Medicinal Plant Drying Using a Superabsorbent Polymer Dryer Incorporated with an Insulated Heater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polymer Hydrogel
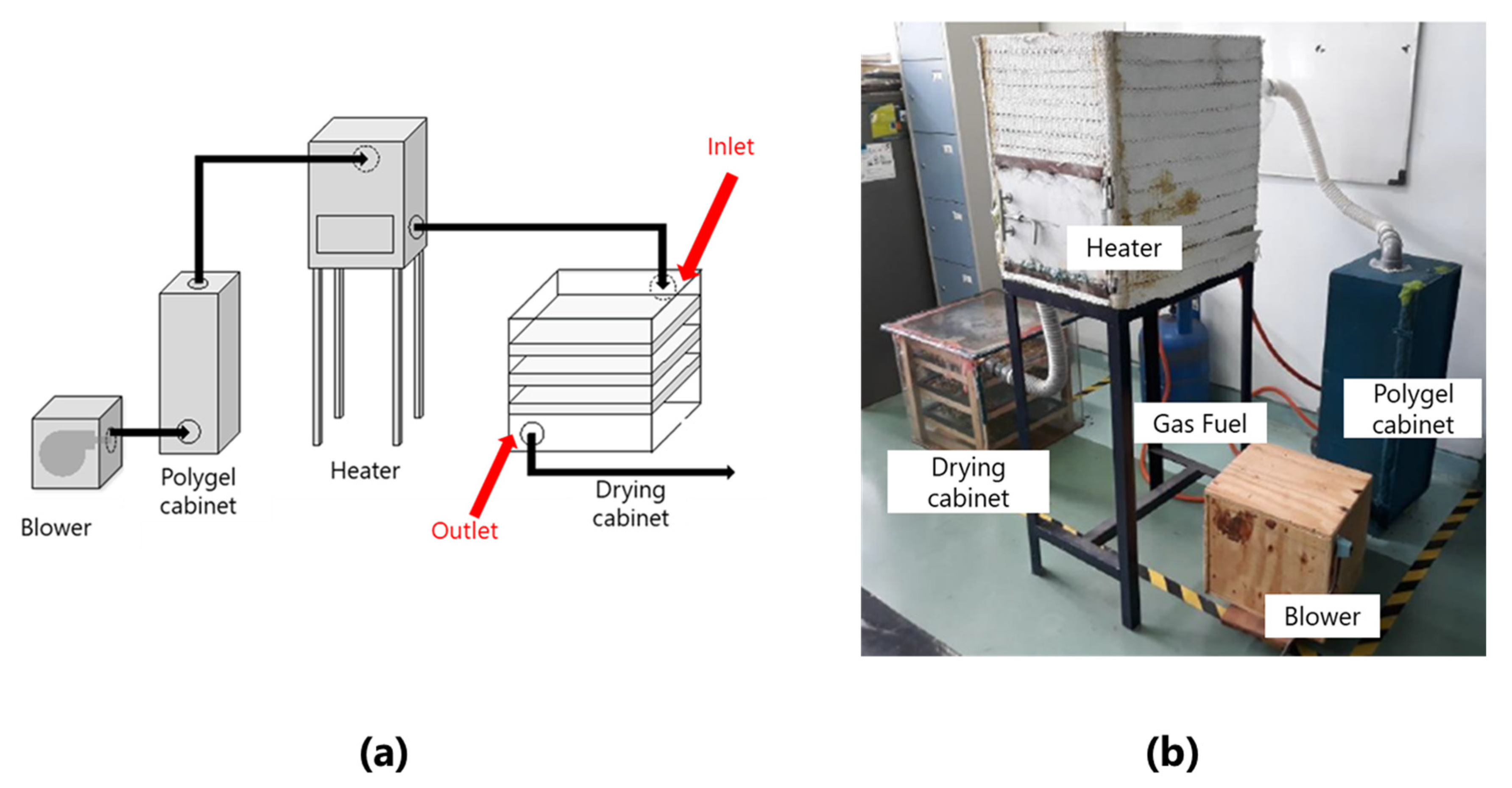
2.3. Design and Construction of Polydryer Incorporated with a Heater
2.4. Operation of Polydryer
2.5. Polygel Regeneration
2.6. Acquisition of Drying Profiles
2.7. Product Characterization
2.8. Drying Model Fitting
3. Results and Discussion
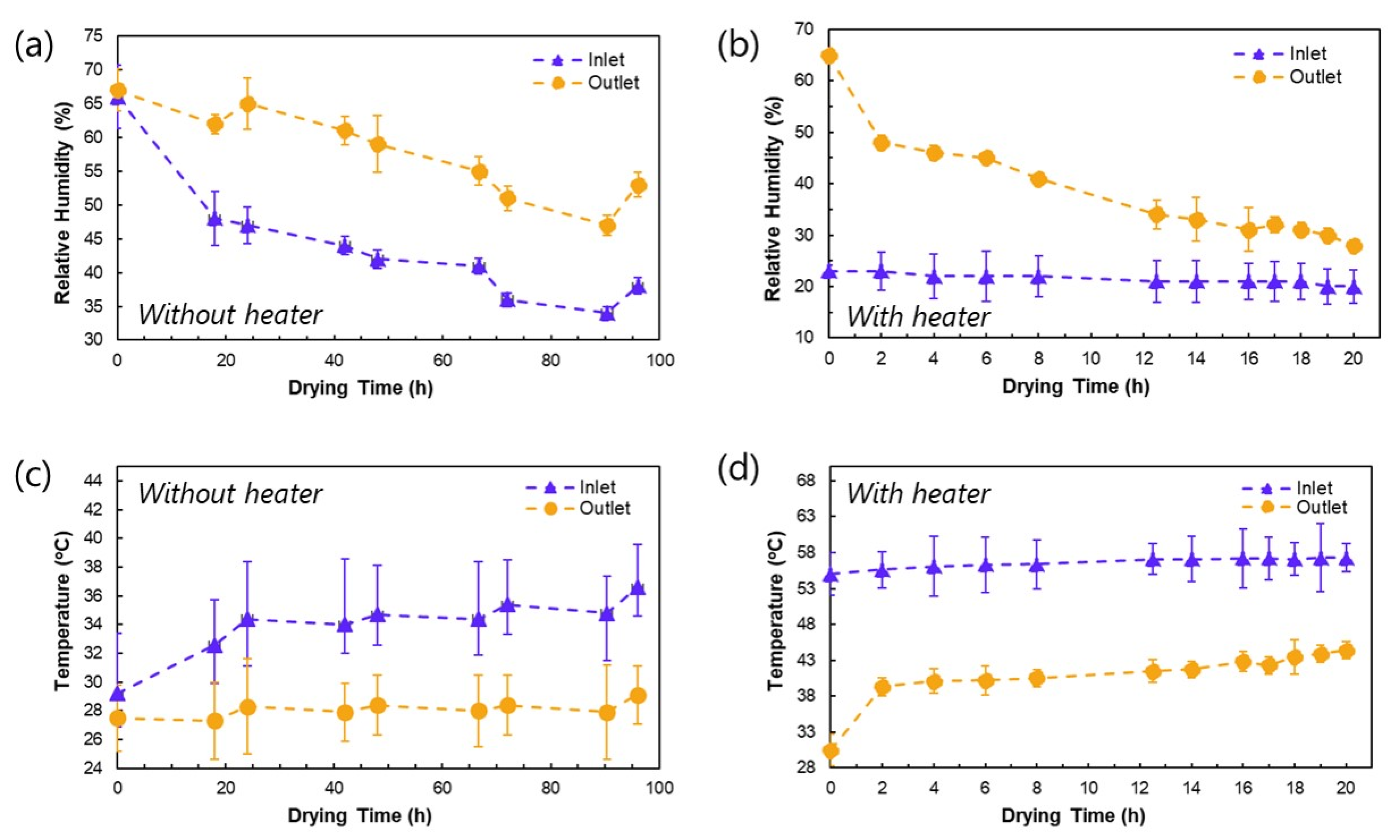
3.1. Temperature and Humidity Profiles in the Drying Cabinet
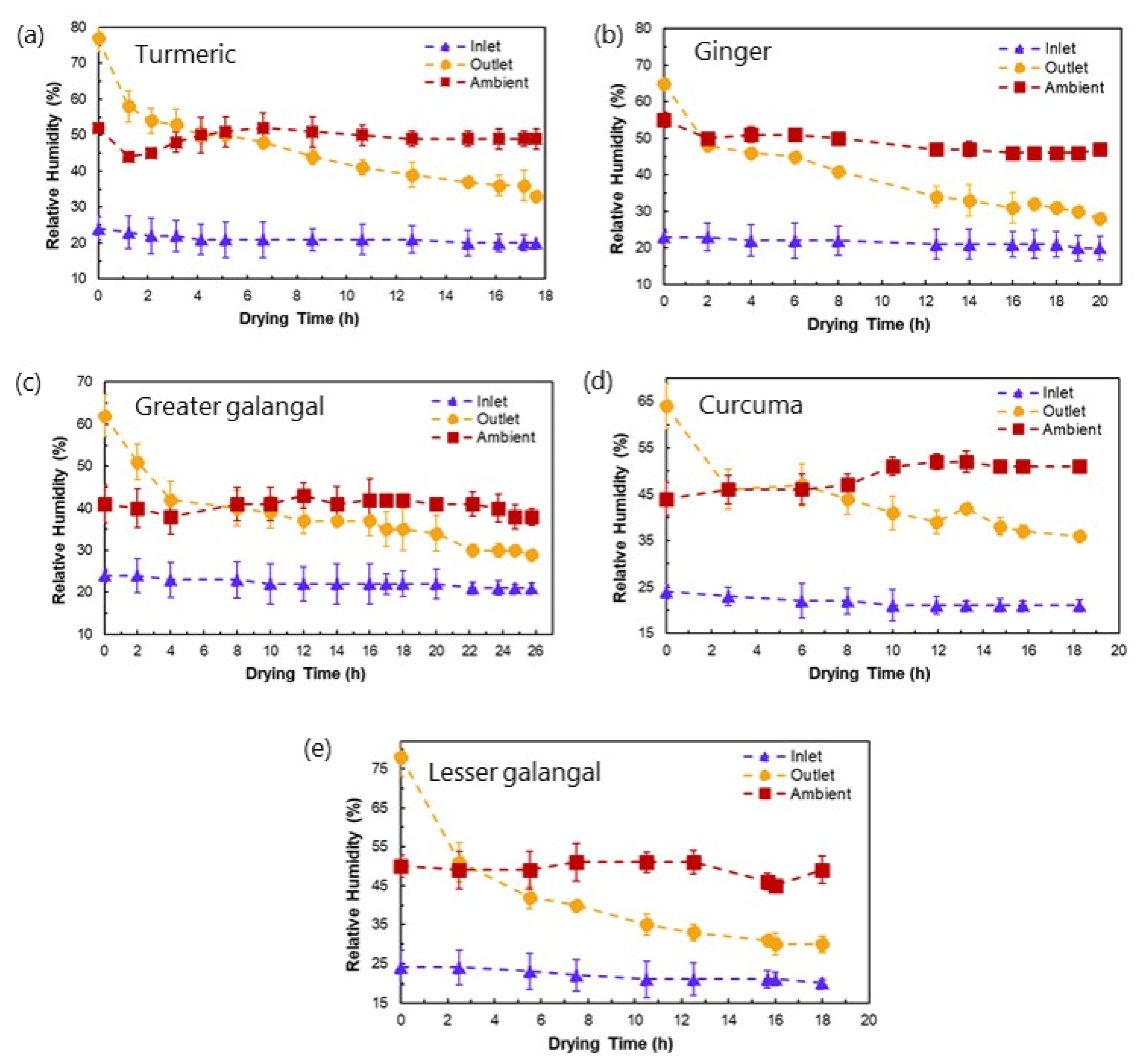
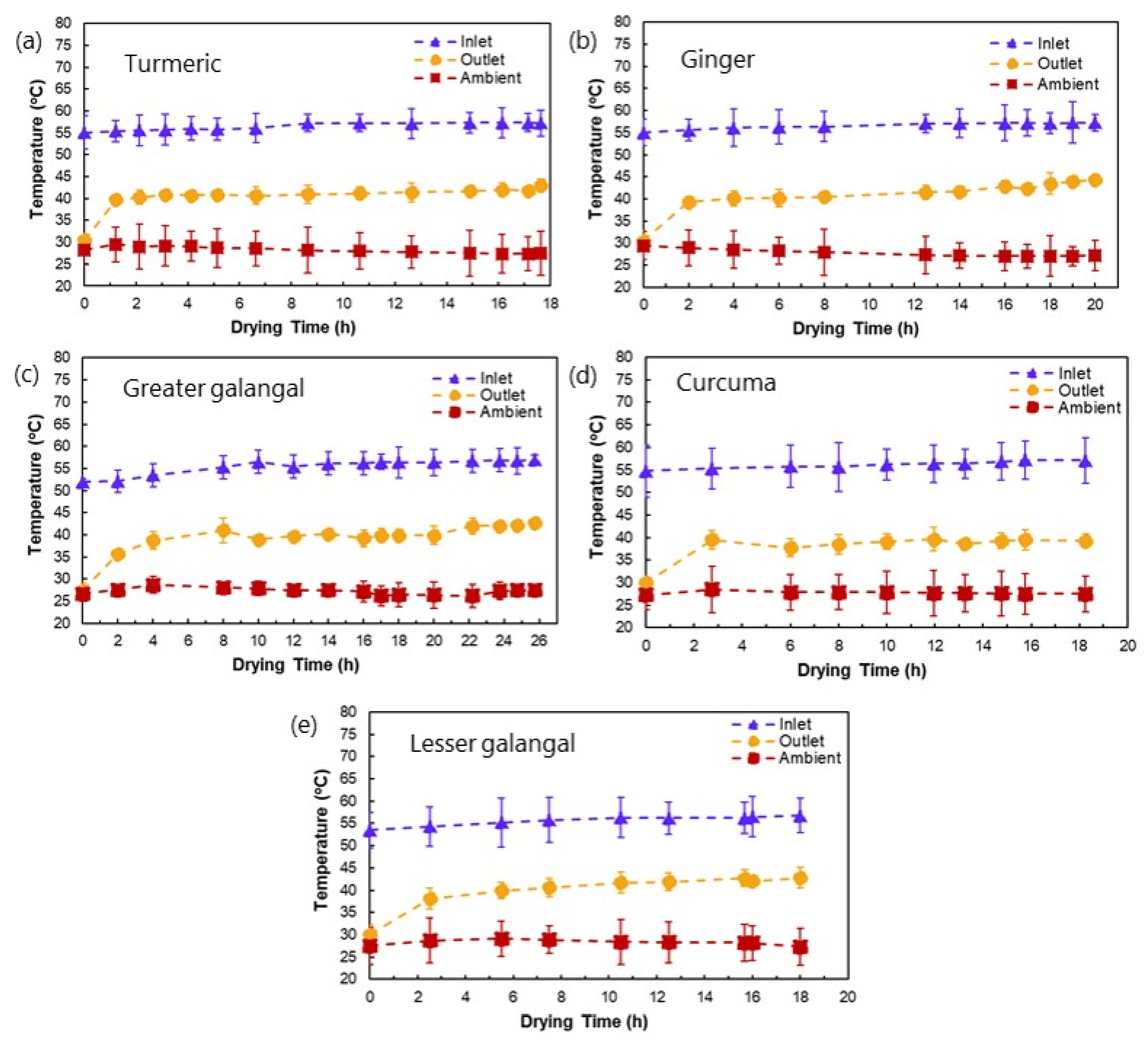
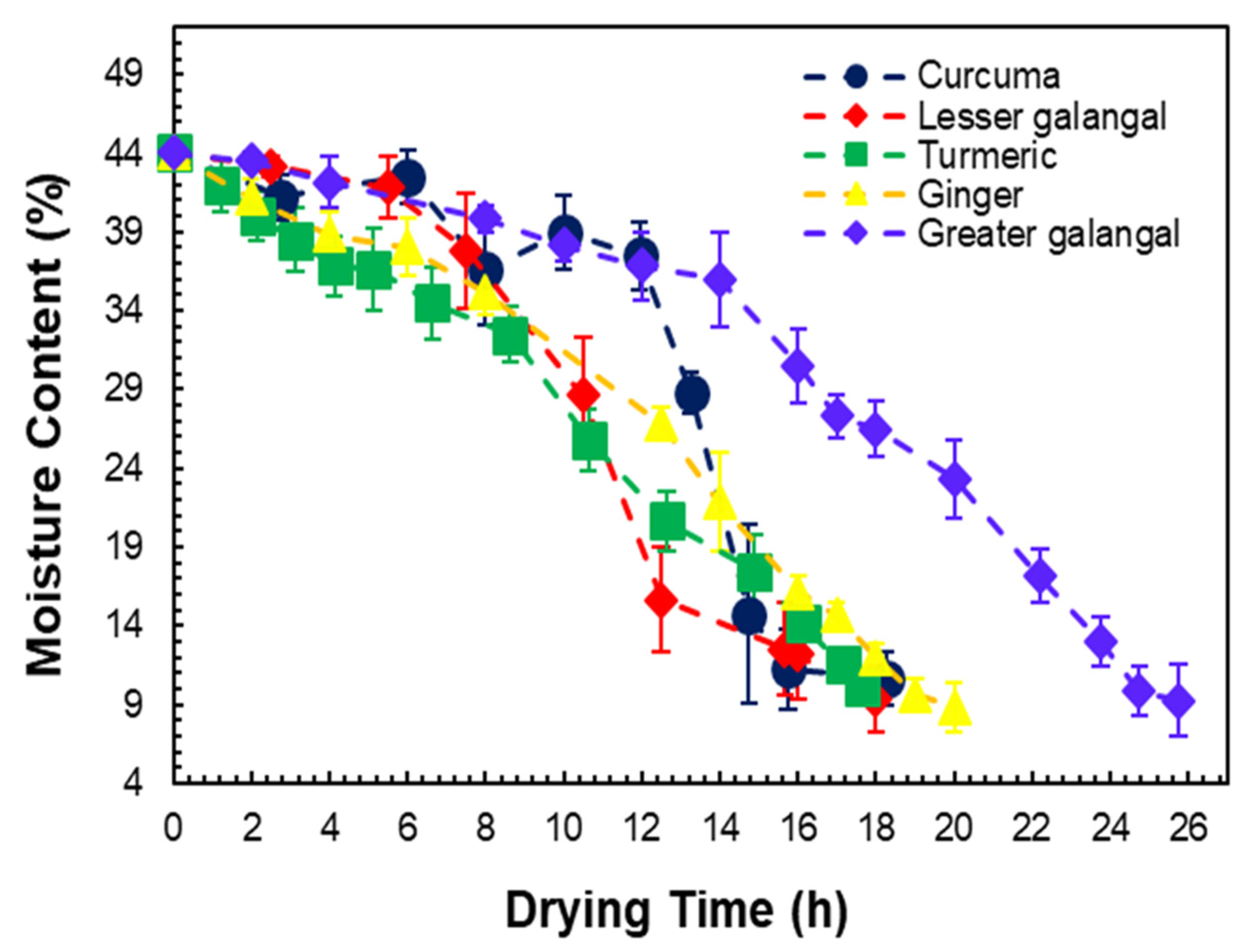
3.2. Drying Profiles of Medicinal Plant Materials
3.3. Drying Models of Medicinal Plant Materials
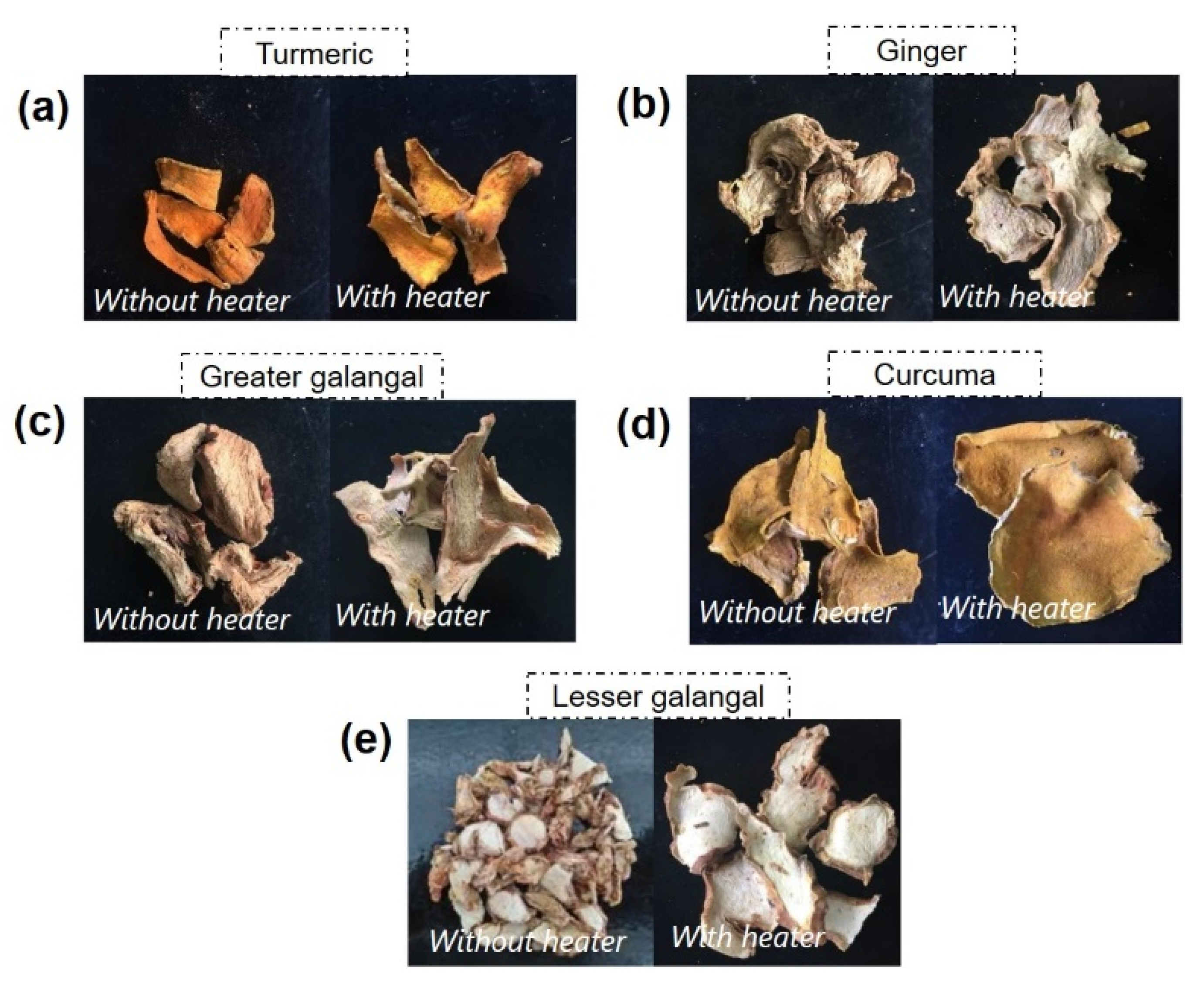
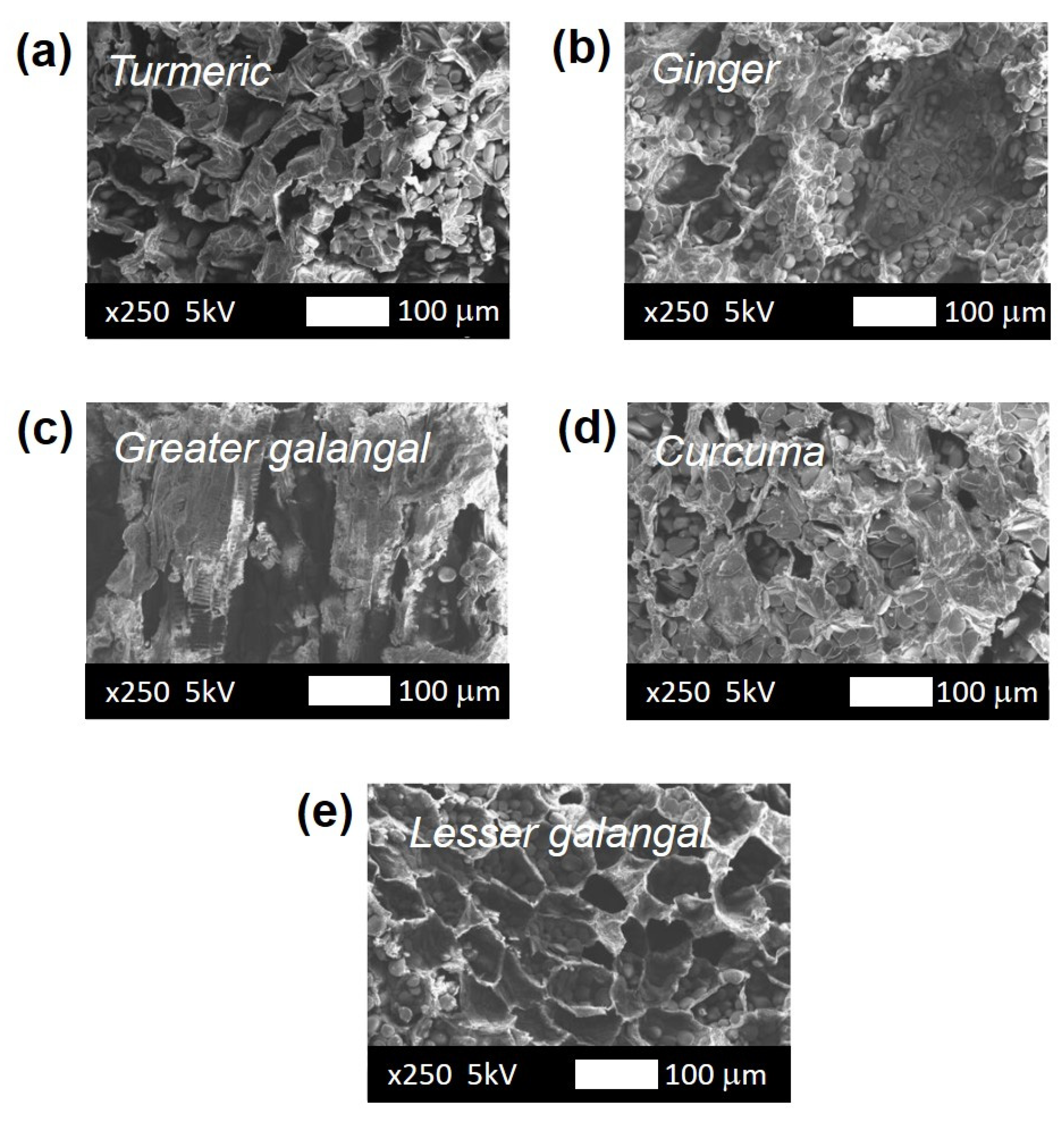
3.4. Quality of Dried Medicinal Materials
3.5. Energy and Economic Aspect of Polydryers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Purnomo, C.W.; Indarti, S. Modification of Indirect Solar Dryer for Simplicia Production. IOP Conf. Ser. Earth Environ. Sci. 2018, 120, 012026. [Google Scholar] [CrossRef]
- Sumarya, I.M.; Suarda, I.W.; Sudaryati, N.L.G.; Sitepu, I. Benefits of biopharmaca products towards healthy Indonesia. J. Phys. Conf. Ser. 2020, 1469, 012133. [Google Scholar] [CrossRef]
- Ministry of Health the Republic of Indonesia. Keputusan Menteri Kesehatan Republik Indonesia No. 261 Tahun 2009 Tentang Farmakope Herbal Indonesia Edisi Pertama. Sekretariat Negara. Jakarta. (Decree of the Minister of Health of the Republic of Indonesia No. 261/MENKES/SK/IV/2009 on the Indonesian Herbal Pharmacopoeia First Edition. Indonesia State Secretariat. Jakarta); Ministry of Health the Republic of Indonesia: Jakarta Selatan, Indonesia, 2009. [Google Scholar]
- Idu, M.; Erhabour, J.O.; Efijuemue, H.M. Documentation on medicinal plants sold in markets in Abeokuta, Nigeria. Trop. J. Pharm. Res. 2010, 9, 110–118. [Google Scholar] [CrossRef]
- Borah, A.; Sethi, L.N.; Sarkar, S.; Hazarika, K. Drying Kinetics of Sliced Turmeric (Curcuma longa L.) in a Solar-Biomass Integrated Drying System. J. Food Processing Preserv. 2017, 41, e12904. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. State of the art on solar drying technology: A review. Int. J. Renew. Energy Technol. 2012, 3, 107–141. [Google Scholar] [CrossRef]
- Shamekhi-Amiri, S.; Gorji, T.B.; Gorji-Bandpy, M.; Jahanshahi, M. Drying behaviour of lemon balm leaves in an indirect double-pass packed bed forced convection solar dryer system. Case Stud. Therm. Eng. 2018, 12, 677–686. [Google Scholar] [CrossRef]
- Sun, R.; Hikosaka, S.; Goto, E.; Sawada, H.; Saito, T.; Kudo, T.; Ohno, T.; Yoshimatsu, K.; Kawano, N.; Inui, T.; et al. Effects of Post-harvest Storage and Drying Temperatures on Four Medicinal Compounds in the Root of Chinese Licorice (Glycyrrhiza uralensis). Environ. Control Biol. 2014, 51, 149–155. [Google Scholar] [CrossRef]
- Maruddin, F.; Hasim, W.T.; Malaka, R.; Ali, H.M.; Taufik, M.; Sabil, S. Effects of drying methods and acidic strength on physicochemical properties of potassium caseinate. Ital. J. Food Sci. 2022, 34, 1–12. [Google Scholar] [CrossRef]
