Insight into the Acidity and Catalytic Performance on Butane Isomerization of Thermal Stable Sulfated Monoclinic Zirconia
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Samples
2.2. Characterization of the Catalysts
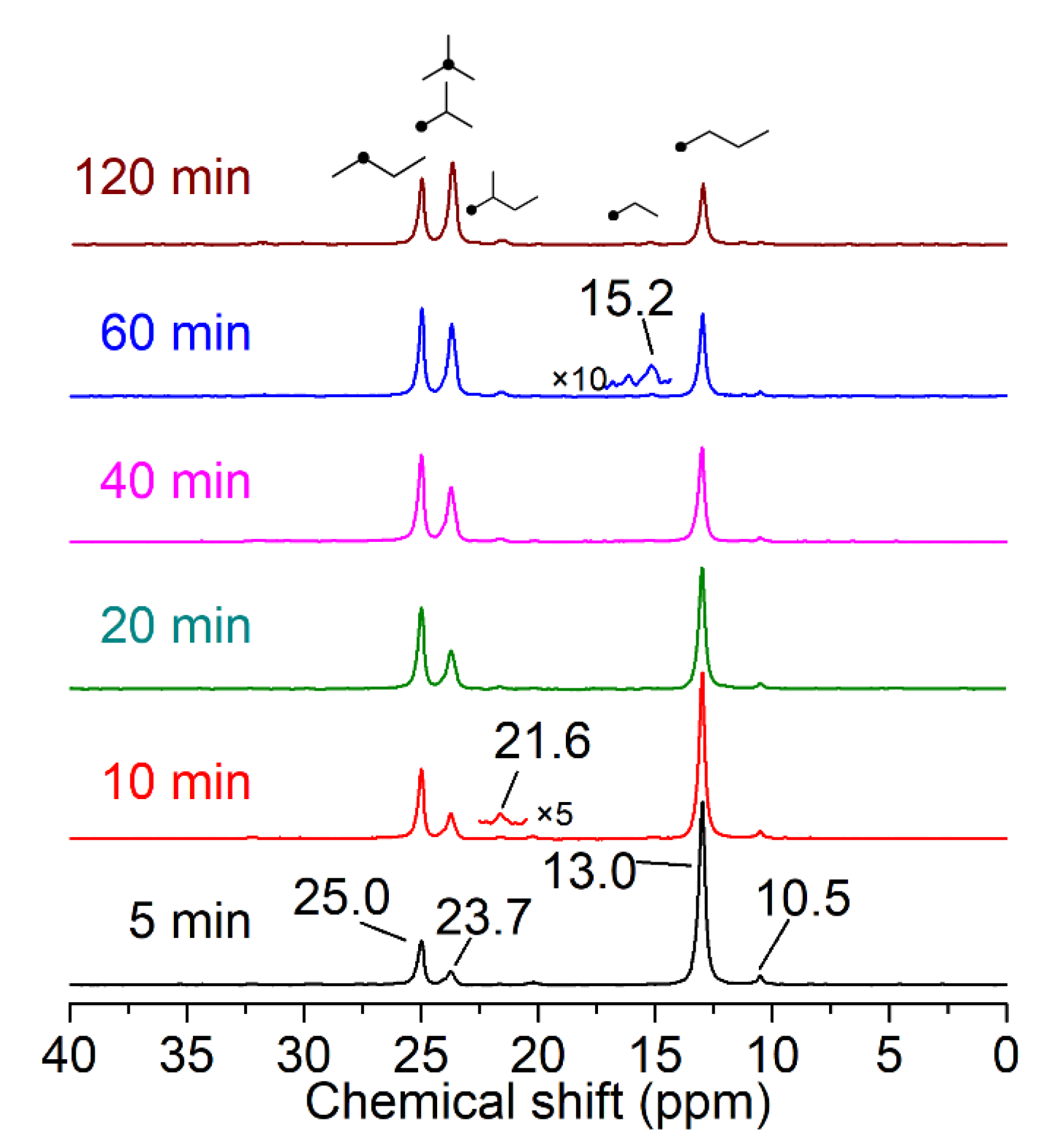
2.3. Solid-State NMR Study on Isomerization of 1-13C-n-Butane
3. Results and Discussion
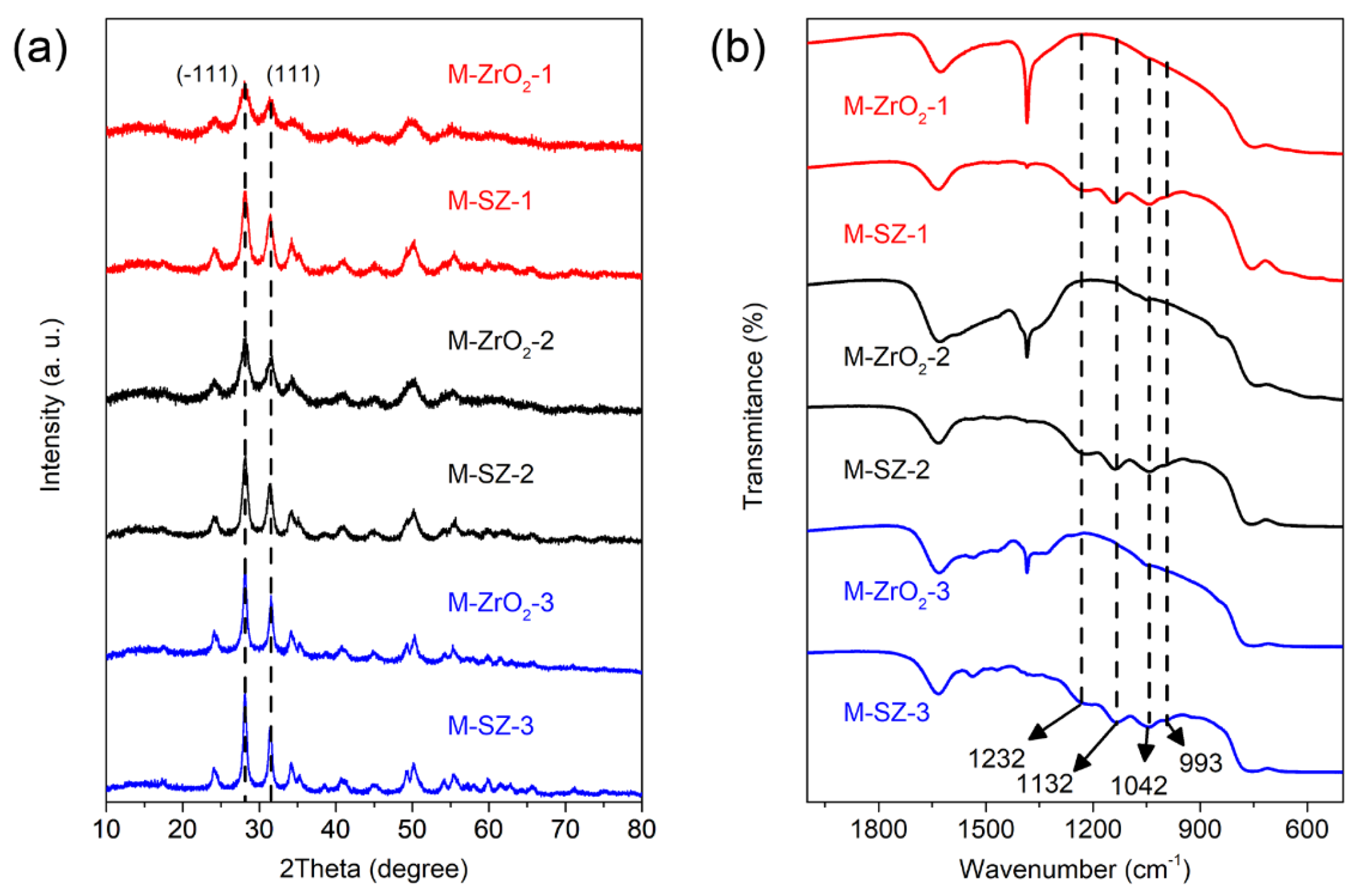
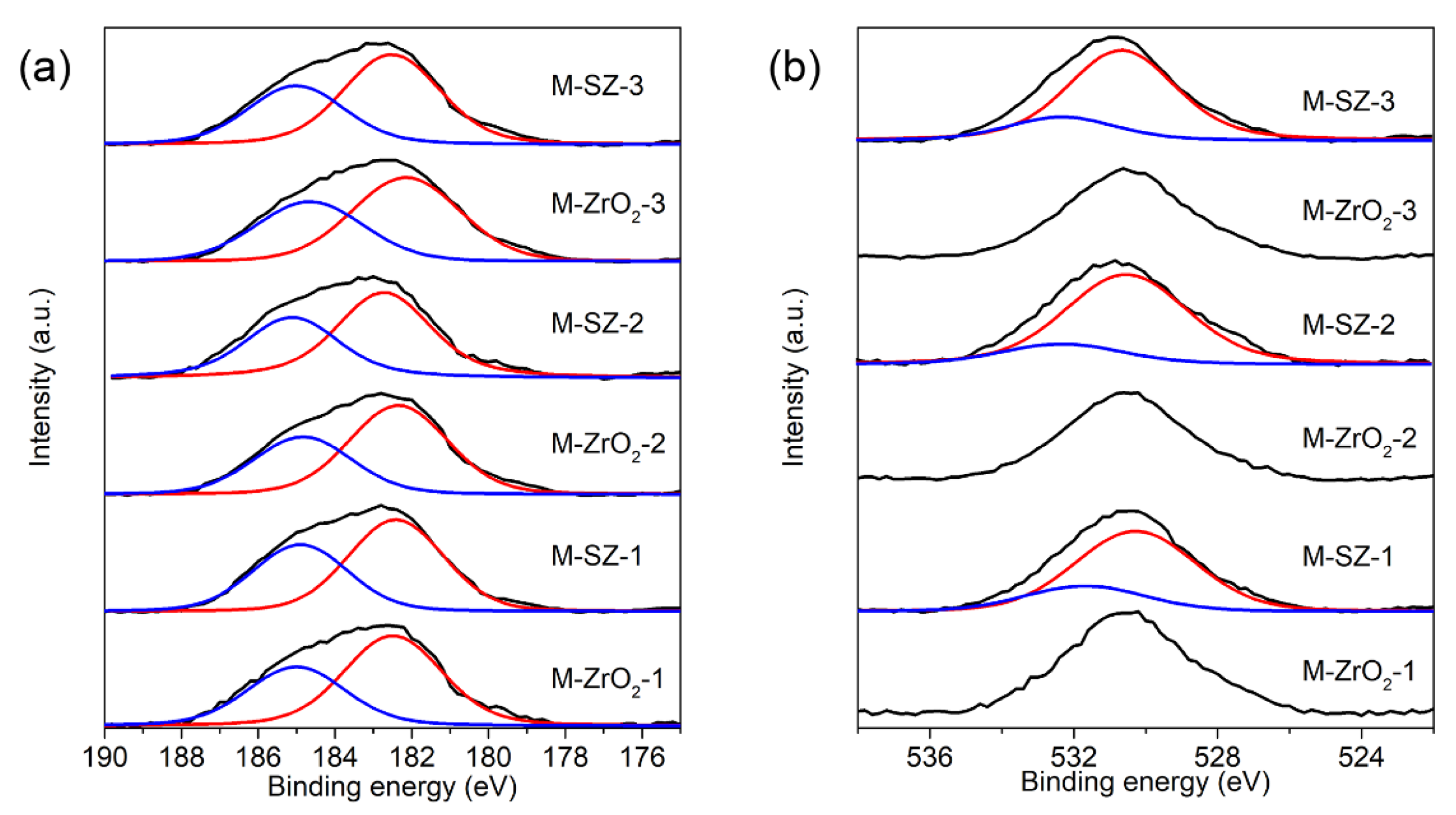
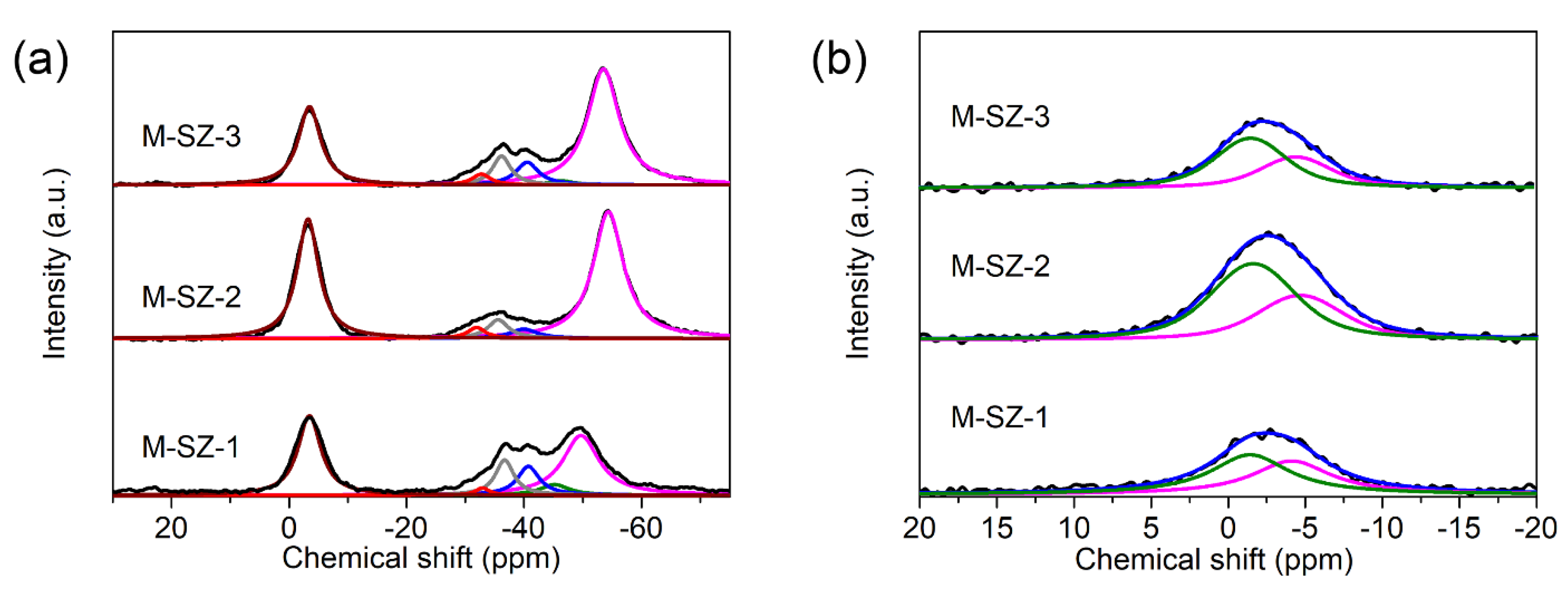
3.1. Characterization
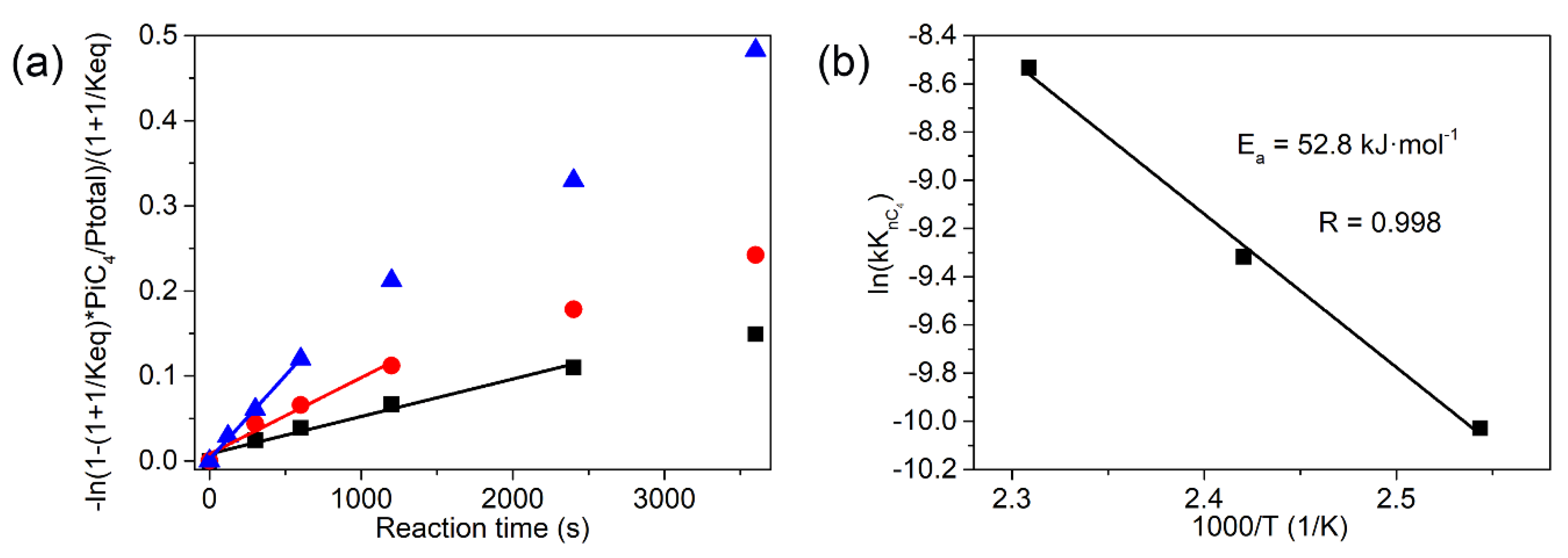
3.2. The Isomerization of Butane Catalyzed by M-SZ Catalysts
3.3. The Stability of M-SZ and T-SZ Catalysts Operated at High Temperature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, P.Z.; Yue, Y.Y.; Wang, T.H.; Bao, X.J. Alkane isomerization over sulfated zirconia solid acid system. Int. J. Energy Res. 2020, 44, 3270–3294. [Google Scholar] [CrossRef]
- Potter, M.E.; Le Brocq, J.J.M.; Oakley, A.E.; McShane, E.B.; Vandegehuchte, B.D.; Raja, R. Butane isomerization as a diagnostic tool in the rational design of solid acid catalysts. Catalysts 2020, 10, 1099. [Google Scholar] [CrossRef]
- Echevskii, G.V.; Aksenov, D.G.; Kodenev, E.G.; Ovchinnikova, E.V.; Chumachenko, V.A. Activity of a sulfated zirconia catalyst in isomerization of n-butane fractions. Pet. Chem. 2019, 59, S101–S107. [Google Scholar] [CrossRef]
