Hydrogen Production in Catalytic Membrane Reactors Based on Porous Ceramic Converters
Abstract
:1. Introduction
- Obtaining highly pure products reduces their cost, and the possibility of placing the reactor and the separator into a single vessel significantly reduces the size of the device;
- The possibility of bypassing the thermodynamic limitations of equilibrium processes, which makes it possible to achieve the same substrate conversions at lower temperatures or higher conversions at the same temperatures as used in a conventional flow reactor;
- Lower process temperatures enable new reactor heating strategies. One of these approaches is the use of turbine exhaust gases, which is an energy efficient technological solution. In addition, this approach reduces the cost of structural materials, which reduces the cost and increases the safety of the processes.
2. Experimental Part
2.1. Investigation Objects
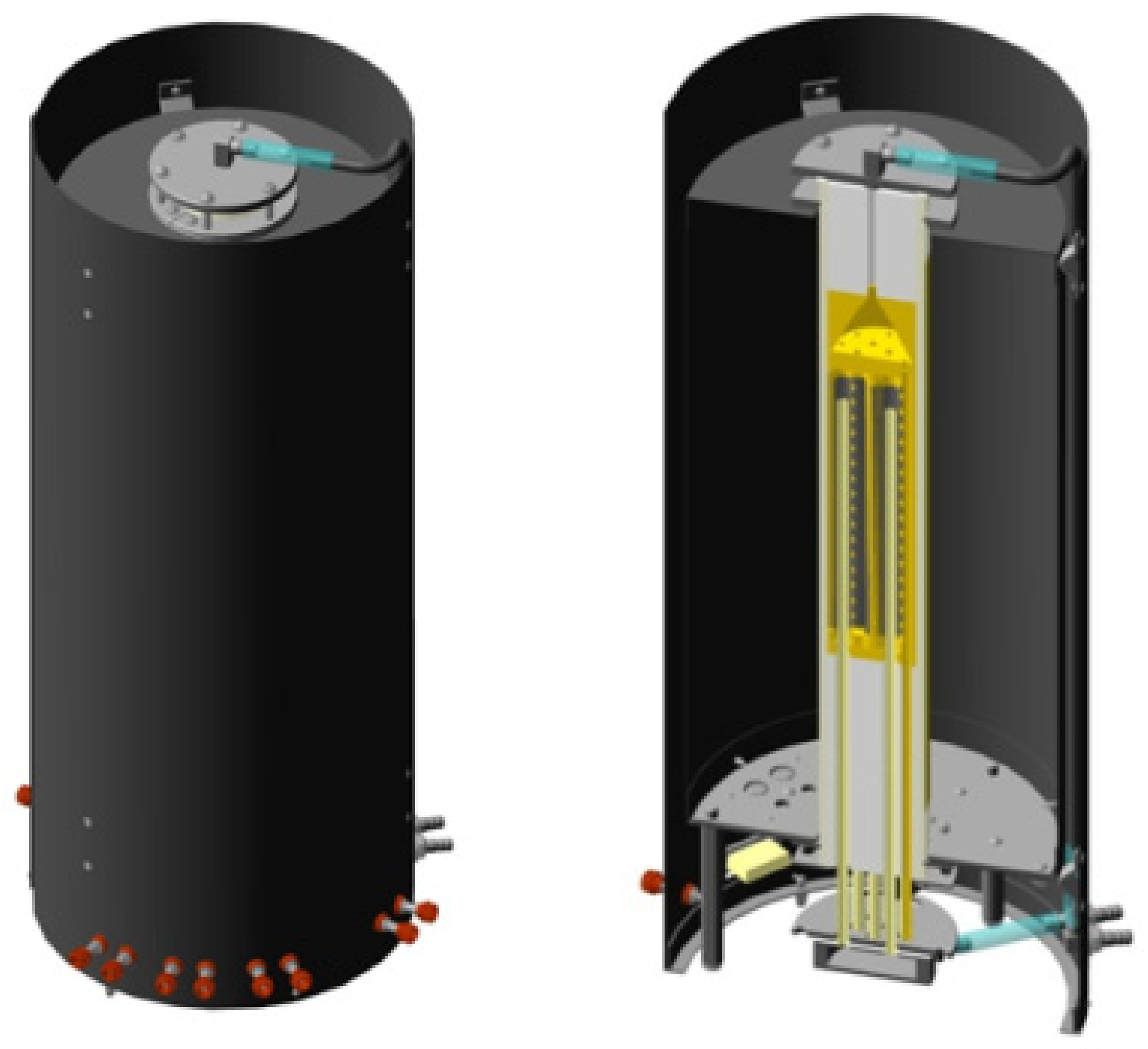
2.2. Design of a Laboratory Setup and a Catalytic Membrane Reactor
2.3. Experimental Procedure
2.4. Calculations
3. Results and Discussion
3.1. Water–Gas Shift Reaction on Porous Catalytic Converters
3.2. Steam Reforming of Carbon Monoxide Mixed with Hydrogen
3.3. Production of Ultrapure Hydrogen Using the Hybrid Catalytic Membrane Reactor
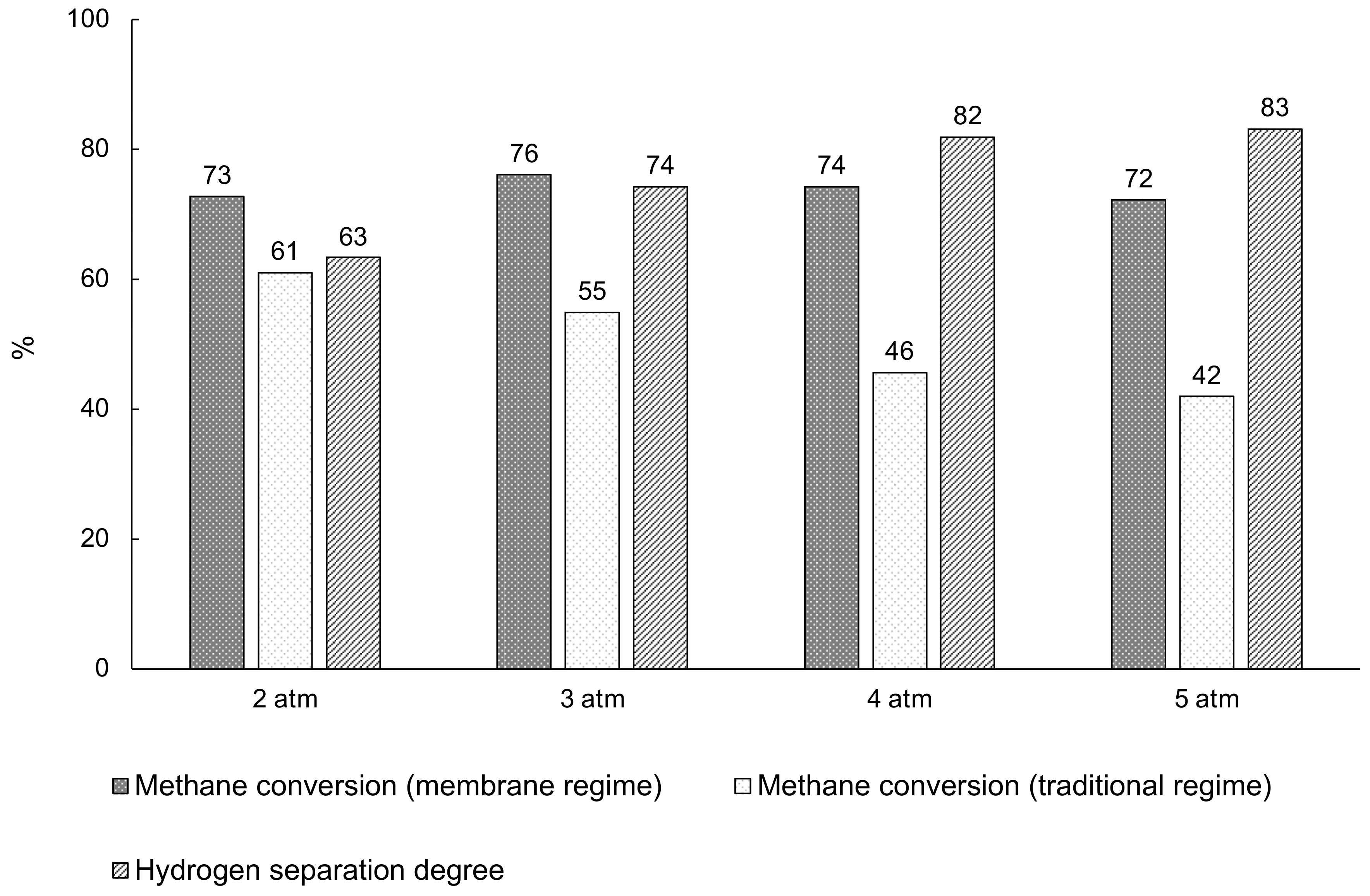
