Analysis of the Corrosion Process with the Application of the Novel Type of Coupon Installation
Abstract
1. Introduction
2. Materials and Methods
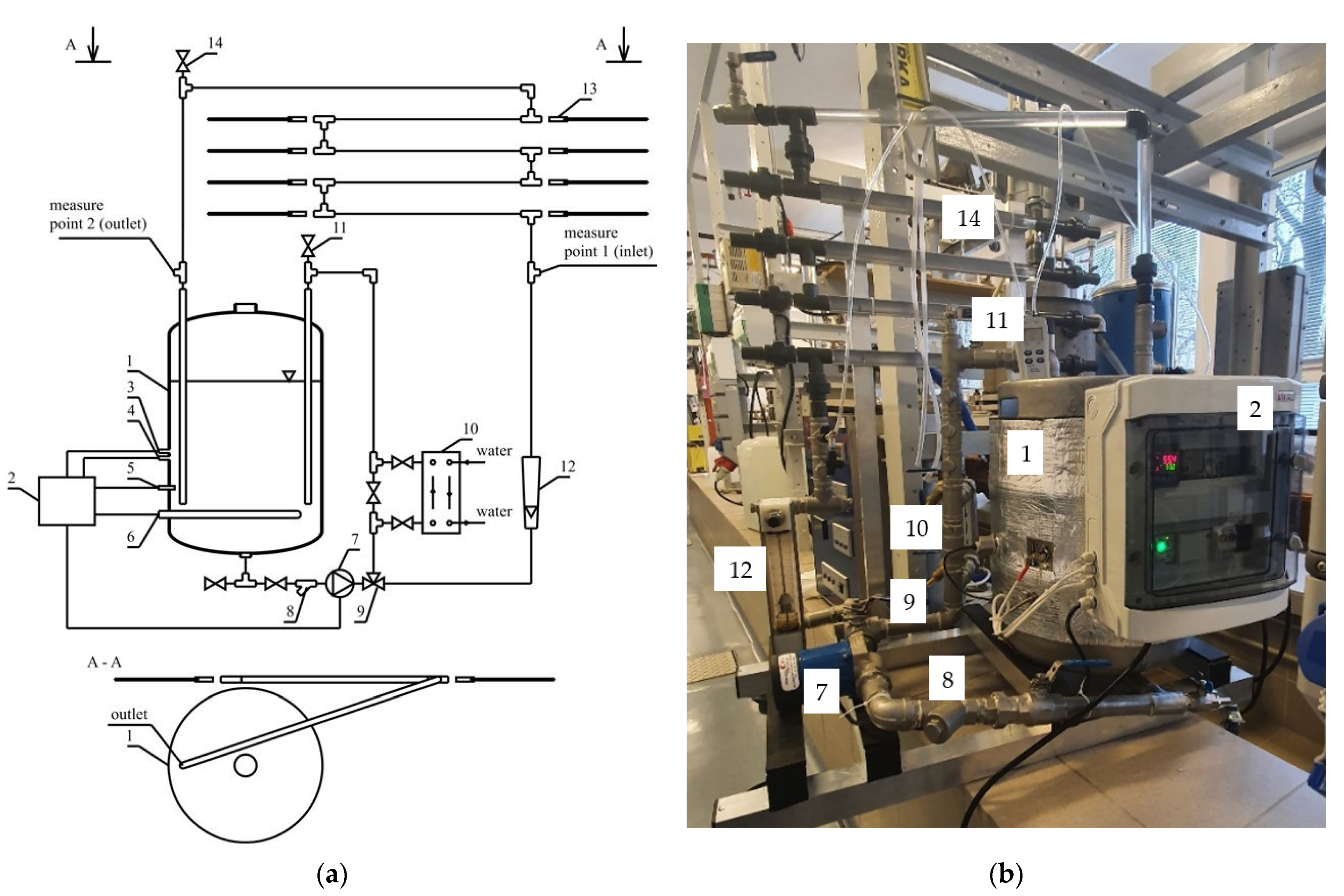
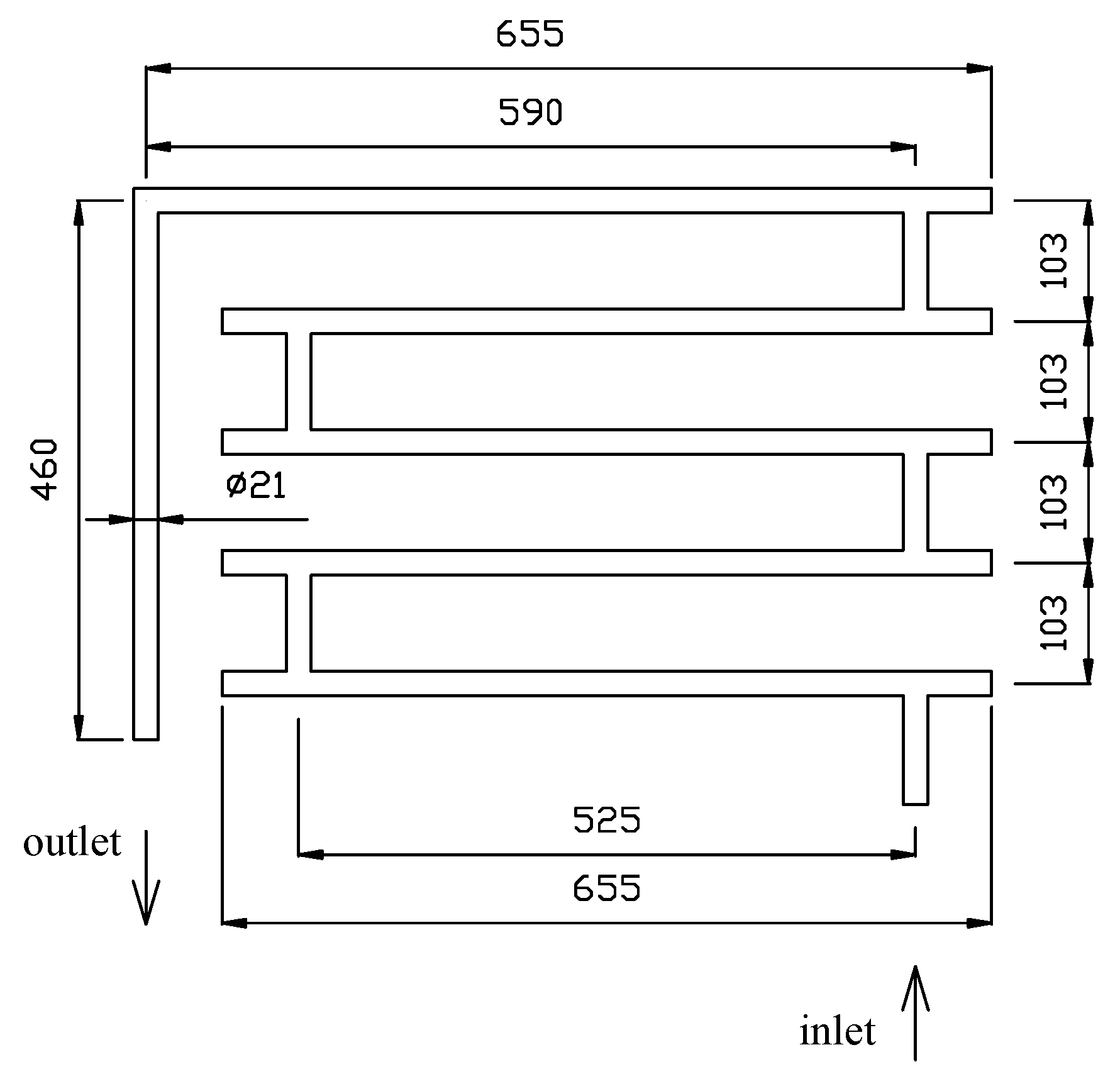
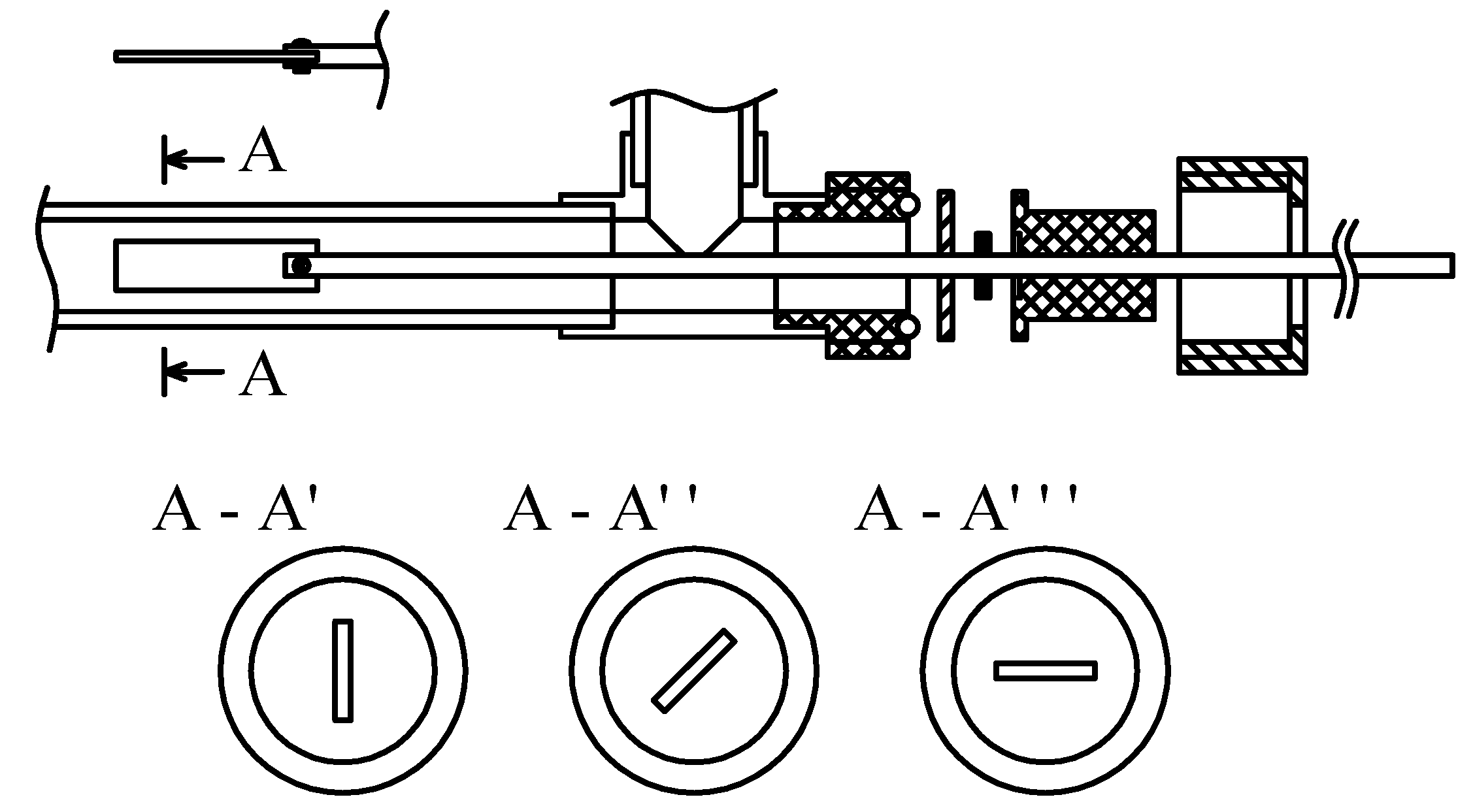
2.1. Experimental Setup
2.2. Temperature Conditions
2.3. Hydrodynamic Conditions
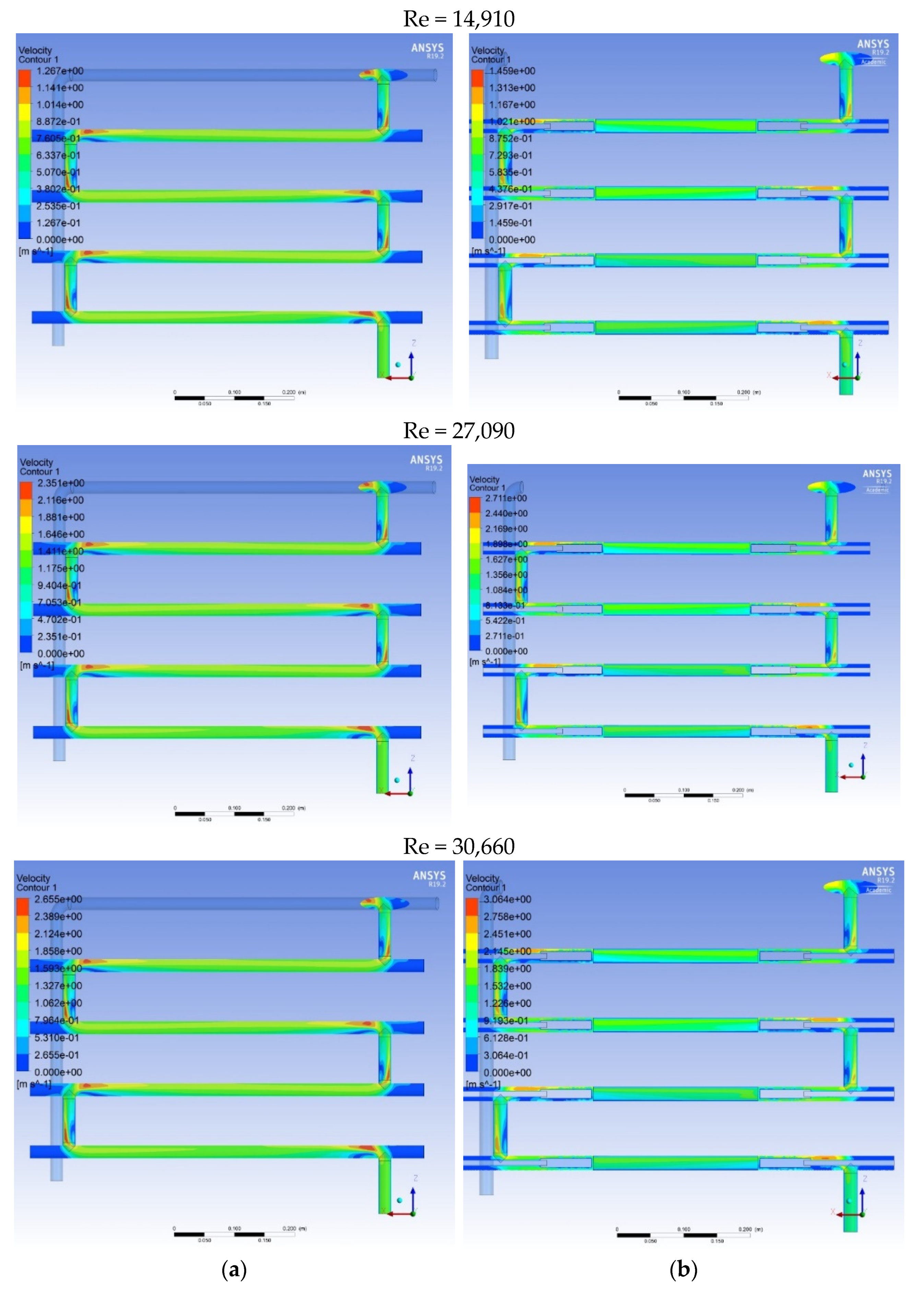
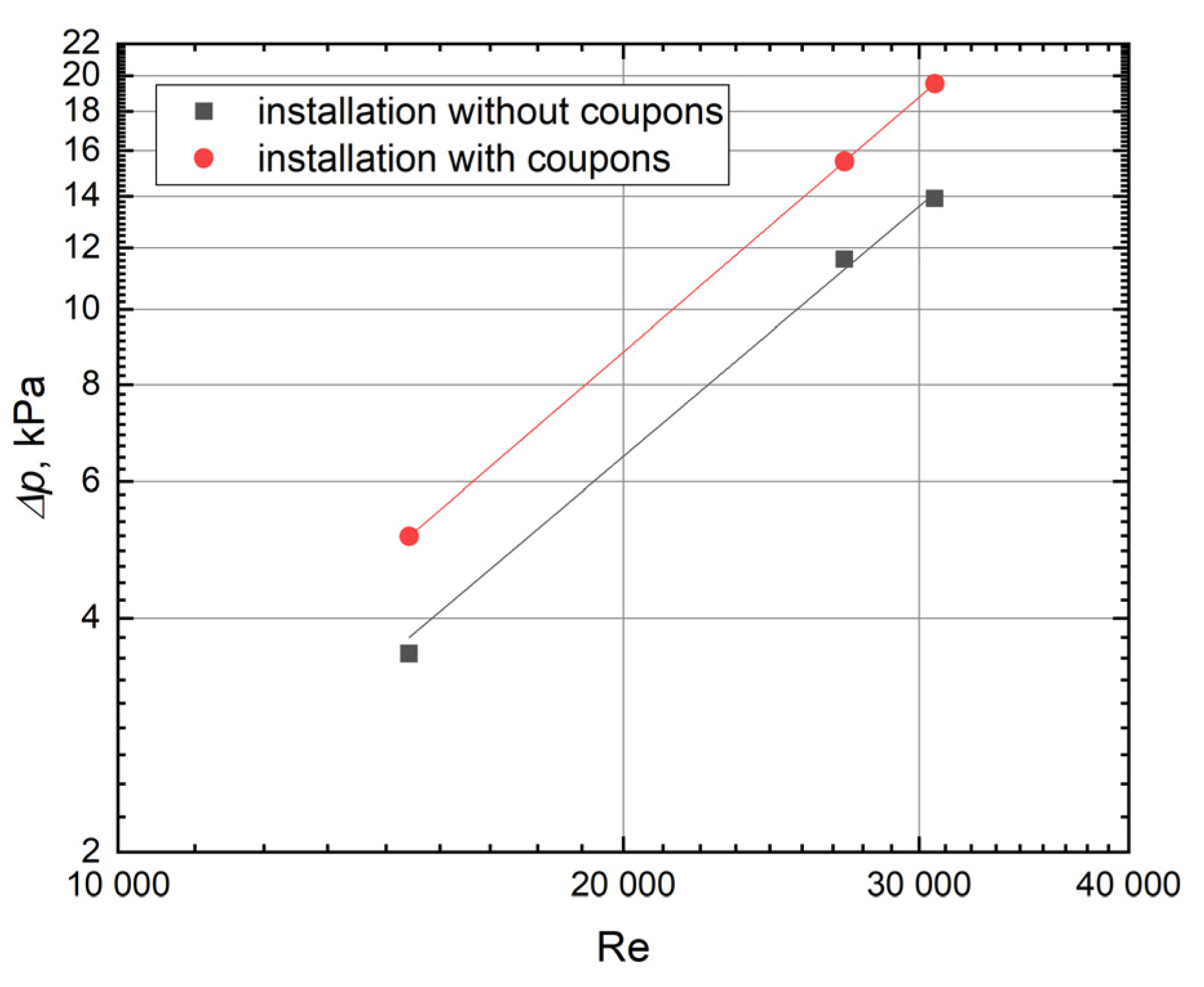
2.4. Investigation of Pressure Drop
2.5. Numerical Simulaiton
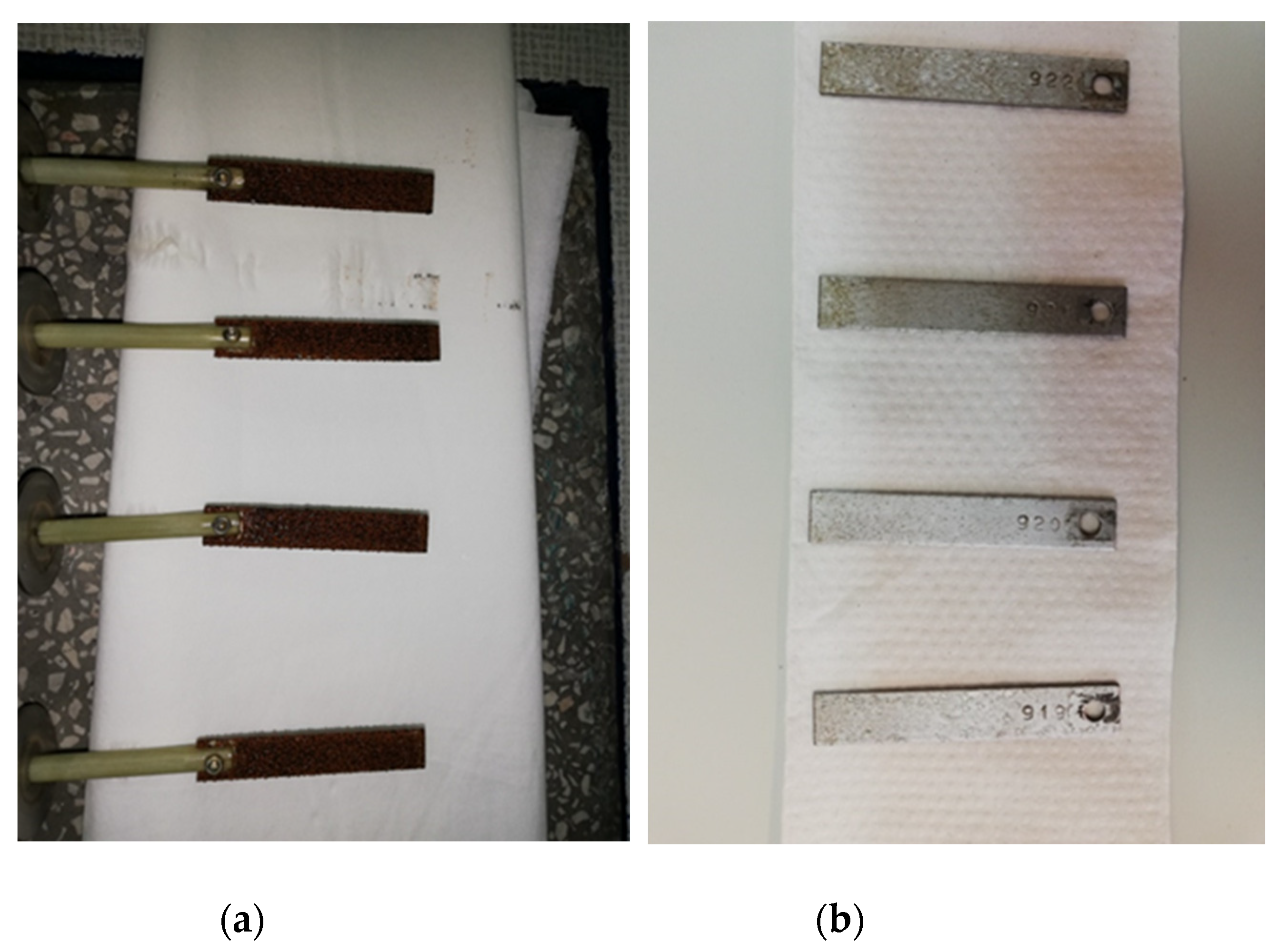
2.6. Experimental Procedure
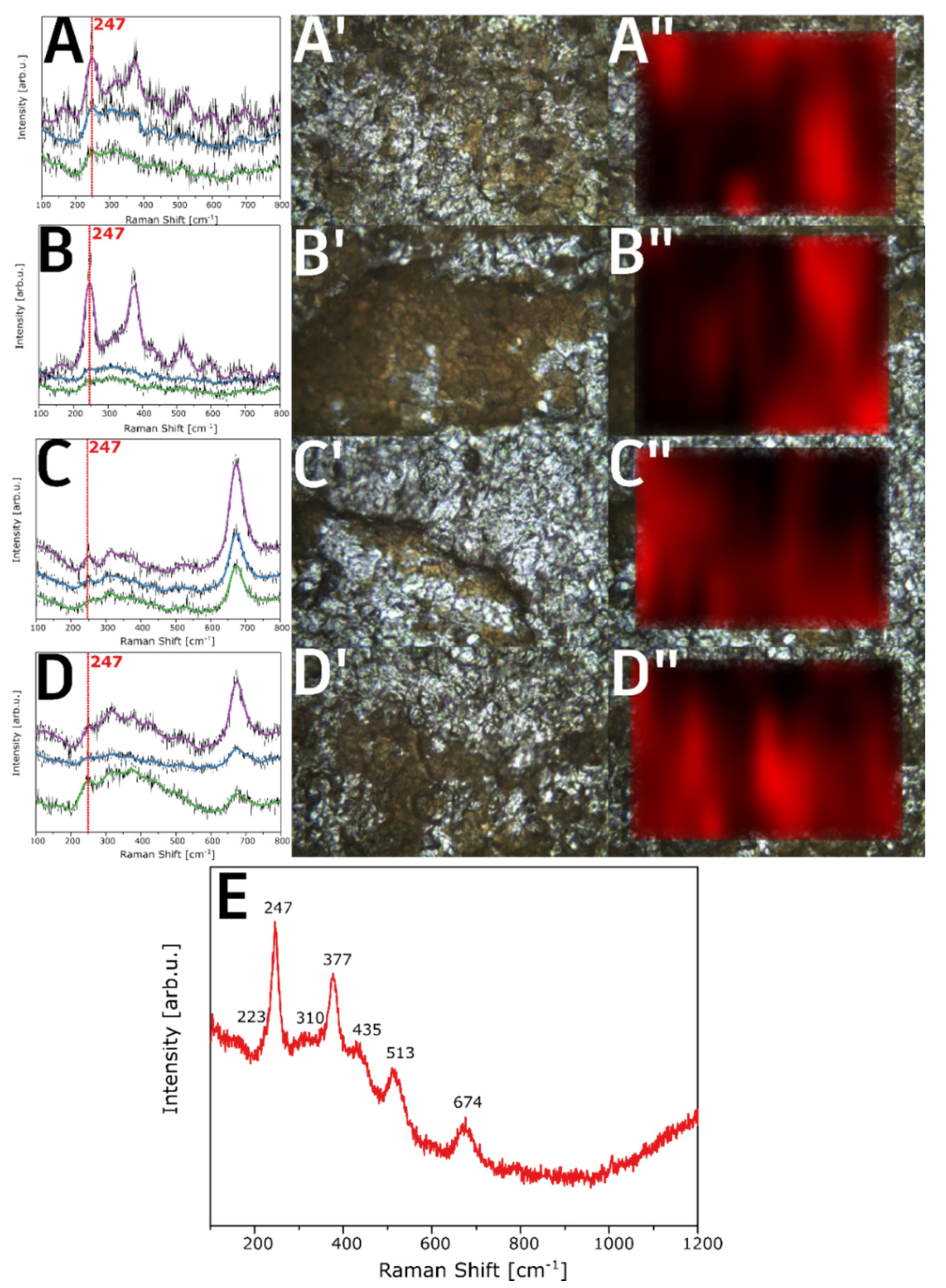
3. Results and Discussion
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wranglen, G. An Introduction to Corrosion and protection of Metals; Springer: Netherlands, The Netherlands, 1985. [Google Scholar]
- Titov, A.I.; Lun-Fu, A.V.; Gayvaronskiy, A.V.; Bubenchikov, M.A.; Bubenchikov, A.M.; Lider, A.M.; Syrtanov, M.S.; Kudiiarov, V.N. Hydrogen Accumulation and Distribution in Pipeline Steel in Intensified Corrosion Conditions. Materials 2019, 12, 1409. [Google Scholar] [CrossRef] [PubMed]
- Comensoli, L.; Albini, M.; Kooli, W.; Maillard, J.; Lombardo, T.; Junier, P.; Joseph, E. Investigation of Biogenic Passivating Layers on Corroded Iron. Materials 2020, 13, 1176. [Google Scholar] [CrossRef] [PubMed]
