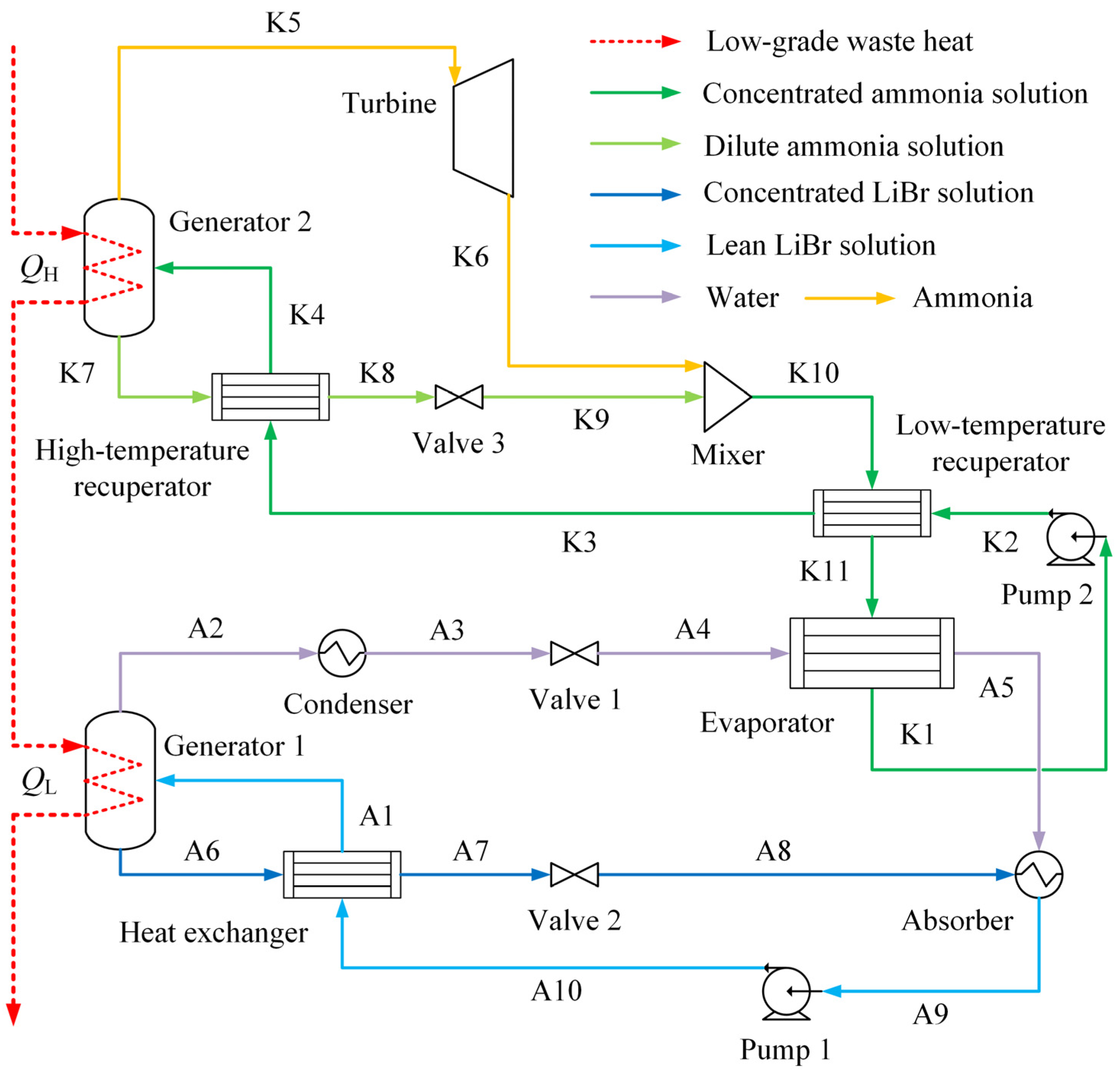
4.1. Exergy Analysis Results
Irreversibility exists in all practical applications and processes due to heat transfer with finite temperature difference, chemical reactions and substances mixing. Exergy analysis helps to find the reason for system inefficiency and quantifies the irreversibility of the whole system.