- Huang, B.; Wang, G.; Chu, Z.; Qin, L. Effect of Oven Drying, Microwave Drying, and Silica Gel Drying Methods on the Volatile Components of Ginger (Zingiber officinale Roscoe) by HS-SPME-GC-MS. Dry. Technol. 2012, 30, 248–255. [Google Scholar] [CrossRef]
- Nijhuis, H.H.; Torringa, H.M.; Muresan, S.; Yuksel, D.; Leguijt, C.; Kloek, W. Approaches to improving the quality of dried fruit and vegetables. Trends Food Sci. Technol. 1998, 9, 13–20. [Google Scholar] [CrossRef]
- Behera, S.; Mahanwar, P.A. Superabsorbent polymers in agriculture and other applications: A review. Polym. Plast. Technol. Mater. 2020, 59, 341–356. [Google Scholar] [CrossRef]
- Chang, L.; Xu, L.; Liu, Y.; Qiu, D. Superabsorbent polymers used for agricultural water retention. Polym. Test. 2021, 94, 107021. [Google Scholar] [CrossRef]
- Fang, S.; Wang, G.; Li, P.; Xing, R.; Liu, S.; Qin, Y.; Yu, H.; Chen, X.; Li, K. Synthesis of chitosan derivative graft acrylic acid superabsorbent polymers and its application as water retaining agent. Int. J. Biol. Macromol. 2018, 115, 754–761. [Google Scholar] [CrossRef]
- Lee, H.X.D.; Wong, H.S.; Buenfeld, N.R. Self-sealing of cracks in concrete using superabsorbent polymers. Cem. Concr. Res. 2016, 79, 194–208. [Google Scholar] [CrossRef]
- Zhong, P.; Wyrzykowski, M.; Toropovs, N.; Li, L.; Liu, J.; Lura, P. Internal curing with superabsorbent polymers of different chemical structures. Cem. Concr. Res. 2019, 123, 105789. [Google Scholar] [CrossRef]
- Draney, K.; Bates, J. Biodegradable Superabsorbent Polymers. In Proceedings of the TMS 2022 TMS 2022 Annual Meeting & Exhibition—Anaheim Convention Center & Anaheim Marriott, Anaheim, CA, USA, 27 February–3 March 2022; Springer International Publishing: Cham, The Netherlands, 2022; pp. 727–735. [Google Scholar]
- Abidin, A.Z.; Putra, R.P.; Izzati, A.U.N.; Christian, Y. Design and performance evaluation of a superabsorbent polymer-based dryer for medicinal plants. J. Food Processing Preserv. 2021, 45, e15988. [Google Scholar] [CrossRef]
- Abidin, A.Z.; Puspasari, T.; Graha, H.P.R. Utilization of cassava starch in copolymerisation of superabsorbent polymer composite (SAPC). J. Eng. Sci. Technol. 2014, 46, 286–298. [Google Scholar] [CrossRef][Green Version]
- Ikechukwu, G.A.; Mallik, S.; Njoku, J.E.E.; Depiver, J. Experimental Analysis of Thin Layer Drying of Ginger Rhizome in Convective Environment. Adv. Sci. Technol. Eng. Syst. J. 2020, 5, 1132–1142. [Google Scholar] [CrossRef]
- Jayashree, E.; Visvanathan, R.; Zachariah, J. Quality of dry ginger (Zingiber officinale) by different drying methods. J. Food Sci. Technol. 2014, 51, 3190–3198. [Google Scholar] [CrossRef]
- Bos, R.; Windono, T.; Woerdenbag, H.J.; Boersma, Y.L.; Koulman, A.; Kayser, O. HPLC-photodiode array detection analysis of curcuminoids in Curcuma species indigenous to Indonesia. Phytochem. Anal. 2007, 18, 118–122. [Google Scholar] [CrossRef]
- Das, G.; Patra, J.K.; Gonçalves, S.; Romano, A.; Gutiérrez-Grijalva, E.P.; Heredia, J.B.; Talukdar, A.D.; Shome, S.; Shin, H.-S. Galangal, the multipotent super spices: A comprehensive review. Trends Food Sci. Technol. 2020, 101, 50–62. [Google Scholar] [CrossRef]
