Bt and G10evo-EPSPS Protein Expressed in ZDAB3 Corn Has No Impact on Nutritional Composition and Toxicological Safety
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. RNA Extraction and RT-PCR Analysis
2.3. Protein Quantification of Cry1Ab, Cry2Ab, and G10evo-EPSPS
2.4. Acute Oral Toxicity Testing of G10evo-EPSPS
2.4.1. Bioethics
2.4.2. Test and Control Substances
2.4.3. Animal Experiment
2.5. Nutritional Composition Analysis
2.5.1. Proximates
2.5.2. Fatty Acids
2.5.3. Amino Acids
2.5.4. Vitamins
2.5.5. Minerals
2.5.6. Anti-Nutrients
2.6. Statistical Analysis
3. Results
3.1. Molecular Character Detection of ZDAB3
3.2. Lack of Acute Toxicity of G10evo-EPSPS Protein
3.3. Nutritional Compositions of ZDAB3 Grain
3.3.1. Proximates
3.3.2. Fatty Acids
3.3.3. Amino Acids
3.3.4. Vitamins
3.3.5. Minerals
3.3.6. Anti-Nutrients
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ISAAA. Global Status of Commercialized Biotech/GM Crops in 2019: Biotech Crops Drive Socio-Economic Development and Sustainable Environment in the New Frontier. 2019. Available online: https://www.isaaa.org (accessed on 2 May 2022).
- Kramer, K.J.; Morgan, T.D.; Throne, J.E.; Dowell, F.E.; Bailey, M.; Howard, J.A. Transgenic avidin maize is resistant to storage insect pests. Nat. Biotechnol. 2000, 18, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Sato, S.J.; Behrens, M.; Jiang, W.Z.; Clemente, T.E.; Weeks, D.P. Genetic engineering of maize (Zea mays) for high-level tolerance to treatment with the herbicide dicamba. J. Agric. Food Chem. 2011, 59, 5830–5834. [Google Scholar] [CrossRef] [PubMed]
- Murry, L.E.; Elliott, L.G.; Capitant, S.A.; West, J.A.; Hanson, K.K.; Scarafia, L.; Johnston, S.; Deluca-Flaherty, C.; Nichols, S.; Cunanan, D. Transgenic corn plants expressing MDMV strain B coat protein are resistant to mixed infections of maize dwarf mosaic virus and maize chlorotic mottle virus. Bio/technology 1993, 11, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- El-Shemy, H.A.; Khalafalla, M.M.; Fujita, K.; Ishimoto, M. Improvement of protein quality in transgenic soybean plants. Biol. Plantarum 2007, 51, 277–284. [Google Scholar] [CrossRef]
- Mclaren, J.S. Crop biotechnology provides an opportunity to develop a sustainable future. Trends Biotechnol. 2005, 23, 339–342. [Google Scholar] [CrossRef]
- Soltani, N.; Dille, A.J.; Burke, I.C.; Everman, W.J.; Vangessel, M.J.; Davis, V.M.; Sikkema, P.H. Potential corn yield losses due to weeds in North America. Weed Technol. 2016, 30, 979–984. [Google Scholar] [CrossRef]
- Oerke, E.-C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Christensen, A.H.; Sharrock, R.A.; Quail, P.H. Maize polyubiquitin genes: Structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol. Biol. 1992, 18, 675–689. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Datta, K.; Zhang, J.; Yang, W.; Raychaudhuri, S.; Miyao, M.; Datta, S.K. Enhanced photosynthesis rate in genetically engineered indica rice expressing pepc gene cloned from maize. Plant Sci. 2007, 172, 1204–1209. [Google Scholar] [CrossRef]
- Odell, J.T.; Nagy, F.; Chua, N.H. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature 1985, 313, 810–812. [Google Scholar] [CrossRef]
