Abstract
Raltitrexed is a classical antifolate drug with antimetabolite and anticancer properties. In this research, we provide its detailed antitrypanosomal inhibition against six Trypanosoma species and investigate its potential mode of action. Molecular dynamics (MD) simulations and in silico analyses were used to track the binding strength and stability. Raltitrexed showed broad-spectrum trypanocidal actions against Trypanosoma brucei brucei GUTat3.1, T. b. rhodesiense IL1501, T. b. gambiense IL1922, T. evansi Tansui, T. equiperdum IVM-t1 and T. congolense IL3000. The estimated IC50 was found to be in the range of 5.18–24.13 µg/mL, indicating inhibition of Trypanosoma in the low micromolar range. Although the co-crystallized ligand had robust hydrogen bonding and lipophilic characteristics, its docking score was only −4.6 compared to raltitrexed’s −7.78, indicating strong binding with T. brucei dihydrofolate reductase-thymidylate synthase (TbDHFR-TS). MD simulations support the strong binding of raltitrexed with TbDHFR-TS evidenced by low root mean square deviation (RMSD), low residues fluctuations, a tight radius of gyration (ROG) and an average of 3.38 ± 1.3 hydrogen bonds during 50 ns MD simulation. The prospective extended spectrum of raltitrexed against Trypanosoma species grants further research for the synthesis of raltitrexed derivatives and repurposing against other protozoa.
1. Introduction
Raltitrexed, a classical antifolate, was the first drug approved for treating advanced colorectal cancer in the United Kingdom in 1996 [1]. It is a quinazoline analog with a 6-6 ring-fused structure, like natural folates, and is considered a “classical antifolate” [2]. The chemical structure of classical antifolates comprises folate analogs with a pterin ring, an aromatic ring and a glutamate tail [3]. Due to their charged glutamate tail, they are unable to passively diffuse across cell membranes and must be actively transported [3].
Folic acid is required for DNA synthesis in bacteria and other organisms because of its role in the production of nitrogenous bases purine and pyrimidine. As the prevalence of infectious diseases rises, there is an urgent need for the discovery of new therapies [4]. Anticancer antifolate drugs can also be tested for antimicrobial potential, as these compounds can target the major enzymes required for folic acid metabolism, which is required to maintain microbial species integrity [5]. As raltitrexed is a thymidylate synthase (TS) inhibitor, it can act as an antibacterial agent because DHFR-TS catalyzes the reduction of folate or 7,8-dihydrofolate to tetrahydrofolate and intimately couples with DHFR-TS. Targeting DHFR-TS eventually inhibits the catalysis of folic acid to its constituents which ultimately stops the synthesis of DNA, and the integrity of microbial cells will be disturbed [6].
Trypanosomiasis is a devastating and fatal blood protozoal disease affecting humans and animals worldwide. The Trypanosoma cruzi parasite that causes Chagas disease is already present in 21 countries across Latin America and the southern United States. More than 7 million individuals are infected with Chagas disease at present, causing 10,000 deaths annually from its complications, and another 70 million are at risk of infection [7]. Human African trypanosomiasis (HAT), often known as sleeping sickness, remains one of Africa’s most dreaded and lethal diseases, affecting an estimated 70 million people across 36 nations in sub-Saharan Africa [8]. The safety and toxicity of many antitrypanosomal medicines are extremely poor. New kinds of antitrypanosomal drugs that are more effective and have lower host toxicity need to be discovered [9]. There is an immediate need for effective, safe and cost-effective antitrypanosomal medications because of microbial resistance to the few traditional antitrypanosomal drugs, increasing vector resistance to insecticides, a lack of effective vaccinations and the side effects of the present drugs [10].