- Madan, S.; Madan, R.; Hussain, A. Evaluation of corrosion and scaling tendency of polyester textile dyeing effluent, Haridwar, Uttarakhand, India. Environ. Sci. Pollut. Res. 2022, 29, 39827–39837. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawajfeh, A.E.; Glade, H.; Ulrich, J. Scaling in multiple-effect distillers: The role of CO2 release. Desalination 2005, 182, 209–219. [Google Scholar] [CrossRef]
- Wojtkowska, M.; Malesińska, A.; Machowska, A.; Puntorieri, P.; Barbaro, G.; Fiamma, V.; Biedugnis, S. The Influence of Water Quality Change on the Corrosion Process in Galvanized Pipes of Fire Protection Installations. Sustainability 2022, 14, 7708. [Google Scholar] [CrossRef]
- Alsaqqar, A.S.; Khudair, B.H.; Ali, S.K. Evaluating Water Stability Indices from Water Treatment Plants in Baghdad City. J. Water Resour. Prot. 2014, 6, 1344–1351. [Google Scholar] [CrossRef]
- Rhem, B.; Haghshenas, A.; Paknejad, A.; Al-Yami, A.; Hughes, J.; Schubert, J. Underbalanced Drilling: Limits and Extremes; Gulf Publishing Company: Houston, TX, USA, 2012; ISBN 978-0128103524. [Google Scholar]
- Sellers, R.S.; Cheng, W.-J.; Kelleher, B.C.; Anderson, M.; Sridharan, K.; Wang, C.-J.; Allen, T. Corrosion of 316L Stainless Steel Alloy and Hastelloy-N Superalloy in Molten Eutectic LiF-NaF-KF Salt and Interaction with Graphite. Nucl. Technol. 2014, 188, 192–199. [Google Scholar] [CrossRef]
- Durrani, F.; Wesley, R.; Srikandarajah, V.; Eftekhari, M.; Munn, S. Predicting Corrosion rate in Chilled HVAC Pipe Network: Coupon vs Linear Polarisation Resistance method. Eng. Fail. Anal. 2019, 109, 104261. [Google Scholar] [CrossRef]
