Abstract
Increases in energy demand and waste are a major cause of natural resource depletion and environmental pollution, and technology capable of processing waste to convert it into energy is required to mitigate this issue. Hydrothermal carbonization (HTC) is an example of this technology that can convert waste into energy, and various studies have been conducted using it for fuel conversion. This study focused on the production of a solid fuel equivalent to coal for power generation through HTC processes using waste wood. Unlike previous work, which consists only of laboratory-scale HTC experiments, we confirmed scalability through pilot-scale HTC experiments. Overall, it was possible to convert waste wood into HTC solid fuel with a calorific value of over 27,000 kJ/kg through the pilot plant HTC process. Additionally, heavy metal and hazardous substance analyses proved that it can be used as a biosolid fuel.
1. Introduction
Increases in energy demand and waste are causing natural resource depletion and environmental pollution, making technologies capable of solving these problems crucial worldwide [1]. Many Asian, North American, and European governments are focusing on developing technologies that convert various organic wastes into energy as a solution to the zero organic waste policy. Among organic waste-to-energy technologies, hydrothermal carbonization (HTC) is used in various fields, such as energy conversion, environmental improvement, and nutrient recovery, and can convert waste into high-energy-density carbon materials [2].
HTC is a reaction that combines dehydration and decarboxylation processes, and its solid fuels have high calorific values with increasing carbon contents [3]. To convert organic waste into solid fuel using HTC, a temperature of approximately 180–250 °C is required to be maintained for 1–12 h [4,5]. It is relatively low compared with other energization technologies, such as pyrolysis requiring 400 °C and gasification requiring 800 °C. Thus, there is an advantage in that less input energy is required. Additionally, HTC has the advantage of producing solid fuel without dehydration and drying in the pretreatment process [6].
Recently, many studies have been conducted to confirm the possibility of biofuel conversion from various wastes using HTC technology. In these studies, various organic wastes have been used including waste wood, paper, MSW, food waste, and animal waste [7,8,9,10]. Additionally, the calorific value of hydrochar after HTC is relatively high in lignocellulosic biomass, such as wood or wood chips, which contain a substantial amount of carbon [11]. However, considering commercial facility design, if the HTC reaction time has to be increased from 4 to 72 h when producing biofuels, it is not an efficient fuel production per hour [7,8,12,13].
Research on HTC biofuel generation using catalysts is also being conducted. The catalysts are made by dissolving in distilled water by mixing with one or more chloride-based metal salts and acids. The catalyst concentration in the aqueous solution is in the range of 2–20 g/L. Hence, the catalyst is used to lower the pH concentration, thereby lowering the activation energy and reducing the hydrothermal carbonization reaction time. When lignocellulosic biomass feedstock HTC was compared according to the presence or absence of catalysts, the reaction time was reduced from 4 to 1 h when catalysts were absent and present, respectively [14,15]. The reaction temperature can be lowered from 10 to 40 °C, and the reaction pressure can be lowered by 1–2 MPa, which reduces the cost when manufacturing the reactor. Additionally, after HTC, the lignin component in the organic material decomposes in the hydrochar, but catalytic HTC showed less lignin degradation by FTIR spectroscopy [15]. Furthermore, when the lignin components are maintained, they have an adsorption effect on each other, making it possible to produce advantageous pellets without the additional attachment of materials during fuel transport. It was confirmed that there was no breakdown through the electrochemical water ingress (EWI) test [16].
Some researchers have expanded their facility capacity for commercialization. For example, Hoekman et al. [17] fabricated an expanded hydrothermal carbonization reactor (40 L) to process 3 kg of lignocellulosic biomass and showed that the experimental results were similar to those of a laboratory-scale 2 L reactor. They compared the experimental results when the HTC reaction temperature was 235 and 275 °C. At 275 °C, the calorific value increased by 40% compared to the supplied raw material. However, considering the effect of increasing the calorific value, when the reaction temperature condition is 275 °C, energy cost and cooling time increase. Furthermore, Ismail et al. [18] numerically analyzed the expected energy and production volume when converting municipal solid waste (MSW) into solid fuel in a pilot-scale facility. They proposed a numerical model for a pilot plant facility that converts MSW to coal through numerical analysis. These studies are meaningful from the perspective of commercial design, but verification has not been conducted through commercialization facilities. Mackintosh et al. [19] designed a pilot plant-scale binary reactor, performed proximate analyses, and determined the calorific value of hydrochar according to the HTC reaction of the catalyst [15] they applied to their lab-scale HTC experiments. They further analyzed the fuel characteristics by expanding the pilot scale, but a comparative study with and without a catalyst is required to verify the effect of the catalyst.