- Hirt, H.; Kögl, M.; Murbacher, T.; Heberle-Bors, E. Evolutionary conservation of transcriptional machinery between yeast and plants as shown by the efficient expression from the CaMV 35S promoter and 35S terminator. Curr. Genet. 1990, 17, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Jiang, Y.Y.; Wang, P.F.; Lai, Y.M.; Chen, X.Y.; Xu, J.F. Resistance evaluation of genetically modified maize GAB-3 expressing Cry1Ab and Cry2Ab against four major lepidopteran pests. J. Agri. Sci. Technol.-Iran 2020, 22, 97–104. [Google Scholar]
- Schauzu, M. The concept of substantial equivalence in safety assessment of foods derived from genetically modified organisms. AgBiotechNet 2000, 2, ABN044. [Google Scholar]
- World Health Organization. Regional Office for Europe. European Centre for Environment and Health, Rome Division WHO/EURO/ECEH. In Safety Aspects of Genetically Modified Foods of Plant Origin: Report of a Joint FAO/WHO Expert Consultation on Foods Derived from Biotechnology: WHO Headquarters, Geneva, Switzerland, 29 May–2 June 2000|Clc; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Rothamsted, A. Review of the strategies for the comprehensive food and feed safety and nutritional assessment of GM plants per se. EFSA Support. Publ. 2013, 10, EN-480. [Google Scholar]
- Flachowsky, H.G. Proposals for nutritional assessments of feeds from genetically modified plants. J. Anim. Feed Sci. 2005, 14, 49–70. [Google Scholar] [CrossRef]
- Chinese Standard GB 5009.3-2016; Methods for Determination of Moisture in Foods. Standards Press of China: Beijing, China, 2017.
- Chinese Standard GB 5009.5-2016; Methods for Determination of Protein in Foods. Standards Press of China: Beijing, China, 2017.
- Chinese Standard GB 5009.6-2016; Methods for Determination of Fat in Foods. Standards Press of China: Beijing, China, 2017.
- Chinese Standard GB/T 5009.10-2003; Methods for Determination of Crude Fiber in Vegetable Foods. Standards Press of China: Beijing, China, 2004.
- Chinese Standard GB 5009.4-2016; Methods for Determination of Ash in Foods. Standards Press of China: Beijing, China, 2017.
- Chinese Standard GB 5009.9-2016; Methods for Determination of Starch in Foods. Standards Press of China: Beijing, China, 2017.
- Chinese Standard GB 5009.168-2016; Methods for Determination of Fatty Acids in Foods. Standards Press of China: Beijing, China, 2017.
- Chinese Standard GB 5009.124-2016; Methods for Determination of Amino Acids in Foods. Standards Press of China: Beijing, China, 2017.
- Chinese Standard GB 5009.84-2016; Methods for Determination of Thiamine (Vitamin B1) in Foods. Standards Press of China: Beijing, China, 2017.
- Chinese Standard GB 5009.85-2016; Methods for Determination of Riboflavin (Vitamin B2) in Foods. Standards Press of China: Beijing, China, 2017.
- Chinese Standard GB 5009.82-2016; Methods for Determination of Tocopherol (Vitamin E) in Foods. Standards Press of China: Beijing, China, 2017.
- Chinese Standard GB 5009.268-2016; Methods for Determination of Minerals in Foods. Standards Press of China: Beijing, China, 2017.
- International Standard: 65.020.99; Safety Assessment of Genetically Modified Plant and Derived Products Parts 2: Assay of Anti-nutrients Pancreatic Trypsin Inhibiter. Standards Press of China: Beijing, China, 2006.