The data regarding the antimicrobial action of classical antifolates as raltitrexed is very limited. Because conventional antifolates are strongly negatively charged chemicals that cannot enter the bacterial cell membrane, their utility as antimicrobials have been limited. As a result, almost all commercially available antibacterial folates fall into the non-classical group of antifolates [11,12]. In protozoal infections, several reports support the value of raltitrexed in this class of parasites. In Toxoplasma gondii (T. gondii)-infected mice, the peritoneal fluid and liver imprints had fewer tachyzoites after treatment with raltitrexed, which also prevented mortality. The findings showed that raltitrexed has significant anti-T. gondii RH strain protective properties [13]. Raltitrexed is a DHFR-TS inhibitor, which might be an important target for its antiprotozoal actions [14]. Although raltitrexed’s antiprotozoal activity appears promising, there are few reports to back up this claim. The antitrypanosomal activities of raltitrexed will be investigated in six Trypanosoma species in this study.
2. Materials and Methods
2.1. The Evaluation of Trypanocidal Activity In Vitro
2.1.1. Materials
Raltitrexed was purchased from ApexBio (Houston, TX, USA), and CultureSure DMSO was obtained from Wako Pure Chemical (Osaka, Japan). T. b. brucei (Tbb) GuTat3.1 [15], T. b. rhodesiense (Tbr) IL1501 [16], T. b. gambiense (Tbg) IL1922 (isolated from Ivory Coast), T. evansi (Tev) Tansui (isolated from Taiwan), T. equiperdum (Teq) IVM-t1 [17] and T. congolense (Tc) IL3000 were also obtained for this study [18]. A 96-well Optical bottom plate was purchased from ThermoFisher SCIENTIFIC (Waltham, MA, USA). CellTiter-Glo Luminescent cell viability reagent and GloMax plate reader were purchased from Promega Corporation (Fitchburg, WI, USA). Hiram’s modified Isocove’s Dulbecco’s medium (HMI-9), HEPES, pyruvic acid sodium salt, BSA, thymidine, 2-β-mercaptoethanol, L-cysteine, bathocuproine and hypoxanthine were purchased from Sigma-Aldrich (Tokyo, Japan). A basic plate shaker was obtained from IKA® JAPAN (K.K., Osaka, Japan).
2.1.2. Antitrypanosomal Assay
The trypanocidal activity of raltitrexed was evaluated following a previous report [19]. The trypanosomes were cultured at 37 °C for Tbb, Tbg, Tbr, Teq and Tev and 33 °C for Tc in an incubator at 5% CO2 using HMI-9 supplemented with 20% heat-inactivated fetal calf serum, 60 mM HEPES, 10 μg/L insulin, 0.1 mM bathocuproine, 5.5 μg/L transferrin, 1 mM pyruvic acid sodium salt, 1 mM hypoxanthine, 16 μM thymidine, 6.7 ng/L sodium selenite, 0.0001% 2-β-mercaptoethanol, 2 mM L-cysteine and 0.4 g/L BSA. Trypanosomes were subcultured every two days. Raltitrexed was dissolved in DMSO:H2O (1:10, v/v). The effect of the solvent was assessed to be negligible on the parasite.
Initially, raltitrexed was examined at two concentrations of 25 and 0.25 µg/mL to check the possible extended spectrum opportunities against six trypanosomes. The initial screening revealed that raltitrexed showed promising trypanocidal activities at 25 µg/mL. As a confirmed inhibitor, the IC50 of raltitrexed was evaluated using serial dilution from 25 µg/mL to 0.78 µg/mL. This range was effective in the accurate estimation of the IC50 of the compound. After three days of cultivation, 25 µL of CellTiter-Glo Luminescent cell viability reagent were aliquoted into each well, and the plate was shaken for 500 shakes/min by an MS3 basic plate shaker for 30 s to facilitate cell lysis and release intracellular ATP. After mixing, the bioluminescence of each well was measured using a GloMax plate reader.
2.2. Docking Studies
Docking studies were performed to determine the binding potential of raltitrexed (Figure 1) with TbDHFR-TS. The protein, ligand and docking processes were carried out as previously described [20,21], with minor alterations. In all docking steps, the Schrodinger Maestro suit (Schrodinger LLC, New York, NY, USA) was employed.