In this study, biofuel with a calorific value equivalent to that of coal for power generation was produced in a pilot plant through waste wood HTC. To verify the scalability, HTC reaction experiments were conducted using lab-scale (0.2 L—3 sphere) and pilot-scale (500 L) reactors, and the pilot-scale reactor was composed of the equipment for the entire process. First, the calorific values and mass yields of solid fuels generated through laboratory-scale HTC experiments were compared according to the presence or absence of catalysts and catalyst concentration. The added catalyst is a mixture of a chloride-based metal salt and an acid, which is a different combination from the catalysts of previous studies. Next, heavy metal and hazardous substance analyses were used to confirm whether the solid fuel that satisfied the desired calorific value and yield condition met the fuel use standard. Based on the determined catalyst conditions, a pilot-scale HTC experiment was performed according to the catalyst concentrations. The effect of catalyst addition was confirmed via comparative analyses of the generated solid fuel according to the presence or absence of catalysts. Overall, the trends of the pilot- and laboratory-scale experimental results were consistent, and their scalability was verified. The biosolid fuel produced through HTC with catalyst additions in the pilot plant proved that it can be used as fuel through heavy metal and hazardous substance analyses.
2. Experimental Setup and Procedure
2.1. Fuel Characteristics of the Waste Wood Raw Material
The waste wood used in the experiment was selected as a product that can be supplied in large quantities because it is required for both laboratory- and pilot-scale HTC experiments. The waste wood is acquired through a company that specializes in recycling. In the test site, only waste wood that has not been stained with adhesives, paints, oil, or preservatives was permitted to be brought in. The same waste wood was used for both the laboratory- and pilot-scale HTC experiments. To compare the fuel properties of the hydrochar after HTC, we analyzed the fuel properties of the raw materials (Table 1). Table 1 shows the results of the fuel characteristic analyses of the waste wood. Detailed fuel characteristic analyses can be divided into proximate, elemental, and calorific value analyses. The collected samples were subjected to proximate analysis before drying, while elemental and calorific value analyses were performed after drying. The analysis method used followed the American Society for Testing and Materials (ASTM), and different equipment were used according to each analysis method. Proximate analysis was performed using the TGA-701 Proximate analyzer, and the ratio of volatile substances, ash, water, and fixed carbon can be known. Elemental analysis was performed using TruSpec Elemental Analyzer. To measure carbon and hydrogen, the sample was burned in a tube furnace and the water and CO2 produced were absorbed and analyzed. The nitrogen concentration was measured by the Kjeldahl–Gunning method [20]. Sulfur was analyzed using an SC-832DR Sulfur Analyzer. Sulfur was measured by conversion to sulfur dioxide, followed by absorption in hydrogen peroxide solution, and titration with barium acetate solution. The calorific value is divided into a Higher Heating Value (HHV) and a Lower Heating Value (LHV). In this study, the calorific value was confirmed to be measurable HHV. The calorific value was analyzed using an AC600 Semi-Auto Calorimeter. The calorific value is confirmed by analyzing the temperature change after the combustion of the sample based on the initial temperature.

Table 1.
Waste wood chip fuel characteristics.
