Study on Three-Stage Counter-Current Water Washing Desalination Characteristics and Mechanism of High Chlorine Waste Incineration Fly Ash
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
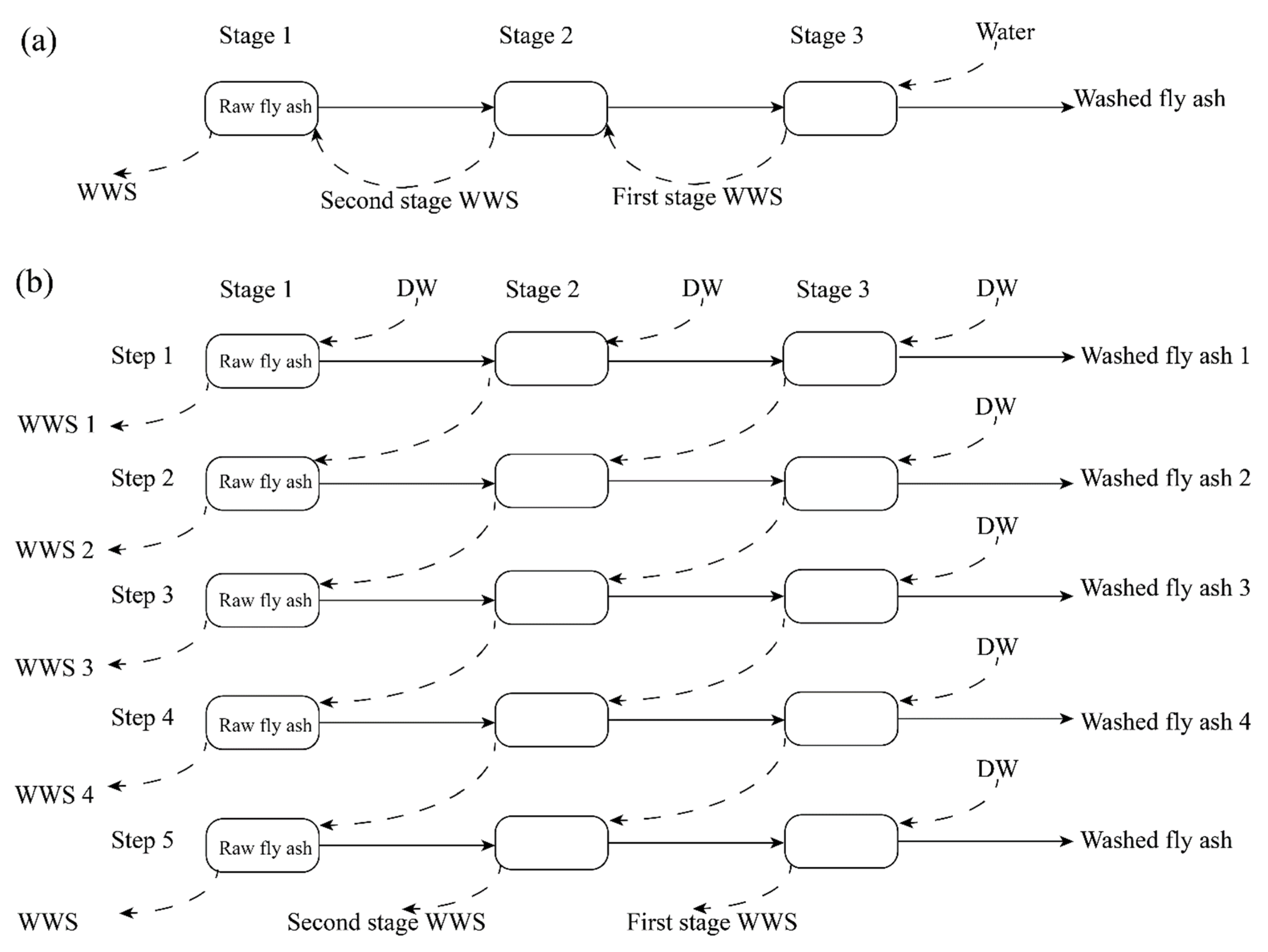
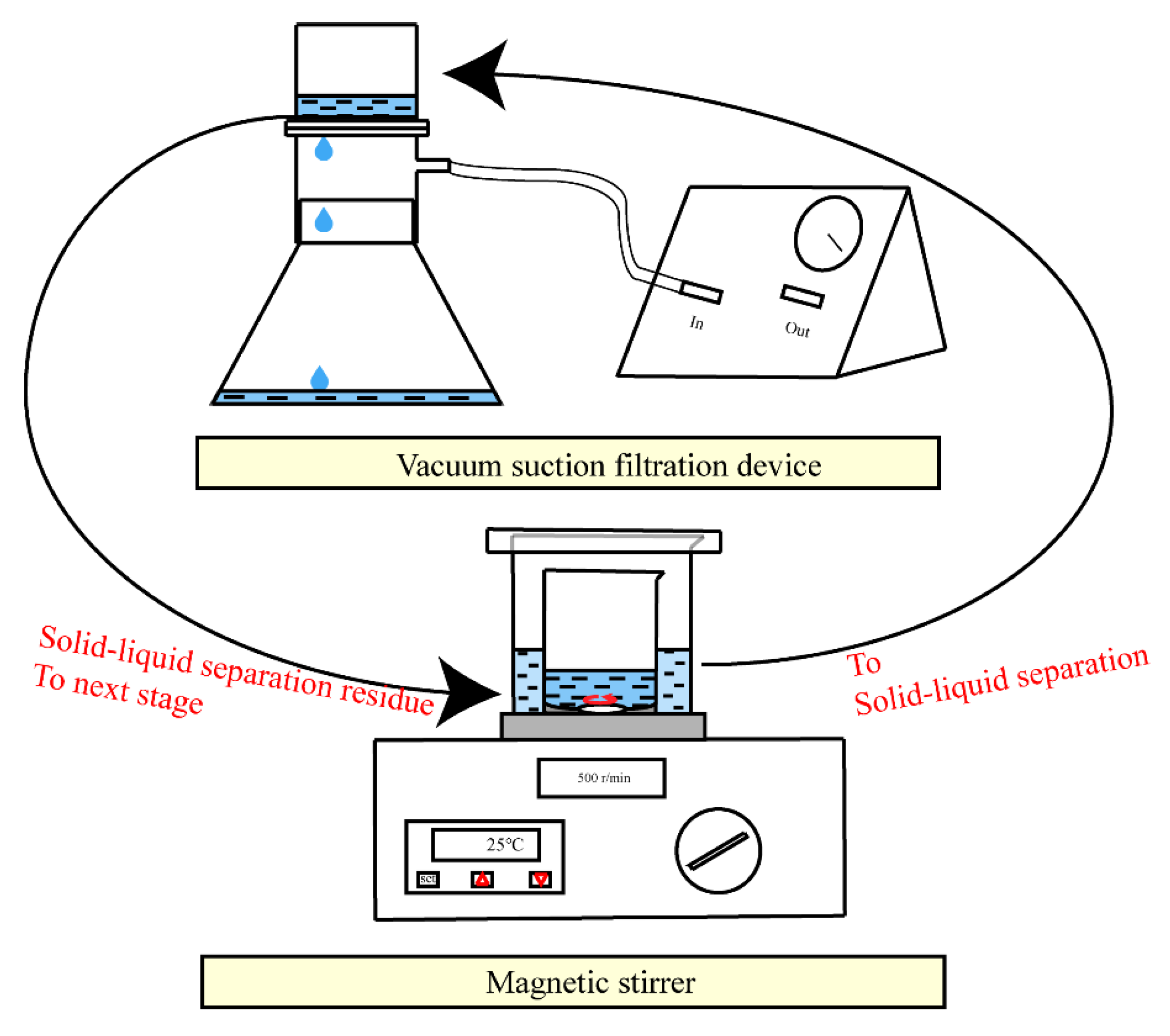
2.2. Experimental Procedures
2.3. Analytical Methods
3. Results and Discussion
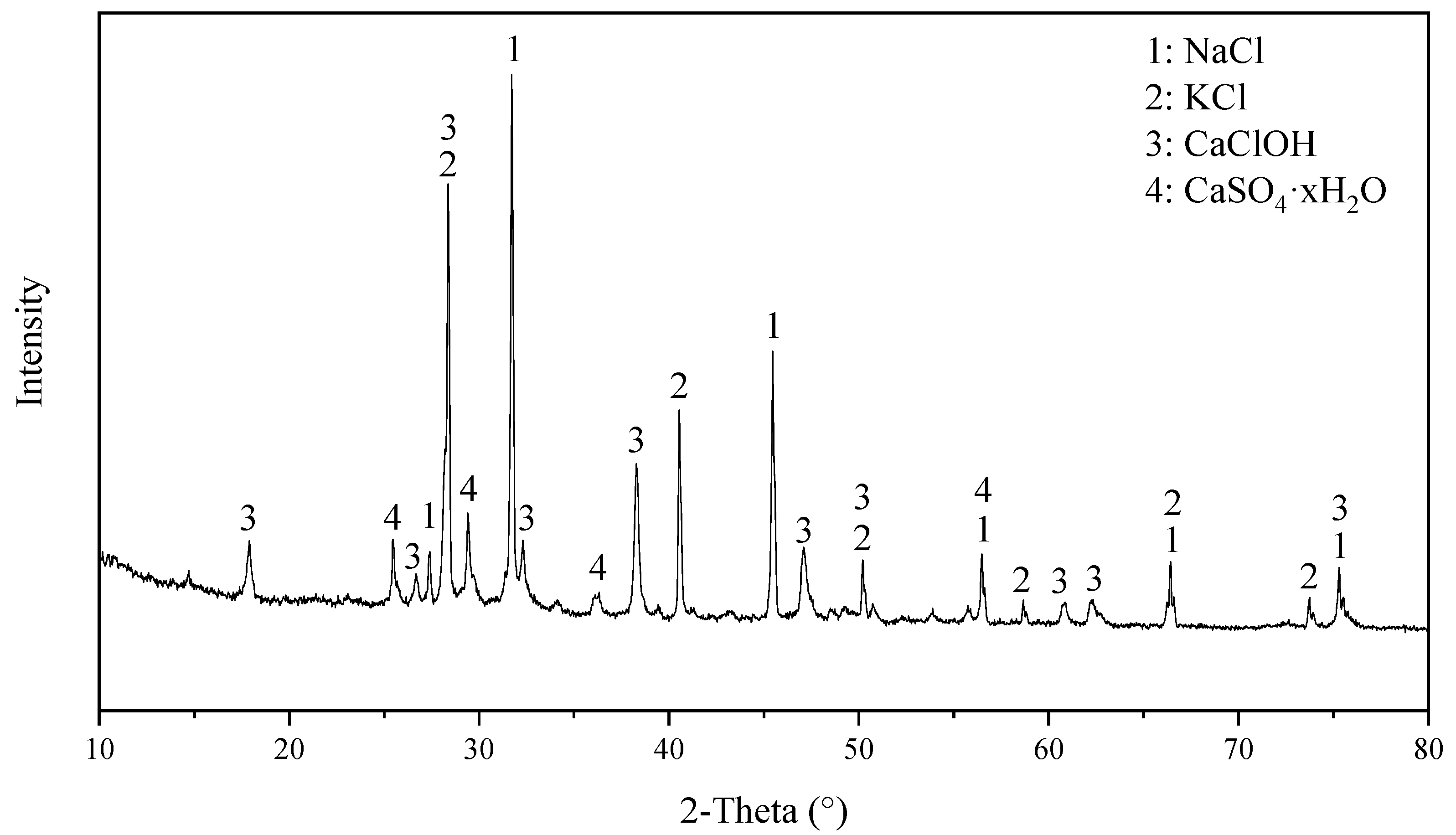
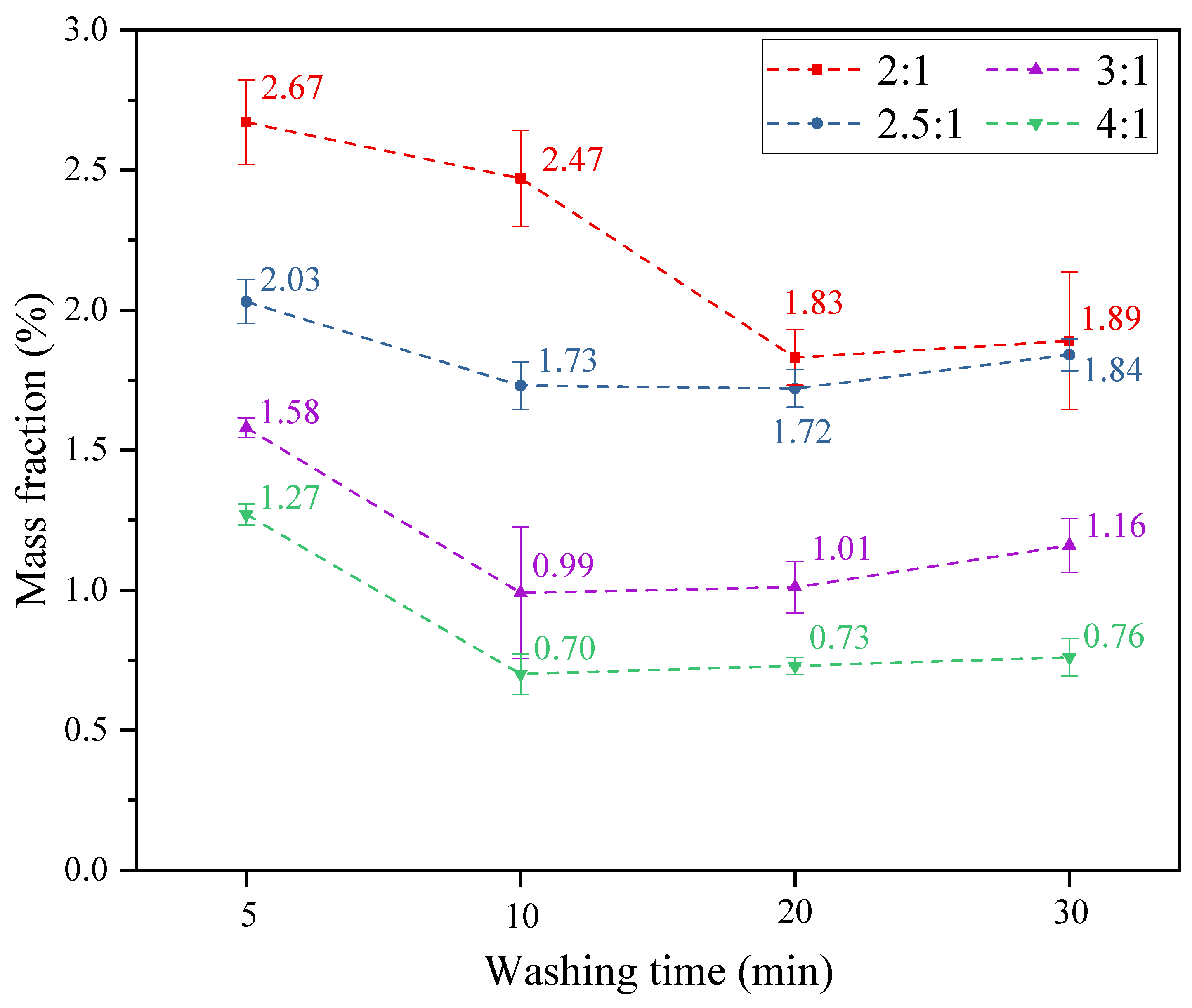
3.1. Main Components in WWS and the Influence of Different Washing Conditions
