Kinetic Study of 4-Chlorophenol Biodegradation by Acclimated Sludge in a Packed Bed Reactor
Abstract
:1. Introduction
2. Kinetic Model
2.1. Kinetic Model for Batch Experiments
2.2. Kinetic Model for PBR
2.3. Kinetic Model Solution
3. Materials and Methods
3.1. Nutrient and Minerals Salt Media
3.2. Inoculum Cultivation of Acclimated Sludge
3.3. Batch Experiments
3.4. Supporting Media
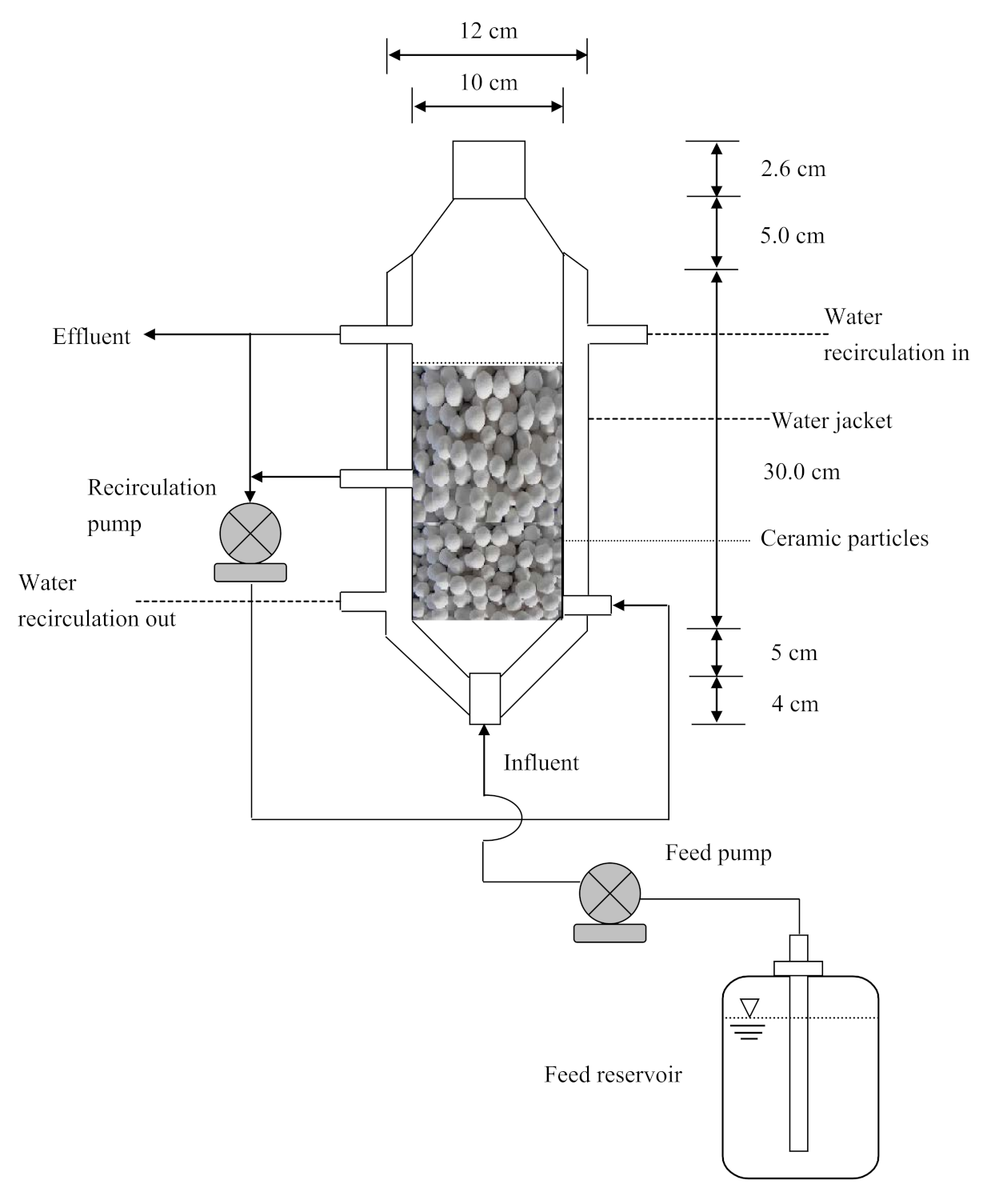
3.5. PBR Experiments
3.6. Analytical Methods
4. Results and Discussion
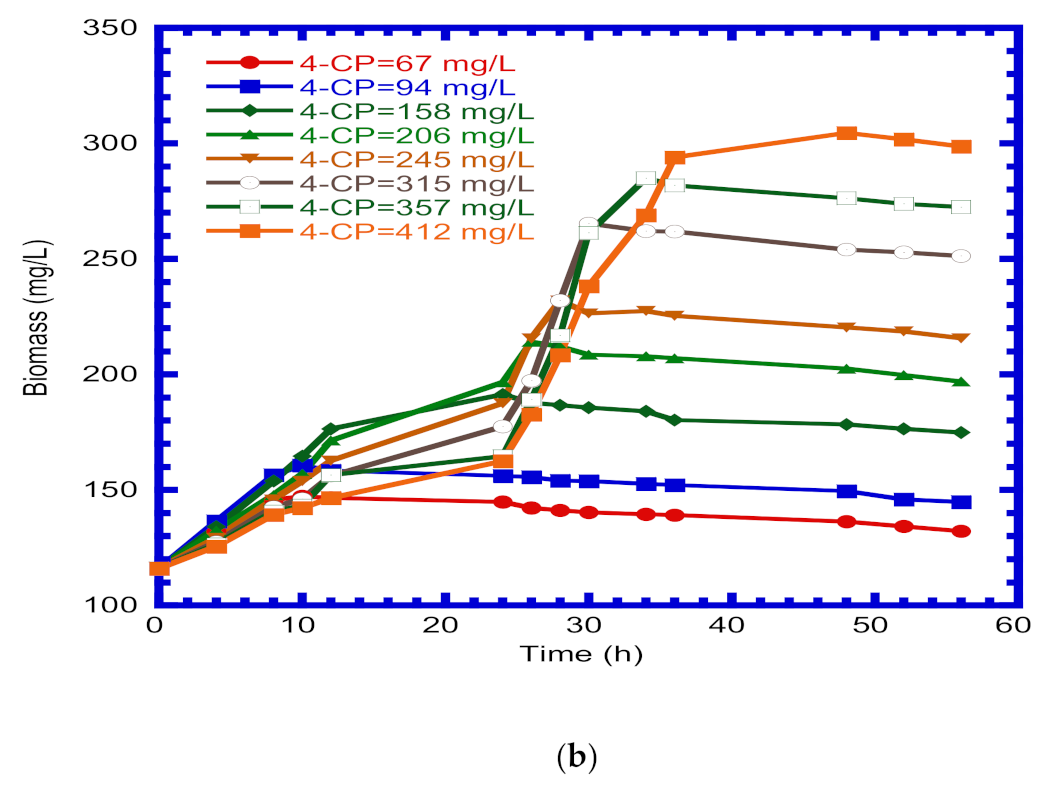
4.1. Degradation of 4-CP and Biomass Growth
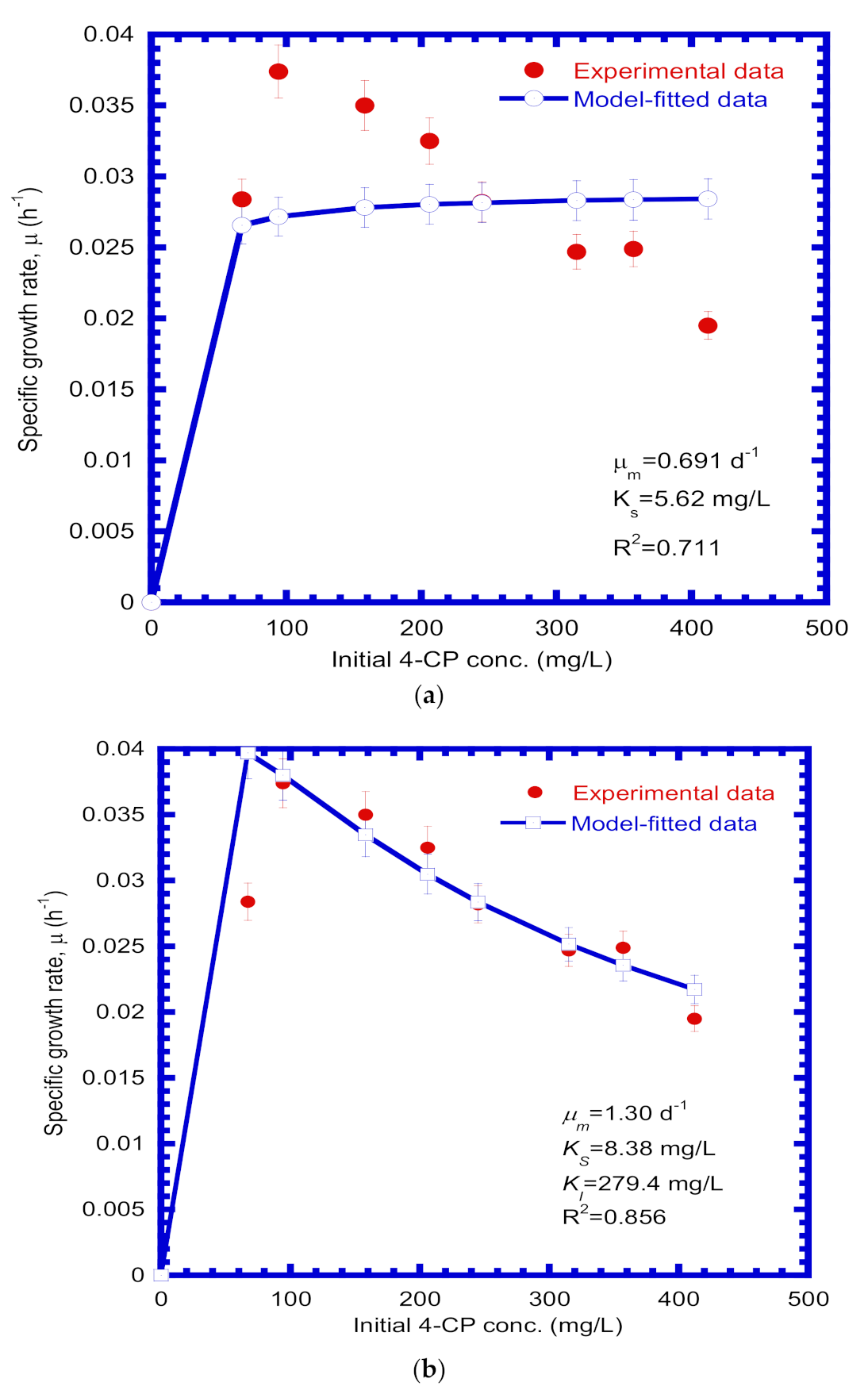
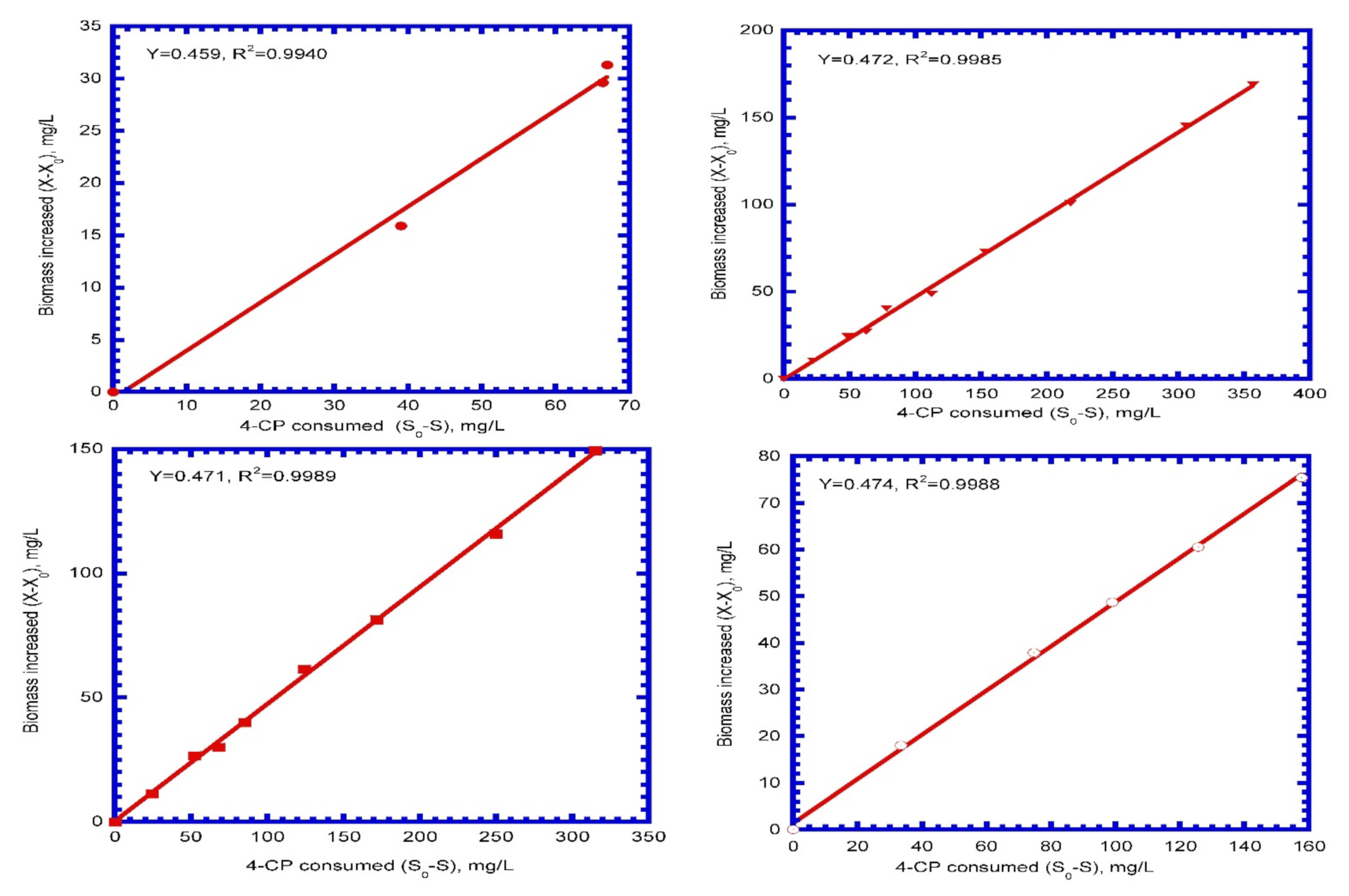
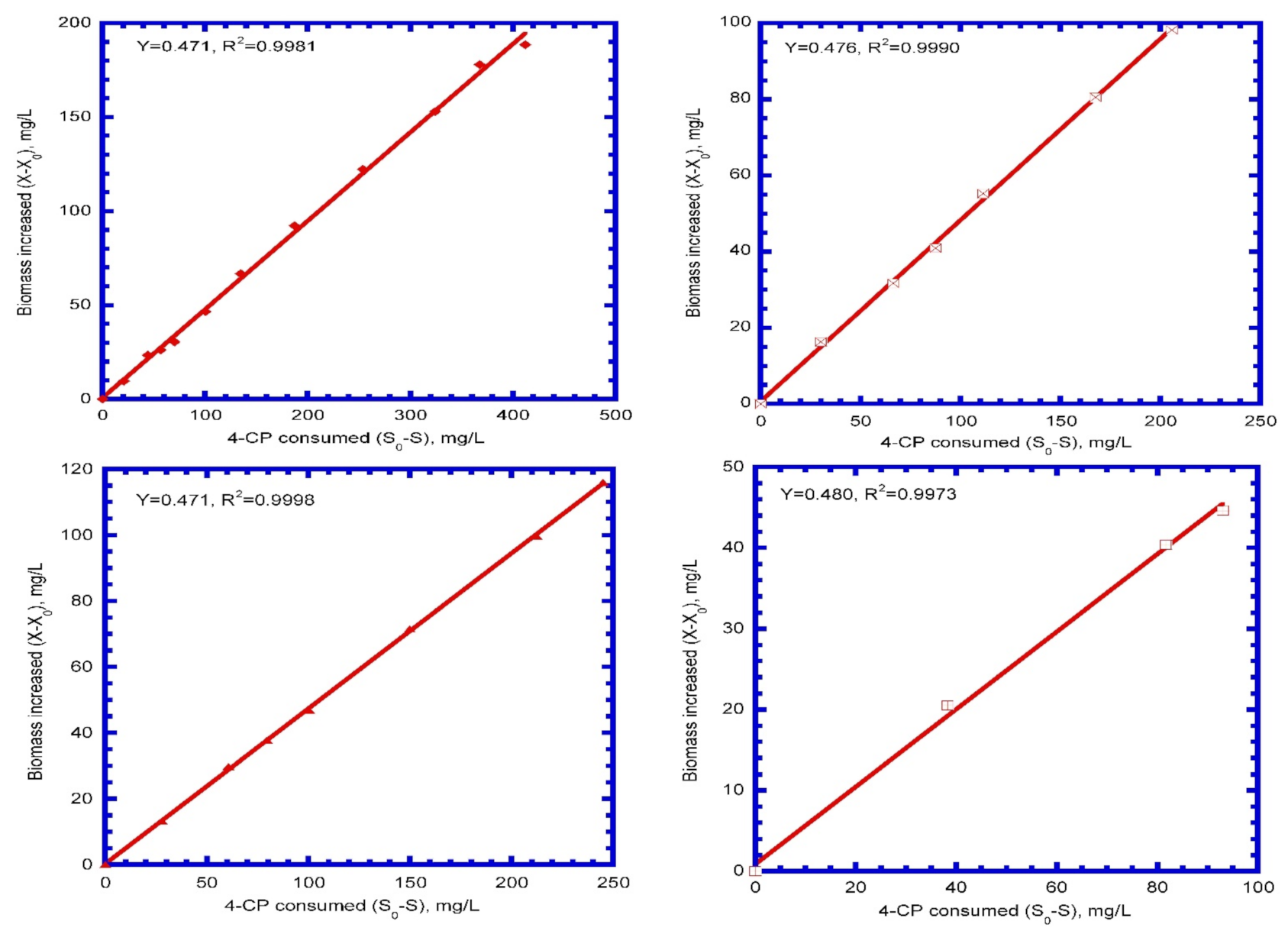
4.2. Kinetic Parameters
4.3. Mass Transfer Coefficients
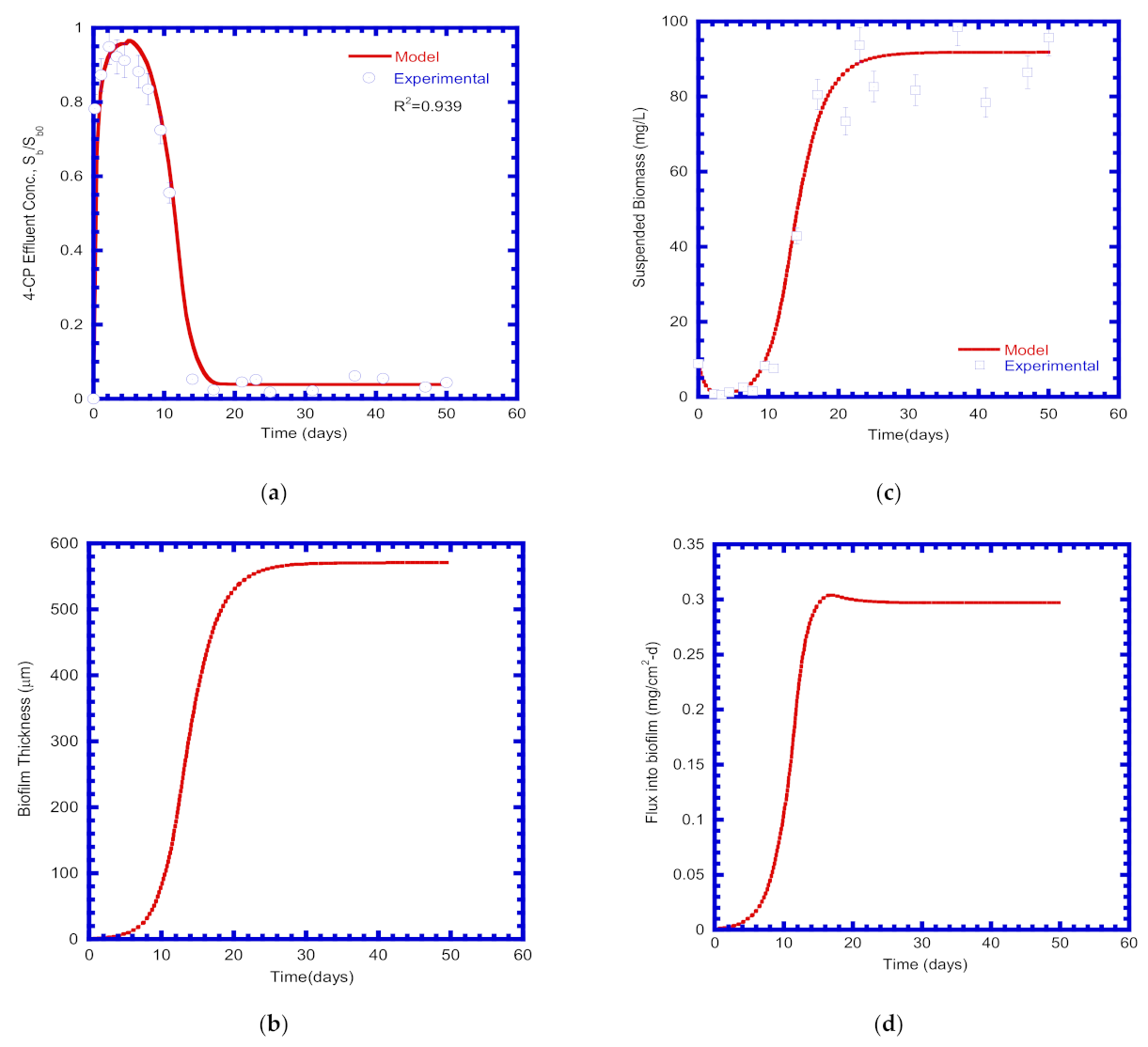
4.4. 4-CP Degradation in PBR
4.5. Biomass Growth
4.6. Flux Diffused into Biofilm Layer
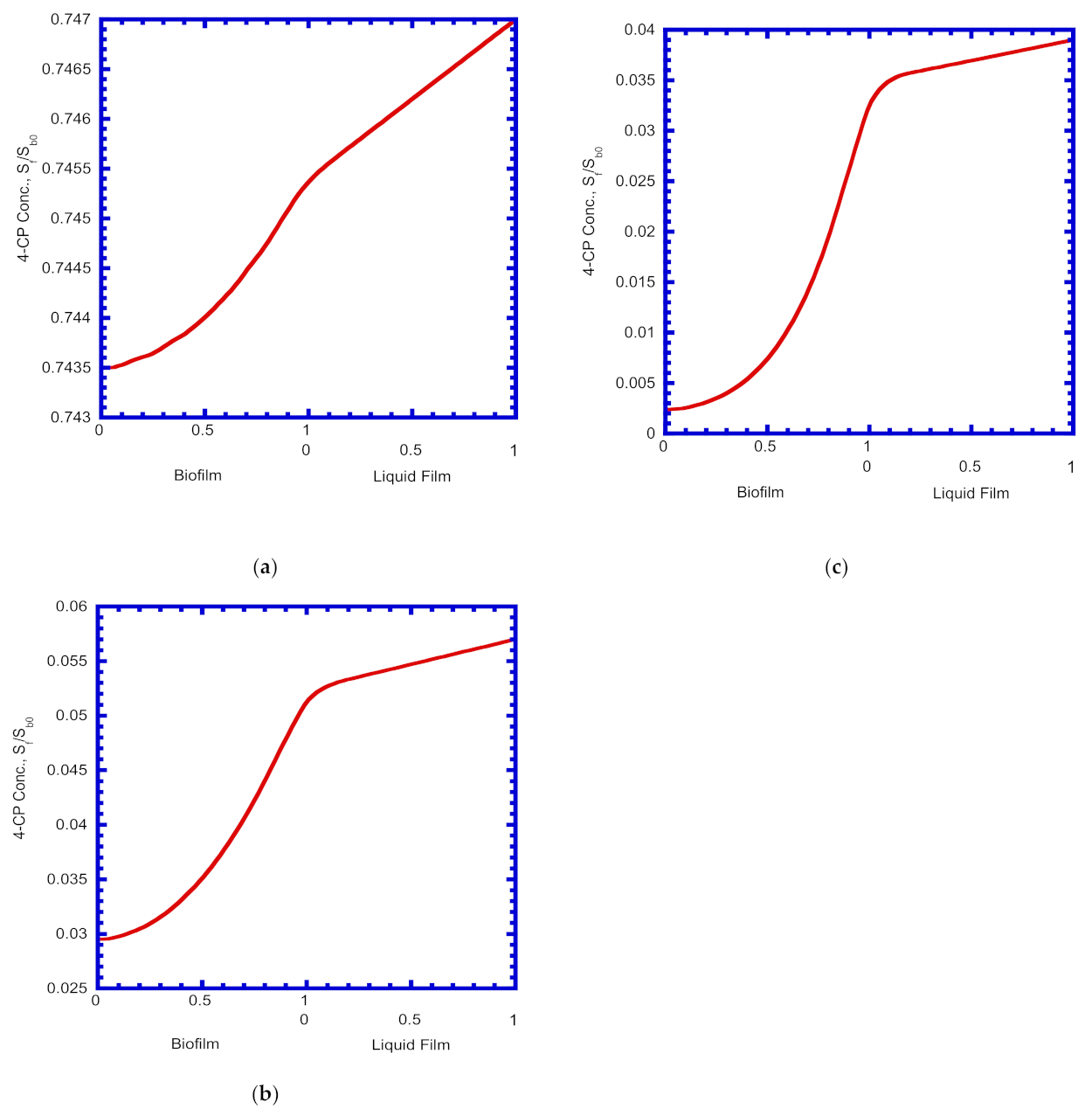
4.7. 4-CP Concentration Profiles in Biofilm and Liquid Film
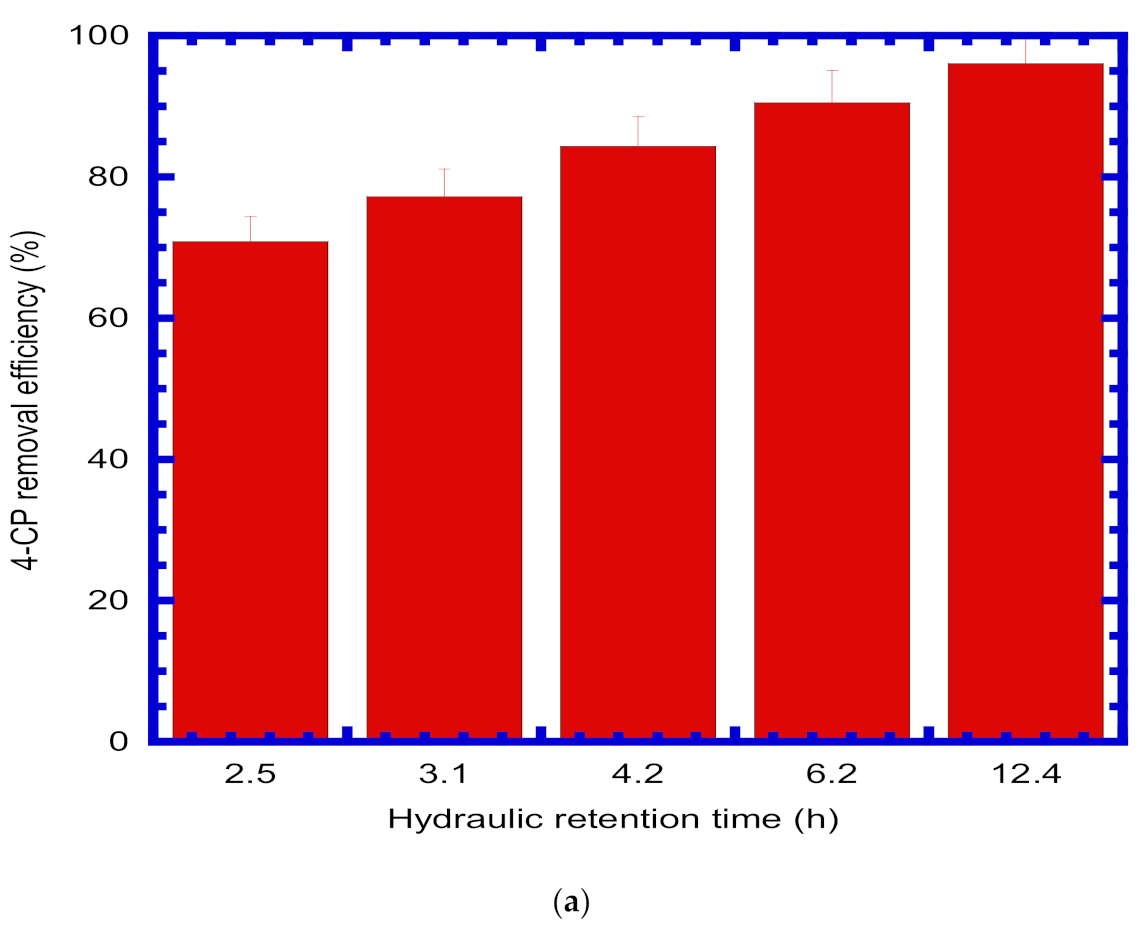
4.8. The Effect of Operation Parameters on 4-CP Removal Efficiency
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Movahedyan, H.; Assadi, A.; Amin, M. Effects of 4-chlorophenol loadings on acclimation of biomass with optimized fixed time sequencing batch reactor. J. Environ. Health Sci. Eng. 2008, 5, 225–234. [Google Scholar]
- Dey, A.; Sarkar, P.; Das, A. View of studies on biodegradation of 4-chlorophenol and 4-nitrophenol by isolated pure cultures. Eur. J. Sustain. Dev. 2019, 8, 281. [Google Scholar] [CrossRef]