2.2. Specification of a Laboratory-Scale Reactor for HTC Experiments
Three 200 mL reactors were installed in the laboratory-scale HTC experiment equipment (Figure 1), and a heating jacket was used as the heat source of the reactor to obtain the appropriate moisture content. The maximum allowable temperature was 250 °C, and the reactor temperature was capable of reaching the reaction temperature using the heating jacket and temperature sensor. The time to reach the reaction temperature is about 50 min. Therefore, the average heating rate of the reactor is 4.5 °C/min. The electric capacity supplied to one unit of the heating jacket was 1.5 kW, and the maximum electric power of the HTC test equipment for the experiment was 5 kW. A temperature sensor that could measure the temperature and a control device that could maintain the reaction temperature were installed. Additionally, a pressure sensor was installed to check the pressure according to the temperature inside the reactor, which was measured to be approximately 2.32 MPa when the temperature inside the reactor was 220 °C. SUS316L, which has strong corrosion resistance, was used as the reactor material in consideration of corrosion and the catalyst, and the maximum allowable pressure of the reactor was designed to be 5.39 MPa considering safety.

Figure 1.
Laboratory-scale hydrothermal carbonization reactor (0.2 L).
2.3. Laboratory-Scale Reactor HTC Experimental Conditions and Process
The HTC process can be divided into pretreatment, reaction, and post-treatment steps. In the pretreatment step, the raw material was pulverized into particles of 2 mm or less to produce uniform particles. The moisture content of the pulverized raw material was then measured and placed into an aqueous solution inside the reactor to obtain the appropriate moisture content. The total volume of the raw material and the aqueous solution was 60–70% of the total volume of the reactor. The amount of catalyst added to the input aqueous solution varied depending on the catalyst concentration, including the case with or without the catalyst. The added catalyst contains inorganic metals and acids. At this time, the case where a catalyst is added to HTC is called catalytic HTC. Additionally, the amount of catalyst added is determined by catalyst conditions. Catalytic conditions according to the experiment were similarly performed according to previous studies [14,15]. The catalyst is a combination of specific inorganic metals and acids. Two combinations designated by the KINAVA Company were used in the above experiment. The first case (Catalyst #1) is a combina-tion of strong acid-based catalysts. The second case (Catalyst #2) is a combination of weak acid-based catalysts. In all cases, they were provided in the form of liquid catalysts prepared by an already specified method. Then, adding the catalyst, the catalyst was diluted in 40% of the total aqueous solution, mixed with the remaining aqueous solution, stirred for 20 min, then added into the reactor. In the reaction step, a heat source was supplied such that the temperature inside the reactor reached the reaction temperature, and the reaction temperature was maintained for the reaction time. In the post-treatment step, it was cooled after the reaction holding time while discharging the vapor inside the reactor. When the temperature reached 80 °C, the HTC product inside the reactor was recovered, and the liquid and solid wastes were separated. Finally, the calorific values and mass yields were measured by drying the solid.
2.4. Pilot Plant Reactor Configuration and Process
The pilot plant facility for the pilot-scale HTC experiment was connected from raw material storage to the pellet production process, as shown in Figure 2. The capacity of the HTC reactor was 500 L, and the heat source was waste heat steam generated at the installation site and electrical energy through the heating jacket. First, waste heat steam with a pressure and temperature of 1.96 MPa and 200 °C raises the reactor temperature to 200 °C. After that, the reactor’s internal temperature is increased through the heating jacket. The temperature could be maintained constant after reaching the target reaction temperature because the PID controller was connected to the heating jacket.
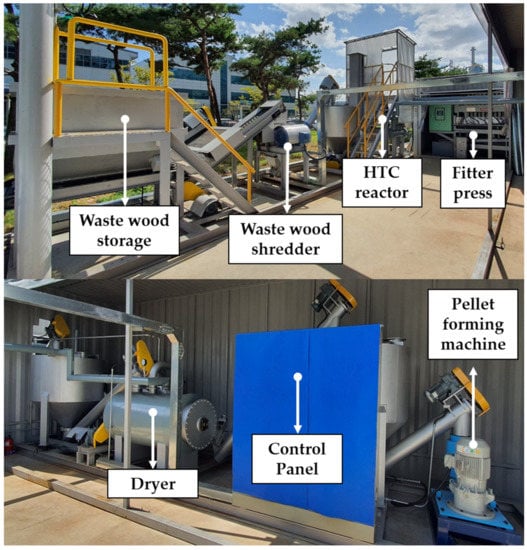
Figure 2.
HTC biofuel production pilot plant.