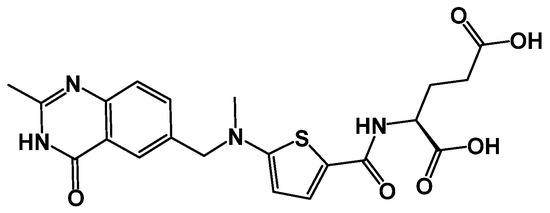
Figure 1.
The chemical structure of raltitrexed.
2.2.1. Macromolecule and Compounds Retrieval and Preparation
The compounds’ 2D structures were obtained from the PubChem website, loaded using Ligprep, and then 3D optimized at optimal physiological pH. The Protein Data Bank was used to retrieve the protein structure file (PDB, 3RG9), which constitutes a TbDHFR structure in complex with WR99210 at 2.00 Å resolution [22].
Using the protein preparation module, the macromolecule structure was optimized for docking. Crystallographic chemicals and water molecules were removed from the solution. The protein was protonated by adding polar hydrogens, and the structures were optimized and the energy was reduced using the OPLS2005 force field. WR99210 was used as the center of a 20 Å grid box for docking grid generation.
2.2.2. Docking Runs
The standard precision SP Glide docking methodology was utilized, and the docking scores were used to rank the output results. Redocking of WR99210 was used to assess the correctness of the docking run, and the docking pose demonstrated complete complementarity and low RMSD when compared to the bound ligand.
2.3. Molecular Dynamics Simulation
A molecular dynamics simulation lasting 50 ns was performed using Desmond software according to the previously performed protocol [21,23]. Docking studies provided the protein–ligand complexes needed for molecular dynamics simulation. In static conditions, the binding status of a ligand can be predicted by molecular docking studies. Predictions of ligand binding in a physiological setting were simulated. The Protein Preparation Wizard or Maestro was used to perform complex optimization and minimization prior to actual protein–ligand complex preparation. The System Builder application was used to set up all the systems. Tip3P opted for the Solvent Model, which features a square orthorhombic box (Transferable Intermolecular Interaction Potential 3 Points). The simulation makes use of the OPLS 2005 force field. When necessary, counter ions were added to the models to achieve electrical neutrality. Physiological conditions were mimicked by adding 0.15 M NaCl. As the simulation was conducted at a constant 300 K and 1 atm, the NPT ensemble was used (Isothermal-Isobaric: moles (N), pressure (P) and temperature (T) are all conserved). Before running the simulation, the models were softened. Trajectories were saved at 100 ps intervals so that the RMSD of the protein and ligand could be calculated over time and used to assess the stability of the simulations.
2.4. Statistical Analysis
MS Excel and GraphPad Prism were used to handle and present all of the data. The data were reported as Mean SD or, in some cases, as the mean plus range. Changes in each isolation parameter were expressed using descriptive statistics.
3. Results and Discussion
3.1. Trypanocidal Assay
Initially, raltitrexed was examined at 0.25 or 25 µg/mL, constituting high and low concentrations of the drug (Table 1). Raltitrexed showed broad-spectrum trypanocidal actions. At 25 µg/mL, raltitrexed suppressed all of the test strains. Stronger action was noticed on TbbGUTat3.1, TbrIL1501, TbgIL1922, Tev Tansui and Teq IVM-t1 by showing more than 99% inhibition rate (Table 1). However, a low trypanocidal rate was observed for TcIL3000.

Table 1.
The inhibition rate of raltitrexed at 0.25 or 25 µg/mL against 6 Trypanosoma species, TcIL3000, TbbGUTat3.1, TbrIL1501, TbgIL1922, Tev Tansui and Teq IVM-t1.