Figure 4 depicts the distribution of exergy input, exergy destruction and electrical power output of this waste heat recovery process. Considering the electricity consumption is only 3.16 kW for maintaining the continuous system operation, the total exergy inflow is calculated as 147.42 kW. It can be found that more than half of the exergy input enters the Kalina cycle to achieve power production. Only 42.06% of the total is recovered by the absorption chiller to realize cooling output. Fluid compression in pumps only accounts for around 2% of the total exergy input. As shown in the distribution of exergy destruction and electrical power output, the total exergy destruction of the integrated system is calculated as 93.02 kW, occupied more than 60% of the total input. This indicates that a larger proportion of the exergy input is lost during the system operation and the electrical power output is obtained as 54.40 kW. Hence, the exergy efficiency of the integrated system is obtained as 35.52%. The Kalina cycle has more exergy destruction than that of the absorption refrigeration cycle. It can be explained as higher temperature of the working medium and larger temperature difference during the heat-transfer process in the Kalina cycle.
Detailed exergy destruction of equipment is shown in
Figure 5. In the LiBr/H
2O absorption refrigeration cycle, the largest exergy destruction is in the absorber and the value is 16.44 kW (accounting for 38.01% of the total destruction in this cycle). Irreversible heat transfer process and working medium mixing result in considerable exergy destruction in the absorber. In total, 32.83%, 22.13% and 6.31% of the total destruction are occupied by Generator 1, the Condenser and the Heat exchanger, respectively. Valve 1, Pump 1 and Valve 2 only hold less than 1% of the total destruction in the refrigeration cycle. In the Kalina cycle, the distribution is also concentrated, but the total amount is larger. Generator 2 occupies a very large proportion (accounting for 42.62% of the total destruction). The second to the fourth largest destruction are in the gas turbine, HTR and Evaporator, accounting for 19.79%, 18.49% and 15.95% of the total, respectively. Exergy destruction at the higher temperature side of the evaporator is found to be larger than that at the lower temperature side. This is because the heat transfer temperature difference is 10 °C at the higher-temperature side, greater than that at the lower-temperature side. In addition, the mass flow rate of the ammonia solution at the higher-temperature side is 0.56 kg/s while the flow rate of refrigerant at the lower temperature side is merely 0.11 kg/s. Only around 3% of the total destruction of the Kalina cycle occurs in the Valve 3, Mixer, LTR and Pump 2. Heat transfer in HTR and LTR are both sensible heat transfer processes, but the heat duty in HTR is larger. In addition, logarithmic mean temperature difference (LMTD) of the heat transfer process in HTR is calculated as approximately 16 °C, while LMTD in LTR is obtained as only 7 °C. For these reasons, exergy destruction in HTR is significantly larger than that in LTR. Thus, it can be concluded that the exergy destruction is basically associated with irreversible heat transfer processes in heat exchangers; hence, reducing the irreversibility of heat transfer processes will be the research focus in future works.
A Sankey diagram, or a Sankey energy distribution diagram, is regarded as an intuitive visual analysis method. It uses the width of branches to present the size of data flow. This diagram can vividly describe the specific exergy-flow balance of the integrated system in this work.
Figure 6 is the Sankey diagram of the integrated system, presenting the detailed distribution of exergy input, exergy destruction and electricity production. The lower part of this diagram represents the exergy inflow and the upper part indicates where the exergy is going. This figure shows that the low-grade waste heat can be efficiently converted into high-grade electrical power in the integrated system by consuming a small amount of electricity. Exergy input and destruction in the Kalina cycle are both larger, and considerable exergy destruction can be found in the generators, absorber, turbine, high-temperature recuperator, condenser and evaporator.