- Azizi, E.; Abbasi, F.; Baghapour, M.A.; Shirdareh, M.R.; Shooshtarian, M.R. 4-chlorophenol removal by air lift packed bed bioreactor and its modeling by kinetics and numerical model (artifical neural network). Sci. Rep. 2021, 11, 670. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.K.; Bae, H. Bacterial degradation of chlorophenols and their derivatives. Microb. Cell Factories 2014, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Sreekrishnan, T.R.; Shaikh, Z.A. Evaluating the effects on performance and biomass of hybrid anaerobic reactor while treating effluents having glucose with increasing concentrations of 4-chlorophenols. J. Environ. Chem. Eng. 2018, 6, 2643–2650. [Google Scholar] [CrossRef]
- Patel, N.; Shahane, S.; Bhunia, B.; Mishra, U.; Chaudhary, V.K.; Srivastav, A.L. Biodegradation of 4-chlorophenol in batch and continuous packed bed reactor by isolated Bacillus subtilis. J. Environ. Manag. 2022, 301, 113851. [Google Scholar] [CrossRef]
- Sandhibigraha, S.; Mandal, S.; Awasthi, M.; Bandyopadhyay, T.; Bhunia, B. Optimization of various process parameters for biodegradation of 4-chlorophenol using Taguchi methodology. Biocatal. Agric. Biotechnol. 2020, 24, 101568–101577. [Google Scholar] [CrossRef]
- Farah, M.A.; Ateeq, B.; Ali, M.N.; Sabir, R.; Ahmad, W. Studies on lethal concentrations and toxicity stress of some xenobiotics on aquatic organisms. Chemosphere 2004, 55, 257–265. [Google Scholar] [CrossRef]
- Dizicheh, A.A.; Bayat, M.; Alimohmmadi, M.; Hashemi, J. Survey bioremediation of 4-chlorophenol by yeast and mold isolated from industrial and petroleum wastewaters (Imam Khomeini seaport, Mahshahr). Int. J. Mol. Clin. Microbiol. 2018, 8, 957–966. [Google Scholar]