For each process of the pilot plant, the conversion of waste wood to HTC solid fuel through the process is shown in Figure 3. The raw material of waste wood was homogenized into particles within 2 mm using a shredder. Pulverized waste wood produced HTC solid fuel through HTC, and its shape changed depending on its moisture content and molding. The process consisted of the following steps: raw material storage and pretreatment, HTC, solid–liquid separation, drying, and pellet production.

Figure 3.
HTC solid fuel according to the pilot plant process.
- Step 1. Raw Material Storage and Pretreatment Process
Waste wood, which is a raw material, is transported to a waste wood storage tank where it could be stored and transported. To pulverize into uniform particles, metal materials that are mixed in the waste wood are first removed using a magnetic separator. The inclusion of non-ferrous metals is minimized in sorting waste wood before transport. However, the reason for installing the magnetic separator is that the residual metal mixed in the waste wood may cause a malfunction of the conveying machine. The waste wood is then pulverized into uniform particles within 2 mm using a grinder. The pulverized waste wood is transported to the pulverized wood storage tank through a screw conveyor and stored for use in the next process.
- Step 2. HTC Process
The pulverized waste wood is moved to the reactor inlet via a bucket conveyor. The feeding speed of the bucket conveyor can be adjusted through the control panel. At this time, the input amount can be known through the mass sensor in the pulverized waste wood storage tank. The reactor is a batch type, and the inlet and outlet can also be opened and closed through the control panel. The water for processing is placed in the reactor, and the stirrer is operated simultaneously so that the pulverized waste wood and water content is appropriate. At this time, approximately 60–70% of the total volume of the HTC reactor is filled. When the input is completed, the input valve of the HTC reactor is shut off, and a heat source is supplied to the HTC tank to increase the target reaction temperature. The reaction temperature is raised by introducing steam up to 200 °C, at which point the waste heat steam is cut off. At 200 °C or higher, the heat source of the heating jacket is used to increase the temperature to the target reaction temperature. After maintaining the reaction temperature for the target reaction time, the HTC reactor is cooled through steam discharge. When the internal temperature of the HTC reactor is 120 °C, it is discharged into the carbide storage tank using the internal pressure of the HTC reactor.
- Step 3. Solid–Liquid Separation Process
The water contained in the hydrothermally carbonized solid is removed by transporting it to a high-pressure filter press using a pump. The solid–liquid separation liquid removed from the solid–liquid separation process is stored in a separate wastewater storage tank. Furthermore, the water content of the hydrothermally carbonized solid is approximately 50%. Subsequently, it is moved to a solid storage tank.
- Step 4. Drying Process and Pellet Production Process
The hydrothermally carbonized solid is dried to a moisture content of 20% after being transferred to a dryer. The moisture that evaporated during the drying process is condensed using a condenser, and the condensed water is moved to the wastewater storage tank. It is transported to the condensed gas treatment facility to remove pollutants and then discharged to the outside. After the drying process, the HTC solid fuel is moved to a pellet-forming machine. The pellet-forming machine produces HTC solid fuel in the form of pellets with a length of 2–3 mm.
3. Results
3.1. Setting of HTC Reaction Conditions of the Pilot Plant through Lab-Scale HTC Effect Analyses
The HTC experiment was conducted (Table 2) using a laboratory-scale reactor. The change in calorific value and mass yield of the HTC solid fuel was confirmed according to the reaction temperature and time. Sawdust has properties similar to those of waste wood, and because the particles are uniform, uniform results can be confirmed when analyzing carbides after HTC. The experimental conditions were the same as 80% moisture content according to the waste wood raw material in a 0.2 L laboratory-scale reactor, and the HTC reaction was performed by changing the reaction temperature and time.

Table 2.
Comparison of calorific value and mass yield after HTC of sawdust.
