Abstract
Sludges from the papermaking industry represent a challenging residue stream that is difficult to dewater using conventional processes. The successful development and scale-up of innovative processes from lab- to pilot- to industrial-scale are required to tackle challenges for waste treatment, including paper sludges. Biological paper sludge was treated via a mild hydrothermal carbonization process (TORWASH®) to improve dewaterability of the sludge, including long-duration, continuous testing. Initial lab-scale experiments indicated the optimal treatment temperature for sludge dewatering was 190 °C. Dewaterability improved with increasing temperature, but the obtained solid yield decreased. Scaling-up to a continuous flow pilot plant required a temperature of 200 °C to achieve optimum dewatering. Pilot-scale hydrothermal treatment and dewatering resulted in solid cakes with an average dry matter content of 38% and a solid yield of 39%. This study demonstrates the benefits of hydrothermal carbonization for the dewatering of biological paper sludge without the use of dewatering aids such as fiber sludge or polyelectrolytes. The results also demonstrate the successful adaptation of a lab-scale batch process to a pilot-scale continuous flow process for hydrothermal carbonization of industrial wastewater sludge.
1. Introduction
The paper industry is one of the largest global industries with an estimated production of >400 Mton of board and paper produced per year. During this production, approximately 400 Mton of wet sludges are generated annually as a residue stream of wastewater treatment at paper mills [1,2]. Of these, the most problematic for the paper mill is biological (bio-) sludge, so named because it is a product of biological wastewater treatment (typically aeration followed by clarification). The composition of biological paper sludge is complex and can contain high concentrations of harmful substances, including heavy metals, inorganics and pathogenic microorganisms [3,4]. Typically, these sludges are disposed of via landfilling or incineration. Landfilling of bio-sludge has been banned in many countries due to associated negative environmental impacts. Incineration is still a common disposal pathway but is difficult and expensive due to the high concentration of inorganic particles in the paper sludges combined with their poor dewaterability [5,6,7]. Dewatering, the separation of solid and liquid components, of biological paper sludge in order to improve the calorific value at incineration, can be so challenging that it can be responsible for over half of the total wastewater treatment costs at a paper mill [8], often requiring the use of costly dewatering aids such as coagulants or polyelectrolytes. A widely applied method to improve the dewaterability of the bio-sludge is the addition of fiber sludge, a by-product of paper making. Fiber sludge is composed of short cellulose fibers and is obtained from primary clarification in the wastewater treatment process. The addition of fiber sludge to bio-sludge to form a mixed sludge enhances the overall dewaterability via mechanical dewatering, which is highly preferred over thermal dewatering [8,9]. However, the use of fiber sludge as a dewatering aid is not ideal as it is rich in cellulose fibers and organics and therefore has the potential for reuse as a raw material in the papermaking process [5]. Therefore, alternative methods to improve the dewaterability of bio-sludge should be explored.
A variety of thermochemical processes have been developed for the upgrading of biomass. The majority of these processes use dry biomass as a feedstock, requiring an energy- and cost-intensive upstream drying step [10]. For hydrothermal carbonization this is not required, as water is the applied reaction medium. Therefore, it is an excellent upgrading process for wet biogenic feedstocks, including sludges [6]. Hydrothermal carbonization (HTC) is typically applied at temperatures in the range of 180–250 °C under autogenous pressure (1–5 MPa). Historically, the higher temperatures in this temperature range in combination with long residence times were targeted, but the focus has been shifting towards the lower temperatures of this temperature range in combination with shorter residence times [11]. At TNO, the TORWASH® technology has been developed as a reactor concept in which a mild HTC process, with relatively low temperatures (150–210 °C) and an adjustable short residence time, is applied for the upgrading of wet low-grade biomass feedstocks towards high-grade solid fuel of a quality suitable for co-firing [12,13].
At the applied conditions, a wide range of chemical reactions take place, including hydrolysis, dehydration, decarboxylation, recondensation and aromatization. These reactions result in lower O/C and H/C ratios for the obtained product compared to the original biomass, with values similar to conventional solid fuels [14,15]. During this carbonization process, organic acids (acetic acid, lactic acid, formic acid, levulinic acid) are generated, which decreases the pH of the reaction media. The decrease in pH further enhances the hydrolysis and decomposition of the polymers, making HTC an autocatalytic process [16,17]. As such, a drop in pH of the product slurry is a good indication for an efficient carbonization reaction, and the resulting decrease in the O/C ratio can therefore be linked to physicochemical properties such as enhanced dewaterability and higher heating value (HHV) [18].
The main product of HTC is a solid fraction (hydrochar), with a carbon-rich aqueous solution and a small amount of gas (70–90% of which is CO2) as side products. The distribution between these components is influenced by the reaction conditions and the feedstock [17,19]. The most influential reaction parameter is the applied temperature. Moderately low temperatures are applied in HTC, as higher temperatures (>250 °C) favor the formation of liquids and gases. With increasing temperature, the atomic ratios of H/C and O/C gradually decrease [20,21], but this comes at the expense of the hydrochar yield [10,22]. Another important parameter is the reaction time. Longer reaction times result in a higher hydrochar yield with better properties such as enhanced porosity, pore volume and surface area and a higher calorific value due to a decrease in the O/C ratio [23,24].
The generated hydrochar has many improved properties compared to the initial biomass, including higher mass and energy density, and improved grindability, hydrophobicity and stability to microbial degradation, and better combustion performance as a solid. The increased hydrophobicity results in better dewaterability of the hydrochar and removal of part of the inorganics, which significantly improves the combustion performance [19,23,25]. The hydrochar has many applications, including as a solid bio-fuel, adsorbent, catalyst, soil improver and carbon sequestration medium [19,21].
Hydrothermal carbonization of paper sludge has been widely reported in recent years [26,27,28,29,30,31]. Studies on the effect of temperature on different paper sludges show similar trends. With increasing temperature, a decrease in oxygen content relative to the carbon content was observed, resulting in a higher heating value (HHV) for the obtained hydrochar. Additionally, a clear decrease in solid yield was observed for both a higher reaction temperature and increased residence time [26,27,28].