In light of raltitrexed’s potential in vitro antitrypanosomal activity, the IC50 was determined in the presence of various doses of the molecule. The IC50 for raltitrexed was found to be in the range of 5.18–24.13 µg/mL, indicating that it possessed potent antitrypanosomal activity (Table 2). The strongest trypanocidal activity was found in Teq IVM-t1 with IC50 = 5.18 ± 0.53 µg/mL, while TcIL3000 showed the highest resistance to the trypanosomal activity of raltitrexed with IC50 > 25 µg/mL. The strength of the trypanocidal actions of raltitrexed was in the following order Teq IVM-t1 > Tev Tansui > TbrIL1501 > TbgIL1922 > TbbGUTat3.1 > TcIL3000.

Table 2.
The IC50 of raltitrexed against six Trypanosoma species, TcIL3000, TbbGUTat3.1, TbrIL1501, TbgIL1922, Tev Tansui and Teq IVM-t1.
Nanomolar potency against T. brucei was determined for methotrexate, pemetrexed and raltitrexed in thymidine and folate-deficient medium. Adding folate and thymidine decreased the effectiveness of the antifolates, with the exception of nolatrexed. The addition of thymidine decreased the effectiveness of raltitrexed and pemetrexed more so than the addition of folate [24]. The medium used to incubate the trypanosomes in our study contained 1 mg/L folic acid and thymidine at a final concentration of 16 µM. Even though these two substances, folic acid and thymidine, were present in the culture media, inhibitory qualities were visible. This may further support the hypothesis that raltitrexed effects include mechanisms other than folate synthesis. This observation might be species-specific and varies according to the nature of DHFR-TS mutation or amino acid changes.
3.2. Molecular Docking
Given the interactions and inhibition of DHFR-TS with raltitrexed, it was modeled with TbDHFR-TS to verify its binding conformations and binding potency. Raltitrexed had a docking score of −7.78, compared to −4.6 for the co-crystalized ligand WR99210 (Table 3), indicating the possibility of significant strong interaction with TbDHFR-TS. The binding of raltitrexed was supported by both favorable hydrogen bonds and lipophilic interaction scores. Because of its superior ligand efficiency, lower hydrogen bond score and lower Lipo score (Table 3), raltitrexed formed more robust contact than WR99210 (Figure 2A,B).

Table 3.
The docking score and binding parameters for raltitrexed and the compound WR99210 with T. brucei dihydrofolate reductase.
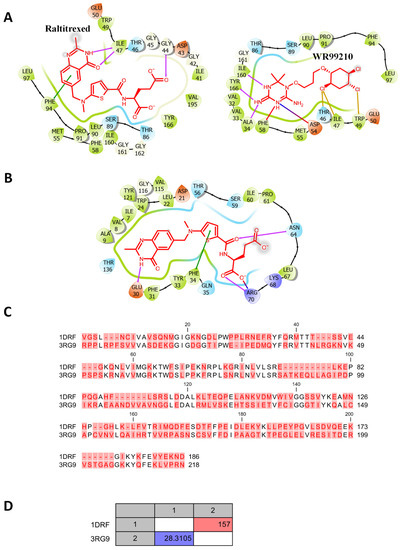
Figure 2.
The ligand interactions and docking site of raltitrexed with DHFR. (A) The ligand interactions of raltitrexed with Trypanosoma brucei dihydrofolate reductase. (B) The docking site of raltitrexed with Humans dihydrofolate reductase. Hydrogen bonds in violet arrows, pi-pi stacking in green sticks, salt bridges in multicolored sticks and halogen bonds in amber. (C) Pairwise alignment of human (1DFR) and T. brucei (3RG9) DHFR. The different residues are highlighted in pink. (D) Pairwise comparison statistics of human (1DFR) and T. brucei (3RG9) DHFR. The number of amino acid differences is highlighted in red. The identity% is highlighted in blue.