Overall, as the reaction temperature and time increased, the calorific value of the hydrothermal carbides increased, and the mass yield decreased. The reaction temperature did not show a sharp change at 180–200 °C but a rapid change at 200–230 °C. The experimental results confirmed that the effective reaction temperature for converting waste wood to hydrochar should be at least 200 °C. In this experiment, as the reaction temperature and time increased, the mass yield of the reactants decreased to 35.1% when the calorific value increased to a maximum of 29,898 kJ/kg. The conditions for producing 23,027 kJ/kg or more, equivalent to coal for power generation with a mass yield of 60% or more, were analyzed. As a result, when the reaction time was 1–2 h, the reaction temperature was 220 °C. Furthermore, When the reaction time is 3 h, the condition is satisfied if the reaction temperature is 210 °C. However, in terms of production per hour, the reaction time is preferably within 1 h. Since the pilot plant may require more reaction time, the reaction time was set in the range of 1–1.5 h.
Under the HTC test conditions using waste wood, the moisture content was 80%, and the reaction temperature was in the range of 200–220 °C. The reaction time was tested in the range of 1–1.5 h to be applied to the pilot plant. As with sawdust, as the reaction temperature and time increased, the calorific value increased and the mass yield decreased. It was confirmed that the change in the reaction temperature had a greater effect on the HTC reaction than the change in the reaction time. Unlike sawdust, in waste wood, the calorific value was low at reaction temperatures of 210 °C or higher. In this experiment (Table 3), a calorific value of 23,000 kJ/kg or more and the conditions for production with a mass yield of 60% or more was when the reaction temperature was 220 °C. Therefore, in the pilot-scale HTC experiment, the equipment were configured such that the heat source of the heating jacket could be raised to at least 220 °C.

Table 3.
Comparison of calorific value and mass yield after HTC of waste wood.
3.2. Laboratory-Scale Catalytic HTC Effect Analysis
A HTC experiment was conducted (Table 4) with reference to the results of a previous study [14,15] for catalyst types and concentrations. When the catalyst concentration ratio was 2 fold, the calorific value and mass yield after HTC were compared. If the calorific value increases after catalytic HTC, it is a combination of the Catalyst #1 (a strong acid-based catalyst). The higher the concentration of Catalyst #1, the better the catalytic HTC reaction. Additionally, as in the previous experiment, the yield tends to decrease as the reaction progresses well. The catalyst concentration of Catalyst #1 was determined to have a calorific value of ≥25,120 kJ/kg that was a minimum of 1.5-fold more than the catalytic density ratio. When the catalyst concentration was reduced to the initial catalytic density ratio, the calorific value decreased to 25,120 kJ/kg or less, which did not reach the target calorific value. Therefore, in the pilot plant HTC experiment, we decided to add the catalyst at a concentration equal to or greater than 2-fold the catalyst density ratio.

Table 4.
Comparison of calorific value and mass yield after catalytic HTC of waste wood at the laboratory scales.
Furthermore, a HTC experiment was conducted with a catalyst with a reaction time of 1.5 h, and as a result, the calorific value increased slightly. This was consistent with the tendency of HTC to occur more easily as the reaction time increased under the same reaction temperature conditions. Although the catalyst concentration ratios were the same, the strong acid was added in a larger amount than the weak acid, considering the purity of the catalyst. When the strong acid-based catalyst (Catalyst #1) was added, the calorific value was high, but the yield was lower and the amount of catalyst added was increased. When the weak acidbased catalyst (Catalyst #2) was added, the calorific value was lower than when Catalyst #1 was used, but a stable yield was obtained.
3.3. Analysis of Heavy Metals and Hazardous Substances on Laboratory-Scale HTC Solid Fuel
Prior to the production of HTC solid fuel in the pilot plant, heavy metals and hazardous substances were analyzed using specific samples in laboratory-scale HTC experiments. Table 5 is the quality standard applied when producing biosolid fuel at the test site where the pilot plant is installed. Therefore, it was a rule that had to be followed when conducting this study. The analysis method was carried out according to ISO 17022-1 biosolid fuel. Chlorine and sulfur were analyzed by Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES), and metals were analyzed by Inductively Coupled Plasma-Mass Spectrometer (ICP-MS). When the HTC reaction proceeded, the mass yield decreased, but heavy metals and harmful substances did not decrease; therefore, the content per unit mass or volume increased. The heavy metals and toxic substances contained in the non-catalyst HTC solid fuel slightly increased from the raw material waste wood standard; however, in the catalyst HTC solid fuel, among the heavy metals, chromium increased by more than 7 fold and lead increased by more than 2 fold. Among the harmful substances, sulfur has a synergistic effect because the amount of raw material is small, but chlorine increased by 8–37 fold. It was confirmed that the Catalyst #1 had the highest calorific value, but it was not a catalyst combination that could be used in the pilot plant because the chlorine content exceeded the standard for hazardous substances. Therefore, we decided to use Catalyst #2, which satisfied the standard of biosolid fuel as being suitable for the pilot plant-scale experiment.