- Hidayat, C.; Chaidir, L.; Supriadin, A.; Kurahman, O.T.; Mulya, A.M. Diversity of morphological characters of 30 local torch ginger accessions from Pangandaran of West Java of Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2019, 334, 012016. [Google Scholar] [CrossRef]
- Anindita, P.A.; Putri, T.K.; Ustari, D.; Maulana, H.; Rachmadi, M.; Concibido, V.; Suganda, T.; Karuniawan, A. Dataset of agromorphological traits in early population of turmeric (Curcuma longa L.) local accessions from Indonesia. Data Brief. 2020, 33, 106552. [Google Scholar] [CrossRef] [PubMed]
- Nagendra Chari, K.L.; Manasa, D.; Srinivas, P.; Sowbhagya, H.B. Enzyme-assisted extraction of bioactive compounds from ginger (Zingiber officinale Roscoe). Food Chem. 2013, 139, 509–514. [Google Scholar] [CrossRef]
- Diastuti, H.; Syah, Y.; Juliawaty, L.; Singgih, M. Antibacterial curcuma xanthorrhiza extract and fractions. J. Math. Fundam. Sci. 2014, 46, 224–234. [Google Scholar] [CrossRef][Green Version]
- Basri, A.M.; Taha, H.; Ahmad, N. A Review on the Pharmacological Activities and Phytochemicals of Alpinia officinarum (Galangal) Extracts Derived from Bioassay-Guided Fractionation and Isolation. Pharmacogn. Rev. 2017, 11, 43–56. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Amjad, M.W.; Hussain, F.; Ahmed, T.A.; Khan, S. Exploring recent developments to improve antioxidant, anti-inflammatory and antimicrobial efficacy of curcumin: A review of new trends and future perspectives. Mater. Sci. Eng. C 2017, 77, 1316–1326. [Google Scholar] [CrossRef]
- Abidin, A.Z.; Izzati, N.; Ridhawati, R. Synthesis and characterization of ssuperabsorbent polymer composites based on acrylic acid, acrylamide and bentonite. J. Sains Mater. Indones. 2011, 12, 114–119. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Janghela, S.; Saraiya, A.; Roy, D.; Mukhopadhyay, K.; Prasad, N.E. Effect of Reinforcement at Length Scale for Polyurethane Cellular Scaffolds by Supramolecular Assemblies. J. Phys. Chem. B 2018, 122, 2683–2693. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ministry of Health of the Republic of Indonesia. Indonesian Herbal Pharmacopoeia; Ministry of Health the Republic of Indonesia: Jakarta Selatan, Indonesia, 2008. [Google Scholar]
- Bruce, D.M. Exposed-layer barley drying: Three models fitted to new data up to 150 °C. J. Agric. Eng. Res. 1985, 32, 337–348. [Google Scholar] [CrossRef]
- Henderson, S.M.; Pabis, S. Grain drying theory: Temperature effect on drying kinetics. J. Food Eng. 1961, 39, 37–44. [Google Scholar]
- Majumder, P.; Sinha, A.; Mishra, L.; Gupta, R. Prediction of Moisture Ratios (MRs) During Fluidized Bed Drying of Ginger (Zingiber Officinale) Cubes by Using Mathematical Modeling and Experimental Validation; Biswal, B.B., Sarkar, B.K., Mahanta, P., Eds.; Springer: Singapore, 2020; pp. 729–740. [Google Scholar]
- Page, G.E. Factors Influencing the Maximum Rates of Air Drying Shelled Corn in Thin Layers; Purdue University: West Lafayette, IN, USA, 1949. [Google Scholar]