- Villegas, J.I.; Kumar, N.; Heikkila, T.; Smieskova, A.; Hudec, P.; Salmi, A.; Murzin, D.Y. A highly stable and selective Pt-modified mordenite catalyst for the skeletal isomerization of n-butane. Appl. Catal. A Gen. 2005, 284, 223–230. [Google Scholar] [CrossRef]
- Yadav, G.D.; Nair, J.J. Sulfated zirconia and its modified versions as promising catalysts for industrial processes. Microporous Mesoporous Mater. 1999, 33, 1–48. [Google Scholar] [CrossRef]
- Song, X.M.; Sayari, A. Sulfated zirconia-based strong solid-acid catalysts: Recent progress. Catal. Rev. Sci. Eng. 1996, 38, 329–412. [Google Scholar] [CrossRef]
- Adeeva, V.; Liu, H.Y.; Xu, B.Q.; Sachtler, W.M.H. Alkane isomerization over sulfated zirconia and other solid acids. Top. Catal. 1998, 6, 61–76. [Google Scholar] [CrossRef]
- Yamaguchi, T. Alkane isomerization and acidity assessment on sulfated ZrO2. Appl. Catal. A Gen. 2001, 222, 237–246. [Google Scholar] [CrossRef]
- Morterra, C.; Cerrato, G.; Pinna, F.; Signoretto, M. Crystal phase, spectral features, and catalytic acitivity of sulfate-doped zirconia systems. J. Catal. 1995, 157, 109–123. [Google Scholar] [CrossRef]
- Comelli, R.A.; Vera, C.R.; Parera, J.M. Influence of ZrO2 crystalline-structure and sulfate ion concentration on the catalytic activity of SO42−-ZrO2. J. Catal. 1995, 151, 96–101. [Google Scholar] [CrossRef]
- Stichert, W.; Schuth, F. Synthesis of catalytically active high surface area monoclinic sulfated zirconia. J. Catal. 1998, 174, 242–245. [Google Scholar] [CrossRef]