- OECD. Consensus document on compositional considerations for new varieties of maize (Zea mays): Key food and feed nutrients, anti-nutrients and secondary plant metabolites. Ser. Saf. Nov. Food Feeds 2002, 6, 1–42. [Google Scholar]
- ILSI. Crop Composition Database; International Life Sciences Institute: Washington, DC, USA, 2014. [Google Scholar]
- Anderson, J.A.; Hong, B.; Moellring, E.; Teronde, S.; Walker, C.; Wang, Y.W.; Maxwell, M. Composition of forage and grain from genetically modified DP202216 maize is equivalent to non-modified conventional maize (Zea mays L.). GM Crops Food 2019, 10, 77–89. [Google Scholar] [CrossRef]
- Lundry, D.R.; Burns, J.A.; Nemeth, M.A.; Riordan, S.G. Composition of grain and forage from insect-protected and herbicide-tolerant corn, MON 89034 × TC1507 × MON 88017 × DAS-59122-7 (SmartStax), is equivalent to that of conventional corn (Zea mays L.). J. Agric. Food Chem. 2013, 61, 1991–1998. [Google Scholar] [CrossRef]
- Heinemann, R.J.B.; Fagundes, P.D.L.; Pinto, E.A.; Penteado, M.D.V.C.; Lanfer-Marquez, U. Comparative study of nutrient composition of commercial brown, parboiled and milled rice from Brazil. J. Food Compos. Anal. 2005, 18, 287–296. [Google Scholar] [CrossRef]
- Parengam, M.; Judprasong, K.; Srianujata, S.; Jittinandana, S.; Laoharojanaphand, S.; Busamongko, A. Study of nutrients and toxic minerals in rice and legumes by instrumental neutron activation analysis and graphite furnace atomic absorption spectrophotometry. J. Food Compos. Anal. 2010, 23, 340–345. [Google Scholar] [CrossRef]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A.; Hart, J.P. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed]
- Abou-Hussein, S.D. Climate change and its impact on the productivity and quality of vegetable crops. J. Appl. Sci. Res. 2012, 8, 4359–4383. [Google Scholar]
- Yin, Y.; Xu, Y.D.; Cao, K.L.; Qin, Z.F.; Zhao, X.X.; Dong, X.H.; Shi, W.P. Impact assessment of Bt maize expressing the Cry1Ab and Cry2Ab protein simultaneously on non-target arthropods. Environ. Sci. Pollut. Res. 2020, 27, 21552–21559. [Google Scholar] [CrossRef] [PubMed]