3.2. Chloride Transfer Model
3.3. Elemental Composition of Fly Ash before and after Water-Washing
4. Conclusions
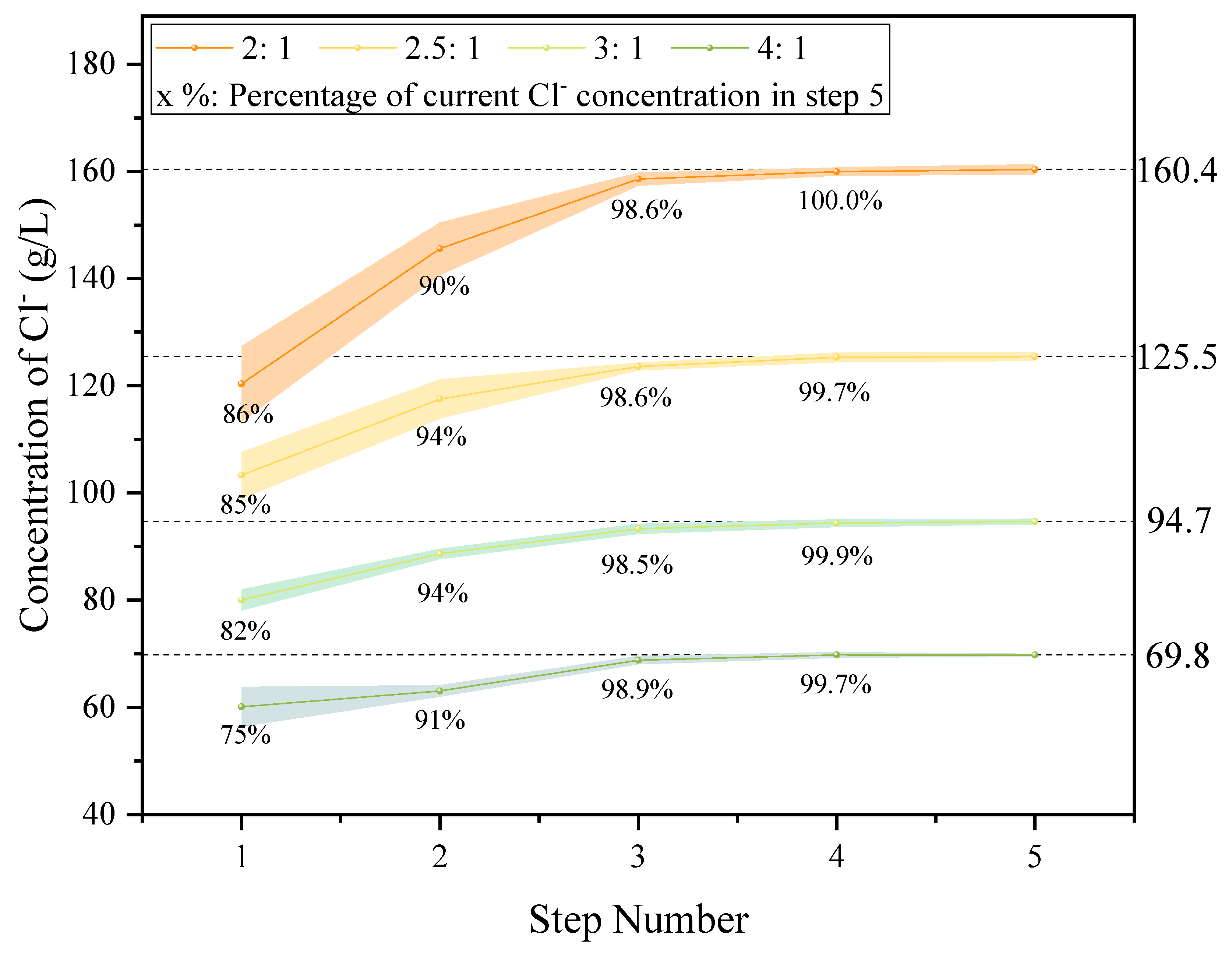
- TSC water washing could achieve a dechlorination effect better than 99% for high-chlorine fly ash with a chlorine content of 30% in a low LSR and reduce the soluble chlorine to less than 1% in washed fly ash. Its dechlorination effect was 76% better than that of single-stage water washing and 21% better than that of two-stage counter-current water washing. The best washing effect could be achieved when LSR = 3:1 and t = 15 min.
- The salt content of the WWS was at the top level in high-salinity wastewater. When LSR = 3 kg/L, the concentration of Cl− in WWS reached 94 g/L, and the concentration of K+, Ca2+, and Na+ also reached 22–33 g/L. The concentration of lead in the WWS was the highest among heavy metals, followed by zinc, copper, and arsenic.
- The chloride-transfer characteristic in the TSC water-washing process is strongly related to the liquid contained in the SR. By introducing the salt content coefficient of the SR, the concentration of WWS at all stages could be calculated through a model. The model revealed that reducing the moisture content of the SR after suction filtration could improve the dechlorination effect. This model can guide the design of the fly ash washing process, but still needs further optimization.