- Srimagal, A.; Mishra, S.; Pradhan, R.C. Effects of ethyl oleate and microwave blanching on drying kinetics of bitter gourd. J. Food Sci. Technol. 2017, 54, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Therdthai, N.; Northongkom, H. Characterization of hot air drying and microwave vacuum drying of fingerroot (Boesenbergia pandurata). Int. J. Food Sci. Technol. 2011, 46, 601–607. [Google Scholar] [CrossRef]
- Damena, O.; Tilahun, L.; Kuyu, C.G.; Bekele, Y.; Yirga, T.; Teka, T.A.; Hailu, D. Design, construction, and testing of passive type solar tunnel for maize grain disinfestations. Heliyon 2022, 8, e08739. [Google Scholar] [CrossRef]
- Yang, K.-S.; Hamid, K.; Wu, S.-K.; Sajjad, U.; Wang, C.-C. Experimental Analysis of a Heat Pump Dryer with an External Desiccant Wheel Dryer. Processes 2021, 9, 1216. [Google Scholar] [CrossRef]
- Villegas-Santiago, J.; Calderon-Santoyo, M.; Ragazzo-Sánchez, A.; Salgado-Cervantes, M.A.; Luna-Solano, G. Fluidized bed and tray drying of thinly sliced mango (Mangifera indica) pretreated with ascorbic and citric acid. Int. J. Food Sci. Technol. 2011, 46, 1296–1302. [Google Scholar] [CrossRef]
- Mozafari, A.A.; Vafaee, Y.; Shahyad, M. Phytochemical composition and in vitro antioxidant potential of Cynodon dactylon leaf and rhizome extracts as affected by drying methods and temperatures. J. Food Sci. Technol. 2018, 55, 2220–2229. [Google Scholar] [CrossRef]
- Zuber, L.; Dunn, I.J.; Deshusses, M.A. Comparative Scale-Up and Cost Estimation of a Biological Trickling Filter and a Three-Phase Airlift Bioreactor for the Removal of Methylene Chloride from Polluted Air. J. Air Waste Manag. Assoc. 1997, 47, 969–975. [Google Scholar] [CrossRef]
- Sindhu, J.L.K.; Mohammed Ibrahim, S.; Reddy, K.P.J. Experimental Investigation of Film Cooling Technique over a Blunt Body in Hypersonic Flow. In Proceedings of the 31st International Symposium on Shock Waves, Nagoya, Japan, 9–14 July 2017; Sasoh, A., Aoki, T., Katayama, M., Eds.; Springer International Publishing: Cham, The Netherlands, 2019; pp. 1127–1133. [Google Scholar]
- Joshi, P.R.; Deshmukh, S.C.; Morone, A.P.; Kanade, G.; Pandey, R.A. Air-lift reactor system for the treatment of waste-gas-containing monochlorobenzene. Environ. Technol. 2013, 34, 3023–3029. [Google Scholar] [CrossRef]
- Maier, D.E.; Bakker-Arkema, F.W. Grain drying systems. In Proceedings of the 2002 Facility Design Conference of the Grain Elevator & Processing Society, St. Charles, IL, USA, 28–31 July 2002; pp. 28–31. [Google Scholar]
- Singh, K.J.; Ahuja, I.S.; Kapoor, J.J.A.E.F. Optimization of Process Parameters for Surface Roughness in Ultrasonic Machining of Polycarbonate Bullet Proof Glass and Acrylic Heat Resistant Glass by Taguchi and Grey Relational Analysis Approach. Adv. Eng. Forum 2017, 23, 21–44. [Google Scholar] [CrossRef]
- Baini, R.; Lai, J.C.H.; Abdul Samat, N.A.; Rahman, M.R.; Mohidi, N.S.A. Performance Evaluation of Solar and Oven Drying for Tropical Fruits. Int. J. Adv. Sci. Res. Eng. (IJASRE) 2018, 4, 215–224. [Google Scholar] [CrossRef]
- Fudholi, A.; Sopian, K.; Ruslan, M.H.; Alghoul, M.A.; Sulaiman, M.Y. Review of solar dryers for agricultural and marine products. Renew. Sustain. Energy Rev. 2010, 14, 1–30. [Google Scholar] [CrossRef]