- Beck, C.L.; Smith, N.P.; Riley, B.J.; Clark, S.B. Adsorption of iodine on metal coupons in humid and dry environments. J. Nucl. Mater. 2021, 556, 153204. [Google Scholar] [CrossRef]
- Bolaji, T.A.; Olumayede, E.G.; Ojo, A.M. Evaluation of corrosion and scaling potentials of oilfield waters in an offshore producing facility, Niger Delta. Water Sci. Technol. 2022, 85, 3493–3509. [Google Scholar] [CrossRef]
- Abbasnia, A.; Yousefi, N.; Mahvi, A.H.; Nabizadeh, R.; Radfard, M.; Yousefi, M.; Alimohammadi, M. Evaluation of groundwater quality using water quality index and its suitability for assessing water for drinking and irrigation purposes: Case study of Sistan and Baluchistan province (Iran). Hum. Ecol. Risk Assess. Int. J. 2018, 25, 988–1005. [Google Scholar] [CrossRef]
- Shammi, R.S.; Hossain, S.; Kabir, H.; Islam, S.; Taj, T.I.; Islam, S.; Sarker, E.; Hossain, S.; Idris, A.M. Hydrochemical appraisal of surface water from a subtropical urban river in southwestern Bangladesh using indices, GIS, and multivariate statistical analysis. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.F.; Lu, P.; Devkota, J.; Lu, F.; Ziomek-Moroz, M.; Ohodnicki, P.R., Jr. Corrosion Sensors for Structural Health Monitoring of Oil and Natural Gas Infrastructure: A Review. Sensors 2019, 19, 3964. [Google Scholar] [CrossRef] [PubMed]
- Konopacki, M.; Kordas, M.; Fijałkowski, K.; Rakoczy, R. Computational Fluid Dynamics and Experimental Studies of a New Mixing Element in a Static Mixer as a Heat Exchanger. Chem. Process Eng. 2015, 36, 59–72. [Google Scholar] [CrossRef]