One of the current gaps in hydrothermal carbonization research is the lack of reported data for pilot- and full-scale processes. Most of the existing research focuses on bench-scale operation and model simulations [11,32,33]. Recent work by Zaccariello et al. [34] demonstrated that the reaction conditions are scalable from a 3 L to a 100 L batch reactor but that the thermal efficiency decreases for the larger reactor. Moving from batch to (semi-)continuous reactors for hydrothermal carbonization is also highly relevant from an industrial point of view. The use of liter-scale semi-continuous reactors has been reported in a few studies and the results tend to be in agreement with small batch experiments [35,36]. Higher degrees of carbonization are reported for the hydrochar obtained by semi-continuous operation, which also lead to a higher hydrophobicity and thermal stability [35]. These results are promising, but additional research is needed on continuous HTC at a larger/industrially relevant (multi-kilogram to ton) scale for longer (days/weeks/months) time periods.
The aim of this project is the scale-up of a mild continuous hydrothermal carbonization of paper sludge from batch lab-scale to a continuous pilot-scale (>25 kg/h) in an industrial setting for a long duration (>400 h). A special focus is given to raw bio-sludge, as this is the problematic waste stream of the paper industry. Improving dewatering of this stream following hydrothermal treatment can forego the use of dewatering aids such as polyelectrolytes and fiber sludge. A reaction condition screening is performed at the lab-scale to optimize the HTC reaction conditions for both good dewaterability (reported as dry matter content of the obtained press cakes) and solid yield, and these conditions are applied at a continuous pilot-scale.
2. Materials and Methods
2.1. Raw Feedstock and Reference Dewatering Process
Biological paper sludge (bio-sludge) and mixed sludge (bio-sludge and fiber sludge) were provided by the Smurfit Kappa paper mill in Piteå, Sweden. The bio-sludge is a by-product of the biological wastewater treatment process of the paper mill. The mixed sludge is bio-sludge (33%) mixed with fiber sludge (67%). Fiber sludge is a by-product of paper production and is a wet stream containing solids in the form of very short cellulose fibers. During the initial lab testing, two different batches of bio-sludge were applied. The first batch had a low dry matter content (1.0%), which was a sample obtained directly after a maintenance stop. The second batch was obtained during normal operation, which resulted in a significantly higher dry matter content (3.4%)
Currently, the Smurfit Kappa paper mill in Piteå treats (dewaters) the mixed sludge as a residue stream, where the addition of fiber sludge aims to improve dewaterability of the bio-sludge. The mixed sludge is first treated with the addition of a polyelectrolyte as a chemical dewatering aid and this mixture is dewatered via a screw press to achieve solids with approximately 30–35% dry matter content. The solids are used on-site in a biomass boiler for heat production.
2.2. TORWASH® Process
This study uses the TORWASH® hydrothermal treatment process for short and mild HTC of bio-sludge. For this specific feedstock a temperature of 200 °C and autogenous pressure are applied. TORWASH® is a patented process that applies hydrothermal carbonization on wet organic residues via TORWASH® reactor technology. This process yields a product slurry that can be dewatered mechanically, whilst part of the inorganic ions are removed. Organic material that is dissolved in the filtrate is biodegradable and can be converted to biogas. Figure 1 shows a schematic representation of the overall process. The TORWASH® reactor technology includes flow operation on a small angle. During the carbonization reaction that takes place in the TORWASH® section (Figure 2), CO2 is generated as a result of dehydration and decarboxylation reactions. The angle of the reactor pipe retains the CO2 in the reactor. This results in a decrease in pH of the reaction media, catalyzing the hydrothermal carbonization [12].
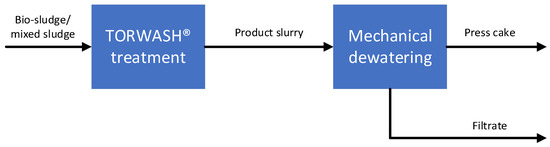
Figure 1.
Schematic representation of the TORWASH® process.
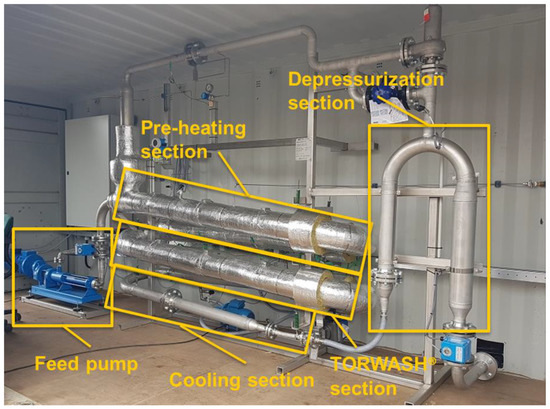
Figure 2.
Containerized pilot plant applied for the continuous flow hydrothermal experiments.
2.3. Lab-Scale Batch Experiments
Initial screening tests were performed in small microclave vessels (125 mL) to determine the optimal temperature for hydrothermal treatment. Six different temperatures (160–210 °C) were selected to test the effect of the reaction temperature on the properties and the dewaterability of the obtained product slurry. In total, 80 g of feedstock was added to the microclave vessel, which was then tightly sealed and inserted into a heating block and heated to a target temperature. A typical test was performed with a stirring speed of 500 rpm and a run time at setpoint of 30 min.
Autoclave experiments were performed in a stainless steel vessel (20 L), which was indirectly heated. These experiments were performed to scale up optimized conditions as determined by the initial screening experiments. A total of 15 kg of feedstock was added to the vessel and the vessel was tightly sealed. The mixture was stirred by a stirrer equipped inside the vessel. Heating to the set temperature required approximately three hours. Upon reaching the temperature set point, the reactor was kept at this temperature for 30 min. Afterwards the reactor was left to cool to room temperature.