Inspection of the mode of binding of raltitrexed with TbDHFR-TS indicated a favorable binding mode supported by hydrogen bonding with the side chain of ILE17 and with LY44 and stacking interaction PHE94, which helps in orientation and fixation into the active site of TbDHFR-TS (Figure 2).
The present findings are in agreement with the previous in vitro activity of raltitrexed. Raltitrexed was found to be an inhibitor for the DHFR and TS activities of TbDHFR-TS with IC50 values of 93.1 and 215 nM, respectively [24,25]. Trimethoprim, pyrimethamine and raltitrexed were reported to function as powerful competitive inhibitors of T. brucei DHFR with Ki values of 11.4 ± 1.2, 17.6 ± 2.3 and 70.4 ± 7.2 nM, respectively [24].
To gain a better understanding of how raltitrexed interacts with humans and T. brucei DHFR, comparative docking, protein sequence alignment and alignment satisfaction were all compared. The docking score, as well as the ligand efficiency, was a little bit higher with TbDHFR (Table 4). The carboxylate group of raltitrexed was more interactive with human DHFR (Figure 2C). Hydrogen bonds and a salt bridge were formed between raltitrexed and the side chains of GLU30, ASN64 and ARG70. The pairwise alignment showed approximately 34 gaps and 175 amino acid differences between the human and T. brucei enzymes (Figure 2C). These distinctions accounted for approximately 28.31% of the identity percentage (Figure 2D). A low homology rate and a significant number of differences could serve as a foundation for designing more selective antitrypanosomal medicines. Further, raltitrexed chemical modifications targeting the terminal charged atoms are likely to reduce the affinity for human DHFR without reducing the affinity for the parasitic enzyme.

Table 4.
The docking score and binding parameters for raltitrexed with human (PDB ID 1DRF) or T. brucei DHFR.
3.3. Molecular Dynamics Simulations
RMSD was calculated for the raltitrexed-TbDHFR-TS complex. The RMSD graph (Figure 3) shows that the structure remained stable throughout the simulation time with some fluctuation within the range of ~1 Å, which is a normal behavior of the globular protein. The raltitrexed-TbDHFR-TS complex reached stability within five ns at the start of the simulation (Figure 3A). These findings support the observed docking scores and the strong binding of raltitrexed with TbDHFR-TS. The raltitrexed-TbDHFR-TS complex showed low RMSF and indicated modest variations of amino acid residues, with the exception of a flexible loop made up of residue no. 45–55 (Figure 3B).
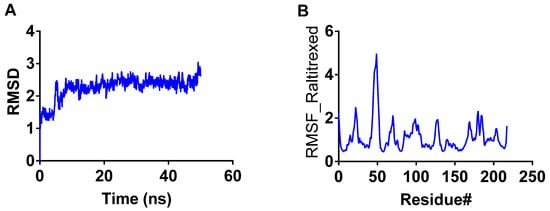
Figure 3.
RMSD and RMSF of the interaction of raltitrexed with Trypanosoma brucei dihydrofolate reductase. (A) RMSD of raltitrexed for 50 ns. (B) RMSF of raltitrexed for 50 ns.
The total number of hydrogen bonds formed between raltitrexed and TbDHFR-TS was traced during 50 ns simulation (Figure 4A). The hydrogen bonding statistics indicated that hydrogen bonds ranged from 0–7 with a mean value of 3.38 ± 1.3. This indicates the fixation and consistent binding of raltitrexed with TbDHFR-TS. The slight changes (Figure 4B) in ROG indicate the general compactness of the raltitrexed–TbDHFR-TS complex.
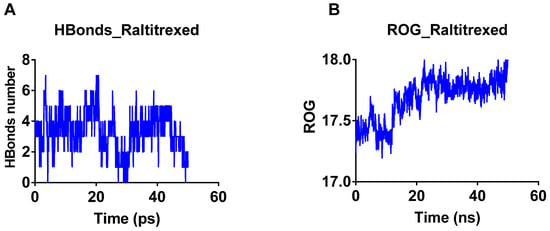
Figure 4.
