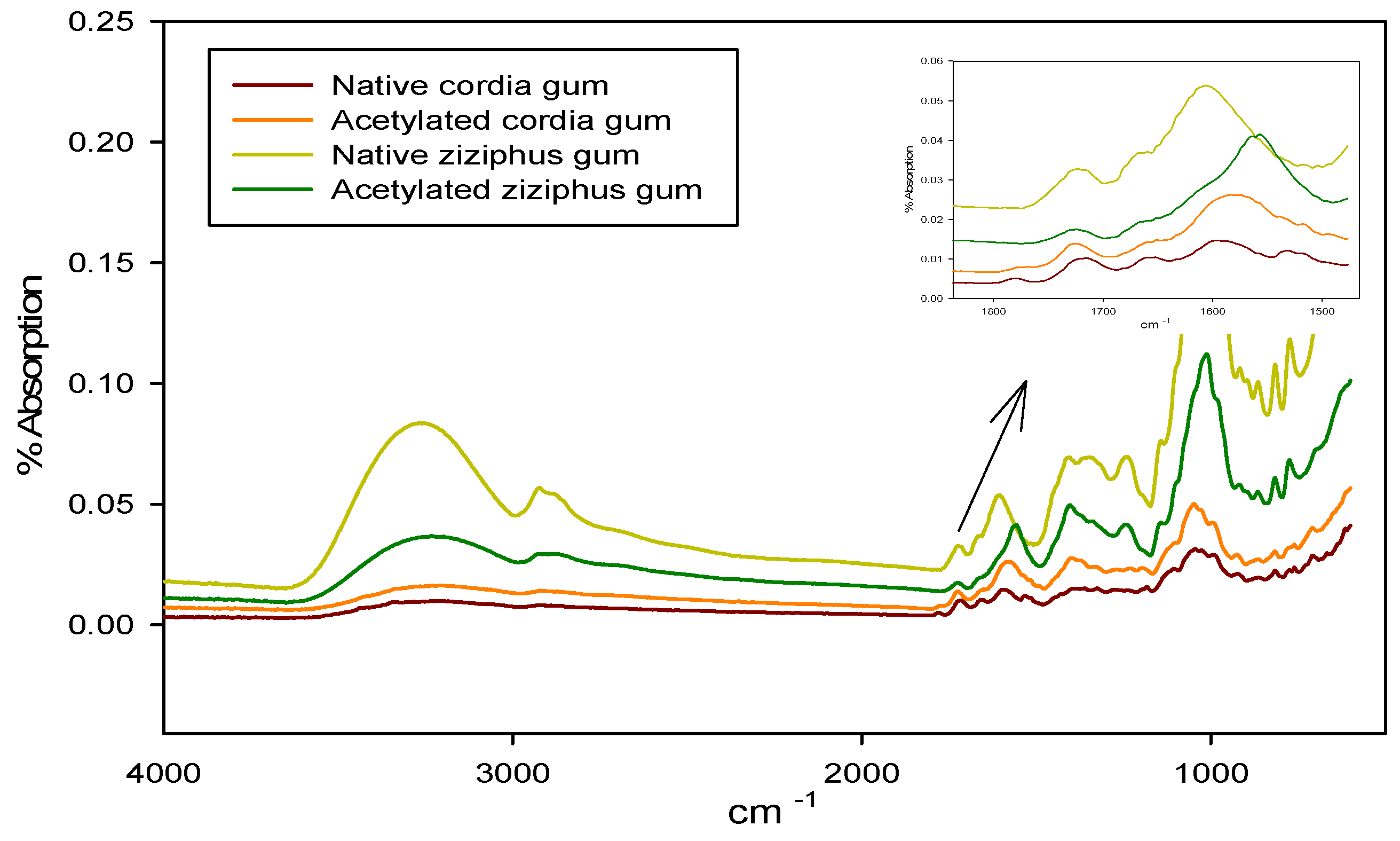
3.1. FTIR
3.2. Degree of Acetylation
3.3. Differential Scanning Calorimetry (DSC)
3.4. Rapid Visco Analyzer (RVA)
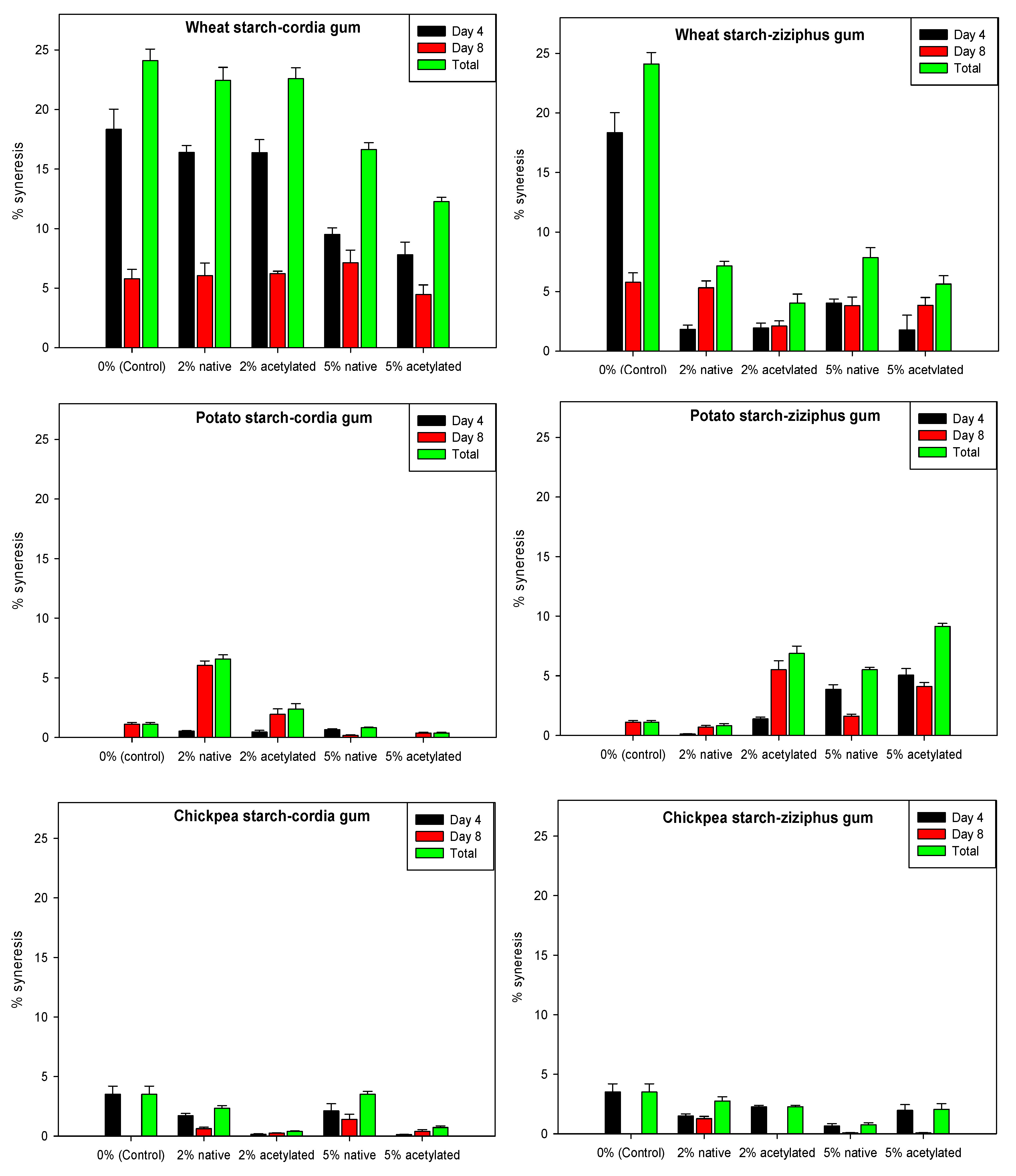
3.5. Freeze–Thaw Stability of Starch Gels
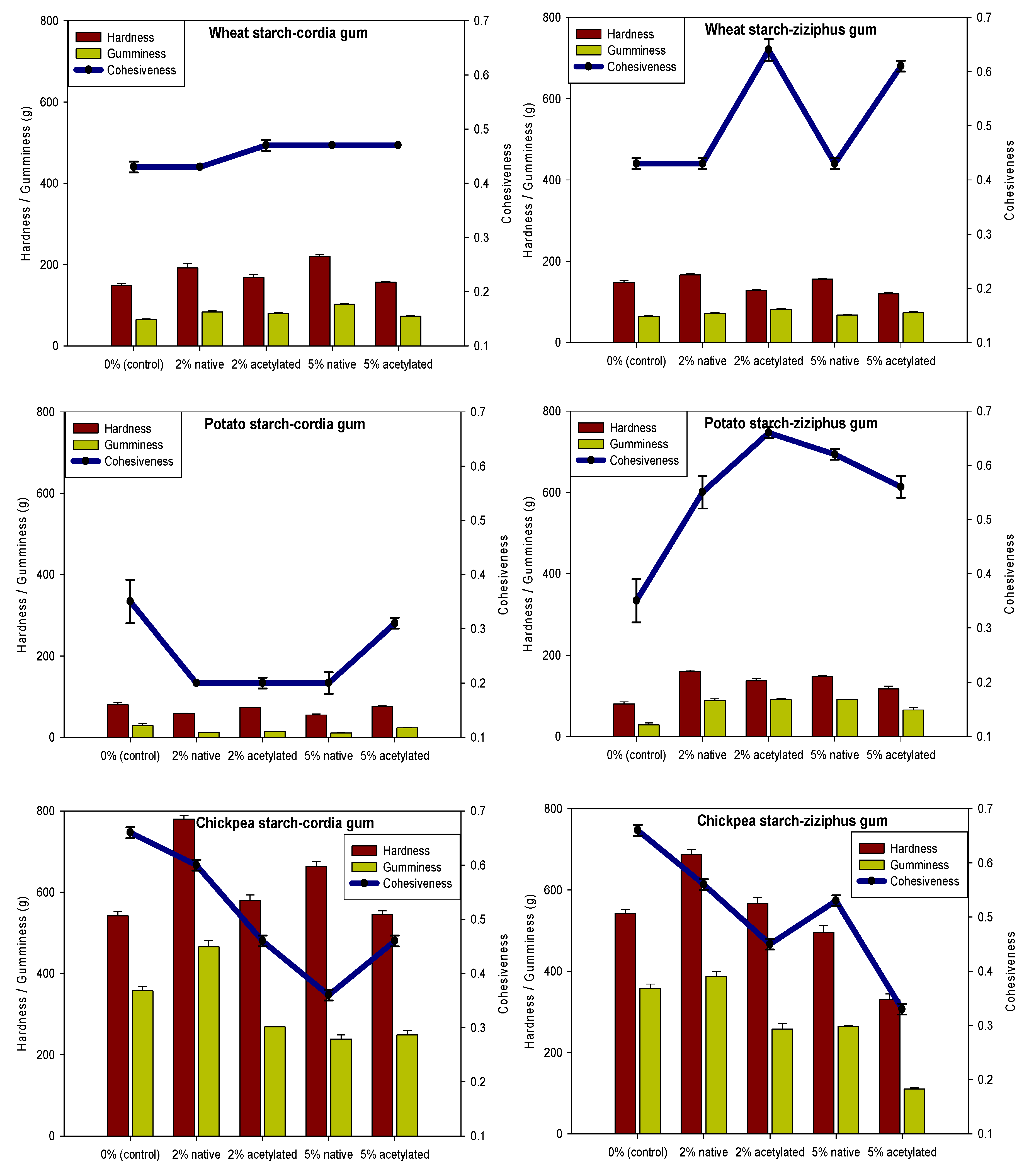
3.6. Gel Texture
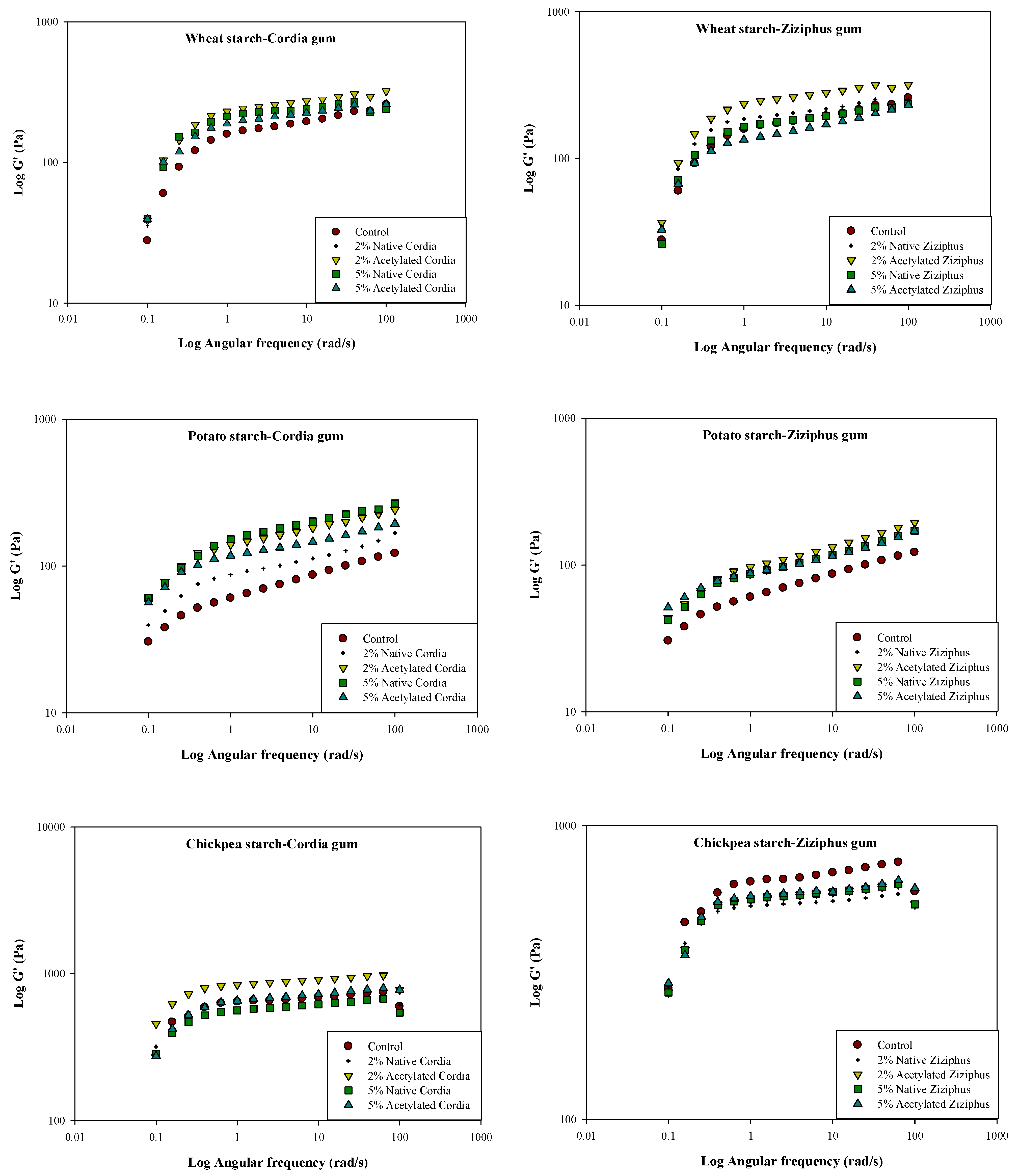
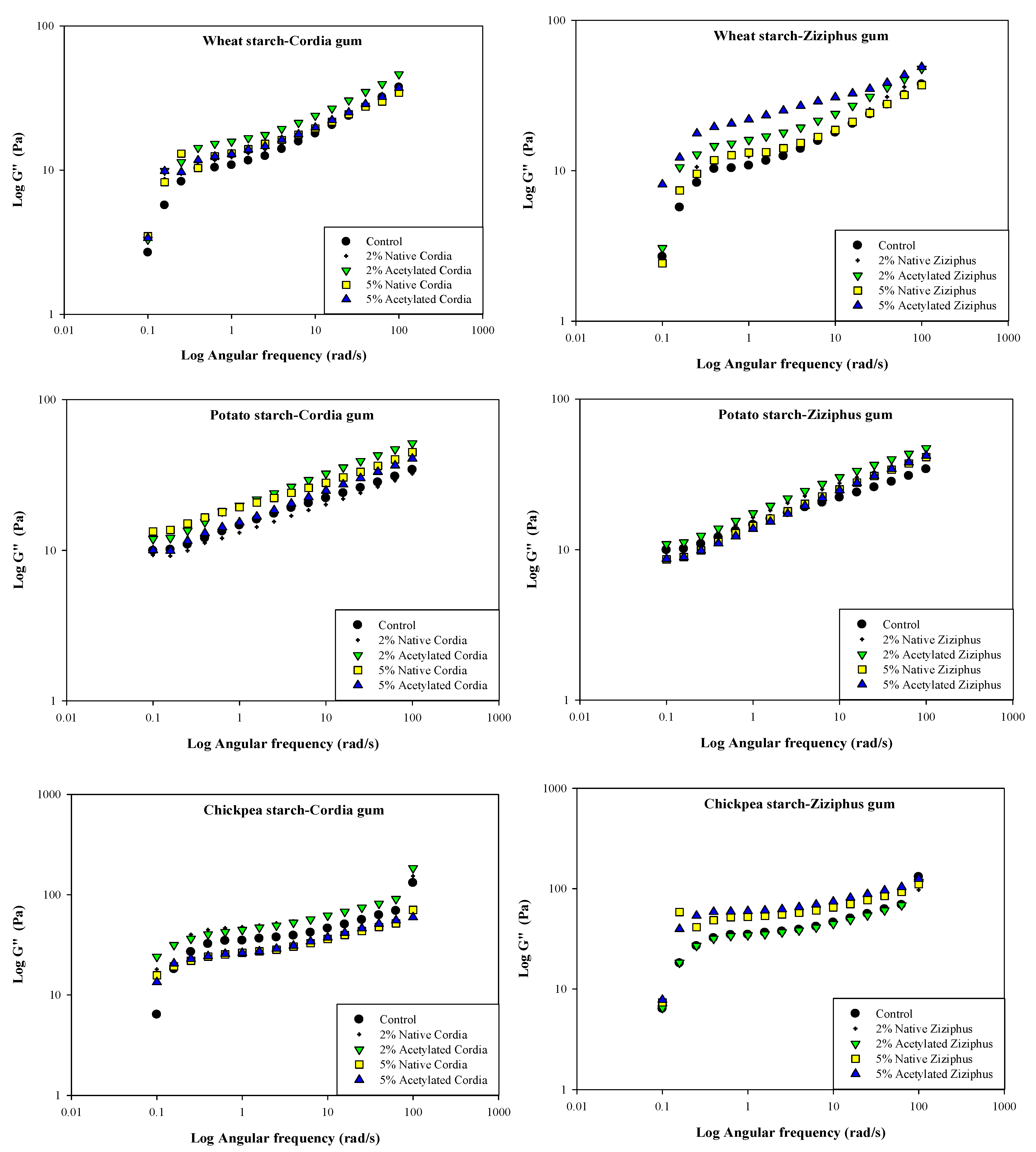
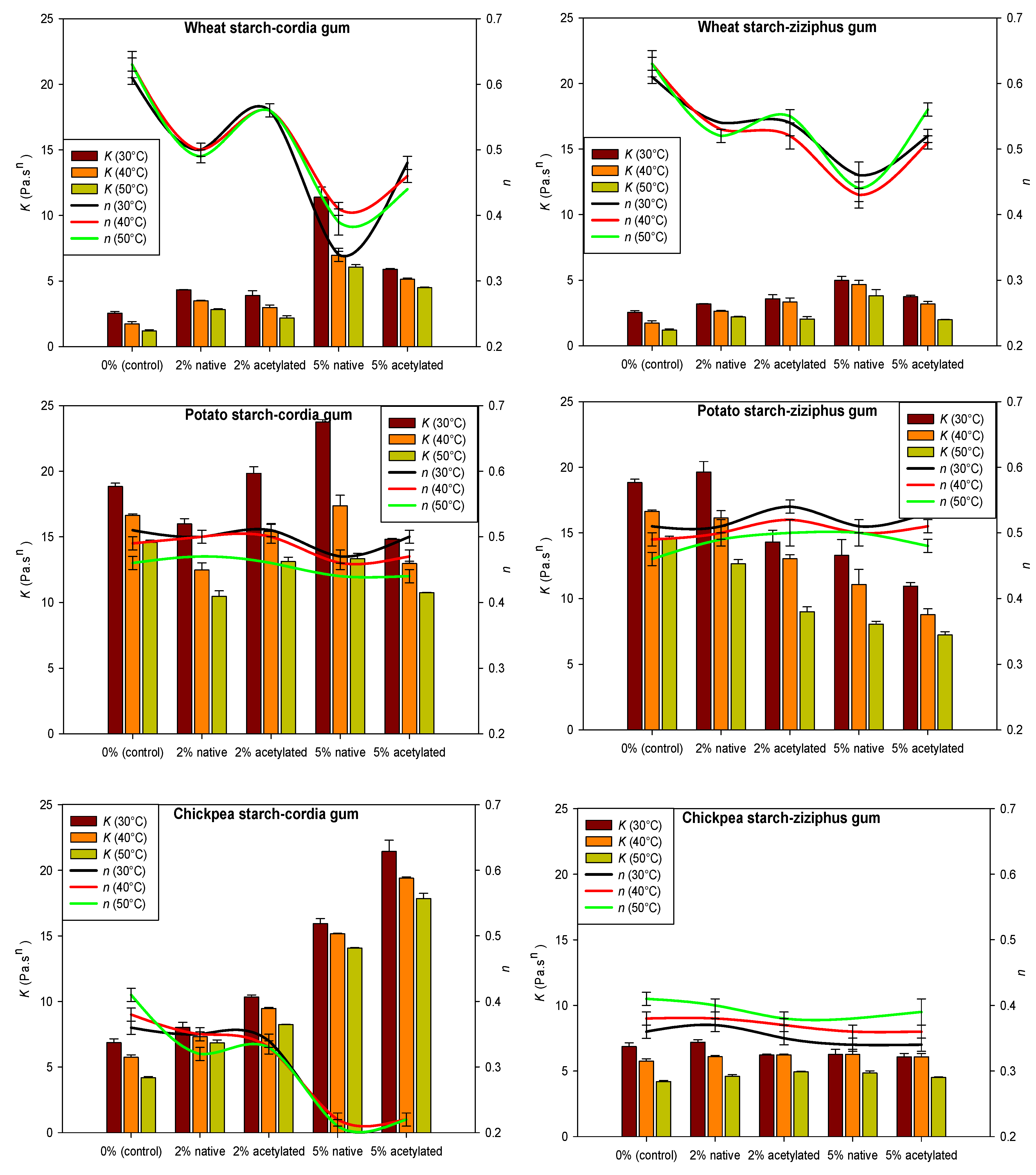
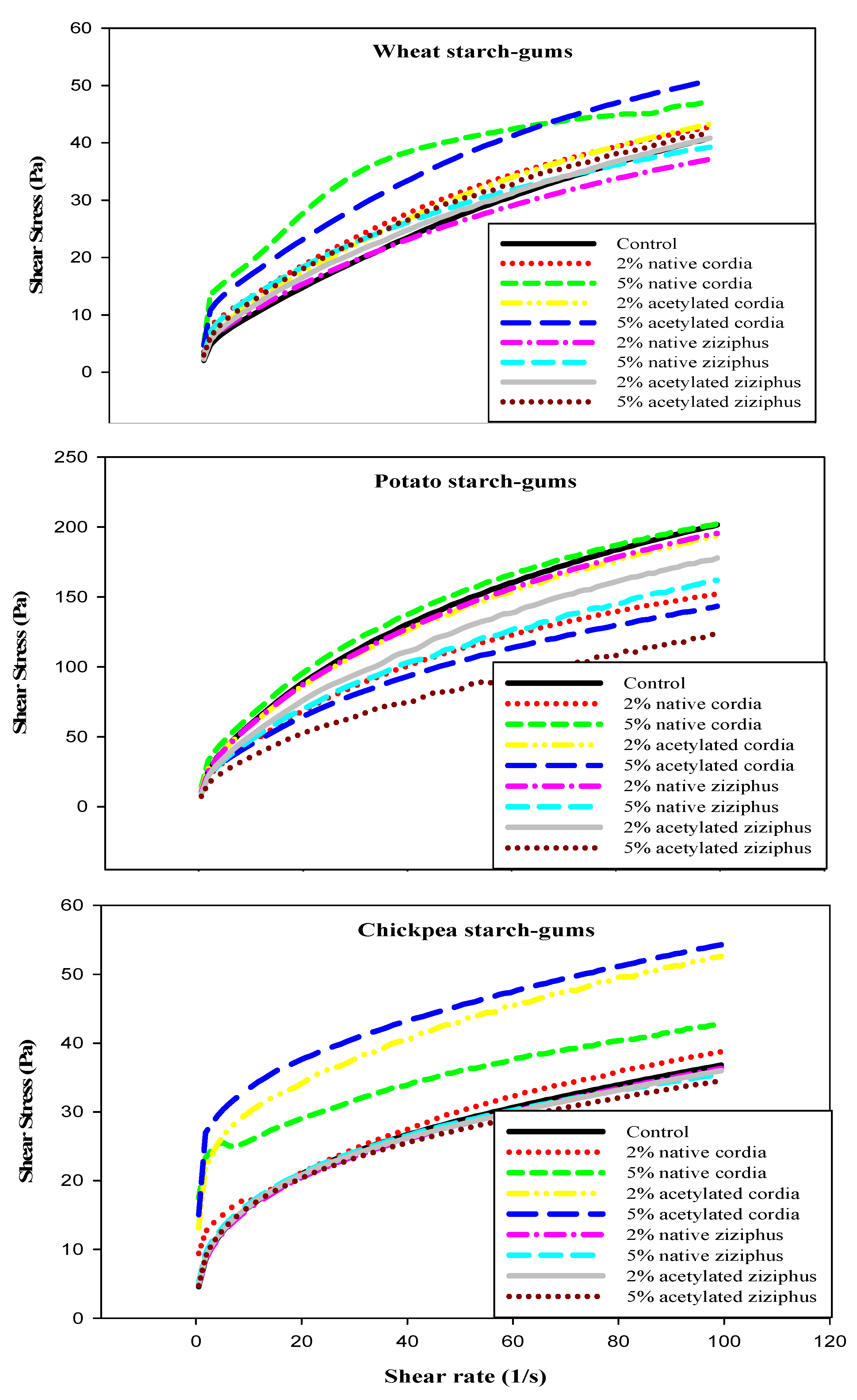
3.7. Rheological Measurements and Temperature Dependency
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoefler, A.C. Hydrocolloids; American Assoc. of Cereal Chemists: Saint Paul, MN, USA, 2004. [Google Scholar]
- Funami, T.; Nakauma, M.; Ishihara, S.; Tanaka, R.; Inoue, T.; Phillips, G.O. Structural modifications of sugar beet pectin and the relationship of structure to functionality. Food Hydrocoll. 2011, 25, 221–229. [Google Scholar] [CrossRef]
- Guarda, A.; Rosell, C.M.; Benedito, C.; Galotto, M. Different hydrocolloids as bread improvers and antistaling agents. Food Hydrocoll. 2004, 18, 241–247. [Google Scholar] [CrossRef]
- Tran, T.; Thitipraphunkul, K.; Piyachomkwan, K.; Sriroth, K. Effect of Starch Modifications and Hydrocolloids on Freezable Water in Cassava Starch Systems. Starch Stärke 2008, 60, 61–69. [Google Scholar] [CrossRef]
- Heflich, L.W. A Baker’s Perspective. In Baked Goods Freshness, Technology, Evaluation and Inhibition of Staling; Hebeda, R.E., Zobel, H., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 239–256. [Google Scholar]
- Gimeno, E.; Moraru, C.I.; Kokini, J.L. Effect of Xanthan Gum and CMC on the Structure and Texture of Corn Flour Pellets Expanded by Microwave Heating. Cereal Chem. J. 2004, 81, 100–107. [Google Scholar] [CrossRef]
- Williams, P.A.; Phillips, G.O. Gums and Stabilisers for the Food Industry 10; Woodhead Publishing: Sawston, UK, 2000; Volume 10. [Google Scholar]
- Gomez-Diaz, D.; Navaza, J.M. Comments about rheological effects of food hydrocolloids addition. J. Food Agric. Environ. 2003, 1, 98–102. [Google Scholar]
- Kislev, M. Archaeobotanical evidence of birdliming at Ashkelon. Ashkelon 2008, 1, 1985–2006. [Google Scholar]
- Aberoumand, A. Determination and comparison of potential nutritive values and mineral elements of three important food edible plants from southern part of Iran. Hrvat. Časopis Za Prehrambenu Tehnol. Biotehnol. Nutr. 2011, 6, 148–151. [Google Scholar]