- Stichert, W.; Schüth, F.; Kuba, S.; Knözinger, H. Monoclinic and tetragonal high surface area sulfated zirconias in butane isomerization: CO adsorption and catalytic results. J. Catal. 2001, 198, 277–285. [Google Scholar] [CrossRef]
- Zhang, X.G.; Rabee, A.I.M.; Isaacs, M.; Lee, A.F.; Wilson, K. Sulfated zirconia catalysts for D-sorbitol cascade cyclodehydration to isosorbide: Impact of zirconia phase. ACS Sustain. Chem. Eng. 2018, 6, 14704–14712. [Google Scholar] [CrossRef]
- Shukla, S.; Seal, S. Mechanisms of room temperature metastable tetragonal phase stabilisation in zirconia. Int. Mater. Rev. 2005, 50, 45–64. [Google Scholar] [CrossRef]
- Morales, M.D.; Infantes-Molina, A.; Lázaro-Martínez, J.M.; Romanelli, G.P.; Pizzio, L.R.; Rodríguez-Castellón, E. Heterogeneous acid catalysts prepared by immobilization of H3PW12O40 on silica through impregnation and inclusion, applied to the synthesis of 3H-1,5-benzodiazepines. Mol. Catal. 2020, 485, 110842. [Google Scholar] [CrossRef]
- Yu, H.G.; Fang, H.J.; Zhang, H.L.; Li, B.J.; Deng, F. Acidity of sulfated tin oxide and sulfated zirconia: A view from solid-state NMR spectroscopy. Catal. Commun. 2009, 10, 920–924. [Google Scholar] [CrossRef]
- Zheng, A.M.; Liu, S.B.; Deng, F. 31P NMR chemical shifts of phosphorus probes as reliable and practical acidity scales for solid and liquid catalysts. Chem. Rev. 2017, 117, 12475–12531. [Google Scholar] [CrossRef]