The number of hydrogen atoms and radius of gyration during the interaction of raltitrexed with Trypanosoma brucei dihydrofolate reductase. (A) ROG of raltitrexed during simulation. (B) The number of hydrogen bonds that were formed during a 50 ns simulation between raltitrexed and T. brucei dihydrofolate reductase.
Several drugs belonging to the sulfonamides family and non-classical antifolates have already been reported as potent antimicrobial agents. Similarly, mafenide is regarded as the first-line antibacterial treatment regime for severe burn wounds and shows greater antibacterial potential against silver sulfadiazine-resistant Pseudomonas spp as it has a higher penetration ability and thus can easily pass through the bacterial membrane and destroy the bacterial cell [11]. Coadministration of DHFR (dihydrofolate reductase) and DHPS (dihydropteroate synthase) inhibitors are the most widely used antifolate-based antibacterial treatment as it has a broad spectrum of action by killing both Gram-negative and Gram-positive types of bacteria effective against drug-resistant bacteria (Stenotrophomonas maltophilia, methicillin-resistant Staphylococcus aureus) with minimum side effects [26,27]. The present findings of raltitrexed suggest further investigations regarding its synergists of actions. The combination of raltitrexed with DHPS and its effect on Trypanosoma species are still to be determined.
In this study, we showed for the first time how raltitrexed affects a wide range of trypanosomes. This range has highly distinct host characteristics; in addition to T. brucei species, T. evansi affects camels, cattle, horses and dogs. Furthermore, T. equierdum is a parasite that infects horses and other equines. The examined trypanosomes impact a wide range of tissue infections. In addition to the well-known blood protozoal illness caused by trypanosomes, T. equiperdum is well-known for its genital tract infection and for being a venereal disease that is not spread by an insect vector. The variety of species included in our investigation, as well as their host range and diverse diseased tissues, indicated that raltitrexed could be used to treat diseases such as dourine in equines and T. evansi in camels and other animals.
4. Conclusions
In this study, raltitrexed was proposed as a possible treatment for trypanosomiasis. As part of this study, we described its efficacy as an antitrypanosomal agent against six different Trypanosoma species and delved into its possible mechanism of action. Binding stability and strength were monitored by MD simulations and in silico studies. Raltitrexed’s trypanocidal effects were observed against a wide variety of parasites, including TbbGUTat3.1, TbrIL1501, TbgIL1922, Tev Tansui and Teq IVM-t1. In silico studies provide credence to the robust binding of raltitrexed with TbDHFR-TS. Research into the manufacture of raltitrexed derivatives and its repurposing against additional protozoa is enabled by the potential broadened range of raltitrexed against several Trypanosoma species.
Author Contributions
Conceptualization, M.K. and K.S.; methodology, M.K. and K.S.; software, M.K.; validation, M.K. and K.S.; formal analysis, K.S.; investigation, M.K. and K.S.; resources, M.K.; data curation, K.S.; writing—original draft preparation, M.K.; writing—review and editing, K.S.; visualization, M.K.; supervision, M.K. and K.S.; project administration, M.K. and K.S.; funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Project# AN000176).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are within the manuscript.