- Aberoumand, A. Preliminary evaluation of some phytochemical and nutrients constituents of Iranian Cordia myxa fruits. Int. J. Agric. Food Sci. 2011, 1, 3033. [Google Scholar]
- Benhura, M.A.N.; Chidewe, C.K. The emulsifying properties of a polysaccharide isolated from the fruit of Cordia abyssinica. Int. J. Food Sci. Technol. 2004, 39, 579–583. [Google Scholar] [CrossRef]
- Rafe, A.; Masood, H. The rheological modeling and effect of temperature on steady shear flow behavior of Cordia abyssinica gum. J. Food Process. Technol. 2014, 5, 1. [Google Scholar]
- Hasani, M.; Yazdanpanah, S. The Effects of Gum Cordia on the Physicochemical, Textural, Rheological, Microstructural, and Sensorial Properties of Apple Jelly. J. Food Qual. 2020, 2020, 8818960. [Google Scholar] [CrossRef]
- Hussain, S.; Mohamed, A.A.; Alamri, M.S.; Ibraheem, M.A.; Qasem, A.A.A.; Shahzad, S.A.; Ababtain, I.A. Use of Gum Cordia (Cordia myxa) as a Natural Starch Modifier; Effect on Pasting, Thermal, Textural, and Rheological Properties of Corn Starch. Foods 2020, 9, 909. [Google Scholar] [CrossRef] [PubMed]
- El-Mogy, M.M.; Parmar, A.; Ali, M.R.; Abdel-Aziz, M.E.; Abdeldaym, E.A. Improving postharvest storage of fresh artichoke bottoms by an edible coating of Cordia myxa gum. Postharvest Biol. Technol. 2020, 163, 111143. [Google Scholar] [CrossRef]
- Haq, M.A.; Alam, M.J.; Hasnain, A. Gum Cordia: A novel edible coating to increase the shelf life of Chilgoza (Pinus gerardiana). LWT-Food Sci. Technol. 2013, 50, 306–311. [Google Scholar] [CrossRef]
- Haq, M.A.; Abid, H. Studies on shelf life extension of chilgoza (Pinus gerardiana). Asian J. Chem. 2013, 25, 343–348. [Google Scholar] [CrossRef]
- Haq, M.A.; Hasnain, A. Antioxidant Containing Gum Cordia Coatings for Control of Peanut Oxidation. J. Food Process. Preserv. 2014, 38, 896–904. [Google Scholar] [CrossRef]
- Delfanian, M.; Kenari, R.E.; Sahari, M.A. Utilization of Jujube Fruit (Ziziphus mauritianaLam.) Extracts as Natural Antioxidants in Stability of Frying Oil. Int. J. Food Prop. 2016, 19, 789–801. [Google Scholar] [CrossRef] [Green Version]
- Obeed, R.S.; Harhash, M.M.; Abdel-Mawgood, A.L. Fruit Properties and Genetic Diversity of Five Ber (Ziziphus mauritiana Lamk) Cultivars. Pak. J. Biol. Sci. 2008, 11, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Muchuweti, M.; Zenda, G.; Ndhlala, A.R.; Kasiyamhuru, A. Sugars, organic acid and phenolic compounds of Ziziphus mau-ritiana fruit. Eur. Food Res. Technol. 2005, 221, 570–574. [Google Scholar] [CrossRef]