- Lunsford, J.H.; Sang, H.; Campbell, S.M.; Liang, C.H.; Anthony, R.G. An NMR study of acid sites on sulfated-zirconia catalysts using trimethylphosphine as a probe. Catal. Lett. 1994, 27, 305–314. [Google Scholar] [CrossRef]
- Barich, D.H.; Nicholas, J.B.; Xu, T.; Haw, J.F. Theoretical and experimental study of the 13C chemical shift tensors of acetone complexed with Brønsted and Lewis acids. J. Am. Chem. Soc. 1998, 120, 12342–12350. [Google Scholar] [CrossRef]
- Hunger, M. Multinuclear solid-state NMR studies of acidic and non-acidic hydroxyl protons in zeolites. Solid State Nucl. Magn. Reson. 1996, 6, 1–29. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Yang, C.; Wei, W.; Li, W.H.; Sun, Y.H. Catalytic performance of copper supported on zirconia polymorphs for CO hydrogenation. J. Mol. Catal. A Chem. 2005, 231, 75–81. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, B.; Ren, Y.H.; Chen, X.Y.; He, H.Y. An aluminum promoted cesium salt of 12-tungstophosphoric acid: A catalyst for butane isomerization. Catal. Sci. Technol. 2013, 3, 2113–2118. [Google Scholar] [CrossRef]
- Chuah, G.K.; Jaenicke, S.; Cheong, S.A.; Chan, K.S. The influence of preparation conditions on the surface area of zirconia. Appl. Catal. A Gen. 1996, 145, 267–284. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Ardizzone, S.; Cappelletti, G. Surface state of sulfated zirconia: The role of the sol-gel reaction parameters. Surf. Interface Anal. 2004, 36, 745–748. [Google Scholar] [CrossRef]