- The mass fraction of chlorine in the fly ash reached around 30%. The calcium content in fly ash mainly depends on the calcium oxide or calcium hydroxide injected in air pollution control process, reaching more than 20%. Potassium and sodium in fly ash mainly come from industrial waste, kitchen waste, or biomass, and the potassium content determines the value of the crystalline salt of the fly ash washing solution. After washing, the pore size and specific surface area of fly ashes increased and the adsorption capacity was promoted.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mian, M.; Zeng, X.; Bin, N.; Al-Hamadani, S. Municipal solid waste management in China: A comparative analysis. J. Mater. Cycles. Waste Manag. 2017, 19, 1127–1135. [Google Scholar] [CrossRef]
- Lin, X.; Chen, J.; Xu, S.; Mao, T.; Liu, W.; Wu, J.; Li, X.; Yan, J. Solidification of heavy metals and PCDD/Fs from municipal solid waste incineration fly ash by the polymerization of calcium carbonate oligomers. Chemosphere 2022, 288, 132420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Chen, L.; Ma, B.; Zhang, Y.; Ni, W.; Tsang, D. Treatment of municipal solid waste incineration fly ash: State-of-the-art technologies and future perspectives. J. Hazard. Mater. 2021, 411, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, Z.; Fang, Z.; Qian, Y.; Zhong, P.; Yan, J. Review of harmless treatment of municipal solid waste incineration fly ash. WDSE 2020, 2, 25. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. Annual Report of China’s Ecological Environment Statistics. Available online: https://www.mee.gov.cn/hjzl/sthjzk/sthjtjnb/202202/W020220218339925977248.pdf (accessed on 20 November 2022). (In Chinese)
- Chen, J.; Lin, X.; Li, M.; Mao, T.; Li, X.; Yan, J. Heavy metal solidification and CO2 sequestration from MSWI fly ash by calcium carbonate oligomer regulation. J. Clean. Prod. 2022, 359, 132044. [Google Scholar] [CrossRef]
- Wang, P.; Hu, P.; Cheng, H. Municipal solid waste (MSW) incineration fly ash as an important source of heavy metal pollution in China. J. Environ. Pollut. 2019, 252, 461–475. [Google Scholar] [CrossRef]
- Zheng, L.; Song, J.; Li, C.; Gao, Y.; Geng, P.; Qu, B.; Lin, L. Preferential policies promote municipal solid waste (MSW) to energy in China: Current status and prospects. Renew. Sustain. Energy Rev. 2014, 36, 135–148. [Google Scholar] [CrossRef]
- Xu, H.; Miao, J.; Chen, P.; Zhang, L.; Wang, Y. Chemical and geotechnical properties of solidified/stabilized MSWI fly ash disposed at a landfill in China. Eng. Geol. 2019, 255, 59–68. [Google Scholar] [CrossRef]
- Fan, C.; Wang, B.; Ai, H.; Qi, Y.; Liu, Z. A comparative study on solidification/stabilization characteristics of coal fly ash-based geopolymer and Portland cement on heavy metals in MSWI fly ash. J. Clean. Prod. 2021, 319, 128790. [Google Scholar] [CrossRef]
- Long, L.; Jiang, X.; Lv, G.; Chen, Q.; Liu, X.; Chi, Y.; Yan, J.; Zhao, X.; Kong, L. Characteristics of fly ash from waste-to-energy plants adopting grate-type or circulating fluidized bed incinerators: A comparative study. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–18. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Guo, G.; Gong, L.; Zhang, L.; Qie, Y.; Hu, H.; Yao, H. Ash formation and the inherent heavy metal partitioning behavior in a 100 t/d hazardous waste incineration plant. Sci. Total Environ. 2022, 814, 151938. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chang, F.; Shen, Y.; Tsai, M.; Ko, C. Removal of chloride from MSWI fly ash. J. Hazard. Mater. 2012, 237–238, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Mao, T.; Chen, Z.; Zhang, S.; Li, X.; Yan, J. Thermal cotreatment of municipal solid waste incineration fly ash with sewage sludge: Phases transformation, kinetics and fusion characteristics, and heavy metals solidification. J. Clean. Prod. 2021, 317, 128429. [Google Scholar] [CrossRef]
- Dontriros, S.; Likitlersuang, S.; Janjaroen, D. Mechanisms of chloride and sulfate removal from municipal-solid-waste-incineration fly ash (MSWI FA): Effect of acid-base solutions. Waste Manag. 2020, 101, 44–53. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, J.; Ning, N.; Yang, Z. Chemical stabilization of heavy metals in municipal solid waste incineration fly ash: A review. Environ. Sci. Pollut. Res. 2022, 29, 40384–40402. [Google Scholar] [CrossRef]
- Zou, D.; Wang, X.; Wu, C.; Li, T.; Wang, M.; Liu, S.; Wang, Q.; Shimaoka, T. Dechlorination of Municipal Solid Waste Incineration Fly Ash by Leaching with Fermentation Liquid of Food Waste. Sustainability 2020, 12, 4389. [Google Scholar] [CrossRef]