3.4. Carbon dioxide Reforming of Methane
3.5. Carbon Dioxide Reforming of Ethanol
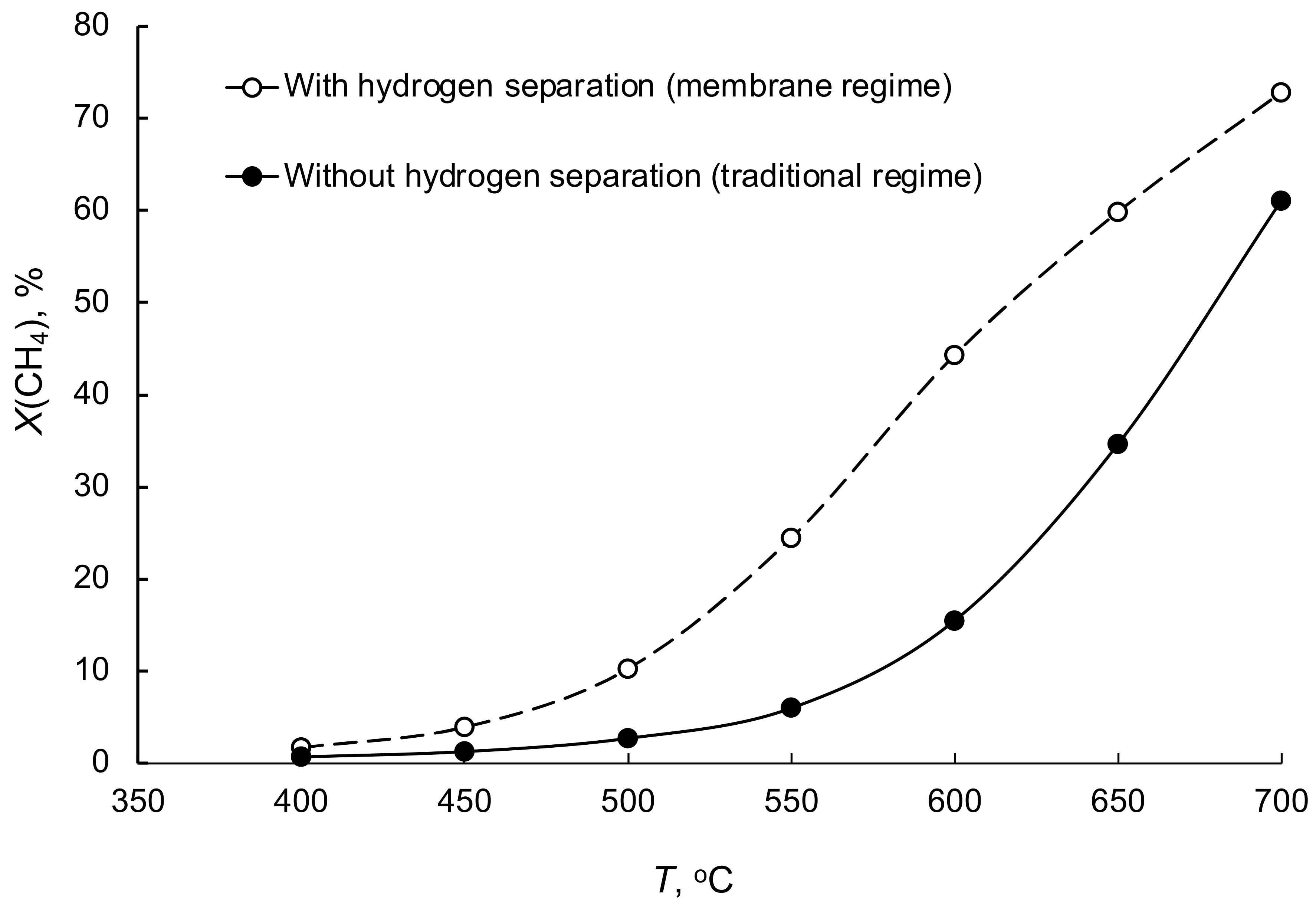
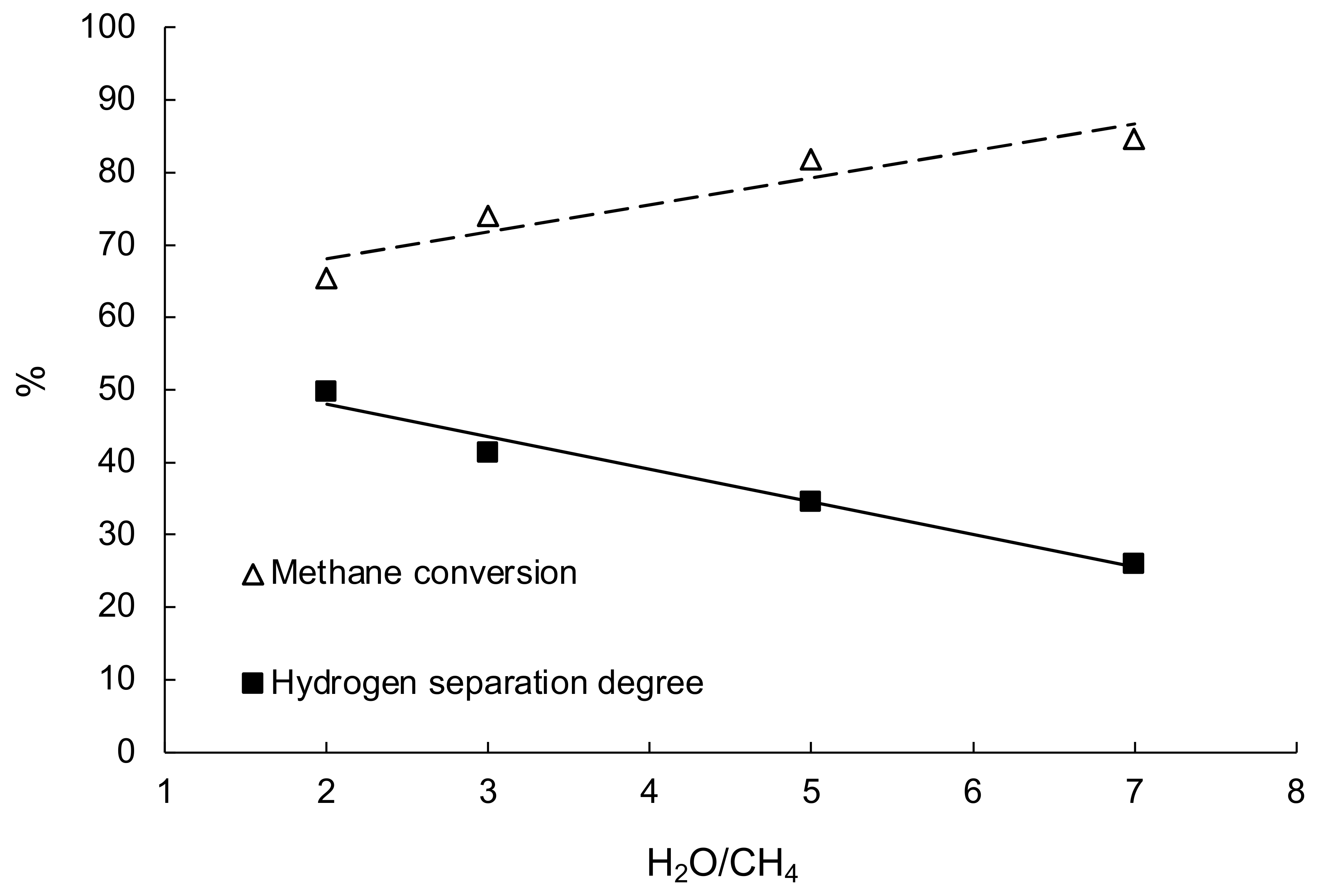
3.6. Steam Reforming of Methane
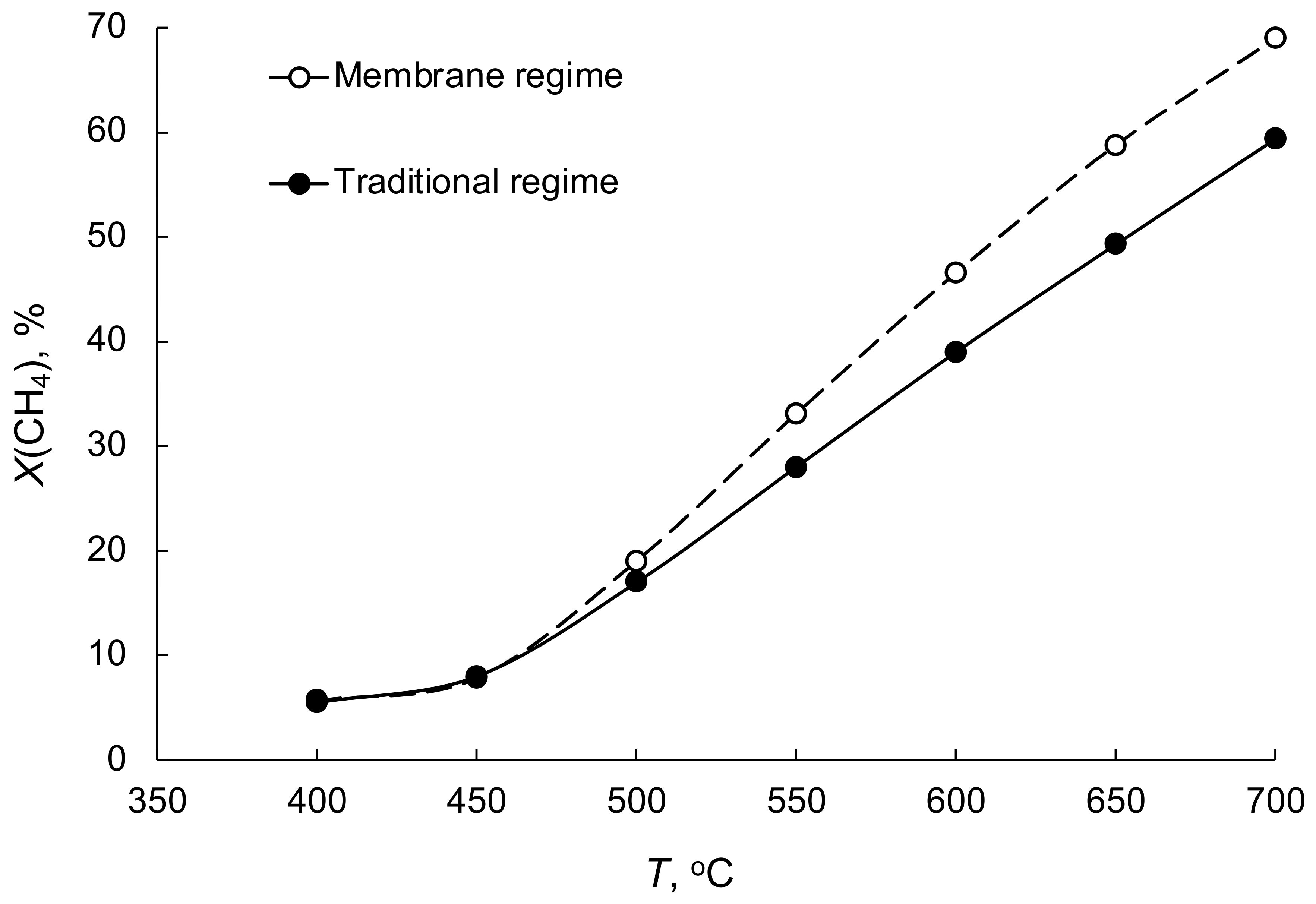
3.7. Steam Reforming of Ethanol and Fermentation Products
3.8. Steam Reforming of Dimethyl Ether
3.9. Combined Carbon Dioxide and Steam Reforming of the Fischer–Tropsch Synthesis By-Products
3.10. Carbon Dioxide and Steam Reforming of Aviation Kerosene Partial Oxidation Products
3.11. Hydrogenation of Carbon Oxides Present in the Products of Ethanol Steam Reforming in a Porous Catalytic Converter