- Lee, H.B.; Kim, S.Y. Studies on the changes of chemical components of dried jujube (Zizyphus jujuba Miller) during storage. Korean J. Agric. Sci. 1988, 15, 95–113. [Google Scholar]
- Alamri, M.S.; Mohamed, A.A.; Hussain, S.; Almania, H.A. Legume starches and okra (Abelmoschus esculentus) gum blends: Pasting, thermal, and viscous properties. Food Sci. Technol. Res. 2013, 19, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Alamri, M.S.; Mohamed, A.A.; Hussain, S. Effects of alkaline-soluble okra gum on rheological and thermal properties of systems with wheat or corn starch. Food Hydrocoll. 2013, 30, 541–551. [Google Scholar] [CrossRef]
- Sodhi, N.S.; Singh, N. Characteristics of acetylated starches prepared using starches separated from different rice cultivars. J. Food Eng. 2005, 70, 117–127. [Google Scholar] [CrossRef]
- Sandhu, K.; Singh, N. Some properties of corn starches II: Physicochemical, gelatinization, retrogradation, pasting and gel textural properties. Food Chem. 2007, 101, 1499–1507. [Google Scholar] [CrossRef]
- Kim, C.; Yoo, B. Rheological properties of rice starch–xanthan gum mixtures. J. Food Eng. 2006, 75, 120–128. [Google Scholar] [CrossRef]
- Ray, P.; Chatterjee, S.; Saha, P. Screening of polysaccharides from fruit pulp of Ziziphus mauritiana L. and Artocarpus heterophyllus L. as natural mucoadhesives. Futur. J. Pharm. Sci. 2021, 7, 1–10. [Google Scholar] [CrossRef]
- Hongbo, T.; Shiqi, G.; Yanping, L.; Siqing, D. Synthesis, characterization and properties of acetylated guar gum. Cell. Chem. Technol. 2017, 51, 237–244. [Google Scholar]
- Tang, M.; Hong, Y.; Gu, Z.; Zhang, Y.; Cai, X. The effect of xanthan on short and long-term retrogradation of rice starch. Starch Stärke 2013, 65, 702–708. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.-S.; Chen, H.-H.; Li, Q.-Q. Effect of sodium alginate on the gelatinization and retrogradation properties of two tuber starches. Cereal Chem. J. 2018, 95, 445–455. [Google Scholar] [CrossRef]
- Feng, M.; Yang, X.; Sun, J.; Xu, X.; Zhou, G. Study on retrogradation of maize starch-flaxseed gum mixture under various storage temperatures. Int. J. Food Sci. Technol. 2018, 53, 1287–1293. [Google Scholar] [CrossRef]