- Norton, T.; Tiwari, B.K.; Sun, D.-W. Computational Fluid Dynamics in the Design and Analysis of Thermal Processes: A Review of Recent Advances. Crit. Rev. Food Sci. Nutr. 2013, 53, 251–275. [Google Scholar] [CrossRef]
- Hu, C.-Y.; Zhang, J.; Xu, B.; Lin, Y.-L.; Zhang, T.-Y.; Tian, F.-X. Effect of pipe corrosion product–goethite–on the formation of disinfection by-products during chlorination. Desalin. Water Treat. 2014, 57, 553–561. [Google Scholar] [CrossRef]
- Tan, H.; He, W.; Han, H.; Lu, Y.; Zhang, N. Speciation of iron and development of iron corrosion scales in drinking water distribution systems. Desalin. Water Treat. 2014, 56, 646–654. [Google Scholar] [CrossRef]
- Sobanska, S.; Deneele, D.; Barbillat, J.; Ledésert, B. Natural weathering of slags from primary Pb–Zn smelting as evidenced by Raman microspectroscopy. Appl. Geochem. 2016, 64, 107–117. [Google Scholar] [CrossRef]
- Bellot-Gurlet, L.; Neff, D.; Réguer, S.; Monnier, J.; Saheb, M.; Dillmann, P. Raman Studies of Corrosion Layers Formed on Archaeological Irons in Various Media. J. Nano Res. 2009, 8, 147–156. [Google Scholar] [CrossRef]
- Morco, R.P.; Musa, A.Y.; Momeni, M.; Wren, J. Corrosion of carbon steel in the [P 14666][Br] ionic liquid: The effects of γ-radiation and cover gas. Corros. Sci. 2016, 102, 1–15. [Google Scholar] [CrossRef]