- Zhao, K.; Hu, Y.; Tian, Y.; Chen, D.; Feng, Y. Chlorine removal from MSWI fly ash by thermal treatment: Effects of iron/aluminum additives. J. Environ. Sci. 2020, 88, 112–121. [Google Scholar] [CrossRef]
- Nguyen, T.; Tsai, C.; Horng, J. Sustainable Recovery of Valuable Nanoporous Materials from High-Chlorine MSWI Fly Ash by Ultrasound with Organic Acids. Molecules 2022, 27, 2289. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, R.; Luo, Z.; Wang, R.; Yang, F.; Wang, Z.; Shu, J.; Chen, M. Heavy Metals Removing from Municipal Solid Waste Incineration Fly Ashes by Electric Field-Enhanced Washing. Materials 2020, 13, 793. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, Y.; Wang, W.; Wang, X.; Li, J.; Jin, Y.; Pang, D. A new co-processing mode of organic anaerobic fermentation liquid and municipal solid waste incineration fly ash. Waste Manag. 2022, 151, 70–80. [Google Scholar] [CrossRef]
- Kang, D.; Son, J.; Yoo, Y.; Park, S.; Huh, I.; Park, J. Heavy-metal reduction and solidification in municipal solid waste incineration (MSWI) fly ash using water, NaOH, KOH, and NH4OH in combination with CO2 uptake procedure. Chem. Eng. J. 2020, 380, 122534. [Google Scholar] [CrossRef]
- Tian, X.; Rao, F.; Leon-Patino, C.; Song, S. Co-disposal of MSWI fly ash and spent caustic through alkaline-activation: Immobilization of heavy metals and organics. Cem. Concr. Compos. 2020, 114, 103824. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, X.; Zhang, S.; Zou, X.; Li, X.; Lu, S.; Yan, J. Thermal cotreatment of municipal solid waste incineration fly ash with sewage sludge for PCDD/Fs decomposition and reformation suppression. J. Hazard. Mater. 2021, 416, 126216. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Hu, H.; Xu, S.; Chen, T.; Huang, Y.; Yang, Y.; Yang, F.; Yao, H. Fate of heavy metals during molten salts thermal treatment of municipal solid waste incineration fly ashes. Waste Manag. 2020, 103, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hu, H.; Guo, G.; Gong, L.; Liu, H.; Yao, H. Investigation of properties change in the reacted molten salts after molten chlorides cyclic thermal treatment of toxic MSWI fly ash. J. Hazard. Mater. 2022, 421, 126536. [Google Scholar] [CrossRef]
- Mangialardi, T.; Paolini, A.; Polettini, A.; Sirini, P. Optimization of the solidification/stabilization process of MSW fly ash in cementitious matrices. J. Hazard. Mater. 1999, 70, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ji, R.; Liu, L.; Wang, X.; Zhang, Z. Recycling of municipal solid waste incineration by-product for cement composites preparation. Constr. Build. Mater. 2018, 162, 794–801. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, S.; Tang, M.; Ding, J.; Buekens, A.; Yang, J.; Qiu, Q.; Yan, J. Mechanical activation of fly ash from MSWI for utilization in cementitious materials. Waste Manag. 2019, 88, 182–190. [Google Scholar] [CrossRef]
- Mangialardi, T. Disposal of MSWI fly ash through a combined washing-immobilisation process. J. Hazard. Mater. 2003, 98, 225–240. [Google Scholar] [CrossRef]
- Xie, Q.; Wang, D.; Fu, D.; Tao, H.; Liu, S. Recovery of soluble chlorides from municipal solid waste incineration fly ash using evaporative crystallisation and flotation methods. Sep. Sci. Technol. 2022, 57, 2276–2286. [Google Scholar] [CrossRef]
- Yan, M.; Jiang, J.; Zheng, R.; Yu, C.; Zhou, Z.; Hantoko, D. Experimental study on the washing characteristics of fly ash from municipal solid waste incineration. Waste Manag. Res. 2022, 40, 1212–1219. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, S.; Yao, R.; Chen, S.; Gao, J.; Shimaoka, T. Removal of harmful components from MSWI fly ash as a pretreatment approach to enhance waste recycling. Waste Manag. 2022, 150, 110–121. [Google Scholar] [CrossRef]
- Mao, Y.; Wu, H.; Wang, W.; Jia, M.; Che, X. Pretreatment of municipal solid waste incineration fly ash and preparation of solid waste source sulphoaluminate cementitious material. J. Hazard. Mater. 2020, 385, 121580. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, C.; Zhang, L. Critical Review on the Chemical Reaction Pathways Underpinning the Primary Decomposition Behavior of Chlorine-Bearing Compounds under Simulated Municipal Solid Waste Incineration Conditions. Energy Fuels 2020, 34, 1–15. [Google Scholar] [CrossRef]
- Wu, B.; Wang, D.; Chai, X.; Takahashi, F.; Shimaoka, T. Characterization of chlorine and heavy metals for the potential recycling of bottom ash from municipal solid waste incinerators as cement additives. Front. Environ. Sci. Eng. 2016, 10, 8. [Google Scholar] [CrossRef]