- Kwean, O.S.; Cho, S.Y.; Yang, J.W.; Cho, W.; Park, S.; Lim, Y.; Shin, M.C.; Kim, H.S.; Park, J.; Kim, H.S. 4-chlorophenol biodegradation facilitator composed of recombinant multi-biocatalysis immobilized onto montmorillonite. Bioresour. Technol. 2018, 259, 268–275. [Google Scholar] [CrossRef]
- Swain, G.; Sonwani, R.K.; Singh, R.S.; Jaiswal, R.P.; Rai, B.N. Removal of 4-chlorophenol by Bacillus flexus as free and immobilized system: Effect of process variables and kinetic study. Environ. Technol. Innov. 2021, 21, 101356. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Lo, W.H.; Sillanpää, M.E. Treatment of contaminated water laden with 4-chlorophenol using coconut shell waste-based activated carbon modified with chemical agents. Sci. Sep. Technol. 2011, 46, 460–472. [Google Scholar] [CrossRef]
- Yang, H.; Ye, S.; Zeng, Z.; Zeng, G.; Tan, X.; Xiao, R.; Wang, J.; Song, B.; Du, L.; Qin, M.; et al. Utilization of biochar for resource recovery from water: A review. Chem. Eng. J. 2020, 397, 125502. [Google Scholar] [CrossRef]
- Xie, S.; Li, M.; Liao, Y.; Qin, Q.; Sun, S.; Tan, Y. In-situ preparation of biochar loaded particle electrode and its application in the electrochemical degradation of 4-chlorophenol in wastewater. Chemosphere 2021, 273, 128506. [Google Scholar] [CrossRef] [PubMed]
- Erol Nalbur, B.; Alkan, U. The inhibitory effects of 2-CP and 2,4 DCP containing effluents on sequencing batch reactors. Int. Biodeterior. Biodegrad. 2007, 60, 178–188. [Google Scholar] [CrossRef]