4.2. Advanced Exergy Analysis Results
Physical properties, including mass fraction, temperature, pressure, vapor fraction, mass flow rate, mass enthalpy, as well as mass entropy of both the lithium bromide/water solution and ammonia/water solution, are obtained in the process simulation. Detailed simulation results of each stream in the real, unavoidable and ideal cycles are given in
Supplementary Tables S1–S3. Mass concentrations of these two basic solutions are kept the same (55.5% for the LiBr/H
2O solution and 70% for the ammonia solution). As the integrated system operates in the real, unavoidable and ideal conditions, working fluids have totally different mass concentrations. In the absorption refrigeration cycle, the strong solution has the highest mass fraction of 69% when the system operates in the ideal condition. This value is obviously larger than that of the real cycle (62%). This indicates that relatively more refrigerant vapor (H
2O) is released from the basic solution in the generator. The mass fraction difference among different concentrated streams in the Kalina cycle is very obvious as well. More ammonia vapor escapes from the liquid solution in the ideal cycle, absorbing more heat in the generator. In the ideal condition, the condenser temperature of the absorption chiller (stream A3) is around 30 °C, lower than the corresponding value of 35 °C in the real condition. This allows the absorption chiller to operate at a lower high pressure. Similarly, the smaller minimum temperature difference of the evaporator in the ideal cycle results in much lower turbine exhaust outlet pressure (backpressure). Thus, the basic solution of the Kalina cycle reaches much lower low pressure (241 kPa lower than 293 kPa), increasing the turbine expansion ratio and electrical power generation. Mass vapor fractions are also influenced by different operation conditions. By comparing the mass flow rates among these three conditions, it can be noticed that for both the absorption refrigeration cycle and the power generation cycle, improving the component efficiency reduces the flow rates of the working fluids. The flow rate determines the size of a certain process which means that this integrated system will have a much compact structure when operating in the ideal condition. Mass enthalpy and mass entropy are obtained based on the status of each stream at different reference state. Thus, there are positive and negative values for these two working solutions. Then, these obtained results are used for exergy calculation of each component.
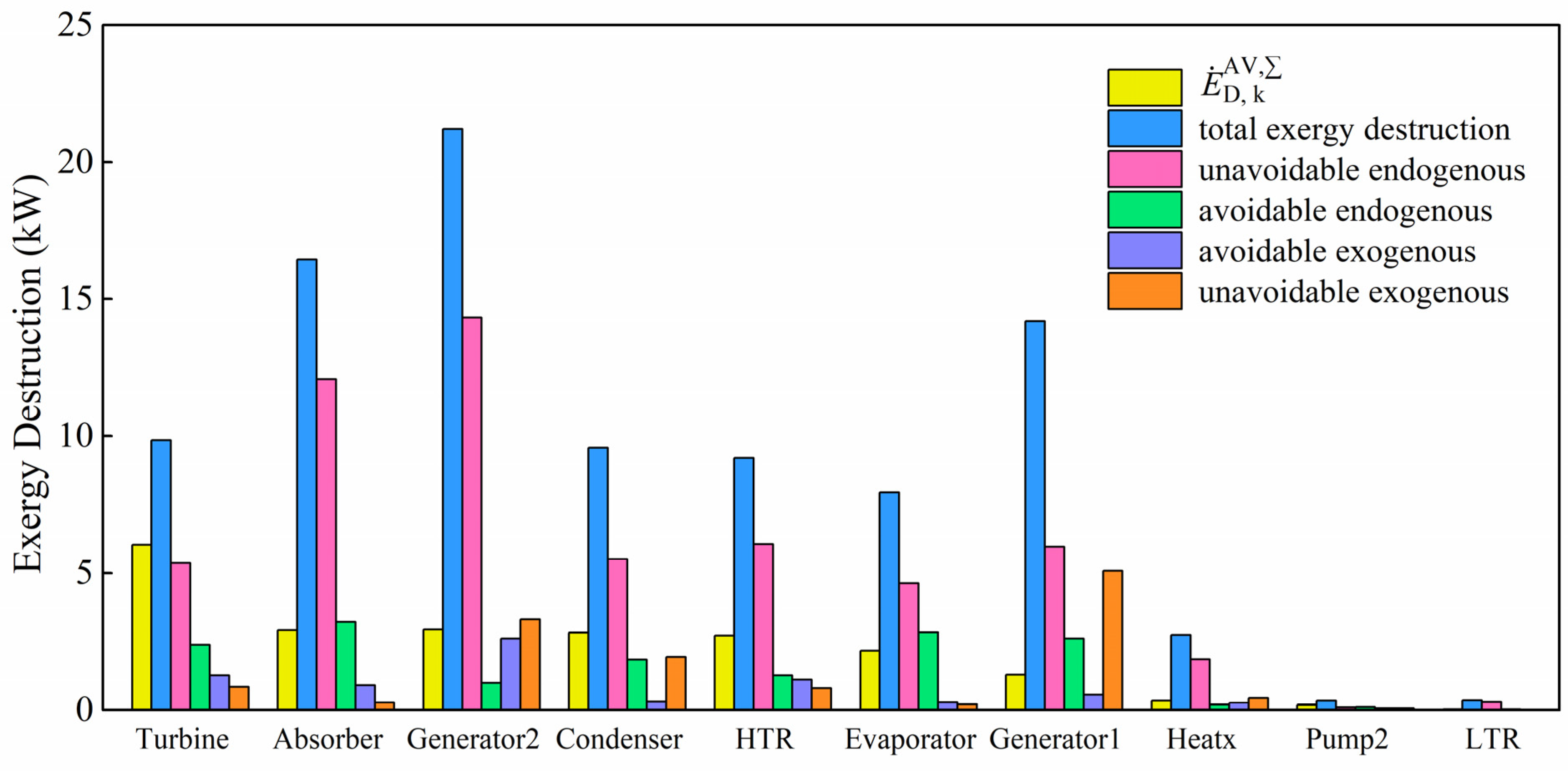
On the basis of exergy calculation for each component, detailed exergy destruction distribution has been obtained. The total exergy destruction, endogenous/exogenous exergy destruction, avoidable/unavoidable exergy destruction and avoidable endogenous/avoidable exogenous/unavoidable endogenous/unavoidable exogenous exergy destruction for each considered component are provided in
Table 4. It should be mentioned that the electrical power consumption of the LiBr/H