- Bosman, H.J.M.; Pijpers, A.P.; Jaspers, A. An X-ray photoelectron spectroscopy study of the acidity of SiO2-ZrO2 mixed oxides. J. Catal. 1996, 161, 551–559. [Google Scholar] [CrossRef]
- Fu, Y.Y.; Zhang, L.; Yue, B.; Chen, X.Y.; He, H.Y. Simultaneous characterization of solid acidity and basicity of metal oxide catalysts via the solid-state NMR technique. J. Phys. Chem. C 2018, 122, 24094–24102. [Google Scholar] [CrossRef]
- Chu, Y.Y.; Yu, Z.W.; Zheng, A.M.; Fang, H.J.; Zhang, H.L.; Huang, S.J.; Liu, S.B.; Deng, F. Acidic strengths of Brønsted and Lewis acid sites in solid acids scaled by 31P NMR chemical shifts of adsorbed trimethylphosphine. J. Phys. Chem. C 2011, 115, 7660–7667. [Google Scholar] [CrossRef]
- Zhao, B.Y.; Pan, H.J.; Lunsford, J.H. Characterization of [(CH3)3P-H]+ complexes in normal H-Y, dealuminated H-Y, and H-ZSM-5 zeolites using 31P solid-state NMR spectroscopy. Langmuir 1999, 15, 2761–2765. [Google Scholar] [CrossRef]
- Lunsford, J.H. Characterization of acidity in zeolites and related oxides using trimethylphosphine as a probe. Top. Catal. 1997, 4, 91–98. [Google Scholar] [CrossRef]
- Wang, S.; Fang, Y.; Huang, Z.; Xu, H.L.; Shen, W. The effects of the crystalline phase of zirconia on C-O activation and C-C coupling in converting syngas into aromatics. Catalysts 2020, 10, 262. [Google Scholar] [CrossRef]
- Zhang, H.L.; Yu, H.G.; Zheng, A.M.; Li, S.H.; Shen, W.L.; Deng, F. Reactivity enhancement of 2-propanol photocatalysis on SO42-/TiO2: Insights from solid-state NMR spectroscopy. Environ. Sci. Technol. 2008, 42, 5316–5321. [Google Scholar] [CrossRef]