- Yadav, K.; Yadav, B.S.; Yadav, R.B.; Dangi, N. Physicochemical, pasting and rheological properties of colocasia starch as influenced by the addition of guar gum and xanthan gum. J. Food Meas. Charact. 2018, 12, 2666–2676. [Google Scholar] [CrossRef]
- Leite, T.D.; Nicoleti, J.F.; Penna, A.L.B.; Franco, C.M.L. Effect of addition of different hydrocolloids on pasting, thermal, and rheological properties of cassava starch. Food Sci. Technol. 2012, 32, 579–587. [Google Scholar] [CrossRef] [Green Version]
- Arocas, A.; Sanz, T.; Fiszman, S. Improving effect of xanthan and locust bean gums on the freeze-thaw stability of white sauces made with different native starches. Food Hydrocoll. 2009, 23, 2478–2484. [Google Scholar] [CrossRef]
- Alqah, H.; Alamri, M.S.; Mohamed, A.A.; Hussain, S.; Qasem, A.A.; Ibraheem, M.A.; Ababtain, I.A. The Effect of Germinated Sorghum Extract on the Pasting Properties and Swelling Power of Different Annealed Starches. Polymers 2020, 12, 1602. [Google Scholar] [CrossRef] [PubMed]
- González-Reyes, E.; Méndez-Montealvo, G.; Solorza-Feria, J.; Toro-Vazquez, J.; Bello-Pérez, L. Rheological and thermal characterization of Okenia hypogaea (Schlech. & Cham.) starch. Carbohydr. Polym. 2003, 52, 297–310. [Google Scholar] [CrossRef]
- Mandala, I.; Bayas, E. Xanthan effect on swelling, solubility and viscosity of wheat starch dispersions. Food Hydrocoll. 2004, 18, 191–201. [Google Scholar] [CrossRef]
- Ji, N.; Qiu, C.; Xu, Y.; Xiong, L.; Sun, Q. Differences in rheological behavior between normal and waxy corn starches modified by dry heating with hydrocolloids. Starch Stärke 2017, 69, 1600332. [Google Scholar] [CrossRef]
- Choi, D.-W.; Chang, Y.-H. Steady and Dynamic Shear Rheological Properties of Buckwheat Starch-galactomannan Mixtures. Prev. Nutr. Food Sci. 2012, 17, 192–196. [Google Scholar] [CrossRef] [Green Version]
| Component | % (per 100 g of Edible Portion) |
|---|---|
| carbohydrates | 57 |
| Crude proteins | 8.32 |
| Total sugars | 3.55 |
| Reducing sugars | 3.41 |
| Non reducing sugars | 0.08 |
| Pectin | 4.5 |
| Minerals (mg/g) dry basis | |
| Sodium | 1.62 |
| Potassium | 7.83 |
| Calcium zinc | 0.46 |
| Zinc | 0.35 |
| Iron | 0.51 |
| Wheat Starch | 100% Wheat Starch |
|---|---|
| 2% native cordia | 2 g native cordia—98 g wheat starch |
| 2% acetylated cordia | 2 g acetylated cordia—98 g wheat starch |
| 5% native cordia | 5 g native cordia—95 g wheat starch |
| 5% acetylated cordia | 5 g acetylated cordia—95 g wheat starch |
| 2% native ziziphus | 2 g native ziziphus—98 g wheat starch |