- Ying, W.; Yan, L.; Huining, D.; Wei, Z.; Zhang, S. Optimization for dechlorination washing of fly ash from municipal solid waste incineration by response surface method. Environ. Pollut. Control 2020, 42, 5. (In Chinese) [Google Scholar]
- Yan, D.; Peng, Z.; Yu, L.; Sun, Y.; Yong, R.; Karstensen, K. Characterization of heavy metals and PCDD/Fs from water-washing pretreatment and a cement kiln co-processing municipal solid waste incinerator fly ash. Waste Manag. 2018, 78, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, R.; Li, Y.; Wei, L. Release of soluble salts and heavy metals during the short-time washing process of MSWI fly ash. Adv. Mater. Res. 2012, 518, 3247–3251. [Google Scholar] [CrossRef]
- Chiang, K.; Hu, Y. Water washing effects on metals emission reduction during municipal solid waste incinerator (MSWI) fly ash melting process. Waste Manag. 2010, 30, 831–838. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. Technical Specification for Pollution Control of Fly-Ash from Municipal Solid Waste Incineration: HJ 1134—2020. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/dqhjbh/xgbz/202009/W020200902556206483862.pdf (accessed on 20 November 2022). (In Chinese)
- Wang, Y. Experimental Study on Removal of Chlorine by Water-Washing and Dioxin Degradation of Grate MSWI Fly Ash; Zhejiang Univ.: Hangzhou, China, 2019. (In Chinese) [Google Scholar]
- Yen, C.; Zhou, S.; Shen, Y. The Recovery of Ca and Zn from the Municipal Solid Waste Incinerator Fly Ash. Sustainability 2020, 12, 9086. [Google Scholar] [CrossRef]
- Frank, V.; Regnery, J.; Chan, K.; Ramey, D.; Spear, J.; Cath, T. Co-treatment of residential and oil and gas production wastewater with a hybrid sequencing batch reactor-membrane bioreactor process. J. Water Process Eng. 2017, 17, 82–94. [Google Scholar] [CrossRef]
- Zhan, Y.; Wei, R.; Zhou, H. Improvement on the treatment of thick oil sewage by using integrated biochemical treatment technology. Int. J. Environ. Sci. Technol. 2018, 15, 81–92. [Google Scholar] [CrossRef]
- Ng, K.; Shi, X.; Ong, S.; Lin, C.; Ng, H. An innovative of aerobic bio-entrapped salt marsh sediment membrane reactor for the treatment of high-saline pharmaceutical wastewater. Chem. Eng. J. 2016, 295, 317–325. [Google Scholar] [CrossRef]
- Tan, X.; Acquah, I.; Liu, H.; Li, W.; Tan, S. A critical review on saline wastewater treatment by membrane bioreactor (MBR) from a microbial perspective. Chemosphere 2019, 220, 1150–1162. [Google Scholar] [CrossRef] [PubMed]
- Nikolaychuk, P. The revised potential—pH diagram for Pb—H2O system. Ovidius Univ. Ann. Chem. 2018, 29, 55–67. [Google Scholar] [CrossRef]
- Wang, Y.; Chai, L.; Chang, H.; Chang, H.; Peng, X.; Shu, Y. Equilibrium of hydroxyl complex ions in Pb2+-H2O system. Trans. Nonferrous Met. Soc. China 2009, 19, 458–462. [Google Scholar] [CrossRef]
- Sudmalis, M.; Sheikholeslami, R. Coprecipitation of CaCO3 and CaSO4. Can. J. Chem. Eng. 2000, 78, 21–31. [Google Scholar] [CrossRef]
- Yan, Y.; Yu, T.; Zhang, H.; Song, J.; Qu, C.; Li, J.; Yang, B. Co-Deposition Mechanisms of Calcium Sulfate and Calcium Carbonate Scale in Produced Water. Crystals 2021, 11, 1494. [Google Scholar] [CrossRef]
- Zhu, F.; Takaoka, M.; Shiota, K.; Oshita, K.; Kitajima, Y. Chloride chemical form in various types of fly ash. Environ. Sci. Technol. 2008, 42, 3932–3937. [Google Scholar] [CrossRef]
- Chen, Z.; Chang, W.; Jiang, X.; Lu, S.; Buekens, A.; Yan, J. Leaching Behavior of Circulating Fluidised Bed MSWI Air Pollution Control Residue in Washing Process. Energies 2016, 9, 743. [Google Scholar] [CrossRef]
- Yuan, B.; Hu, H.; Huang, Y.; Guo, G.; Gong, L.; Zou, C.; Dong, L.; Yao, H. Effect of hydrated lime on the migration behavior of selenium in the MSWI plants: Experimental and theoretical study. Chem. Eng. J. 2022, 433, 134603. [Google Scholar] [CrossRef]


| No. | 1 | 2 |
|---|---|---|
| Element | Raw FA α | Raw FA β |
| % | ||
| Ca | 32.06 | 22.83 |
| Cl | 30.26 | 28.58 |
| Na | 8.02 | 5.87 |
| K | 5.33 | 4.18 |
| S | 2.34 | 1.83 |
| Si | 1.27 | 1.27 |
| Mg | 0.76 | 0.77 |
| Al | 0.59 | 0.34 |
| Zn | 0.85 | 0.56 |
| Fe | 0.56 | 0.35 |
| Ti | 0.104 | 0.101 |
| Pb | 0.130 | 0.046 |
| Fly Ash | Experiment | Stirring Rate r/min | Temperature °C | LSR g/mL | Washing Time min |
|---|---|---|---|---|---|
| FA α | Washing Condition | 500 | 25 | 2, 2.5, 3, 4 | 5, 10, 20, 30 |
| FA α | Washing Time | 500 | 25 | 3 | 5~300 |
| FA β | Supplementary Experiment | 500 | 25 | 3 | 20 |
| LSR L/kg | Washing Time min | Main Ions g/L | Main Heavy Metals mg/L | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K+ | Ca2+ | Na+ | Cl− | Pb | Ba | Cu | Zn | As | ||