- Chen, B.; Munson, E.J. Investigation of the mechanism of n-butane oxidation on vanadium phosphorus oxide catalysts: Evidence from isotopic labeling studies. J. Am. Chem. Soc. 2002, 124, 1638–1652. [Google Scholar] [CrossRef]
- Zarkalis, A.S.; Hsu, C.Y.; Gates, B.C. Solid superacid catalysis: Kinetics of butane isomerization catalyzed by a sulfated oxide containing iron, manganese, and zirconium. Catal. Lett. 1994, 29, 235–239. [Google Scholar] [CrossRef]
- Ma, Z.N.; Zou, Y.; Hua, W.M.; He, H.Y.; Gao, Z. In situ 13C MAS NMR study on the mechanism of butane isomerization over catalysts with different acid strength. Top. Catal. 2005, 35, 141–153. [Google Scholar] [CrossRef]
- Ma, Z.N.; Hua, W.M.; Ren, Y.; He, H.Y.; Gao, Z. n-Butane isomerization over Cs-salts of H3PW12O40: A mechanistic study by 13C MAS NMR. Appl. Catal. A Gen. 2003, 256, 243–250. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Heimbuch, C.R.; Armes, C.T.; Gates, B.C. A highly active solid superacid catalyst for n-butane isomerization: A sulfated oxide containing iron manganese and zirconium. J. Chem. Soc. Chem. Commun. 1992, 22, 1645–1646. [Google Scholar] [CrossRef]
- Na, K.; Okuhara, T.; Misono, M. Skeletal isomerization of n-butane over caesium hydrogen salts of 12-tungstophosphoric acid. J. Chem. Soc. Faraday Trans. 1995, 91, 367–373. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Yuan, L.N.; Ma, S.Q.; Han, Y.; Zhao, L.; Wang, W.; Chen, C.L.; Xiao, F.S. Improved catalytic activity and stability of mesostructured sulfated zirconia by Al promoter. Appl. Catal. A Gen. 2004, 268, 17–24. [Google Scholar] [CrossRef]