2.4. Pilot-Scale Continuous Flow Experiments
The TORWASH® continuous flow pilot plant (Figure 2) was designed for a maximum throughput of 50 kg/h, maximum temperature of 250 °C and a maximum pressure of 25 bar. The feedstock passes through three distinct temperature regimes in the pilot plant. The first section is the preheat section (~30 min residence time), in which the feed is gradually heated from ambient temperature to approximately 180 °C. Hydrolysis reactions occur in this section, but dehydration reactions are limited. In the TORWASH® section the temperature is increased to the desired temperature (200 °C for bio-sludge, ~1 h residence time) to achieve hydrothermal carbonization. In the cooling section the mixture is rapidly cooled to 40 °C to stop the reaction. The product slurry is depressurized and afterwards collected in external tanks. For the heating sections, electrical heating is applied, whereas the cooling section applies cooling water.
The typical throughput during the pilot tests was 25 kg/h, which corresponds to an overall residence time of ~2.5 h. The pilot plant is containerized and mobile and was used for long-duration (>400 h) experiments performed on-site at the Smurfit Kappa paper mill in Piteå, Sweden.
2.5. Dewatering Experiments
For lab-scale experiments and initial testing of the TORWASH® reactor, the obtained product slurry after hydrothermal carbonization was filtered (Whatman GF/D glass microfiber filter, 2.7 μm pore size) to remove the bulk of the liquid from the product slurry, yielding a transparent orange–brown filtrate and a solid filter cake. The filter cake was further mechanically dewatered with a carver die unit. For the carver die (58 mm diameter), nylon filters (20 μm pore size) were used at the top and bottom of the die. The loaded die was pressed unidirectionally in a hydraulic press, operated at 65 bar for 1 min to generate a solid press cake.
For on-site pilot-scale testing, dewatering of the large quantities of product slurry obtained during the long-duration test was performed by a pilot-scale membrane filter press of Limburg Filter, which employs their Leaktite technology. Briefly, the product slurry was pumped into the chambers of the membrane filter press, which was set at a (adjustable) pressure. The pressure slowly increased, which removed the bulk of the water from the solids. Next, the membranes were squeezed to dewater the solids as much as possible, yielding the solid press cake.
2.6. Analysis
The dry matter content of the feedstocks, TORWASH® product slurry, press cakes and filtrate was determined by drying overnight in an oven at 105 °C at atmospheric pressure. The dry matter content of press cakes was used to evaluate the dewaterability of the solids following the hydrothermal treatment.
Elemental analysis was performed on all streams (dried) with the Element Analyser Flash 2000 (Thermo Fisher Scientific, Lelystad, The Netherlands) and the ash content was determined using a Nabertherm LV5/11/B180 oven. CHN content was determined according to EN 15104 and the ash content according to EN 14775. Volatile matter was measured according to EN 15148. The higher heating value (HHV) was determined according to EN 14918. Inorganics were measured by Inductively Coupled Plasma with an ICAP6300 ICP-OES destruction according to NEN 6963 standard and ICP measurements according to NEN 6966. F, Cl and Br were analyzed according to NEN-EN-ISO-10304-1. Mercury was analyzed by Cold Vapour Atomic Fluorescence Spectroscopy. Dissolved chemical oxygen demand (COD) and dissolved phosphorus and nitrogen in the liquid fractions were measured with the Hach Lange cuvette test method.
3. Results and Discussion
3.1. Feedstock Analysis
Analysis of the two feedstocks showed that both streams are similar in composition (Table 1). Both feedstocks have close to neutral pH and are very dilute (<3% dry solids) with approximately 42% carbon content in the solids. The oxygen content of the mixed sludge is higher (38%) than in the bio-sludge (31%) due to the presence of cellulose fibers in the mixed sludge. The ash content of both feedstocks is high (up to 16%) due to the presence of a wide range of metals. Heavy metals are common in paper mill wastewater, mainly from the black liquor by-product of papermaking. These metals are concentrated in the sludge during wastewater treatment. The sulfur content in both sludges is particularly high, which is a result of the use of sulfur in the pulping process. The obtained values are within the range of other reported sludges derived from various paper mills [26].

Table 1.
Characteristics of biological sludge and mixed sludge (33% biological sludge and 67% fiber sludge) as received. 1 db = dry basis; analysis on solids that were dried at 105 °C.
3.2. Lab-Scale Experiments
3.2.1. Microclave Tests
A good indication of an efficient hydrothermal carbonization process, and thus dewaterability, is the decrease in pH of the resulting product slurry [17]. For both mixed sludge and bio-sludge, a clear decrease in pH was observed with hydrothermal treatment in the screening tests (Table 2 and Table 3). For bio-sludge, the optimum temperature for pH reduction was 180 °C. For mixed sludge, the pH decreased with increasing temperature to 180 °C and then did not further decrease with higher temperatures. The chemical oxygen demand (COD) of the product slurry increased with increasing reaction temperature, confirming the increase in organics in the liquid fraction by hydrolysis reactions. Hydrothermally treated mixed sludge had increased concentrations of dissolved ammonium, total nitrogen and orthophosphate compared to the feedstock, and a strong relationship between the reaction temperature and the total dissolved matter was observed.

Table 2.
Characteristics of the mixed sludge feedstock before and after processing, and the effect of hydrothermal treatment reaction temperature. Reported values for COD, NH4+, total nitrogen and PO43− correspond to the dissolved fraction.

Table 3.
Characteristics of two bio-sludge feedstocks before and after processing, and the effect of hydrothermal treatment reaction temperature. * Second bio-sludge batch used, n.d. = not determined. Reported values for COD, NH4+, total nitrogen and PO43− correspond to the dissolved fraction.