- Schrøder, M.; Poulsen, M.; Wilcks, A.; Kroghsbo, S.; Miller, A.; Frenzel, T.; Danier, J.; Rychlik, M.; Emami, K.; Gatehouse, A. A 90-day safety study of genetically modified rice expressing Cry1Ab protein (Bacillus thuringiensis toxin) in Wistar rats. Food Chem. Toxicol. 2007, 45, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Onose, J.-i.; Imai, T.; Hasumura, M.; Ueda, M.; Ozeki, Y.; Hirose, M. Evaluation of subchronic toxicity of dietary administered Cry1Ab protein from Bacillus thuringiensis var. Kurustaki HD-1 in F344 male rats with chemically induced gastrointestinal impairment. Food Chem. Toxicol. 2008, 46, 2184–2189. [Google Scholar] [CrossRef]
- Singhal, K.; Tyagi, A.; Rajput, Y.; Singh, M.; Kaur, H.; Perez, T.; Hartnell, G. Feed intake, milk production and composition of crossbred cows fed with insect-protected Bollgard II® cottonseed containing Cry1Ac and Cry2Ab proteins. Animal 2011, 5, 1769–1773. [Google Scholar] [CrossRef][Green Version]
- Rahman, M.; Zaman, M.; Shaheen, T.; Irem, S.; Zafar, Y. Safe use of Cry genes in genetically modified crops. Environ. Chem. Lett. 2015, 13, 239–249. [Google Scholar] [CrossRef]
- Koch, M.S.; Ward, J.M.; Levine, S.L.; Baum, J.A.; Vicini, J.L.; Hammond, B.G. The food and environmental safety of Bt crops. Front. Plant Sci. 2015, 6, 283. [Google Scholar] [CrossRef]
- Gómez, I.; Pardo-López, L.; Muoz-Garay, C.; Fernandez, L.E.; Bravo, A. Role of receptor interaction in the mode of action of insecticidal Cry and Cyt toxins produced by Bacillus thuringiensis. Peptides 2007, 28, 169–173. [Google Scholar] [CrossRef]
- Rubio-Infante, N.; Moreno-Fierros, L. An overview of the safety and biological effects of Bacillus thuringiensis Cry toxins in mammals. J. Appl. Toxicol. 2016, 36, 630–648. [Google Scholar] [CrossRef]
- Hammond, B.G.; Koch, M.S. A Review of the Food Safety of Bt Crops. In Bacillus thuringiensis Biotechnology; Sansinenea, E., Ed.; Springer: Dordrecht, Netherlands, 2012; pp. 305–325. [Google Scholar]
- Harrison, L.A.; Bailey, M.R.; Naylor, M.W.; Ream, J.E.; Hammond, B.G.; Nida, D.L.; Burnette, B.L.; Nickson, T.E.; Mitsky, T.A.; Taylor, M.L. The expressed protein in glyphosate-tolerant soybean, 5-enolypyruvylshikimate-3-phosphate synthase from Agrobacterium sp. strain CP4, is rapidly digested in vitro and is not toxic to acutely gavaged mice. J. Nutr. 1996, 126, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.A.; Launis, K.L.; Bauman, P.A.; Juba, N.C. Double-mutated 5-Enol Pyruvylshikimate-3-phosphate synthase protein expressed in MZHG0JG corn (Zea mays L.) has no impact on toxicological safety and nutritional composition. J. Agric. Food Chem. 2017, 65, 8459–8465. [Google Scholar] [CrossRef] [PubMed]