| 2% acetylated ziziphus | 2 g acetylated ziziphus—98 g wheat starch |
| 5% native ziziphus | 5 g native ziziphus—95 g wheat starch |
| 5% acetylated ziziphus | 5 g acetylated ziziphus—95 g wheat starch |
| Potato Starch | 100% potato Starch |
| 2% native cordia | 2 g native cordia—98 g potato starch |
| 2% acetylated cordia | 2 g acetylated cordia—98 g potato starch |
| 5% native cordia | 5 g native cordia—95 g potato starch |
| 5% acetylated cordia | 5 g acetylated cordia—95 g potato starch |
| 2% native ziziphus | 2 g native ziziphus—98 g potato starch |
| 2% acetylated ziziphus | 2 g acetylated ziziphus—98 g potato starch |
| 5% native ziziphus | 5 g native ziziphus—95 g potato starch |
| 5% acetylated ziziphus | 5 g acetylated ziziphus—95 g potato starch |
| Chickpea Starch | 100% Chickpea Starch |
| 2% native cordia | 2 g native cordia—98 g chickpea starch |
| 2% acetylated cordia | 2 g acetylated cordia—98 g chickpea starch |
| 5% native cordia | 5 g native cordia—95 g chickpea starch |
| 5% acetylated cordia | 5 g acetylated cordia—95 g chickpea starch |
| 2% native ziziphus | 2 g native ziziphus—98 g chickpea starch |
| 2% acetylated ziziphus | 2 g acetylated ziziphus—98 g chickpea starch |
| 5% native ziziphus | 5 g native ziziphus—95 g chickpea starch |
| 5% acetylated ziziphus | 5 g acetylated ziziphus—95 g chickpea starch |
| 1 ∆H (J/g) | 2 T0 (°C) | 3 PT (°C) | |
|---|---|---|---|
| Wheat Starch | |||
| Cordia gum | |||
| 0 | 9.34 ± 0.12 b | 55.35 ± 0.11 d | 62.59 ± 0.23 d |
| 2% native | 9.55 ± 0.10 b | 56.44 ± 0.16 c | 64.42 ± 0.27 b |
| 2% acetylated | 6.64 ± 0.06 c | 57.78 ± 0.26 b | 63.50 ± 0.06 c |
| 5% native | 10.09 ± 0.05 a | 58.72 ± 0.23 a | 65.61 ± 0.28 a |
| 5% acetylated | 5.56 ± 0.21 d | 58.84 ± 0.29 a | 65.43 ± 0.14 a |
| Ziziphus gum | |||
| 0 | 9.34 ± 0.12 a | 55.35 ± 0.11 c | 62.59 ± 0.23 bc |
| 2% native | 6.04 ± 0.04 c | 56.09 ± 0.59 bc | 62.33 ± 0.31 c |
| 2% acetylated | 6.76 ± 0.45 b | 58.06 ± 0.14 a | 64.01 ± 0.26 a |
| 5% native | 5.49 ± 0.20 c | 58.12 ± 0.20 a | 63.54 ± 0.15 a |
| 5% acetylated | 4.83 ± 0.33 d | 56.51 ± 0.45 b | 62.90 ± 0.13 b |
| Potato starch | |||
| Cordia gum | |||
| 0 | 15.97 ± 0.15 a | 59.19 ± 0.04 c | 63.33 ± 0.07 c |
| 2% native | 15.39 ± 0.16 b | 61.14 ± 0.04 a | 65.83 ± 0.05 a |
| 2% acetylated | 14.33 ± 0.16 c | 58.96 ± 0.16 c | 62.80 ± 0.06 d |
| 5% native | 14.20 ± 0.13 c | 59.76 ± 0.07 b | 64.05 ± 0.14 b |