2O pump (Pump 1) is less than 0.01 kW due to small pressure increase. Hence, the exergy destruction distribution of this pump is not given as well. Among all the considered components, Generator 2, Absorber and Generator 1 have the highest total exergy destruction, followed by Turbine, Condenser, High-temperature recuperator, Evaporator, Heat exchanger, etc. Significant temperature mismatching between two different working streams could be the major reason for large exergy destruction in these heat exchangers [
22]. Phase change and fluid mixing might also cause considerable exergy destruction in components such as generators, absorber and turbine, etc. Low-temperature recuperator and Pump 2 have the least total exergy destruction. Endogenous exergy destruction is the part that relies on the inefficiency of the considered component and the exogeneous part relies on the system structure and the inefficiency of the rest of the components. From the endogenous/exogenous exergy destruction results, the endogenous part is much more significant than the other one. Exergy destruction of all the considered components is almost endogenous. Generator 1 and Generator 2 have obviously higher exogenous exergy destruction, which indicates that these two generators are greatly influenced by the rest of the components. In the absorption refrigeration cycle, the largest amount of avoidable exergy destruction is in Absorber and Generator 1. Among all the components in the Kalina cycle, Generator 2 and Turbine have the most avoidable exergy destruction. Although the Condenser and High-temperature recuperator have nearly the same amount of total exergy destruction with Turbine, the avoidable exergy destruction of these two components is relatively lower. The unavoidable exergy destruction refers to the exergy destruction that is inevitable because of technological and manufacturing limitations. For instance, the limitations could be poor equipment design, failure to conform to design standards, unsatisfactory flow dynamics or gas–liquid interaction, inert gases in equipment, fouling problem and sizes limitation of equipment such as heat transfer area of heat exchangers, volume of storage tanks, etc. In present research, the unavoidable part is found to be larger than the avoidable part. It can be concluded that a large proportion of the destruction cannot be avoided by any means. By combining the two concepts, the total exergy destruction is further divided into four parts for a better understanding of the inefficiency of this integrated system. Those with higher avoidable endogenous destruction are worth exergy improvement investigations [
41]. This means that more attention should be paid to the Absorber, Evaporator, Generator 1 and Turbine. The avoidable endogenous part of exergy destruction can be directly reduced by improving the considered component itself. The highest avoidable exogenous exergy destruction can be found in Generator 2, the Turbine and the High-temperature recuperator. To avoid this part in the considered component, structure optimization of the whole process or performance improvement on the rest components is recommended. Endogenous/exogenous distribution of the unavoidable exergy destruction provides the information about this inevitable part due to limitations of the considered component or limitations of the rest of the components and overall system design.
Detailed exergy destruction distribution of each considered component in the absorption chiller and the Kalina cycle is illustrated in