- Herman, R.A.; Phillips, A.M.; Collins, R.A.; Tagliani, L.A.; Prochaska, L.M. Compositional equivalency of Cry1F corn event TC6275 and conventional corn (Zea mays L.). J. Agric. Food Chem. 2004, 52, 2726. [Google Scholar] [CrossRef] [PubMed]
- Herman, R.A.; Storer, N.P.; Phillips, A.M.; Prochaska, L.M.; Windels, P. Compositional assessment of event DAS-59122-7 maize using substantial equivalence. Regul. Toxicol. Pharm. 2007, 47, 37–47. [Google Scholar] [CrossRef] [PubMed]
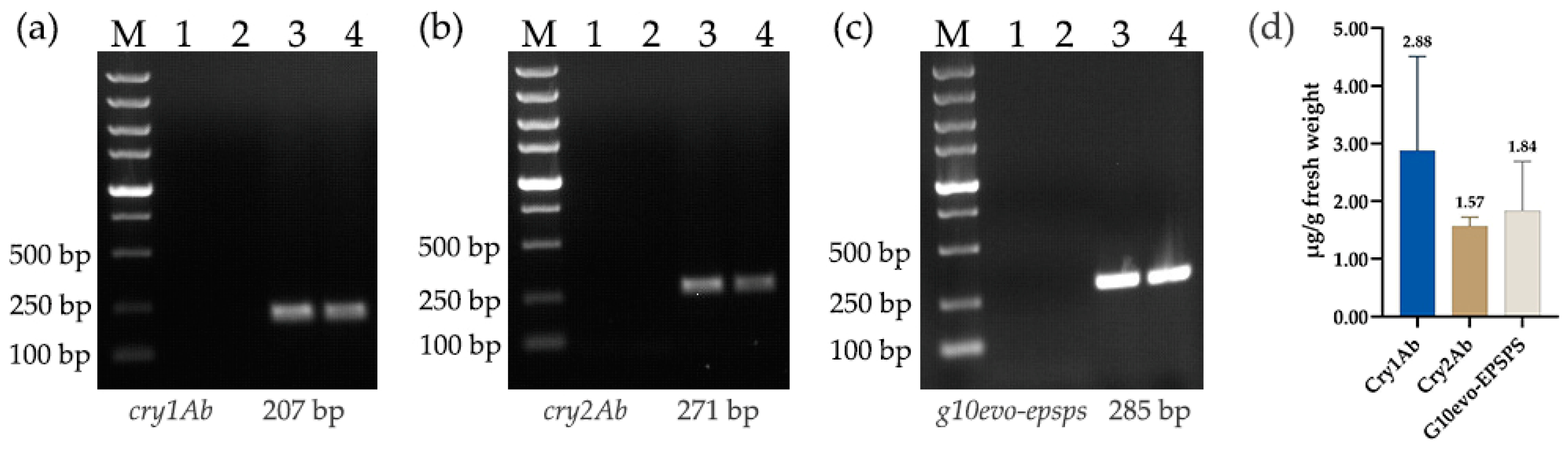
| Target Fragment | Primer Name | Sequence (5′- 3′) | Size (bp) |
|---|---|---|---|
| cry1Ab | cry1Ab-RT-F | GCTGGACATCGTGAGCCTGTTC | 207 |
| cry1Ab-RT-R | GCGTCGGTGTAGATGGTGATGC | ||
| cry2Ab | cry2Ab-RT-F | GCACAACCGCAAGAACAACATCC | 271 |
| cry2Ab-RT-R | GATGGTGGAGTTGCCGATGGAAG | ||
| g10evo-epsps | G10-RT-F | CGCTCAGCCATCCAAGAACTACAC | 285 |
| G10-RT-R | GTCACGAAAGTGGTGCCAGAGG |
| Treatment | Animal | Day 0 BW 1 (g) | Day 7 BW (g) | Day 15 BW (g) | BW (g) Increase |
|---|---|---|---|---|---|
| Control | Male 1 | 17.6 | 29.1 | 33.7 | 16.1 |
| Male 2 | 16.5 | 28.9 | 34.3 | 17.8 | |
| Male 3 | 16.8 | 27.0 | 31.7 | 14.9 | |
| Male 4 | 18.8 | 31.7 | 34.4 | 15.6 | |
| Male 5 | 18.0 | 29.1 | 36.0 | 18.0 | |
| Male 6 | 16.7 | 29.2 | 31.8 | 15.1 | |
| Average | 17.40 ± 0.90 | 29.17 ± 1.50 | 33.65 ± 1.66 | 16.25 ± 1.35 | |
| G10evo-EPSPS | Male 7 | 15.4 | 28.2 | 32.7 | 17.3 |
| Male 8 | 16.6 | 27.6 | 32.3 | 15.7 | |
| Male 9 | 16.6 | 28.0 | 34.1 | 17.5 | |
| Male 10 | 15.7 | 28.3 | 34.0 | 18.3 | |
| Male 11 | 17.6 | 29.9 | 35.0 | 17.4 | |
| Male 12 | 16.7 | 31.5 | 34.0 | 17.3 | |
| Average | 16.43 ± 0.79 | 28.92 ± 1.49 | 33.68 ± 1.00 | 17.25 ± 0.85 | |
| Control | Female 1 | 17.9 | 23.6 | 25.3 | 7.4 |
| Female 2 | 18.0 | 22.6 | 24.4 | 6.4 | |
| Female 3 | 18.3 | 23.6 | 24.6 | 6.3 | |
| Female 4 | 19.2 | 24.7 | 26.1 | 6.9 | |
| Female 5 | 18.5 | 25.0 | 25.4 | 6.9 | |
| Female 6 | 18.8 | 25.3 | 26.6 | 7.8 | |
| Average | 18.45 ± 0.49 | 24.13 ± 1.03 | 25.40 ± 0.85 | 6.95 ± 0.58 | |
| G10evo-EPSPS | Female 7 | 17.7 | 23.2 | 22.9 | 5.2 |
| Female 8 | 17.4 | 21.7 | 25.5 | 8.1 | |
| Female 9 | 16.6 | 21.9 | 24.3 | 7.7 | |
| Female 10 | 17.6 | 22.6 | 25.8 | 8.2 | |
| Female 11 | 17.3 | 24.3 | 25.7 | 8.4 | |
| Female 12 | 18.3 | 26.6 | 28.4 | 10.1 | |
| Average | 17.48 ± 0.56 | 23.38 ± 1.84 | 25.43 ± 1.83 | 7.95 ± 1.58 |
| Proximate | p-Value 1 | Content (g/100 g) | ||