Hydrothermally treated bio-sludge and mixed sludge from the screening tests were pressed with the carver die to test the dewaterability of the obtained product slurries. Sludges that were treated at higher reaction temperatures were more easily pressable, which is closely related to a drop in pH (Table 2 and Table 3). For mixed sludge, the dewaterability was consistent as a good press cake could be obtained in combination with a clear liquid. The dry matter content of the obtained press cake was in the range of 59–63% at treatment temperatures between 160–190 °C (Table S1). The dry matter content in the press cake did show a small (and unexpected) decrease at higher temperatures, to 54% at 200 and 210 °C, as dewaterability is expected to increase for hydrochar obtained at higher temperatures [23]. For bio-sludge, no reliable data for the dry matter content could be obtained due to the small quantity of solids obtained in each experiment. The product slurry obtained at 160 °C was not dewaterable, whereas all the other samples yielded a solid and a clear liquid after mechanical dewatering.
Determination of the total solid yield did show a clear trend for both feedstocks (Figure 3) as a decrease in the solid yield (as a percentage of the total initial solids) was observed with increasing reaction temperature. For mixed sludge, this decrease was gradual from 160 °C (83% solid yield) to 190 °C (69% solid yield). Increasing the temperature to 200 °C resulted in a sharp drop to 56% solid yield. For bio-sludge treated at 170 °C and 180 °C, the solid yield was comparable (42% and 38%, respectively). Higher temperatures resulted in a sharp decrease in solid yield, ranging from 26% at 190 °C to 10% at 210 °C, as was also observed in comparable studies [26,27]. The very low values obtained for bio-sludge are also a result of the small quantities of solids obtained (less than 0.4 g), which makes any product loss during work-up result in a significant yield loss percentage-wise.
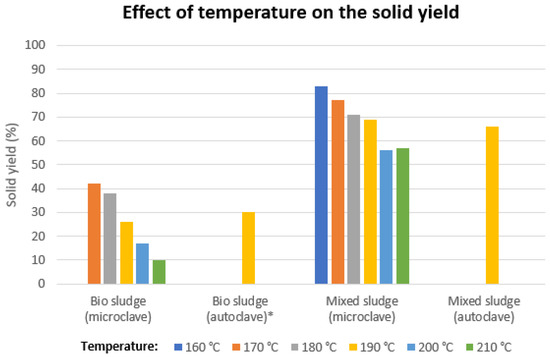
Figure 3.
Solid yield of the press cakes from the obtained product slurry from bio-sludge and mixed sludge after mild hydrothermal carbonization at different temperatures and carver die dewatering. * Second batch of bio-sludge.
The differences between both feedstocks confirm the positive effect of the addition of fiber sludge to bio-sludge for both the dewaterability and the solid yield of the obtained product. Based on these results the optimal reaction temperature for mixed sludge was determined to be 190 °C. It should be noted that the results (and trends) observed for the experiments at the higher temperatures are likely an actual overestimation of the temperature effect. This is due to the relatively slow heating and cooling profile of the microclave, causing a longer exposure to reactive conditions compared to the experiments performed at lower temperatures.
Some follow-up experiments were performed with a different (second) batch of bio-sludge from Smurfit Kappa, which had different properties compared to the first batch (Table 3). In particular the dry matter content (3.4% vs. 1%) and the dissolved organics (COD of 2.7 g/L vs. 0.2 g/L) were significantly higher than those of the original bio-sludge sample. Two microclave experiments were performed for this new bio-sludge, at 180 °C and 200 °C, and confirmed the trends observed with the previous feedstock. The result for the total solid yield was similar at 180 °C (40% solid yield), but a significant improvement was observed at 200 °C (28% solid yield vs. 17% for the first batch). This is likely a result of the higher amount of dry matter content in the second batch and the lower pH of both the feedstock and the resulting product slurry, resulting in more efficient carbonization.
3.2.2. Autoclave Tests
Based on the results obtained with the microclave experiments, a reaction temperature of 190 °C was determined as a setpoint for the autoclave tests for mixed sludge and the second batch of bio-sludge. The dewaterability of both product slurries was very good, with consistent quality of the solid press cake and a clear liquid. For mixed sludge, the obtained results were similar to the microclave experiment, although the pH of the resulting mixture was slightly more acidic (pH 5.4) and the COD slightly higher (9.2 g/L) (Table 4). Due to the high volume of product slurry created, 18 press cakes were pressed in order to obtain good average values. A total solid yield of 66% and an average dry matter content of 61% were obtained for the press cakes, which is in correspondence with the results obtained with the microclave experiments.

Table 4.
Characteristics of the product slurry obtained with the hydrothermal treatment at 190 °C with the autoclave. Reported values for COD, NH4+, total nitrogen and PO43− correspond to the dissolved fraction.
For bio-sludge, the results were also in line with the microclave experiments. The characteristics of the obtained product slurry are similar to the results with the microclave at 200 °C, with a slightly higher amount of dissolved components. This is likely a result of the longer heating and cooling time of the autoclave compared to the microclave, effectively resulting in a longer reaction time. A total solid yield of 30% was obtained and an average dry matter content of 61% was obtained for the press cakes (average of six press cakes). These results are also comparable to the results obtained with the microclave at 200 °C.