Table 5.
Laboratory-scale HTC solid fuel analysis according to heavy metal and hazardous substance standards of biosolid fuel.
3.4. Pilot-Scale HTC Effect Analysis and Scalability Verification
In the pilot-scale HTC experiment, the analysis was performed based on the HTC solid fuel discharged from the filler press during the process. The analyzed results were compared with the laboratory-scale experimental results, as shown in Table 6. The reaction temperature was fixed at 220 °C, and the reaction times were 1 and 1.5 h, and it was confirmed that there was no significant difference with the laboratory-scale test result. The calorific value was 23,027 kJ/kg and the mass yield was 60% or more.

Table 6.
Comparison of calorific value and mass yield after HTC of waste wood at the laboratory and pilot scales.
The catalytic HTC experiment was performed according to the reaction conditions listed in Table 7. Unlike the laboratory-scale HTC experiment, the calorific value did not exceed 25,120 kJ/kg at a catalyst concentration of the same condition. The reduction in calorific value and mass yield is an effect of catalyst addition, thus additional experiments were conducted by increasing the catalyst concentration. It was increased by the initial density ratio, and the calorific value was measured to be higher than 25,120 kJ/kg at a 3-fold density ratio. It was also confirmed that the mass yield was more than 60% up to a 4-fold density ratio.

Table 7.
Comparison of calorific value and mass yield after catalytic HTC of waste wood according to catalytic density ratio at the pilot scale.
The chemical positions of the waste wood used as a reactant in these experiments and the biosolid fuels produced from the pilot-scale HTC processes were compared with a Van Krevelen diagram (Figure 4). The atomic H/C and O/C ratios of waste wood were 1.65 and 0.51, which are similar to that of general biomass. After HTC of waste wood at 220 °C for 1.5 h without a catalyst, the atomic H/C ratios of the biosolid fuel decreased from 1.65 to 1.13, and the atomic O/C ratios decreased from 0.51 to 0.39. This reduced the atomic H/C and O/C ratios by 31.5% and 23.5%, respectively, compared to those of the raw material, and showed intermediate levels of peat and lignite. On the other hand, the atomic H/C and O/C ratios of the biosolid fuel produced from the catalytic HTC (Catalyst #2) under the same conditions were reduced to 0.83 and 0.24, respectively. These figures showed reduction rates of 49.7% and 52.9%, respectively, compared to those of the raw material, and showed a degree of carbonization similar to that of general coal. In addition, the atomic H/C and O/C ratios decreased by 26.5% and 38.5%, respectively, compared to the biosolid fuel produced from the HTC without a catalyst. From these results, it was confirmed that Catalyst #2 provided by KINAVA greatly increased the selectivity for dehydration even in the HTC reaction under the same conditions, enabling the production of biosolid fuel with a high calorific value due to a high degree of carbonization.
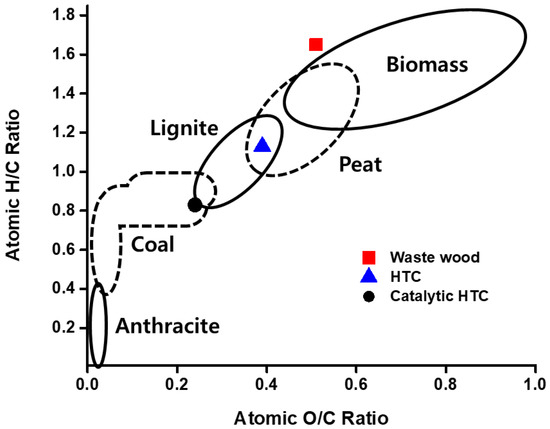
Figure 4.