Acknowledgments
The authors would appreciate the financial support from the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaye, S.B. New antimetabolites in cancer chemotherapy and their clinical impact. Br. J. Cancer 1998, 78, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gangjee, A.; Jain, H.D.; Phan, J.; Lin, X.; Song, X.; McGuire, J.J.; Kisliuk, R.L. Dual inhibitors of thymidylate synthase and dihydrofolate reductase as antitumor agents: Design, synthesis, and biological evaluation of classical and nonclassical pyrrolo [2, 3-d] pyrimidine antifolates. J. Med. Chem. 2006, 49, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, M.V.; Randazzo, O.; La Franca, M.; Barone, G.; Vignoni, E.; Rossi, D.; Collina, S. DHFR inhibitors: Reading the past for discovering novel anticancer agents. Molecules 2019, 24, 1140. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.; Arciszewska, K.; Maliszewski, D.; Drozdowska, D. Trimethoprim and other nonclassical antifolates an excellent template for searching modifications of dihydrofolate reductase enzyme inhibitors. J. Antibiot. 2020, 73, 5–27. [Google Scholar] [CrossRef]
- Zhou, W.; Scocchera, E.W.; Wright, D.L.; Anderson, A.C. Antifolates as effective antimicrobial agents: New generations of trimethoprim analogs. MedChemComm 2013, 4, 908–915. [Google Scholar] [CrossRef]
- He, J.; Qiao, W.; An, Q.; Yang, T.; Luo, Y. Dihydrofolate reductase inhibitors for use as antimicrobial agents. Eur. J. Med. Chem. 2020, 195, 112268. [Google Scholar] [CrossRef]
- Cerny, N.; Bivona, A.E.; Sanchez Alberti, A.; Trinitario, S.N.; Morales, C.; Cardoso Landaburu, A.; Cazorla, S.I.; Malchiodi, E.L. Cruzipain and Its Physiological Inhibitor, Chagasin, as a DNA-Based Therapeutic Vaccine Against Trypanosoma cruzi. Front. Immunol. 2020, 11, 565142. [Google Scholar] [CrossRef]
- Kennedy, P.G. Clinical features, diagnosis, and treatment of human African trypanosomiasis (sleeping sickness). Lancet Neurol. 2013, 12, 186–194. [Google Scholar] [CrossRef]
- Kourbeli, V.; Chontzopoulou, E.; Moschovou, K.; Pavlos, D.; Mavromoustakos, T.; Papanastasiou, I.P. An overview on target-based drug design against kinetoplastid protozoan infections: Human African trypanosomiasis, Chagas disease and leishmaniases. Molecules 2021, 26, 4629. [Google Scholar] [CrossRef]
- Abdeta, D.; Kebede, N.; Giday, M.; Terefe, G.; Abay, S.M. In Vitro and In Vivo Antitrypanosomal Activities of Methanol Extract of Echinops kebericho Roots. Evid. Based Complementary Altern. Med. eCAM 2020, 2020, 8146756. [Google Scholar] [CrossRef]
- Fernández-Villa, D.; Aguilar, M.R.; Rojo, L. Folic acid antagonists: Antimicrobial and immunomodulating mechanisms and applications. Int. J. Mol. Sci. 2019, 20, 4996. [Google Scholar] [CrossRef] [PubMed]
- Scocchera, E.; Reeve, S.M.; Keshipeddy, S.; Lombardo, M.N.; Hajian, B.; Sochia, A.E.; Alverson, J.B.; Priestley, N.D.; Anderson, A.C.; Wright, D.L. Charged nonclassical antifolates with activity against gram-positive and gram-negative pathogens. ACS Med. Chem. Lett. 2016, 7, 692–696. [Google Scholar] [CrossRef] [PubMed]
- de Paula Reis, M.; de Lima, D.A.; Pauli, K.B.; Andreotti, C.E.L.; de Moraes, A.L.S.; Gonçalves, D.D.; Navarro, I.T.; Bueno, P.S.A.; Seixas, F.A.V.; Gasparotto Junior, A.; et al. Molecular docking to Toxoplasma gondii thymidylate synthase-dihydrofolate reductase and efficacy of raltitrexed in infected mice. Parasitol. Res. 2018, 117, 1465–1471. [Google Scholar] [CrossRef]