3.3. Pilot-Scale Continuous Flow Experiments
The first experiments in the pilot plant were performed at TNO in an effort to prepare for and de-risk the long-duration experiments on-site. The first experiment was performed with mixed sludge as the feedstock, during which a range of temperatures were tested. A clear improvement in dewaterability was observed with increasing temperature. The liquid obtained after pressing the product slurry obtained at 185 °C contained solids, whilst at higher reaction temperatures, a clear liquid was obtained. The press cake obtained from the mixed sludge treated at 185 °C showed large fluctuations in the dry matter content, ranging from 20–46%. At temperatures of 195 °C and 205 °C, more consistent results for the press cakes were obtained with an average dry matter content of 61% and 63%, respectively (Table 5), which is in correspondence with the results obtained with the microclave and autoclave at 190 °C. The improved dewaterabilty at elevated temperatures is in accordance with the reported literature [23]. The solid yields at 195 °C and 205 °C were 69% and 68%, respectively, which was similar to the result with the microclave at 190 °C (69%). pH analysis of the product slurries confirmed the trend of improved dewaterability with decreasing pH. The product slurry obtained at 185 °C had a pH of 6.0, compared to a pH of 5.8 for treatment at 195 °C and 5.6 at 205 °C. The pH of the obtained product slurries was less acidic compared to the corresponding results with the microclave, indicating a less efficient carbonization reaction. Overall, it was noticed that a higher temperature (~10 °C) was required for the pilot plant to obtain similar results to those for the microclave and autoclave tests. This could be a result of a low degree of mixing in the pilot plant, which limits the heat transfer in the reaction mixture. The requirement for higher temperatures could also be a result of the shorter residence time in the pilot plant compared to the autoclave and microclave. Therefore, the carbonization reaction is not as effective in the pilot plant compared to the batch systems at the same temperature, and a higher temperature is required in the pilot reactor to obtain similar results to the batch system.

Table 5.
Results obtained for the press cake and the pH of the obtained product slurry.
Following 8.5 h of continuous pilot plant operation with mixed sludge, the flow profile became irregular and the experiment was stopped. Subsequent inspection of the pilot plant showed the accumulation of fibrous material inside the reactor. This would be problematic for continuous flow operation for an extended time period.
Initial pilot experiments with bio-sludge were performed at the same temperatures as mixed sludge. As with the mixed sludge, a clear increase in dewaterability was observed with increasing temperature. Bio-sludge treated at 185 °C was not dewaterable. A temperature increase to 195 °C yielded a product slurry that was pressable, albeit with difficulty. The product slurry obtained at 205 °C was significantly easier and more consistent to press. The obtained press cakes from the product slurry generated at 195 °C and 205 °C had average dry matter contents of 48% and 50%, respectively, which were lower than the 61% obtained from the autoclave experiment at 190 °C. The solid yields at 195 °C and 205 °C were 49% and 44%, respectively, which were markedly higher compared to the microclave and autoclave experiments, which had a solid yield of around 30%, which could be an effect of the shorter residence time in the flow setup [26,27,28]. The pH of the product slurry followed the same trend of decreasing with increasing reaction temperature. Inspection of the pilot plant following the experiments did not show any accumulation of solids, demonstrating the suitability of bio-sludge for long duration experiments. The improved dewaterability with increasing temperature is in good accordance with literature reports. Enhanced dewaterability is directly linked to the polarity index ((O+N)/C), where a low value (due to a high carbon content) corresponds to hydrophobicity [37,38]. At higher temperatures, the carbon content of the obtained hydrochar is higher, resulting in enhanced dewaterability.
Based on the results of the initial pilot plant experiments with mixed sludge and bio-sludge, bio-sludge was selected as the feedstock for long duration (>400 h) testing on-site at the Smurfit Kappa paper mill. The preliminary experiments showed that hydrothermal treatment of mixed sludge does not offer many benefits in terms of dewaterability over the bio-sludge and, in fact, is detrimental at the pilot-scale due to the fibers settling in the reactor. The use of bio-sludge as the sole feedstock for hydrothermal treatment is also beneficial from an industrial perspective, as this material is the problematic waste stream for the paper industry. Fiber sludge is typically added as a dewatering aid, but it could be used instead for higher value applications [8].
The long-duration pilot-scale experiment was performed on the site of the wastewater treatment plant at the paper mill of Smurfit Kappa in Piteå, Sweden. This allowed for pilot testing on the actual location where the residue stream was generated and ensured a constant supply of fresh bio-sludge for the entire duration of the test, providing a better approximation of actual scale-up conditions. The long-duration experiment was performed over a period of 24 days, with a pilot plant uptime of 75% (410 h). The reactor down-time was mainly caused by the unusual flow behavior of heated bio-sludge and fouling within the reactor, more details of which can be found in the Supporting Information.
During the long-duration experiment, the target temperature was set at 200 °C, and throughout the experiment the temperature ranged between 195 and 205 °C. Nearly 11,000 kg of product slurry was produced in this experiment. The obtained product slurry was easily dewaterable with a membrane filter press, yielding a clear filtrate. In total, 142 kg of press cake was obtained from the product slurry with the membrane filter press (Figure 4). The press cakes had an average dry matter content of 38% (54.4 kg dry solid) and a total solid yield of 39%. The dry matter content of the press cake was consistent throughout the entire test with values ranging from 33–42% (9 filter press runs). The solid yield is comparable but slightly lower than those obtained in the preliminary pilot plant experiments. The values for the dry matter content of the press cakes obtained during continuous operation were approximately 10% lower compared to the values obtained during the lab and pilot tests. This is due to the difference in operating pressure of the applied filter press. The carver die operates at a higher pressure compared to the membrane filter press, resulting in a more compressed cake. The dry matter content of the obtained press cakes was higher than the values obtained with the currently applied dewatering method at the paper mill, showing the potential of the hydrothermal treatment process.

Figure 4.
Press cake obtained with the membrane filter press during the long duration experiment.
Analysis of the bio-sludge feedstock on-site showed values that were an intermediary of the two bio-sludge samples used in the lab-scale testing. This demonstrates the variability within this feedstock and the requirement for a robust process to treat it. Hydrothermally treated bio-sludge had a slightly smaller dry matter content compared to untreated bio-sludge (Table 6). This loss is likely a combined effect of loss due to the formation of gases (~5% of the initial organic content) and some settling of solids in both the pilot plant and the collection tanks. The high dry matter content of the filtrate (53% of the feed) indicates that a large fraction of the initial feed is dissolved in the liquid fraction as a result of the hydrothermal treatment and valorization of this stream is essential. The decrease in pH of the initial feedstock compared to both the obtained product slurry and filtrate and the marked increase in dissolved COD are indicators for efficient hydrothermal carbonization.

Table 6.