- Kain, V. Flow Accelerated Corrosion: Forms, Mechanisms and Case Studies. Procedia Eng. 2014, 86, 576–588. [Google Scholar] [CrossRef]
- Buecker, B. Flow-accelerated corrosion: A critical issue revisited. Power Eng. 2007, 111, 20–23. [Google Scholar]
- Uchida, S.; Naitoh, M.; Uehara, Y.; Okada, H.; Hiranuma, N.; Sugino, W.; Koshizuka, S.; Lister, D.H. Evaluation Methods for Corrosion Damage of Components in Cooling Systems of Nuclear Power Plants by Coupling Analysis of Corrosion and Flow Dynamics (III). J. Nucl. Sci. Technol. 2009, 46, 31–40. [Google Scholar] [CrossRef]
- De Seranno, T.; Lambrechts, E.; De Meyer, E.; Hater, W.; De Geyter, N.; Verliefde, A.R.D.; DePover, T.; Verbeken, K. Effect of Film-Forming Amines on the Acidic Stress-Corrosion Cracking Resistance of Steam Turbine Steel. Metals 2020, 10, 1628. [Google Scholar] [CrossRef]
- Moed, D.; Verliefde, A.; Heijman, S.; Rietveld, L. Organic acid formation in steam–water cycles: Influence of temperature, retention time, heating rate and O2. Appl. Therm. Eng. 2014, 65, 194–200. [Google Scholar] [CrossRef]
- Moed, D.H.; Verliefde, A.R.D.; Rietveld, L.C. Effects of Temperature and Pressure on the Thermolysis of Morpholine, Ethanolamine, Cyclohexylamine, Dimethylamine, and 3-Methoxypropylamine in Superheated Steam. Ind. Eng. Chem. Res. 2015, 54, 2606–2612. [Google Scholar] [CrossRef]