- Sahinkaya, E.; Dilek, F.B. Biodegradation kinetics of 2,4-dichlorophenol by acclimated mixed culture. J. Biotechnol. 2007, 127, 716–726. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Li, J.; Wang, Y.; Wang, C.; Wang, P. Experimental and kinetic study on the cometabolic biodegradation of phenol and 4-chlorophenol by psychrotrophic Pseudomonas putida LY1. Environ. Sci. Pollut. Res. 2015, 22, 565–573. [Google Scholar] [CrossRef]
- Li, S.; Ma, B.; She, Z.; Guo, L.; Zhao, Y.; Jin, C.; Gao, M. Effect of norfloxacin on performance, microbial enzymatic activity and microbial community of a sequencing batch reactor. Environ. Technol. Innov. 2020, 18, 100726. [Google Scholar] [CrossRef]
- Loh, K.C.; Yu, Y.G. Kinetics of carbazole degradation by Pseudomonas putida in presence of sodium salicylate. Water Res. 2000, 34, 4131–4138. [Google Scholar] [CrossRef]
- Wang, S.J.; Loh, K.C. Growth kinetics of Pseudomonas putida in cometabolism of phenol and 4-chlorophenol in the presence of a conventional carbon source. Biotechnol. Bioeng. 2000, 68, 437–447. [Google Scholar] [CrossRef]
- Hao, O.J.; Kim, M.H.; Seagren, E.A.; Kim, H. Kinetics of phenol and chlorophenol utilization by Acinetobacter species. Chemosphere 2002, 46, 797–807. [Google Scholar] [CrossRef]
- Gąszczak, A.; Bartelmus, G.; Rotkegel, A.; Greñ, I.; Janecki, D. Kinetics of cometabolic biodegradation of 4-chlorophenol and phenol by stenotrophomonas maltophilia KB2. Chem. Process Eng. 2018, 39, 395–410. [Google Scholar]
- Sahinkaya, E.; Dilek, F.B. Biodegradation of 4-chlorophenol by acclimated and unacclimated activated sludge-Evaluation of biokinetic coefficients. Environ. Res. 2005, 99, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Buitron, G.; Gonzalez, A. Characterization of the microorganisms from an acclimated activated sludge degrading phenolic compounds. Water Sci. Technol. 1996, 34, 289–294. [Google Scholar] [CrossRef]