Characteristics of the bio-sludge feed, product slurry and filtrate obtained with the pilot plant operated at 200 °C. Reported values for COD, NH4+, total nitrogen and PO43− correspond to the dissolved fraction. Early stage denotes the first two weeks of long duration testing, late stage denotes the final two weeks of long duration testing.
In order to obtain more insight into the chemical changes during the process and the final composition of the obtained products, an extensive analysis was performed on the feedstock, hydrothermal carbonization product slurry, the obtained press cake from the membrane filter press and the resulting filtrate of the membrane filter press (Table 7). One of the main observations is the high ash content (24.9%) of the obtained press cake. More specifically, the press cake has a high affinity for metals such as aluminum, barium, cadmium, chromium, copper, iron, lead, manganese, mercury, silicon, strontium, titanium, vanadium and zinc compared to the obtained filtrate. This makes the hydrothermal carbonization of bio-sludge an efficient method to concentrate and trap metals in a wastewater stream [39]. Calcium, magnesium, phosphorus and sulfur were also detected in the press cake, but these were more evenly distributed between the press cake and filtrate. The filtrate has high affinity for boron, bromine, chlorine, potassium and sodium and is also rich in calcium and sulfur. Another trend is the increase in the higher heating value (HHV) for the obtained product slurry and press cake (19.2 and 20.8 MJ/kg db) compared to the feedstock (18.1 MJ/kg db). This is associated with an increase in the carbon content relative to oxygen in the product slurry and press cake, a direct effect of the carbonization of the feedstock. The increase in the HHV and the removal of most of the water (99.2% of the initial feedstock) could make this a suitable process to produce solid fuel. Further investigation to see if the press cake can be used directly as a solid fuel (e.g., in the steelmaking industry) should be performed [40], whereas the effluent could be used for nutrient recovery [41] and biogas formation via anaerobic digestion [42]. The effect of the high ash content of the hydrochar obtained from this specific feedstock should be carefully evaluated as this will exclude the use as, e.g., soil remediation. Even if no direct applications are possible for this feedstock, this process is still of high interest for the paper industry as the enhanced dewaterability of bio-sludge can lead to improved energy efficiency of the sludge and cost savings. Preliminary techno-economic analysis indicates the proposed process can save three times the primary fuel and reduce treatment costs by 28% when compared to the conventional dewatering and incineration process at the mill. Detailed techno-economic and environmental assessments are ongoing and represent topics for future research.

Table 7.
Analysis results of bio-sludge, the hydrothermal carbonization product slurry, the press cake obtained with the membrane press and the resulting filtrate.
4. Conclusions
This study reports the successful scale-up from a batch lab-scale process to a continuous flow pilot plant for the mild hydrothermal carbonization of biological sludge from the wastewater treatment plant of a paper mill. Initial experiments were performed at lab-scale on bio-sludge and mixed sludge (mix of bio- and fiber sludge, with the latter as a dewatering aid) to determine the optimal reaction temperature for dewaterability of the sludge whilst maintaining a good solid yield. At lab-scale, a temperature of around 190 °C was established as the optimal temperature as determined by the overall solid yield (26% for bio-sludge and 69% for mixed sludge) and ease of mechanical dewaterability (pressing). The experiments could be increased in scale from a 100 mL microclave to a 20 L autoclave with good and comparable results for both bio-sludge (30% solid yield, 61% dry matter content) and mixed sludge (66% solid yield, 61% dry matter content) at 190 °C. In short tests at the pilot-scale, it was possible to achieve the same results at a small increase in temperature to approximately 200 °C. This increase in temperature was likely required due to poorer mixing and uneven heating in the pilot reactor. For mixed sludge, the pilot-scale test showed that the accumulation of cellulose fibers in the reactor would be problematic. For bio-sludge, a significant improvement in solid yield to over 40% was achieved by hydrothermal treatment in combination with a dry matter content close to 50%, which is higher than the current applied methods for enhancing bio-sludge dewaterability. Therefore, bio-sludge was selected as feedstock for the long duration testing. A long duration experiment, 24 days, 410 h for a total uptime of 75%, was performed on-site at a paper mill with bio-sludge as the feedstock. The results were in good correspondence with the lab tests, with a solid yield of 39% and a dry matter content of 38% for the obtained press cakes (solids). Compared to the current practice at the paper mill, which uses valuable fiber sludge and polymer as dewatering aids to achieve 30% dry matter content, this study highlights the potential of hydrothermal treatment to improve the dewaterability of wastewater sludges without the use of dewatering aids. The obtained press cake shows potential as a means to concentrate metals present in wastewater, and due to the enhanced dewaterability of bio-sludge, it should have enhanced potential as a solid fuel. Furthermore, 99.2% of the initial water content was removed from the bio-sludge during the process of hydrothermal carbonization and dewatering. Applying a more concentrated feed and valorization of the filtrate are topics that should be addressed in follow-up research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr10122702/s1, Figure S1: Build-up and blockages of sludge in the reactor (left) and unexpected phase separation of floating sludge upon heating of bio-sludge (right) to explain the blockages at the top of the reactor tubes; Figure S2: Fouling on the inside of the TORWASH pilot plant upon inspection and cleaning at the end of the long-duration tests; Table S1: Results for the microclave and autoclave experiments at different reaction temperatures on bio-sludge and mixed sludge as feedstock.