Table 5. This table clearly presents the percentage of each part of the exergy destruction. The Evaporator, Absorber and Low-temperature recuperator have the highest proportion of endogenous exergy destruction and less than 10% of the exergy destruction is caused by extrinsic causes. Pump 2, Evaporator and Turbine are the components with more than 1/3 of the exergy destruction that can be avoided. The highest proportion of the avoidable endogenous part is in Evaporator, Pump 2 and Turbine. For the avoidable exogenous part, Pump 2, Turbine, Generator 2 and the High-temperature recuperator have the highest percentage of this part that is caused by external factors. The unavoidable endogenous part always occupies the highest proportion of the total amount, except for Pump 2.
To investigate how one component affects other components, detailed splitting on the exogenous exergy destruction has been achieved for each component, as given in
Supplementary Table S4. The avoidable part of the exogenous exergy destruction has also been obtained. The splitting results for each considered component show that the interconnections among different components are not crucially important but rather complicated. It is the introduction of inefficiency in the rth component that changes the flow rates and thermodynamic properties of the working medium in the kth component. The values of exogenous exergy destruction caused by other components can be positive or negative [
51]. The positive values indicate that introducing the irreversibility of the rth component into the process makes the exergetic performance of the kth component worse. The negative values stand for the opposite situation. If the exogenous exergy destruction corresponding to the rth component equals zero, that means this considered component is not influenced by the rth component at all. The expression ‘<0.01’ indicates a weak interaction between two components and that interaction can be neglected. It can be noticed that components in the Kalina cycle are basically not affected by those components completely in the absorption chiller (i.e., Generator 1, Condenser, Absorber and Heat exchanger). This can be explained as the absorption chiller recovers the thermal energy from the Kalina cycle, and hence it is the bottoming cycle of this power generation cycle. The working status of the components in the topping cycle determines the working status of the follow-up components, but not vice versa. The turbine seems to have considerable effect on the rest of the components. LTR causes negligible changes to other components. It is not obviously influenced by others either. Heat exchanger and Pump 2 also have no obvious influence on the rest of the components. The mexogenous exergy destruction is then calculated using the exogenous exergy destruction of the kth component and the sum of all the (n − 1) parts caused by inefficiency of the rth components. This mexogenous part is noteworthy in components such as Generator 1, Generator 2 and Turbine. The avoidable exogenous part is also very important because it indicates the achievable exogenous exergy-saving potential. The avoidable exogenous parts of Generator 1, Evaporator, Absorber and HTR are highly related to the generator of the Kalina cycle.