- Gangjee, A.; Li, W.; Yang, J.; Kisliuk, R.L. Design, synthesis, and biological evaluation of classical and nonclassical 2-amino-4-oxo-5-substituted-6-methylpyrrolo [3, 2-d] pyrimidines as dual thymidylate synthase and dihydrofolate reductase inhibitors. J. Med. Chem. 2008, 51, 68–76. [Google Scholar] [CrossRef]
- Hirumi, H.; Hirumi, K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 1989, 75, 985–989. [Google Scholar] [CrossRef]
- Nakamura, K.; Fujioka, S.; Fukumoto, S.; Inoue, N.; Sakamoto, K.; Hirata, H.; Kido, Y.; Yabu, Y.; Suzuki, T.; Watanabe, Y.-i.; et al. Trypanosome alternative oxidase, a potential therapeutic target for sleeping sickness, is conserved among Trypanosoma brucei subspecies. Parasitol. Int. 2010, 59, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, K.; Narantsatsral, S.; Battur, B.; Yamasaki, S.; Otgonsuren, D.; Musinguzi, S.P.; Davaasuren, B.; Battsetseg, B.; Inoue, N. Isolation, cultivation and molecular characterization of a new Trypanosoma equiperdum strain in Mongolia. Parasites Vectors 2016, 9, 481. [Google Scholar] [CrossRef] [PubMed]
- Gibson, W. The origins of the trypanosome genome strains Trypanosoma brucei brucei TREU 927, T. b. gambiense DAL 972, T. vivax Y486 and T. congolense IL3000. Parasites Vectors 2012, 5, 71. [Google Scholar] [CrossRef]
- Suganuma, K.; Allamanda, P.; Hakimi, H.; Zhou, M.; Angeles, J.M.; Kawazu, S.; Inoue, N. Establishment of ATP-based luciferase viability assay in 96-well plate for Trypanosoma congolense. J. Vet. Med. Sci. 2014, 76, 1437–1441. [Google Scholar] [CrossRef]
- Burayk, S.; Oh-Hashi, K.; Kandeel, M. Drug Discovery of New Anti-Inflammatory Compounds by Targeting Cyclooxygenases. Pharmaceuticals 2022, 15, 282. [Google Scholar] [CrossRef]
- Kandeel, M.; Park, B.K.; Morsy, M.A.; Venugopala, K.N.; Oh-hashi, K.; Al-Nazawi, M.; Kwon, H.-J. Virtual Screening and Inhibition of Middle East Respiratory Syndrome Coronavirus Replication by Targeting Papain-like Protease. Dr. Sulaiman Al Habib Med. J. 2021, 3, 179–187. [Google Scholar] [CrossRef]
- Vanichtanankul, J.; Taweechai, S.; Yuvaniyama, J.; Vilaivan, T.; Chitnumsub, P.; Kamchonwongpaisan, S.; Yuthavong, Y. Trypanosomal dihydrofolate reductase reveals natural antifolate resistance. ACS Chem. Biol. 2011, 6, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Kandeel, M.; Kitade, Y.; Almubarak, A. Repurposing FDA-approved phytomedicines, natural products, antivirals and cell protectives against SARS-CoV-2 (COVID-19) RNA-dependent RNA polymerase. PeerJ 2020, 8, e10480. [Google Scholar] [CrossRef]
- Gibson, M.W.; Dewar, S.; Ong, H.B.; Sienkiewicz, N.; Fairlamb, A.H. Trypanosoma brucei DHFR-TS Revisited: Characterisation of a Bifunctional and Highly Unstable Recombinant Dihydrofolate Reductase-Thymidylate Synthase. PLoS Negl. Trop. Dis. 2016, 10, e0004714. [Google Scholar] [CrossRef] [PubMed]
- Cullia, G.; Tamborini, L.; Conti, P.; De Micheli, C.; Pinto, A. Folates in Trypanosoma brucei: Achievements and opportunities. ChemMedChem 2018, 13, 2150–2158. [Google Scholar] [CrossRef]
- Fisher, J.F.; Mobashery, S.; Miller, M.J. Antibacterials; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Palomino, J.C.; Martin, A. The potential role of trimethoprim-sulfamethoxazole in the treatment of drug-resistant tuberculosis. Future Microbiol. 2016, 11, 539–547. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).