Author Contributions
Conceptualization., P.N., J.R.P. and H.E.W.; methodology, E.C.-P., M.V. and D.J.S.; validation. P.N., J.R.P. and H.E.W.; investigation. E.C.-P., M.V. and D.J.S.; resources, P.N. and H.E.W.; data curation. D.S.Z.; writing—original draft preparation. D.S.Z.; writing—review and editing. H.E.W., J.R.P. and P.N.; supervision, H.E.W. and P.N.; project administration. H.E.W.; funding acquisition, P.N. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No. 884226.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge Stefan Lundqvist, Ingemar Lundstrom, Frank Kruip, Martin Viklund, Ludvig Ånnhagen and Peter Skemark for assistance during the on-site trials in Piteå. We also acknowledge Sayujya Shah and Jan Wilco Dijkstra for preliminary techno-economic information.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Turner, T.; Wheeler, R.; Oliver, I.W. Evaluating land application of pulp and paper mill sludge: A review. J. Environ. Manag. 2022, 317, 115439. [Google Scholar] [CrossRef] [PubMed]
- Oumabady, S.; Selvaraj, P.S.; Periasamy, K.; Veeraswamy, D.; Ramesh, P.T.; Palanisami, T.; Ramasamy, S.P. Kinetic and isotherm insights of Diclofenac removal by sludge derived hydrochar. Sci. Rep. 2022, 12, 2184. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Wang, G.; Li, R.; Xu, K.; Wu, J.; Li, D.; Liu, J. Co-Combustion Behavior of Paper Sludge Hydrochar and Pulverized Coal: Low Rank Coal and Its Product by Hydrothermal Carbonization. Energies 2022, 15, 5619. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, X.; Peng, X.; Hu, S.; Yu, Z.; Fang, S. Effect of hydrothermal carbonization temperature on combustion behavior of hydrochar fuel from paper sludge. Appl. Therm. Eng. 2015, 91, 574–582. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Van, H.T.; Chu, T.H.H.; Nguyen, T.H.V.; Nguyen, T.D.; Hoang, L.P.; Hoang, V.H. Paper waste sludge-derived hydrochar modified by iron (III) chloride for enhancement of ammonium adsorption: An adsorption mechanism study. Environ. Technol. Innov. 2021, 21, 101223. [Google Scholar] [CrossRef]
- Makela, M.; Benavente, V.; Fullana, A. Hydrothermal carbonization of industrial mixed sludge from a pulp and paper mill. Bioresour. Technol. 2016, 200, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Makela, M.; Forsberg, J.; Soderberg, C.; Larsson, S.H.; Dahl, O. Process water properties from hydrothermal carbonization of chemical sludge from a pulp and board mill. Bioresour. Technol. 2018, 263, 654–659. [Google Scholar] [CrossRef]
- Meyer, T.; Amin, P.; Allen, D.G.; Tran, H. Dewatering of pulp and paper mill biosludge and primary sludge. J. Environ. Chem. Eng. 2018, 6, 6317–6321. [Google Scholar] [CrossRef]
- Jarnerud, T.; Karasev, A.V.; Wang, C.; Bäck, F.; Jönsson, P.G. Utilization of Organic Mixed Biosludge from Pulp and Paper Industries and Green Waste as Carbon Sources in Blast Furnace Hot Metal Production. Sustainability 2021, 13, 7706. [Google Scholar] [CrossRef]
- Khan, T.A.; Saud, A.S.; Jamari, S.S.; Rahim, M.H.A.; Park, J.-W.; Kim, H.-J. Hydrothermal carbonization of lignocellulosic biomass for carbon rich material preparation: A review. Biomass Bioenergy 2019, 130, 105384. [Google Scholar] [CrossRef]
- Román, S.; Libra, J.; Berge, N.; Sabio, E.; Ro, K.; Li, L.; Ledesma, B.; Álvarez, A.; Bae, S. Hydrothermal Carbonization: Modeling, Final Properties Design and Applications: A Review. Energies 2018, 11, 216. [Google Scholar] [CrossRef]
- Meijden, C.M.V.D.; Pels, J.R. Reactor for the Hydrothermal Treatment of Biomass. WIPO (PCT) Patent WO 2021/032842 A1, 25 February 2021. [Google Scholar]
- Pels, J.R.; Blijendaal, L.P.J.; Nijman, M.N.W.; Zandvoort, M.H.; Cieplik, M.K. Conversion of water plants to biomass fuel using torwash. Proceedings of 22nd European Biomass Conference and Exhibitions, Hamburg, Germany, 23–26 June 2014. [Google Scholar]
- Ischia, G.; Fiori, L. Hydrothermal Carbonization of Organic Waste and Biomass: A Review on Process, Reactor, and Plant Modeling. Waste Biomass Valorization 2020, 12, 2797–2824. [Google Scholar] [CrossRef]
- Pauline, A.L.; Joseph, K. Hydrothermal carbonization of organic wastes to carbonaceous solid fuel—A review of mechanisms and process parameters. Fuel 2020, 279, 118472. [Google Scholar] [CrossRef]
- Sharma, H.B.; Sarmah, A.K.; Dubey, B. Hydrothermal carbonization of renewable waste biomass for solid biofuel production: A discussion on process mechanism, the influence of process parameters, environmental performance and fuel properties of hydrochar. Renew. Sustain. Energy Rev. 2020, 123, 109761. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Xu, J.; Flora, J.R.V.; Hoque, S.; Berge, N.D. Quantifying the sensitivity of feedstock properties and process conditions on hydrochar yield, carbon content, and energy content. Bioresour. Technol. 2018, 262, 284–293. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.I.; Pozarlik, A.K.; Bramer, E.A.; Niedzwiecki, L.; Pawlak-Kruczek, H.; Brem, G. Hydrothermal carbonization of wet biomass from nitrogen and phosphorus approach: A review. Renew. Energy 2021, 171, 401–415. [Google Scholar] [CrossRef]
- Jamari, S.S.; Howse, J.R. The effect of the hydrothermal carbonization process on palm oil empty fruit bunch. Biomass Bioenergy 2012, 47, 82–90. [Google Scholar] [CrossRef]