- Hamdan, M.; Sabudin, S.; Faizal, M.; Raghavan, V.R. Experimental studies on oil palm frond drying using swirling fluidized bed dryer. AIP Conf. Proc. 2012, 1440, 1212–1219. [Google Scholar] [CrossRef]
- Gagare, S.; Mudgal, V.D.; Champawat, P.S. Convective Drying of Turmeric Rhizome. J. Agric. Eng. Res. 2017, 54, 33–39. [Google Scholar]
- Hoque, M.A.; Bala, B.K.; Hossain, M.A.; Uddin, M.B. Drying Kinetics Of Ginger Rhizome (Zingiber officinale). Bangladesh J. Agric. Res. 2013, 38, 301–319. [Google Scholar] [CrossRef]
- Canabarro, N.I.; Mazutti, M.A.; do Carmo Ferreira, M. Drying of olive (Olea europaea L.) leaves on a conveyor belt for supercritical extraction of bioactive compounds: Mathematical modeling of drying/extraction operations and analysis of extracts. Ind. Crops Prod. 2019, 136, 140–151. [Google Scholar] [CrossRef]
- Gan, H.; Charters, E.; Driscoll, R.; Srzednicki, G. Effects of Drying and Blanching on the Retention of Bioactive Compounds in Ginger and Turmeric. Horticulturae 2017, 3, 13. [Google Scholar] [CrossRef]
- Namkanisorn, A.; Murathathunyaluk, S. Sustainable drying of galangal through combination of low relative humidity, temperature and air velocity. Energy Rep. 2020, 6, 748–753. [Google Scholar] [CrossRef]
- Lv, W.; Zhang, M.; Wang, Y.; Adhikari, B. Online measurement of moisture content, moisture distribution, and state of water in corn kernels during microwave vacuum drying using novel smart NMR/MRI detection system. Dry. Technol. 2018, 36, 1592–1602. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, M.; Mujumdar, A.S.; Zhong, Q.; Wang, Z. Measurement of water mobility and distribution in vacuum microwave-dried barley grass using Low-Field-NMR. Dry. Technol. 2018, 36, 1892–1899. [Google Scholar] [CrossRef]
- Aritesty, E.; Wulandani, D. Performance of the Rack Type-greenhouse Effect Solar Dryer for Wild Ginger (Curcuma xanthorizza Roxb.) Drying. Energy Procedia 2014, 47, 94–100. [Google Scholar] [CrossRef]
- Hailu, A.T. Design, Fabrication and Performance Evaluation of Solar Tunnel Dryer for Ginger Drying; Mettu University: Metu, Ethiopia, 2021. [Google Scholar] [CrossRef]
- Lakshmi, D.V.N.; Muthukumar, P.; Ekka, J.P.; Nayak, P.K.; Layek, A. Performance comparison of mixed mode and indirect mode parallel flow forced convection solar driers for drying Curcuma zedoaria. J. Food Process Eng. 2019, 42, e13045. [Google Scholar] [CrossRef]
- Gill, R.S.; Singh, S.; Hans, V.S.; Mittal, T.C. Turmeric (Curcuma longa) drying in natural circulation solar dryer: An experimental evaluation. J. Food Process Eng. 2021, 44, e13765. [Google Scholar] [CrossRef]
- Ghazanfari, A.; Emami, S.; Tabil, L.G.; Panigrahi, S. Thin-Layer Drying of Flax Fiber: II. Modeling Drying Process Using Semi-Theoretical and Empirical Models. Dry. Technol. 2006, 24, 1637–1642. [Google Scholar] [CrossRef]
- Danish, M.; Jing, H.; Pin, Z.; Ziyang, L.; Pansheng, Q. A new drying kinetic model for sewage sludge drying in presence of CaO and NaClO. Appl. Therm. Eng. 2016, 106, 141–152. [Google Scholar] [CrossRef]