- De Meyer, E.; Van Overstraeten, T.; Heyse, J.; Uddin, M.R.; Vanoppen, M.; Boon, N.; De Gusseme, B.; Verbeken, K.; Verliefde, A.R.D. Organic Matter and Microbial Cell Density Behavior during Ion Exchange Demineralization of Surface Water for Boiler Feedwater. Ind. Eng. Chem. Res. 2019, 58, 14368–14379. [Google Scholar] [CrossRef]
- Frey, M.; Harris, S.; Holmes, J.; Nation, D.; Parsons, S.; Tasker, P.; Winpenny, R. Elucidating the Mode of Action of a Corrosion Inhibitor for Iron. Chemistry 2000, 6, 1407–1415. [Google Scholar] [CrossRef]
- Udabe, E.; Somers, A.; Forsyth, M.; Mecerreyes, D. Design of Polymeric Corrosion Inhibitors Based on Ionic Coumarate Groups. ACS Appl. Polym. Mater. 2021, 3, 1739–1746. [Google Scholar] [CrossRef]



| Ref. | Description |
|---|---|
| [8] | The installation for testing the corrosion process is placed in an autoclave (a hermetically sealed tank in which the chemical process under the created conditions is carried out). These conditions are created considering the tested parameter, i.e., temperature, total pressure, gas mixture, brine, time, types of coupons and rotational speed. The coupons are connected to the rod and placed in the setup’s central part. The installation is used to test the resistance of materials to corrosion, which is used in the production of drilling pipes and covers. |
| [9] | The installation for testing corrosion of FLiNaK salt (46.5% LiF-11.5% NaF-42% KF (mol%)) is presented. This molten salt is proposed as a coolant for reactors and a heat transfer carrier for nuclear reactor plants. The installation contained a 316L steel crucible with steel and graphite coupons. |