To evaluate the improvement priority for all the considered components, the avoidable exogenous part of other components that is caused by the considered component is summed up. This value is then combined with the avoidable endogenous part of this component as the major criterion of improvement priority in the whole system. All these exergy calculation results are shown in
Table 6. Absorber, Evaporator and Generator 1 have the highest avoidable endogenous exergy destruction among all the considered components, while the summed values of these three components are negative. Turbine and Generator 2 have the highest and the second highest summed values of avoidable exogenous exergy destruction. As a result, the turbine possesses the highest
value of more than 6 kW, which far surpasses those values of all the other components. Thus, the first priority of improvement should be given to the turbine, and then Generator 2, Absorber, Condenser, High-temperature recuperator, Evaporator, etc. In contrast, Heat exchanger, Pump 2 and the Low-temperature recuperator hold the lowest
values of all. This indicates that the improvement on these components seems to be less important.
Figure 7 depicts the improvement priority and detailed exergy destruction. The total destruction and the avoidable endogenous part of the Turbine are both not highly ranked. However, it has a significant impact on the remaining components. Thus, efficiency improvement of the Turbine should be the primary consideration. Generator 2, Absorber, Condenser and the High-temperature recuperator have nearly the same level of improvement priority. The Absorber has the highest avoidable endogenous part, which can be avoided by improving the component itself. Generator 2 possesses a considerable amount of avoidable exogenous destruction that can be saved through efficiency improvement of the rest of the components. Hence, improvement of these two components could be placed at the second and the third position, following the turbine. Although Generator 1 has the third-largest exergy destruction among all the considered components, this generator does not have a strong effect on the overall exergy efficiency of this integrated system. However, improvement of Generator 1 is very necessary because it still has a considerable amount of avoidable exergy destruction. Compared with other considered components, the exergy-saving potential in the Heat exchanger is very poor and the total exergy destruction in Pump 2 and Low-temperature recuperator are absolutely insignificant.