| 5% acetylated | 13.02 ± 0.07 d | 57.69 ± 0.26 d | 62.90 ± 0.19 d |
| Ziziphus gum | |||
| 0 | 15.97 ± 0.15 a | 59.19 ± 0.04 b | 63.33 ± 0.07 b |
| 2% native | 16.30 ± 0.47 a | 57.57 ± 0.88 c | 62.42 ± 0.39 c |
| 2% acetylated | 11.33 ± 0.16 b | 55.44 ± 0.10 d | 60.85 ± 0.27 d |
| 5% native | 16.16 ± 0.10 a | 60.45 ± 0.08 a | 65.10 ± 0.24 a |
| 5% acetylated | 10.08 ± 0.39 c | 57.80 ± 0.34 c | 63.14 ± 0.30 b |
| Chickpea starch | |||
| Cordia gum | |||
| 0 | 4.78 ± 0.13 a | 54.87 ± 0.15 e | 63.04 ± 0.45 c |
| 2% native | 3.03 ± 0.05 c | 57.99 ± 0.21 c | 65.34 ± 0.11 b |
| 2% acetylated | 3.09 ± 0.10 c | 57.68 ± 0.15 d | 65.43 ± 0.27 b |
| 5% native | 5.00 ± 0.06 a | 59.23 ± 0.08 b | 66.78 ± 0.13 a |
| 5% acetylated | 3.53 ± 0.26 b | 59.59 ± 0.03 a | 66.94 ± 0.41 a |
| Ziziphus gum | |||
| 0 | 4.78 ± 0.13 c | 54.87 ± 0.15 d | 63.04 ± 0.45 c |
| 2% native | 6.49 ± 0.23 a | 57.67 ± 0.16 b | 65.14 ± 0.13 b |
| 2% acetylated | 5.44 ± 0.15 b | 57.28 ± 0.03 c | 65.27 ± 0.04 b |
| 5% native | 5.79 ± 0.04 b | 59.34 ± 0.08 a | 66.03 ± 0.22 a |
| 5% acetylated | 5.46 ± 0.17 b | 59.41 ± 0.14 a | 66.20 ± 0.07 a |
| 1 PV (cP) | 2 FV (cP) | 3 SB (cP) | 4 PT (°C) | |
|---|---|---|---|---|
| Wheat starch | ||||
| Cordia gum | ||||
| 0 | 2970 ± 18 e | 3725 ± 38 e | 1384 ± 25 c | 86.53 ± 0.09 a |
| 2% native | 3363 ± 07 d | 4044 ± 20 d | 1538 ± 22 b | 64.38 ± 0.08 d |
| 2% acetylated | 3707 ± 20 c | 4483 ± 52 a | 1672 ± 39 a | 66.45 ± 0.35 b |
| 5% native | 4172 ± 16 a | 4465 ± 0.5 b | 1336 ± 19 c | 65.99 ± 0.07 c |
| 5% acetylated | 3876 ± 04 b | 4240 ± 0.4 c | 1386 ± 0.7 c | 66.05 ± 0.04 c |
| Ziziphus gum | ||||
| 0 | 2970 ± 18 d | 3725 ± 38 b | 1384 ± 25 a | 86.53 ± 0.09 b |
| 2% native | 3025 ± 04 c | 3634 ± 0.4 c | 1277 ± 10 b | 86.50 ± 0.03 b |
| 2% acetylated | 3271 ± 08 a | 3964 ± 0.7 a | 1274 ± 0.7 b | 84.90 ± 0.04 c |
| 5% native | 3039 ± 15 c | 3452 ± 27 c | 1209 ± 0.9 c | 88.11 ± 0.02 a |
| 5% acetylated | 3162 ± 34 b | 3730 ± 0.4 b | 1122 ± 23 d | 84.90 ± 0.04 c |
| Potato starch | ||||
| Cordia gum | ||||
| 0 | 9829 ± 171 a | 4395 ± 77 ab | 644 ± 14 ab | 64.47 ± 0.02 c |
| 2% native | 7005 ± 37 d | 3904 ± 77 c | 742 ± 48 a | 66.13 ± 0.04 a |
| 2% acetylated | 8573 ± 85 b | 4465 ± 0.5 a | 402 ± 33 c | 64.23 ± 0.55 b c |
| 5% native | 8136 ± 89 c | 4352 ± 10 ab | 232 ± 14 d | 65.19 ± 0.03 b |
| 5% acetylated | 8175 ± 49 c | 4305 ± 70 b | 554 ± 88 b | 64.83 ± 0.33 c |