- Dutta, P.P.; Baruah, D.C. Drying modelling and experimentation of Assam black tea (Camellia sinensis) with producer gas as a fuel. Appl. Therm. Eng. 2014, 63, 495–502. [Google Scholar] [CrossRef]
- Marinos-Kouris, D.; Maroulis, Z.B. Transport Properties in the Drying of Solids; CRC Press: Boca Raton, FL, USA, 2020; pp. 113–159. Volume 113. [Google Scholar]
- Krokida, M.K.; Karathanos, V.T.; Maroulis, Z.B.; Marinos-Kouris, D. Drying kinetics of some vegetables. J. Food Eng. 2003, 59, 391–403. [Google Scholar] [CrossRef]
- Djaeni, M.; Sari, D.A. Low Temperature Seaweed Drying Using Dehumidified Air. Procedia Environ. Sci. 2015, 23, 2–10. [Google Scholar] [CrossRef]
- Göğüş, F.; Maskan, M. Air drying characteristics of solid waste (pomace) of olive oil processing. J. Food Eng. 2006, 72, 378–382. [Google Scholar] [CrossRef]
- Sripinyowanich, J.; Noomhorm, A. A New Model and Quality of Unfrozen and Frozen Cooked Rice Dried in a Microwave Vibro-Fluidized Bed Dryer. Dry. Technol. 2011, 29, 735–748. [Google Scholar] [CrossRef]
- Obregon, F.I.V.; Silvano, M.B.C.; Vicencio, J.G.; Pestaño, L.D.B. Numerical Simulation of the Drying Kinetics of Sweet Potato to Prevent the Growth of the Fungi Rhizopus oryzae. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012076. [Google Scholar] [CrossRef]
- Santos, N.C.; da Silva, W.P.; Barros, S.L.; Almeida, R.L.J.; de Brito Araújo, A.J.; da Silva Nascimento, A.P. Red rice (Oryza sativa L.) use in flour production: Convective drying and bioactive quality. J. Food Process Eng. 2020, 43, e13490. [Google Scholar] [CrossRef]
- Acar, B.; Sadikoglu, H.; Doymaz, I. Freeze-Drying Kinetics and Diffusion Modeling of Saffron (Crocus sativus L.). J. Food Process. Preserv. 2015, 39, 142–149. [Google Scholar] [CrossRef]
- Kumar, Y.; Sharanagat, V.S.; Singh, L.; Nema, P.K. Convective drying of spine gourd (Momordica dioica): Effect of ultrasound pre-treatment on drying characteristics, color, and texture attributes. J. Food Processing Preserv. 2020, 44, e14639. [Google Scholar] [CrossRef]
- Kumari, V.; Yadav, B.S.; Yadav, R.B.; Nema, P.K. Effect of osmotic agents and ultasonication on osmo-convective drying of sweet lime (Citrus limetta) peel. J. Food Process Eng. 2020, 43, e13371. [Google Scholar] [CrossRef]
- Khaerunnisa; Mahendradatta, M.; Asfar, M. Characteristics of simplicia ginger (Zingiber officinale) and lemongrass (Cymbopogon citratus) powder by different drying method. IOP Conf. Ser. Earth Environ. Sci. 2021, 807, 022052. [Google Scholar] [CrossRef]
- Dehghannya, J.; Gorbani, R.; Ghanbarzadeh, B. Influence of combined pretreatments on color parameters during convective drying of Mirabelle plum (Prunus domestica subsp. syriaca). Heat Mass Transf. 2017, 53, 2425–2433. [Google Scholar] [CrossRef]
- Nimmanpipug, N.; Therdthai, N.; Dhamvithee, P. Characterisation of osmotically dehydrated papaya with further hot air drying and microwave vacuum drying. Int. J. Food Sci. Technol. 2013, 48, 1193–1200. [Google Scholar] [CrossRef]
- Ukegbu, P.O.; Okereke, C.J. Effect of solar and sun drying methods on the nutrient composition and microbial load in selected vegetables, African spinach (Amaranthus hybridus), fluted pumpkin (Telferia occidentalis), and okra (Hibiscus esculentus). Sky J. Food Sci. 2013, 2, 35–40. [Google Scholar]