- Wang, J.H.; Mou, C.Y. Alumina-promoted mesoporous sulfated zirconia: A catalyst for n-butane isomerization. Appl. Catal. A Gen. 2005, 286, 128–136. [Google Scholar] [CrossRef]
- Gao, Z.; Xia, Y.D.; Hua, W.M.; Miao, C.X. New catalyst of SO42-/Al2O3-ZrO2 for n-butane isomerization. Top. Catal. 1998, 6, 101–106. [Google Scholar] [CrossRef]

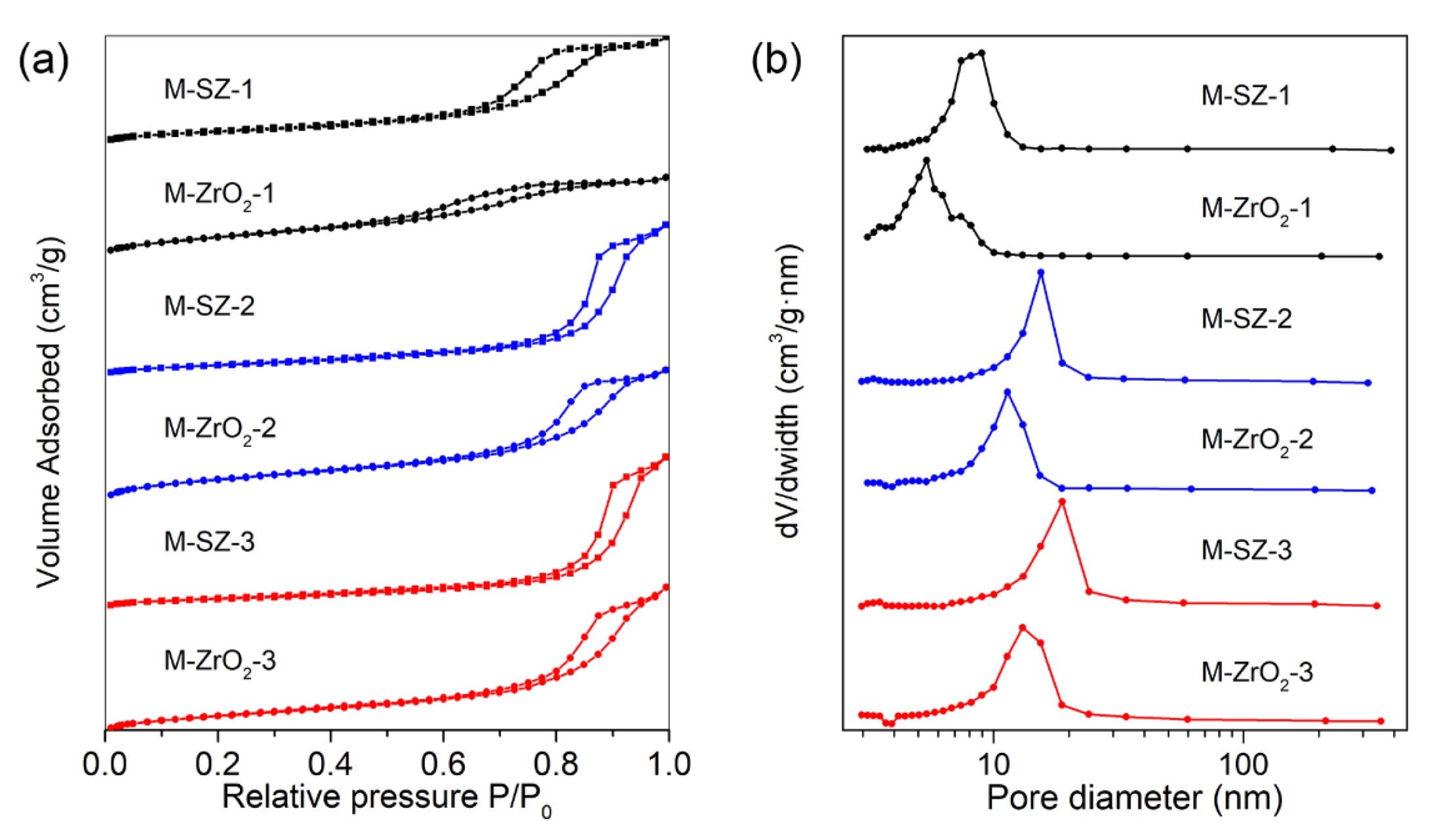

| Samples | BET Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| M-ZrO2-1 | 119 | 0.129 | 5.4 |
| M-SZ-1 | 76 | 0.195 | 7.4 |
| M-ZrO2-2 | 169 | 0.213 | 11.4 |
| M-SZ-2 | 67 | 0.265 | 15.5 |
| M-ZrO2-3 | 136 | 0.246 | 13.1 |
| M-SZ-3 | 57 | 0.263 | 18.8 |
| Sample | Acid Type | Chemical Shift (ppm) | Acid Amount (μmol·g−1) | JP-H (Hz) |
|---|---|---|---|---|
| M-SZ-1 | BA | −3.4 | 68 | 437 |
| LA1, LA2, LA3, LA4, LA5 | −32.9, −36.7, −40.8, −45.2, −49.7 | 4, 27, 26, 12, 89 | - | |
| M-SZ-2 | BA | −3.2 | 112 | 494 |
| LA1, LA2, LA3, LA4, LA5 | −31.8, −35.6, −40.5, -, −54.2 | 9, 19, 12, -, 187 | - | |
| M-SZ-3 | BA | −3.3 | 69 | 474 |
| LA1, LA2, LA3, LA4, LA5 | −32.7, −36.2, −40.6, −46.2, −53.5 | 9, 22, 22, 4, 157 | - |
| Catalysts | X60 (%) | 2-13C-n-Butane (%) | 13C-Isobutane (%) |
|---|---|---|---|
| M-SZ-1 | 0 | 0 | 0 |
| M-SZ-2 | 69.2 | 28.7 | 29.5 |