| Ziziphus gum | ||||
| 0 | 9829 ± 171 a | 4395 ± 77 bc | 644 ± 14 a | 64.47 ± 0.02 b |
| 2% native | 7673 ± 0.5 b | 4463 ± 69 ab | 350 ± 50 b | 64.83 ± 0.36 b |
| 2% acetylated | 7509 ± 41 b | 4563 ± 18 a | 538 ± 70 a | 64.88 ± 0.38 b |
| 5% native | 5756 ± 48 d | 4159 ± 72 d | 619 ± 71 a | 66.39 ± 0.31 a |
| 5% acetylated | 6274 ± 0.3 c | 4268 ± 70 cd | 659 ± 76 a | 66.13 ± 0.04 a |
| Chickpea starch | ||||
| Cordia gum | ||||
| 0 | 4331 ± 27 c | 5822 ± 0.8 d | 2896 ± 63 d | 69.83 ± 0.28 a |
| 2% native | 5991 ± 36 a | 5592 ± 29 e | 3777 ± 14 c | 64.39 ± 0.03 d |
| 2% acetylated | 5576 ± 64 b | 6442 ± 54 b | 5172 ± 32 b | 63.88 ± 0.31 d |
| 5% native | 5519 ± 85 b | 6044 ± 40 c | 2644 ± 22 e | 66.48 ± 0.33 b |
| 5% acetylated | 5338 ± 30 c | 6873 ± 20 a | 5298 ± 0.7 a | 65.22 ± 0.02 c |
| Ziziphus gum | ||||
| 0 | 4331 ± 27 a | 5822 ± 0.8 a | 2896 ± 63 a | 69.83 ± 0.28 c |
| 2% native | 3927 ± 20 c | 5160 ±3 7 c | 2534 ± 29 c | 70.87 ± 0.02 b |
| 2% acetylated | 4153 ± 22 b | 5522 ± 32 b | 2650 ± 56 b | 70.93 ± 0.10 b |
| 5% native | 3541 ± 0.3 e | 4532 ± 20 d | 2060 ± 24 d | 69.36 ± 0.02 c |
| 5% acetylated | 3607 ± 10 d | 4550 ± 21 d | 2007 ± 28 d | 72.28 ± 0.35 a |
| Wheat Starch | |
|---|---|
| Gum Type | G′ Rank |
| GC | 2% AC > 5% NA > 2% NA > 5% AC > C |
| GZ | 2% AC > 2% NA > 5% NA > C > 5% AC |
| Potato starch | |
| GC | 5% NA > 2% AC > 5% AC > 2% NA > C |
| GZ | 2% AC > 2% NA > 5% AC > 2% NA > C |
| Chickpea starch | |
| GC | 2% AC > 5% NA > 2% NA > 5% AC > C |
| GZ | C > 5% AC > 2% AC > 5% NA > 2% NA |
| Starch Blends | Upward Curves | Downward Curves | ||||
|---|---|---|---|---|---|---|
| µ (Pa sn) a | Ea (J/mol K−1) b | R2 | µ (Pa sn) a | Ea (J/mol K−1) b | R2 | |
| Wheat Starch | ||||||
| Cordia gum | ||||||
| 0 | 7.26 × 10−12 | 30,420 | 0.99 | 2.86 × 10−6 | 16,474 | 0.98 |
| 2% native | 4.22 × 10−6 | 17,235 | 0.99 | 8.72 × 10−4 | 11,434 | 0.97 |
| 2% acetylated | 1.17 × 10−8 | 23,437 | 0.92 | 1.01 × 10−4 | 13,516 | 0.98 |
| 5% native | 1.78 × 10−8 | 25,798 | 0.92 | 1.74 × 10−5 | 17,810 | 0.84 |
| 5% acetylated | 2.60 × 10−3 | 10,982 | 0.99 | 0.06 | 7616 | 0.94 |
| Ziziphus gum | ||||||
| 2% native | 1.57 × 10−5 | 15,023 | 0.99 | 1.89 × 10−4 | 12,460 | 0.99 |
| 2% acetylated | 2.08 × 10−8 | 19,430 | 0.84 | 1.29 × 10−3 | 10,548 | 0.94 |
| 5% native | 2.07 × 10−3 | 10,866 | 0.91 | 0.01 | 9005 | 0.99 |
| 5% acetylated | 1.38 × 10−9 | 25,782 | 0.92 | 2.81 × 10−4 | 12,430 | 0.98 |