- Assadi, A.; Alimoradzadeh, R.; Movahedyan, H.; Amin, M. Intensified 4-chlorophenol biodegradation in an aerobic sequencing batch reactor: Microbial and kinetic properties evaluation. Environ. Technol. Innov. 2021, 21, 101243. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, K.K.; Lee, S.T.; Kim, S.W.; Hong, S.I. Biodegradation of phenol and chlorophenols with defined mixed culture in shake-flasks and a packed bed reactor. Process Biochem. 2002, 37, 1367–1373. [Google Scholar] [CrossRef]
- Buitron, G.; Gonzalez, A.; Lopez-Marin, L.M. Biodegradation of phenolic compounds by an activated sludge and isolated bacteria. Water Sci. Technol. 1998, 37, 371–378. [Google Scholar] [CrossRef]
- Chung, J.; Choi, J.; Chung, S. Pilot study of specific microbe immobilization cells (SMICs) technology in removing tetramethyl ammonium hydroxide for reuse of low strength electronics wastewater. J. Hazard. Mater. 2020, 384, 120829. [Google Scholar] [CrossRef]
- Turan, V. Calcite in combination with olive pulp biochar reduces Ni mobility in soil and its distribution in chili plant. Int. J. Phytoremediat. 2022, 24, 166–176. [Google Scholar] [CrossRef]
- Patel, B.P.; Kumar, A. Biodegradation of 4-chlorophenol in an airlift inner loop bioreactor with mixed consortium: Effect of HRT, loading rate and biogenic substrate. Biotech 2016, 6, 117. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Liu, Y.; Wu, M.; Zong, W.; Yi, X.; Zhan, J.; Liu, L.; Zhou, H. Coupling the phenolic oxidation capacities of a bacterial consortium and in situ-generated manganese oxides in a moving bed biofilm reactor (MBBR). Water Res. 2019, 166, 115047. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Singh, R.P.; Yang, X.; Ojha, C.S.P.; Surampalli, R.Y.; Kumar, A.J. Sediment in-situ bioremediation by immobilized microbial activated bawds: Pilot-scale study. J. Environ. Manag. 2018, 226, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Kurade, M.B.; Waghmode, T.R.; Xiong, J.Q.; Govindwar, S.P.; Jeon, B.H. Decolorization of textile industry effluent using immobilized consortium cells in upflow fixed bed reactor. J. Clean. Prod. 2019, 213, 884–891. [Google Scholar] [CrossRef]
- Geed, S.R.; Kureel, M.K.; Giri, B.S.; Singh, R.S.; Rai, B.N. Performance evaluation of malathion biodegradation in batch and continuous packed bed bioreactor (PBBR). Bioresour. Technol. 2017, 227, 56–65. [Google Scholar] [CrossRef]
- Saravanan, V.; Sreekrishnan, T.R. Modelling anaerobic biofilm reactors—A review. J. Environ. Manag. 2006, 81, 1–18. [Google Scholar] [CrossRef]
- Wang, S.; Boyjoo, Y.; Choueib, A.; Zhu, Z.H. Removal of dyes from aqueous solution using fly ash and red mud. Water Res. 2005, 39, 129–138. [Google Scholar] [CrossRef]
- Swain, G.; Sonwani, R.K.; Singh, R.S.; Jaiswal, R.P.; Rai, B.N. A comparative study of 4-chlorophenol biodegradation in a packed bed and moving bed bioreactor: Performance evaluation and toxicity analysis. Environ. Technol. Innov. 2021, 24, 101820. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, X.; Wang, L.; Xu, Y.; Li, J.; Li, Y. Effects of elevated 4-chlorophenol loads on components of polysaccharides and proteins and toxicity in an activated sludge process. Chem. Eng. J. 2017, 330, 236–244. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Kumar, A.; Kumar, A.; Kumar, S. Biodegradation kinetics of phenol and catechol using Pseudomonas putida MTCC 1194. Biochem. Eng. J. 2005, 22, 151–159. [Google Scholar] [CrossRef]
- Konya, I.; Eker, S.; Kargi, F. Mathematical modelling of 4-chlorophenol inhibition on COD and 4-chlorophenol removals in an activated sludge unit. J. Hazard. Mater. 2007, 143, 233–239. [Google Scholar] [CrossRef]
- Arvin, E.; Kristensen, G.H. Effect of denitrification on the pH in biofilms. Water Sci. Technol. 1982, 14, 833–848. [Google Scholar] [CrossRef]
- Wilke, C.E.; Chang, P. Correlation of diffusion coefficients in dilute solutions. AICHE J. 1955, 1, 264–270. [Google Scholar] [CrossRef]
- Williamson, K.; McCarty, P.L. Verification studies of the biofilm model for bacterial substrate utilization. J. Water Pollut. Control Fed. 1976, 48, 281–296. [Google Scholar]
- Dwivedi, P.N.; Upadhyay, S.N. Particle-fluid mass transfer in fixed and fluidized bed. Ind. Eng. Chem. Proc. Des. Dev. 1977, 16, 157–165. [Google Scholar] [CrossRef]
- Eker, S.; Kargi, F. COD, para-chlorophenol and toxicity removal from synthetic wastewater using rotating tubes biofilm reactor (RTBR). Bioresour. Technol. 2010, 101, 9020–9024. [Google Scholar] [CrossRef]
- Kureel, M.K.; Geed, S.R.; Rai, B.N.; Singh, R.S. Novel investigation of the performance of continuous packed bed bioreactor (CPBBR) by isolated Bacillus sp. M4 and proteomic study. Bioresour. Technol. 2018, 266, 335–342. [Google Scholar] [CrossRef] [PubMed]