|---|---|---|---|---|
| ZDAB3 | Control 2 | Literature Range 3 | ||
| Moisture (fw) 4 | 0.80 | 9.14 ± 0.16 | 9.17 ± 0.20 | 7.00–23.00 |
| Protein (dw) 5 | 0.09 | 7.46 ± 0.56 | 8.20 ± 0.18 | 5.72–17.26 |
| Crude fat (dw) | 0.06 | 3.17 ± 0.06 | 3.37 ± 0.12 | 1.36–7.83 |
| Ash (dw) | 0.77 | 1.43 ± 0.15 | 1.40 ± 0.10 | 0.62–6.28 |
| Crude fiber (dw) | 0.12 | 2.00 ± 0.10 | 1.87 ± 0.12 | 0.49–5.50 |
| Starch (dw) | 0.21 | 69.20 ± 0.26 | 68.47 ± 0.59 | na |
| Fatty Acid | p-Value (Adjusted p-Value) 1 | Content (%) | ||
|---|---|---|---|---|
| ZDAB3 | Control 2 | Literature Range 3 | ||
| Myristic (C14:0) | 0.16 | 0.05 ± 0.002 | 0.05 ± 0.001 | ND-0.29 |
| Heptadecanoic (C17:0) | 0.06 | 0.09 ± 0.003 | 0.10 ± 0.001 | ND-0.20 |
| Stearic (C18:0) | 0.20 | 2.33 ± 0.03 | 2.37 ± 0.04 | ND-4.90 |
| Linolenic (C18:3) | 0.34 | 1.56 ± 0.06 | 1.52 ± 0.05 | ND-2.33 |
| Behenic (C22:0) | 0.53 | 0.33 ± 0.04 | 0.31 ± 0.003 | ND-0.50 |
| Palmitic (C16:0) | 0.01 (0.07) | 14.73 ± 0.12 | 14.27 ± 0.15 | 6.81–39.00 |
| Palmitoleic (C16:1) | 0.01 (0.06) | 0.14 ± 0.002 | 0.13 ± 0.002 | ND-0.67 |
| Oleic (C18:1) | 0.002 (0.02) * | 25.77 ± 0.15 | 24.53 ± 0.25 | 16.38–42.81 |
| Linoleic (C18:2) | 0.000 (0.002) * | 53.77 ± 0.12 | 55.53 ± 0.15 | 13.10–67.68 |
| Arachidic (C20:0) | 0.000 (0.002) * | 0.65 ± 0.004 | 0.61 ± 0.002 | 0.27–1.20 |
| Amino Acid | p-Value (Adjusted p-Value) 1 | Content (g/100 g) | ||
|---|---|---|---|---|
| ZDAB3 | Control 2 | Literature Range 3 | ||
| Arginine | 0.05 | 0.31 ± 0.04 | 0.37 ± 0.02 | 0.12–0.71 |
| Aspartic acid | 0.19 | 0.53 ± 0.08 | 0.61 ± 0.03 | 0.33–1.21 |
| Glutamic acid | 0.05 | 1.69 ± 0.18 | 1.99 ± 0.03 | 0.97–3.54 |
| Lysine | 0.09 | 0.26 ± 0.03 | 0.30 ± 0.01 | 0.05–0.67 |
| Methionine | 0.08 | 0.08 ± 0.01 | 0.09 ± 0.003 | 0.10–0.47 |
| Proline | 0.11 | 0.57 ± 0.09 | 0.68 ± 0.03 | 0.46–1.75 |
| Tyrosine | 0.49 | 0.12 ± 0.00 | 0.11 ± 0.02 | 0.10–0.79 |
| Alanine | 0.04 (0.09) | 0.59 ± 0.06 | 0.69 ± 0.02 | 0.44–1.48 |
| Glycine | 0.02 (0.08) | 0.32 ± 0.03 | 0.38 ± 0.01 | 0.18–0.69 |
| Histidine | 0.01 (0.06) | 0.16 ± 0.02 | 0.22 ± 0.02 | 0.14–0.46 |
| Isoleucine | 0.04 (0.09) | 0.22 ± 0.02 | 0.25 ± 0.00 | 0.18–0.71 |
| Leucine | 0.04 (0.09) | 0.71 ± 0.07 | 0.84 ± 0.03 | 0.64–2.49 |
| Phenylalanine | 0.02 (0.07) | 0.30 ± 0.02 | 0.36 ± 0.02 | 0.24–0.93 |
| Valine | 0.02 (0.08) | 0.31 ± 0.03 | 0.37 ± 0.01 | 0.21–0.86 |