Van Krevelen diagram of waste wood and biosolid fuels produced by different HTC processes at the pilot scales.
In the next step, heavy metals and hazardous substances were analyzed to determine whether the HTC solid fuel produced by the pilot plant satisfied the biosolid fuel standard. As shown in Table 8, it was confirmed that the HTC solid fuel produced in the laboratory satisfied the prescribed conditions. As a result, it did not exceed the standard for hazardous substances in the test site, and it was analyzed with a result similar to that of the laboratory-scale HTC solid fuel.

Table 8.
Laboratory- and pilot-scale hydrothermal carbonization solid fuel comparison according to heavy metal and hazardous substance standards of biosolid fuel.
4. Conclusions
The purpose of this study was to convert waste wood into solid fuel with a calorific value equivalent to coal for power generation using HTC. First, a laboratory-scale HTC experiment confirmed that the mass yield was lowered with a higher calorific value than when no catalyst was added under the same reaction conditions. Then, to verify the scalability of the HTC effect, pilot-scale HTC test conditions were determined based on laboratory-scale HTC test results. The pilot-scale HTC experiment confirmed that the reaction temperature was 220 °C, and the reaction time was increased to 1.5 h, similar to the laboratory-scale HTC experiment result. However, in the catalytic HTC experiment, the laboratory-scale experimental results were similar when the catalyst concentration was increased by 1.5 fold or more. Therefore, it was confirmed that optimization of the catalyst concentration is necessary for a commercial HTC plant to which a catalyst is added. In conclusion, the optimal catalyst combination and concentration through the pilot plant produced solid fuel with a calorific value 18% higher than coal for power generation. In addition, it was verified through scalability that it could be used as a biosolid fuel.
Author Contributions
Conceptualization, T.-S.S., K.C. and H.-I.Y.; methodology, T.-S.S., K.C. and H.-I.Y.; validation, T.-S.S.; formal analysis, T.-S.S. and J.-C.L.; investigation, J.-C.L.; Resources, I.-K.K.; data curation, S.-Y.Y. and I.-K.K.; Pilot Plant design verification, H.-B.L.; Pilot Plant test results analysis and verification, N.K. and S.K.; writing—original draft preparation T.-S.S.; writing—review and editing, K.C., J.-C.L. and H.-I.Y.; supervision, H.-I.Y.; Project administration, K.C.; funding acquisition, N.K. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Korea East-West Power Company of the Republic of Korea (Pilot Plant Development for Green Pellet Production from Woodwaste Using Hydrothermal Polymeri-zation Technology (2019)). The funding sponsors had a role in the Pilot Plant design of the study.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This experimental data is a rough representation of what was derived from the joint experiment between the lead author and KINAVA Company. The data presented in this study are available on request from the corresponding authors and KINAVA Company.
Acknowledgments
We appreciate Korea East-West Power Company and KINAVA Company for their assistance in this study.
Conflicts of Interest
S.-Y.Y., I.-K.K. and K.C. are employees of the KINAVA Company. N.K. and S.K. are employees of Republic of Korea East-West Power Company.