| M-SZ-3 | 35.0 | 21.4 | 10.1 |
| Reaction Temperature (K) | kKnC4 × 105 (mol·g−1·s−1) | R |
|---|---|---|
| 393 | 4.41 | 0.990 |
| 413 | 8.98 | 0.986 |
| 433 | 19.66 | 0.998 |
| Catalysts | Reaction Temperature (K) | Uncalcined | Calcined at 673 K for 240 h | ||
|---|---|---|---|---|---|
| X60 (%) | Isobutane (%) | X60 (%) | Isobutane (%) | ||
| M-SZ-2 | 433 | 69.2 | 29.5 | 63.8 | 25.7 |
| T-SZ-general | 338 | 61.1 | 32.7 | 50.2 | 22.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, D.; Feng, W.; Zhang, L.; Yue, B.; He, H. Insight into the Acidity and Catalytic Performance on Butane Isomerization of Thermal Stable Sulfated Monoclinic Zirconia. Processes 2022, 10, 2693. https://doi.org/10.3390/pr10122693
Huang D, Feng W, Zhang L, Yue B, He H. Insight into the Acidity and Catalytic Performance on Butane Isomerization of Thermal Stable Sulfated Monoclinic Zirconia. Processes. 2022; 10(12):2693. https://doi.org/10.3390/pr10122693
Chicago/Turabian StyleHuang, Daofeng, Wenhua Feng, Li Zhang, Bin Yue, and Heyong He. 2022. "Insight into the Acidity and Catalytic Performance on Butane Isomerization of Thermal Stable Sulfated Monoclinic Zirconia" Processes 10, no. 12: 2693. https://doi.org/10.3390/pr10122693
APA StyleHuang, D., Feng, W., Zhang, L., Yue, B., & He, H. (2022). Insight into the Acidity and Catalytic Performance on Butane Isomerization of Thermal Stable Sulfated Monoclinic Zirconia. Processes, 10(12), 2693. https://doi.org/10.3390/pr10122693