| [10] | The corrosion installation consisted of a network of parallel pipes connected via pipe fitting (bends, t-joints, valves etc.). The mass flow rate, pressure, pH, temperature, and humidity sensors are used to monitor corrosion conditions. |
| [11] | Using different carrier gasses, a dynamic flow-through system was assembled to allow for dynamic iodine exposures on hanging substrates. The metal coupons were tested in the setup by monitoring temperature, iodine concentration, flow rate, atmosphere, and relative humidity. |
| Type of Coupon Installation | Parameters | |||
|---|---|---|---|---|
| Number of Cells | Number of Nodes | Skewness | Orthogonal Quality | |
| Without coupons (empty) | 90 601 | 98 584 | 0.177 | 0.953 |
| With coupons | 679 776 | 187 412 | 0.230 | 0.779 |
| Coupon Serial No | Material | Dimensions [in] | Weight |
|---|---|---|---|
| 919 | Mild steel | 3 × 1/2 × 1/16 | 10.6710 g |
| 920 | 10.6579 g | ||
| 921 | 10.6779 g | ||
| 922 | 10.6979 g |
| Metal | After 24 h | After 52 h | After 69 h |
|---|---|---|---|
| Ag | 0 | 0 | 0 |
| Al | 0.019 | 0.0059 | 0.0041 |
| Ba | 0.0003 | 0.0002 | 0.0002 |
| Ca | 0.8859 | 0.59 | 0.6467 |
| Cd | 0 | 0.0003 | 0.0001 |
| Cr | 0.0004 | 0.0004 | 0.0004 |
| Cu | 0.0249 | 0.0176 | 0.017 |
| Fe | 0.2875 | 0.3753 | 0.3967 |
| K | 0.0547 | 0.1114 | 0.1644 |
| Mg | 0.0435 | 0.0239 | 0.028 |
| Mn | 0.0029 | 0.0047 | 0.0048 |
| Na | 7.989 | 8.1361 | 8.1127 |