| Symbol | Kinetic Parameters (Unit) | Value |
|---|---|---|
| Sb0 | Influent concentration of 4-CP (mg/L) | 246.7 |
| μm | Maximum specific growth rate (d−1) | 1.30 |
| KS | Half-saturation constant (mg/L) | 8.38 |
| KI | Inhibition constant (mg/L) | 279.4 |
| Y | Biomass yield (mg SS/mg 4-CP) | 0.472 |
| b | Decay constant (d−1) | 0.054 |
| bs | Shear-loss coefficient (d−1) | 0.223 |
| Df | Diffusion coefficient (cm2/d) | 0.684 |
| kf | Mass transfer coefficient (cm/d) | 282.5 |
| Lf0 | Initial biofilm thickness (μm) | 0.75 |
| Xf | Biofilm density (mg/mL) | 8.86 |
| Xb0 | Initial suspended biomass (mg SS/L) | 8.9 |
| Q | Influent flow rate (mL/d) | 3038–15,192 |
| V | Working volume (mL) | 1568 |
| ε | Reactor porosity (dimensionless) | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-H. Kinetic Study of 4-Chlorophenol Biodegradation by Acclimated Sludge in a Packed Bed Reactor. Processes 2022, 10, 2130. https://doi.org/10.3390/pr10102130
Lin Y-H. Kinetic Study of 4-Chlorophenol Biodegradation by Acclimated Sludge in a Packed Bed Reactor. Processes. 2022; 10(10):2130. https://doi.org/10.3390/pr10102130
Chicago/Turabian StyleLin, Yen-Hui. 2022. "Kinetic Study of 4-Chlorophenol Biodegradation by Acclimated Sludge in a Packed Bed Reactor" Processes 10, no. 10: 2130. https://doi.org/10.3390/pr10102130
APA StyleLin, Y.-H. (2022). Kinetic Study of 4-Chlorophenol Biodegradation by Acclimated Sludge in a Packed Bed Reactor. Processes, 10(10), 2130. https://doi.org/10.3390/pr10102130