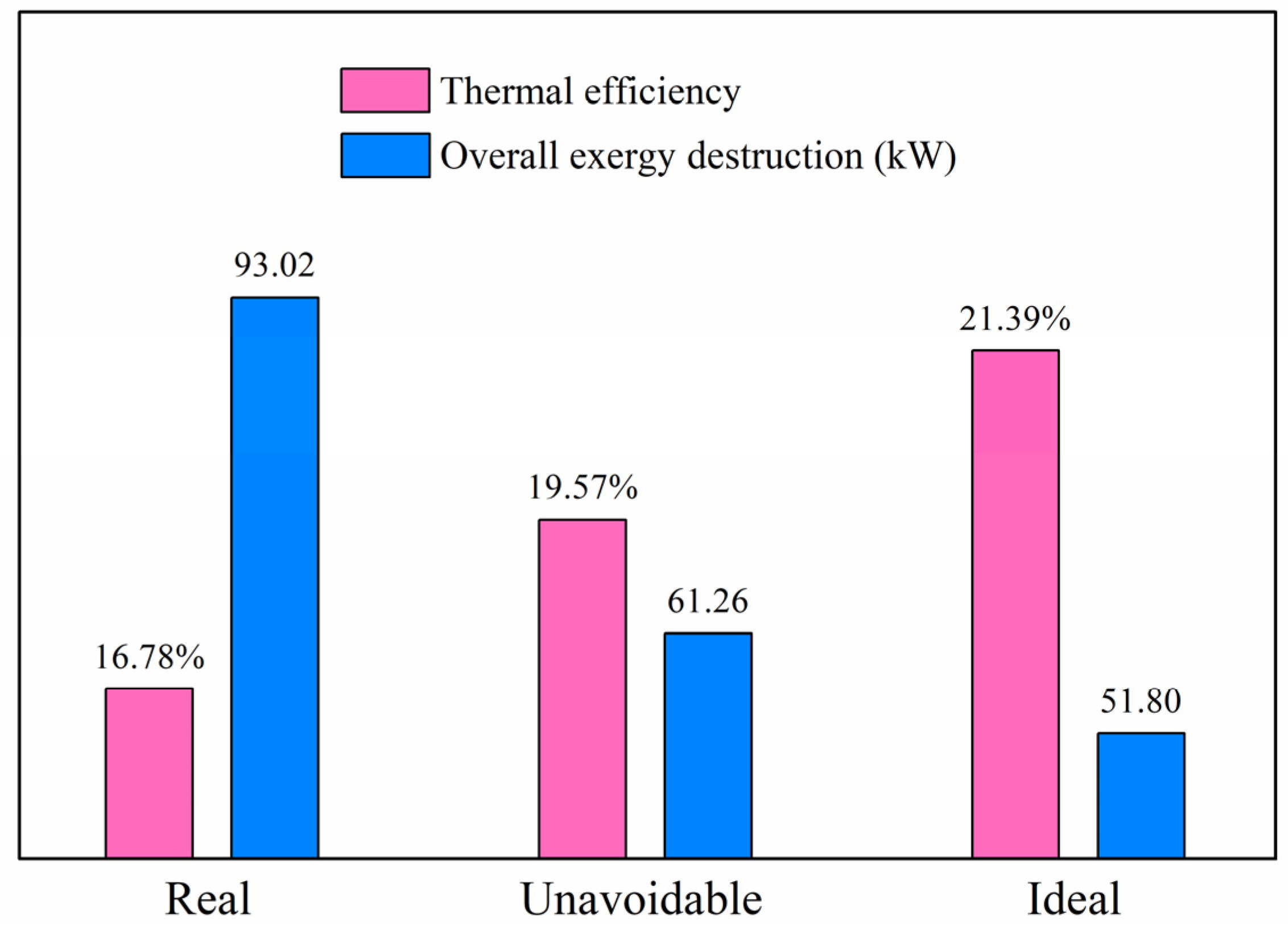
There are several important criteria for evaluating all the considered components and the whole system, including thermal efficiency and exergy destruction of the overall system (as shown in
Figure 8), exergy efficiency, exergy destruction ratio, modified exergy efficiency, modified exergy destruction ratio and
EIP (as given in
Table 7). In the real condition, the thermal efficiency of this integrated system is 16.78%, with overall exergy destruction of 93.02 kW. The corresponding values are 19.57% and 61.26 kW for the unavoidable condition, and 21.39% and 51.80 kW for the ideal condition. When the integrated system operates in the improved conditions of the unavoidable and ideal cycles, the waste heat is recovered and utilized with higher efficiency. Additionally, the overall exergy destruction dramatically decreases.
By comparing the exergy efficiencies with the modified exergy efficiencies in
Table 7, it can be found that the modified efficiencies of both individual components and the overall system have higher values. The modified values in the advanced exergy analysis are more reasonable because these values are calculated by removing the unavoidable and avoidable exogenous exergy destruction. These modified values refer to the exergetic performance with the utmost improvement on components or overall system. The modified relative exergy destruction ratio
of the component is found to be much lower than the relative exergy destruction ratio. The modified ratio indicates the relative avoidable endogenous exergy destruction of the considered component. For instance, Generator 2 has the highest value of relative exergy destruction ratio, but a relatively low modified relative exergy destruction ratio. This is because a huge amount of destruction of this generator is unavoidable and exogenous.
EIP of the advanced analysis represents the unavoidable and avoidable exogenous exergy-destruction ratio of the considered component. For example, the Condenser has a low modified exergy efficiency but a high
EIP value. This means that although the exergy destruction in the condenser is considerable, this destruction cannot be easily reduced because most of it is unavoidable or exogenous. Absorber and Generator 1 also have relatively high
EIP values. In general, these modified values provide more useful information about the exergetic performance and improvement potential of both individual component and the overall system.