- Maniscalco, M.P.; Volpe, M.; Messineo, A. Hydrothermal Carbonization as a Valuable Tool for Energy and Environmental Applications: A Review. Energies 2020, 13, 4098. [Google Scholar] [CrossRef]
- Saqib, N.U.; Sharma, H.B.; Baroutian, S.; Dubey, B.; Sarmah, A.K. Valorisation of food waste via hydrothermal carbonisation and techno-economic feasibility assessment. Sci. Total Environ. 2019, 690, 261–276. [Google Scholar] [CrossRef]
- Heidari, M.; Dutta, A.; Acharya, B.; Mahmud, S. A review of the current knowledge and challenges of hydrothermal carbonization for biomass conversion. J. Energy Inst. 2019, 92, 1779–1799. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Baloch, H.A.; Griffin, G.J.; Mubarak, N.M.; Bhutto, A.W.; Abro, R.; Mazari, S.A.; Ali, B.S. An overview of effect of process parameters on hydrothermal carbonization of biomass. Renew. Sustain. Energy Rev. 2017, 73, 1289–1299. [Google Scholar] [CrossRef]
- Tasca, A.L.; Puccini, M.; Gori, R.; Corsi, I.; Galletti, A.M.R.; Vitolo, S. Hydrothermal carbonization of sewage sludge: A critical analysis of process severity, hydrochar properties and environmental implications. Waste Manag. 2019, 93, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.; Saba, A.; Saha, P.; McGaughy, K.; Franqui-Villanueva, D.; Orts, W.; Hart-Cooper, W.; Reza, M. Hydrothermal Carbonization of Various Paper Mill Sludges: An Observation of Solid Fuel Properties. Energies 2019, 12, 858. [Google Scholar] [CrossRef]
- Hamalainen, A.; Kokko, M.; Kinnunen, V.; Hilli, T.; Rintala, J. Hydrothermal carbonization of pulp and paper industry wastewater treatment sludges—Characterization and potential use of hydrochars and filtrates. Bioresour. Technol. 2022, 355, 127258. [Google Scholar] [CrossRef]
- Sadish, O.; Paul Sebastian, S.; Banu, K.S.P.; Mahendran, R. Hydrochar as an Energy Alternative to Coal: Effect of Temperature on Hydrothermal Carbonization of Paper Board Mill Sludge. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1668–1675. [Google Scholar] [CrossRef]
- Ejilane, J.; Balasubramaniam, P.; Maheswari, M.; Sebastian, S.P.; Sabarish, K. Production and Characterization of Paper Board Mill ETP Sludge Derived Hydrochar. Int. J. Environ. Clim. Change 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Mäkelä, M.; Benavente, V.; Fullana, A. Hydrothermal carbonization of lignocellulosic biomass: Effect of process conditions on hydrochar properties. Appl. Energy 2015, 155, 576–584. [Google Scholar] [CrossRef]
- Alatalo, S.M.; Repo, E.; Makila, E.; Salonen, J.; Vakkilainen, E.; Sillanpaa, M. Adsorption behavior of hydrothermally treated municipal sludge & pulp and paper industry sludge. Bioresour. Technol. 2013, 147, 71–76. [Google Scholar] [CrossRef]
- Briongos, J.V.; Taramona, S.; Gómez-Hernández, J.; Mulone, V.; Santana, D. Solar and biomass hybridization through hydrothermal carbonization. Renew. Energy 2021, 177, 268–279. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Holdich, R.G.; Martin, S.J.; Shama, G.; Wheatley, A.D. Process energetics for the hydrothermal carbonisation of human faecal wastes. Energy Convers. Manag. 2015, 105, 1115–1124. [Google Scholar] [CrossRef]
- Zaccariello, L.; Battaglia, D.; Morrone, B.; Mastellone, M.L. Hydrothermal Carbonization: A Pilot-Scale Reactor Design for Bio-waste and Sludge Pre-treatment. Waste Biomass Valorization 2022, 13, 3865–3876. [Google Scholar] [CrossRef]
- Heidari, M.; Norouzi, O.; MacDermid-Watts, K.; Acharya, B.; Zhang, Y.; Dutta, A. Product evaluation of hydrothermal carbonization of biomass: Semi-continuous vs. batch feeding. Biomass Convers. Biorefinery 2020, 12, 15–25. [Google Scholar] [CrossRef]
- Güleç, F.; Riesco, L.M.G.; Williams, O.; Kostas, E.T.; Samson, A.; Lester, E. Hydrothermal conversion of different lignocellulosic biomass feedstocks—Effect of the process conditions on hydrochar structures. Fuel 2021, 302, 121166. [Google Scholar] [CrossRef]
- Sliz, M.; Tuci, F.; Czerwinska, K.; Fabrizi, S.; Lombardi, L.; Wilk, M. Hydrothermal carbonization of the wet fraction from mixed municipal solid waste: Hydrochar characteristics and energy balance. Waste Manag. 2022, 151, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Namioka, T.; Yoshikawa, K. Effects of hydrothermal treatment on characteristics and combustion behaviors of municipal solid wastes. Appl. Energy 2011, 88, 3659–3664. [Google Scholar] [CrossRef]
- Masoumi, S.; Borugadda, V.B.; Nanda, S.; Dalai, A.K. Hydrochar: A Review on Its Production Technologies and Applications. Catalysts 2021, 11, 939. [Google Scholar] [CrossRef]
- Liang, W.; Nanou, P.; Wray, H.; Zhang, J.; Lundstrom, I.; Lundqvist, S.; Wang, C. Feasibility Study of Bio-Sludge Hydrochar as Blast Furnace Injectant. Sustainability 2022, 14, 5510. [Google Scholar] [CrossRef]
- Gerner, G.; Meyer, L.; Wanner, R.; Keller, T.; Krebs, R. Sewage Sludge Treatment by Hydrothermal Carbonization: Feasibility Study for Sustainable Nutrient Recovery and Fuel Production. Energies 2021, 14, 2697. [Google Scholar] [CrossRef]
- Lucian, M.; Volpe, M.; Merzari, F.; Wust, D.; Kruse, A.; Andreottola, G.; Fiori, L. Hydrothermal carbonization coupled with anaerobic digestion for the valorization of the organic fraction of municipal solid waste. Bioresour. Technol. 2020, 314, 123734. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).