| 2:1 | 5 | 35.5 | 30.9 | 41.1 | 134.4 | 130.13 | 20.68 | 0.906 | 2.325 | 0.081 |
| 10 | 48.6 | 34.8 | 56.6 | 152.7 | 169.64 | 22.08 | 0.836 | 2.575 | 0.072 | |
| 20 | 53.0 | 35.3 | 55.6 | 160.4 | 192.22 | 21.58 | 0.851 | 2.484 | 0.075 | |
| 30 | 49.8 | 33.5 | 50.3 | 150.9 | 207.29 | 22.03 | 0.858 | 2.140 | 0.071 | |
| 2.5:1 | 5 | 23.1 | 20.8 | 35.3 | 110.6 | 121.04 | 15.21 | 0.637 | 2.029 | 0.075 |
| 10 | 33.1 | 23.5 | 41.4 | 127.1 | 134.37 | 18.67 | 0.679 | 2.175 | 0.103 | |
| 20 | 31.9 | 23.2 | 39.2 | 125.5 | 137.74 | 18.58 | 0.596 | 1.804 | 0.083 | |
| 30 | 28.2 | 21.1 | 34.8 | 113.0 | 140.06 | 20.77 | 0.586 | 2.094 | 0.069 | |
| 3:1 | 5 | 17.7 | 22.8 | 24.2 | 92.3 | 70.97 | 12.22 | 0.457 | 1.274 | 0.038 |
| 10 | 26.1 | 22.1 | 32.9 | 94.0 | 76.26 | 14.22 | 0.475 | 1.394 | 0.052 | |
| 20 | 26.2 | 22.0 | 32.0 | 94.7 | 77.19 | 15.08 | 0.447 | 1.355 | 0.019 | |
| 30 | 26.2 | 22.7 | 30.1 | 93.9 | 79.12 | 15.10 | 0.556 | 1.319 | 0.046 | |
| 4:1 | 5 | 14.0 | 16.1 | 19.9 | 67.5 | 42.74 | 8.82 | 0.374 | 0.885 | 0.034 |
| 10 | 19.4 | 17.2 | 23.6 | 70.6 | 42.33 | 7.05 | 0.386 | 0.829 | 0.033 | |
| 20 | 19.0 | 17.0 | 22.3 | 69.8 | 48.49 | 6.74 | 0.364 | 0.803 | 0.034 | |
| 30 | 17.9 | 15.7 | 21.5 | 64.3 | 59.66 | 7.49 | 0.383 | 0.911 | 0.012 | |
| WWS 2 (LSR = 3:1, t = 20 min) | 23.2 | 20.8 | 22.9 | 92.3 | 63.87 | 17.81 | 1.720 | 1.185 | 0.313 | |
| Liquid Solid Ratio | Washing Stage | K+ | Ca2+ | Na+ | Cl− | Dechlorination Effect |
|---|---|---|---|---|---|---|
| g/L | % | |||||
| 2:1 | 1 | 53 | 35.3 | 55.6 | 160.4 | 97.5% |
| 2 | 13.7 | 17.7 | 15.5 | 56.2 | 34.2% | |
| 3 | 3.3 | 7.0 | 4.1 | 16.4 | 10.0% | |
| 2.5:1 | 1 | 31.9 | 23.2 | 39.2 | 125.5 | 98.3% |
| 2 | 9.5 | 13.3 | 10.8 | 39.5 | 30.9% | |
| 3 | 1.9 | 4.5 | 2.5 | 9.8 | 7.7% | |
| 3:1 | 1 | 26.2 | 22.0 | 32.0 | 94.7 | 99.0% |
| 2 | 5.8 | 11.3 | 7.7 | 30.5 | 28.9% | |
| 3 | 1.1 | 4.3 | 1.4 | 7.5 | 7.8% | |
| 4:1 | 1 | 19 | 17 | 22.3 | 69.8 | 99.3% |
| 2 | 2.6 | 4.0 | 3.3 | 12.7 | 18.1% | |
| 3 | 0.4 | 1.6 | 0.5 | 2.6 | 3.7% | |
| Liquid Solid Ratio | Washing Stage | ca | k = 1 | k = 2.5 |
|---|---|---|---|---|
| % | ||||
| 2:1 | 1 | 30.83 | 25.61 | 27.95 |
| 2 | 10.71 | 4.63 | 17.43 | |
| 3 | 3.48 | 8.18 | 9.77 | |
| 2.5:1 | 1 | 22.38 | 19.81 | 20.58 |
| 2 | 7.71 | 2.73 | 8.75 | |
| 3 | 2.27 | 0.54 | 3.99 | |
| 3:1 | 1 | 17.89 | 16.64 | 17.02 |
| 2 | 5.93 | 1.82 | 5.48 | |
| 3 | 1.83 | 0.30 | 1.18 | |
| 4:1 | 1 | 13.21 | 12.39 | 12.51 |
| 2 | 2.66 | 0.97 | 2.74 | |
| 3 | 0.91 | 0.02 | 0.81 | |
| No. | 1-1 | 1-2 | 1-3 | 1-4 | 2 |
|---|---|---|---|---|---|
| Element | Washed FA α (LSR = 3:1 L/kg) Washing Time (min) | Washed FA β (LSR = 3:1 L/kg, t = 20 min) | |||
| 5 | 10 | 20 | 30 | ||
| % | |||||
| Ca | 46.17 | 41.97 | 43.40 | 45.83 | 30.11 |
| Cl | 3.80 | 1.54 | 2.82 | 3.03 | 1.15 |
| Na | 0.89 | 0.49 | 0.69 | 0.91 | 0.50 |
| K | 0.58 | 0.32 | 0.42 | 0.68 | 0.24 |
| S | 4.21 | 3.54 | 4.24 | 4.40 | 2.56 |
| Si | 3.46 | 3.34 | 3.55 | 3.20 | 2.54 |
| Mg | 2.22 | 2.16 | 2.34 | 2.16 | 1.58 |
| Al | 1.09 | 1.08 | 1.18 | 1.06 | 0.86 |
| Zn | 1.53 | 1.56 | 1.57 | 1.54 | 0.93 |
| Fe | 1.08 | 1.16 | 1.15 | 0.98 | 0.64 |
| Ti | 0.328 | 0.323 | 0.341 | 0.285 | 0.185 |
| Pb | 0.252 | 0.283 | 0.262 | 0.284 | 0.096 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Chen, J.; Lin, X.; Mao, T.; Zhu, Z.; Lv, J.; Fu, C.; Chen, S.; Wu, A.; Li, X.; et al. Study on Three-Stage Counter-Current Water Washing Desalination Characteristics and Mechanism of High Chlorine Waste Incineration Fly Ash. Processes 2022, 10, 2540. https://doi.org/10.3390/pr10122540
Li M, Chen J, Lin X, Mao T, Zhu Z, Lv J, Fu C, Chen S, Wu A, Li X, et al. Study on Three-Stage Counter-Current Water Washing Desalination Characteristics and Mechanism of High Chlorine Waste Incineration Fly Ash. Processes. 2022; 10(12):2540. https://doi.org/10.3390/pr10122540
Chicago/Turabian StyleLi, Minjie, Jie Chen, Xiaoqing Lin, Tieying Mao, Zhongxu Zhu, Jiabao Lv, Congkai Fu, Siyu Chen, Angjian Wu, Xiaodong Li, and et al. 2022. "Study on Three-Stage Counter-Current Water Washing Desalination Characteristics and Mechanism of High Chlorine Waste Incineration Fly Ash" Processes 10, no. 12: 2540. https://doi.org/10.3390/pr10122540
APA StyleLi, M., Chen, J., Lin, X., Mao, T., Zhu, Z., Lv, J., Fu, C., Chen, S., Wu, A., Li, X., & Yan, J. (2022). Study on Three-Stage Counter-Current Water Washing Desalination Characteristics and Mechanism of High Chlorine Waste Incineration Fly Ash. Processes, 10(12), 2540. https://doi.org/10.3390/pr10122540