| Ni | 0.0017 | 0.0024 | 0.0027 |
| P | 0.0026 | 0.0072 | 0.0053 |
| Pb | 0.0027 | 0.0019 | 0.0016 |
| S | 0.407 | 0.2467 | 0.2623 |
| Si | 0.19 | 0.1433 | 0.1466 |
| Zn | 0.0189 | 0.0108 | 0.0106 |
| Coupon Serial No | Weight after Process Wa [g] | Wb − Wa [g] | CR [g·m−2·h−1] |
|---|---|---|---|
| 919 | 10.4077 | 0.2633 | 1.8779 |
| 920 | 10.3914 | 0.2665 | 1.9007 |
| 921 | 10.4016 | 0.2763 | 1.9706 |
| 922 | 10.4250 | 0.2726 | 1.9443 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musik, D.; Wójcik, K.; Sekuła-Wybańska, M.; Konopacki, M.; Rakoczy, R. Analysis of the Corrosion Process with the Application of the Novel Type of Coupon Installation. Processes 2022, 10, 2468. https://doi.org/10.3390/pr10122468
Musik D, Wójcik K, Sekuła-Wybańska M, Konopacki M, Rakoczy R. Analysis of the Corrosion Process with the Application of the Novel Type of Coupon Installation. Processes. 2022; 10(12):2468. https://doi.org/10.3390/pr10122468
Chicago/Turabian StyleMusik, Daniel, Krzysztof Wójcik, Małgorzata Sekuła-Wybańska, Maciej Konopacki, and Rafał Rakoczy. 2022. "Analysis of the Corrosion Process with the Application of the Novel Type of Coupon Installation" Processes 10, no. 12: 2468. https://doi.org/10.3390/pr10122468
APA StyleMusik, D., Wójcik, K., Sekuła-Wybańska, M., Konopacki, M., & Rakoczy, R. (2022). Analysis of the Corrosion Process with the Application of the Novel Type of Coupon Installation. Processes, 10(12), 2468. https://doi.org/10.3390/pr10122468