- Hasibuan, R.; Zamzami, M.A. The Effect of Operating Conditions on Drying Characteristics and Quality of Ginger (Zingiber Officinale Roscoe) Using Combination of Solar Energy-Molecular Sieve Drying System. IOP Conf. Ser. Mater. Sci. Eng. 2017, 180, 012153. [Google Scholar] [CrossRef]
- Halilah, N.A.; Febrina, L.; Ramadhan, A.M. Standarisasi Ekstrak Daun Nona Makan Sirih (Clerodendrum x speciosum Dombrain). Proc. Mulawarman Pharm. Conf. 2017, 6, 36–40. [Google Scholar] [CrossRef]
- Santana, Á.L.; Zabot, G.L.; Osorio-Tobón, J.F.; Johner, J.C.F.; Coelho, A.S.; Schmiele, M.; Steel, C.J.; Meireles, M.A.A. Starch recovery from turmeric wastes using supercritical technology. J. Food Eng. 2017, 214, 266–276. [Google Scholar] [CrossRef]
- Chakraborty, I.; Pallen, S.; Shetty, Y.; Roy, N.; Mazumder, N. Advanced microscopy techniques for revealing molecular structure of starch granules. Biophys. Rev. 2020, 12, 105–122. [Google Scholar] [CrossRef]
- Izli, N.; Polat, A. Effect of convective and microwave methods on drying characteristics, color, rehydration and microstructure properties of ginger. Food Sci. Technol. 2019, 39, 652–659. [Google Scholar] [CrossRef]
- Duy, V.N.; Duc, K.N.; Van, N.C. Real-time driving cycle measurements of fuel consumption and pollutant emissions of a bi-fuel LPG-gasoline motorcycle. Energy Convers. Manag. X 2021, 12, 100135. [Google Scholar] [CrossRef]
- Parbowo, H.S.; Ardy, A.; Susanto, H. Techno-economic analysis of Dimethyl Ether production using Oil Palm Empty Fruit Bunches as feedstock—A case study for Riau. IOP Conf. Ser. Mater. Sci. Eng. 2019, 543, 012060. [Google Scholar] [CrossRef]
- Naimah, D.Y.N.; Novitasari, D.; Indartono, Y.S.; Wulandari, E. Technoeconomic and environment assessment of rural electrification using solar photovoltaic (Case study in Parang Island, Indonesia). Int. J. Smart Grid Clean Energy 2020, 9, 383–389. [Google Scholar] [CrossRef]



| Model | Mathematical Equation | Linear Logarithmic Equation | Reference |
|---|---|---|---|
| Lewis | MR = exp (−kt) | ln MR = −kt | [34,39] |
| Henderson & Pabis | MR = a exp (−kt) | ln MR = −kt + ln(a) | [35,36] |
| Page | MR = exp (−ktn) | ln (−ln(MR)) = ln(k) + n ln(t) | [37,38] |
| No. | Material | Initial Moisture Content (%) | Final Moisture Content (%) | Drying Time (h) | ||
|---|---|---|---|---|---|---|
| without Heater | with Heater | without Heater | with Heater | |||
| 1 | Turmeric | 83.1 ± 1.43 | 9.0 ± 2.10 | 10.2 ± 1.07 | 117 ± 1.00 | 18 ± 0.50 |
| 2 | Ginger | 83.2 ± 1.14 | 13.2 ± 1.17 | 8.8 ± 1.57 | 96 ± 0.50 | 20 ± 0.50 |
| 3 | Greater galangal | 89.2 ± 1.45 | 16.0 ± 0.63 | 9.3 ± 2.24 | 119 ± 1.00 | 25 ± 0.65 |
| 4 | Curcuma | 73.2 ± 1.50 | 13.6 ± 0.80 | 10.7 ± 1.70 | 95 ± 0.25 | 18 ± 0.40 |
| 5 | Lesser galangal | 75.2 ± 1.25 | 9.33 ± 2.13 | 9.3 ± 2.13 | 97 ± 0.30 | 18 ± 0.45 |
| No. | Material | Final Moisture Content Using Moisture Content Meter (%) | Final Moisture Content Using Distillation Method (%) | Maximum Moisture Content in Simplicia * (%) |
|---|---|---|---|---|
| 1 | Ginger | 8.8 ± 1.57 | 7.66 ± 0.58 | ≤10 |
| 2 | Greater galangal | 9.3 ± 2.24 | 7.32 ± 1.16 | ≤10 |