| Vitamin | p-Value (Adjusted p-Value) 1 | Content (mg/100 g) | ||
|---|---|---|---|---|
| ZDAB3 | Control 2 | Literature Range 3 | ||
| Vitamin B2 | 0.12 | 0.13 ± 0.01 | 0.11 ± 0.01 | ND-0.74 |
| Vitamin E | 0.26 | 3.35 ± 0.41 | 3.67 ± 0.06 | ND-8.99 |
| Vitamin B1 | 0.04 (0.09) | 0.09 ± 0.004 | 0.08 ± 0.01 | ND-4.00 |
| Mineral | p-Value (Adjusted p-Value) 1 | Content (mg/kg) | ||
|---|---|---|---|---|
| ZDAB3 | Control 2 | Literature Range 3 | ||
| Calcium | 0.32 | 192.00 ± 67.44 | 146.33 ± 15.04 | 21.50–1000.00 |
| Copper | 0.87 | 3.26 ± 0.51 | 3.33 ± 0.39 | 0.73–18.50 |
| Iron | 0.67 | 23.40 ± 2.26 | 22.80 ± 0.10 | 1.00–100.00 |
| Kalium | 0.05 | 3086.67 ± 335.01 | 3640.00 ± 91.65 | 2730.00–7200.00 |
| Magnesium | 0.74 | 745.33 ± 110.55 | 768.67 ± 25.77 | 82.00–1940.00 |
| Sodium | 0.07 | 98.90 ± 6.66 | 88.17 ± 3.77 | 0.00–150.00 |
| Phosphorus | 0.16 | 2406.67 ± 361.16 | 2780.00 ± 105.36 | 1470.00–7500.00 |
| Selenium | 0.58 | 0.02 ± 0.003 | 0.02 ± 0.005 | 0.01–1.00 |
| Zinc | 0.001 (0.02) * | 14.80 ± 0.44 | 18.23 ± 0.55 | 6.50–39.60 |
| Anti-Nutrient | p-Value 1 | Content | ||
|---|---|---|---|---|
| ZDAB3 | Control 2 | Literature Range 3 | ||
| Phytic acid (g/kg) | 0.77 | 14.20 ± 2.85 | 14.83 ± 2.14 | ND-19.40 |
| Trypsin inhibitors (TIU/g) | 0.07 | 2176.67 ± 469.18 | 1430.00 ± 223.38 | ND-8420.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Chen, G.; Zhou, Z.; Chen, X.; He, X.; Jiao, Y.; Wang, P. Bt and G10evo-EPSPS Protein Expressed in ZDAB3 Corn Has No Impact on Nutritional Composition and Toxicological Safety. Processes 2022, 10, 2739. https://doi.org/10.3390/pr10122739
Yu X, Chen G, Zhou Z, Chen X, He X, Jiao Y, Wang P. Bt and G10evo-EPSPS Protein Expressed in ZDAB3 Corn Has No Impact on Nutritional Composition and Toxicological Safety. Processes. 2022; 10(12):2739. https://doi.org/10.3390/pr10122739
Chicago/Turabian StyleYu, Xiaoxing, Guo Chen, Ziying Zhou, Xiaoyun Chen, Xiaoyun He, Yue Jiao, and Pengfei Wang. 2022. "Bt and G10evo-EPSPS Protein Expressed in ZDAB3 Corn Has No Impact on Nutritional Composition and Toxicological Safety" Processes 10, no. 12: 2739. https://doi.org/10.3390/pr10122739
APA StyleYu, X., Chen, G., Zhou, Z., Chen, X., He, X., Jiao, Y., & Wang, P. (2022). Bt and G10evo-EPSPS Protein Expressed in ZDAB3 Corn Has No Impact on Nutritional Composition and Toxicological Safety. Processes, 10(12), 2739. https://doi.org/10.3390/pr10122739