References
- Gil, A. Challenges on Waste-to-Energy for the Valorization of Industrial Wastes: Electricity, Heat and Cold, Bioliquids and Biofuels. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100615. [Google Scholar] [CrossRef]
- Maniscalco, M.P.; Volpe, M.; Messineo, A. Hydrothermal Carbonization as a Valuable Tool for Energy and Environmental Applications: A Review. Energies 2020, 13, 4098. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal Carbonization of Biomass: A Summary and Discussion of Chemical Mechanisms for Process Engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Wu, L.M.; Tong, D.S.; Li, C.S.; Ji, S.F.; Lin, C.X.; Yang, H.M.; Zhong, Z.K.; Xu, C.Y.; Yu, W.H.; Zhou, C.H. Insight into Formation of Montmorillonite-Hydrochar Nanocomposite under Hydrothermal Conditions. Appl. Clay Sci. 2016, 119, 116–125. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal Carbonization of Biomass Residuals: A Comparative Review of the Chemistry, Processes and Applications of Wet and Dry Pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Fakkaew, K.; Koottatep, T.; Pussayanavin, T.; Polprasert, C. Hydrochar Production by Hydrothermal Carbonization of Faecal Sludge. J. Water Sanit. Hyg. Dev. 2015, 5, 439–447. [Google Scholar] [CrossRef]
- Berge, N.D.; Ro, K.S.; Mao, J.; Flora, J.R.V.; Chappell, M.A.; Bae, S. Hydrothermal Carbonization of Municipal Waste Streams. Environ. Sci. Technol. 2011, 45, 5696–5703. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.-H.; Aoyama, H.; Matsuto, T.; Nakagishi, T.; Matsuo, T. Recovery of Solid Fuel from Municipal Solid Waste by Hydrothermal Treatment Using Subcritical Water. Waste Manag. 2012, 32, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ro, K.S.; Chappell, M.; Li, Y.; Mao, J. Chemical Structures of Swine-Manure Chars Produced under Different Carbonization Conditions Investigated by Advanced Solid-State 13C Nuclear Magnetic Resonance (NMR) Spectroscopy. Energy Fuels 2011, 25, 388–397. [Google Scholar] [CrossRef]
- Goto, M.; Obuchi, R.; Hirose, T.; Sakaki, T.; Shibata, M. Hydrothermal Conversion of Municipal Organic Waste into Resources. Bioresour. Technol. 2004, 93, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Simsir, H.; Eltugral, N.; Karagoz, S. Hydrothermal Carbonization for The Preparation of Hydrochars from Glucose, Cellulose, Chitin, Chitosan and Wood Chips via Low-temperature and Their Characterization. Bioresour. Technol. 2017, 246, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Pellechia, P.J.; Flora, J.R.V.; Berge, N.D. Influence of Reaction Time and Temperature on Product Formation and Characteristics Associated with the Hydrothermal Carbonization of Cellulose. Bioresour. Technol. 2013, 138, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Lian, Y.; Yan, L.; Smith, R.L. One-step Preparation of Carbonaceous Solid Acid Catalysts by Hydrothermal Carbonization of Glucose for Cellulose Hydrolysis. Catal. Commun. 2014, 57, 50–54. [Google Scholar] [CrossRef]
- Joo, B.; Yeon, H.; Lee, S.; Ahn, S.; Lee, K.; Jang, E.; Won, J. Conversion of Wood Waste into Solid Biofuel Using Catalytic HTC Process. New Renew. Energy 2014, 10, 12–18. (In Korean) [Google Scholar] [CrossRef][Green Version]
- Mackintosh, A.F.; Shin, T.; Yang, H.; Choe, K. Hydrothermal Polymerization Catalytic Process Effect of Various Organic Wastes on Reaction Time, Yield, and Temperature. Processes 2020, 8, 303. [Google Scholar] [CrossRef]
- Ghaziaskar, A.; McRae, G.A.; Mackintosh, A.; Basu, O.D. Catalyzed Hydrothermal Carbonization with Process Liquid Recycling. Energy Fuels 2019, 33, 1167–1174. (In English) [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Purcell, R.; Zielinska, B.; Felix, L.; Irvin, J. Process Development Unit (PDU) for Hydrothermal Carbonization (HTC) of Lignocellulosic Biomass. Waste Biomass Valoriz. 2013, 5, 669–678. [Google Scholar] [CrossRef]
- Ismail, T.M.; Yoshikawa, K.; Sherif, H.; El-Salam, M.A. Hydrothermal treatment of municipal solid waste into coal in a commercial Plant: Numerical assessment of process parameters. Appl. Energy 2019, 250, 653–664. [Google Scholar] [CrossRef]
- Mackintosh, A.F.; Jung, H.; Kang, I.-K.; Yoo, S.; Kim, S.; Choe, K. Experimental Study on Hydrothermal Polymerization Catalytic Process Effect of Various Biomass through a Pilot Plant. Processes 2021, 9, 758. [Google Scholar] [CrossRef]
- Liu, J.I.; Paode, R.D.; Holsen, T.M. Modeling the Energy Content of Municipal Solid Waste Using Multiple Regression Analysis. J. Air Waste Manag. Assoc. 1996, 46, 650–656. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).