| Potato starch | ||||||
| Cordia gum | ||||||
| 0 | 0.06 | 10,426 | 0.99 | 0.01 | 12,533 | 0.99 |
| 2% native | 7.99 × 10−5 | 17,285 | 0.99 | 1.06 × 10−3 | 14,660 | 0.99 |
| 2% acetylated | 1.87 × 10−4 | 16,894 | 0.98 | 8.15 × 10−4 | 15,693 | 0.99 |
| 5% native | 6.77 × 10−7 | 19,277 | 0.99 | 2.99 × 10−3 | 14,556 | 0.99 |
| 5% acetylated | 3.41 × 10−3 | 13,036 | 0.98 | 0.02 | 11,468 | 0.98 |
| Ziziphus gum | ||||||
| 2% native | 7.98 × 10−5 | 17,858 | 0.99 | 6.44 × 10−3 | 13,341 | 0.99 |
| 2% acetylated | 1.78 × 10−5 | 18,790 | 0.88 | 6.70 × 10−4 | 15,213 | 0.99 |
| 5% native | 3.12 × 10−6 | 20,461 | 0.97 | 1.50 × 10−3 | 14,251 | 0.99 |
| 5% acetylated | 1.00 | 16,936 | 0.99 | 6.97 × 10−4 | 14,673 | 0.99 |
| Chickpea starch | ||||||
| Cordia Gum | ||||||
| 0 | 1.08 × 10−6 | 19,945 | 0.97 | 7.99 × 10−8 | 10,369 | 0.99 |
| 2% native | 0.33 | 7218 | 0.99 | 0.23 | 7115 | 0.99 |
| 2% acetylated | 0.05 | 9245 | 0.98 | 2.20 | 5168 | 0.96 |
| 5% native | 5.96 | 5038 | 0.98 | 0.94 | 7230 | 0.99 |
| 5% acetylated | 1.27 | 7458 | 0.99 | 22.17 | 4404 | 0.99 |
| Ziziphus gum | ||||||
| 2% native | 6.08 × 10−6 | 18,174 | 0.97 | 0.02 | 9599 | 0.99 |
| 2% acetylated | 5.13 × 10−5 | 15,854 | 0.98 | 0.03 | 9141 | 0.99 |
| 5% native | 2.32 × 10−5 | 16,744 | 0.97 | 0.02 | 9591 | 0.98 |
| 5% acetylated | 2.96 × 10−6 | 18,964 | 0.97 | 5.10 × 10−3 | 11,026 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.; Hussain, S.; Alamri, M.S.; Ibraheem, M.A.; Qasem, A.A.A.; Ababtain, I.A. Physicochemical Properties of Starch Binary Mixtures with Cordia and Ziziphus Gums. Processes 2022, 10, 180. https://doi.org/10.3390/pr10020180
Mohamed A, Hussain S, Alamri MS, Ibraheem MA, Qasem AAA, Ababtain IA. Physicochemical Properties of Starch Binary Mixtures with Cordia and Ziziphus Gums. Processes. 2022; 10(2):180. https://doi.org/10.3390/pr10020180
Chicago/Turabian StyleMohamed, Abdellatif, Shahzad Hussain, Mohammed S. Alamri, Mohammed A. Ibraheem, Akram A. Abdo Qasem, and Ibrahim A. Ababtain. 2022. "Physicochemical Properties of Starch Binary Mixtures with Cordia and Ziziphus Gums" Processes 10, no. 2: 180. https://doi.org/10.3390/pr10020180
APA StyleMohamed, A., Hussain, S., Alamri, M. S., Ibraheem, M. A., Qasem, A. A. A., & Ababtain, I. A. (2022). Physicochemical Properties of Starch Binary Mixtures with Cordia and Ziziphus Gums. Processes, 10(2), 180. https://doi.org/10.3390/pr10020180