| 3 | Curcuma | 10.7 ± 1.70 | 7.98 ± 0.01 | ≤13 |
| 4 | Lesser galangal | 9.3 ± 2.13 | 11.3 ± 1.15 | ≤12 |
| No. | Material | Lewis | Henderson & Pabis | Page | |||||
|---|---|---|---|---|---|---|---|---|---|
| k | R2 | k | a | R2 | k | n | R2 | ||
| 1 | Turmeric | 0.132 | 0.997 | 0.148 | 1.302 | 0.978 | 0.017 | 1.725 | 0.945 |
| 2 | Ginger | 0.048 | 0.872 | 0.056 | 1.035 | 0.797 | 0.027 | 1.361 | 0.963 |
| 3 | Greater galangal | 0.061 | 0.951 | 0.077 | 1.389 | 0.903 | 0.004 | 1.799 | 0.985 |
| 4 | Curcuma | 0.055 | 0.939 | 0.070 | 1.100 | 0.964 | 0.036 | 1.222 | 0.963 |
| 5 | Lesser galangal | 0.022 | 0.185 | 0.065 | 1.162 | 0.387 | 0.001 | 2.701 | 0.973 |
| No. | Material | Average Pixel Count | |
|---|---|---|---|
| with Heater | without Heater | ||
| 1 | Turmeric | 121.31 | 124.79 |
| 2 | Ginger | 161.43 | 141.09 |
| 3 | Greater galangal | 139.04 | 130.30 |
| 4 | Curcuma | 122.11 | 139.74 |
| 5 | Lesser galangal | 211.99 | 194.81 |
| No. | Material | Ash Content (%) | Maximum Ash Content in Simplicia (%) * |
|---|---|---|---|
| 1 | Ginger | 6.49 ± 0.170 | ≤4.2 |
| 2 | Greater galangal | 4.04 ± 0.023 | ≤3.9 |
| 3 | Curcuma | 4.53 ± 0.044 | ≤4.8 |
| 4 | Lesser galangal | 4.29 ± 0.012 | ≤8.7 |
| No. | Material | Drying Shrinkage (%) |
|---|---|---|
| 1 | Ginger | 12.35 ± 1.05 |
| 2 | Greater galangal | 18.71 ± 0.30 |
| 3 | Curcuma | 18.18 ± 0.97 |
| 4 | Lesser galangal | 16.73 ± 0.23 |
| No. | Material | Gas Fuel Consumption (kg) | Electricity Consumption (kWh) | Total Power Consumption (kWh) | Total Power Cost (USD) | ||||
|---|---|---|---|---|---|---|---|---|---|
| without Heater | with Heater | without Heater | with Heater | without Heater | with Heater | without Heater | with Heater | ||
| 1 | Turmeric | 0 | 2.30 | 43.29 | 6.66 | 43.29 | 38.65 | 3.89 | 2.44 |
| 2 | Ginger | 0 | 2.56 | 35.52 | 7.40 | 35.52 | 42.95 | 3.19 | 2.71 |
| 3 | Greater galangal | 0 | 3.20 | 44.03 | 9.25 | 44.03 | 53.68 | 3.96 | 3.39 |
| 4 | Curcuma | 0 | 2.30 | 35.15 | 6.66 | 35.15 | 38.65 | 3.16 | 2.44 |
| 5 | Lesser galangal | 0 | 2.30 | 35.89 | 6.66 | 35.89 | 38.65 | 3.23 | 2.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abidin, A.Z.; Gunawan, D.A.; Putra, R.P.; Theodric, D.; Abidin, T. Medicinal Plant Drying Using a Superabsorbent Polymer Dryer Incorporated with an Insulated Heater. Processes 2022, 10, 2319. https://doi.org/10.3390/pr10112319
Abidin AZ, Gunawan DA, Putra RP, Theodric D, Abidin T. Medicinal Plant Drying Using a Superabsorbent Polymer Dryer Incorporated with an Insulated Heater. Processes. 2022; 10(11):2319. https://doi.org/10.3390/pr10112319
Chicago/Turabian StyleAbidin, Akhmad Zainal, Dwi Ananda Gunawan, Ridwan P. Putra, Darien Theodric, and Taufik Abidin. 2022. "Medicinal Plant Drying Using a Superabsorbent Polymer Dryer Incorporated with an Insulated Heater" Processes 10, no. 11: 2319. https://doi.org/10.3390/pr10112319
APA StyleAbidin, A. Z., Gunawan, D. A., Putra, R. P., Theodric, D., & Abidin, T. (2022). Medicinal Plant Drying Using a Superabsorbent Polymer Dryer Incorporated with an Insulated Heater. Processes, 10(11), 2319. https://doi.org/10.3390/pr10112319