3.12. Implementation of a Catalytic Membrane Reactor in Combination with a Solid Oxide Fuel Cell in Small-Sized Power Generators
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ajanovic, A.; Haas, R. Prospects and impediments for hydrogen and fuel cell vehicles in the transport sector. Int. J. Hydrog. Energy 2020, 46, 10049–10058. [Google Scholar] [CrossRef]
- Kovač, A.; Paranos, M.; Marciuš, D. Hydrogen in energy transition: A review. Int. J. Hydrog. Energy 2021, 46, 10016–10035. [Google Scholar] [CrossRef]
- Ogden, J.M. Prospects for Hydrogen in the Future Energy System; Research Report—UCD-ITS-RR-18-07; Institute of Transportation Studies, University of California: Davis, CA, USA, 2018; Available online: https://escholarship.org/uc/item/52s28641#main (accessed on 18 September 2022).
- Filippov, S.P.; Yaroslavtsev, A.B. Hydrogen energy: Development prospects and materials. Russ. Chem. Rev. 2021, 90, 627–643. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dash, S.K.; Elavarasan, R.M.; Kaur, A.; Elangovan, D.; Meraj, S.T.; Kasinathan, P.; Said, Z. Hydrogen Energy as Future of Sustainable Mobility. Front. Energy Res. 2022, 10, 893475. [Google Scholar] [CrossRef]
- Garland, N.L.; Papageorgopoulos, D.C.; Stanford, J.M. Hydrogen and Fuel Cell Technology: Progress, Challenges, and Future Directions. Energy Procedia 2012, 28, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Tu, Z.; Chan, S.H. Recent development of hydrogen and fuel cell technologies: A review. Energy Rep. 2021, 7, 8421–8446. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Staffell, I.; Dodds, P.; Scamman, D.; Abad, A.V.; Dowell, N.M.; Ward, K.; Agnolucci, P.; Papageorgiou, L.; Shah, N.; Ekins, P. The Role of Hydrogen and Fuel Cells in Future Energy Systems. H2FC Supergen. 2017. Available online: https://www.h2fcsupergen.com/wp-content/uploads/2015/08/J5212_H2FC_Supergen_Energy_Systems_WEB.pdf (accessed on 18 September 2022).
- Sundén, B. Chapter 8—Fuel Cell Types—Overview. In Hydrogen, Batteries and Fuel Cells; Academic Press: Cambridge, MA, USA, 2019; pp. 123–144. ISBN 9780128169506. [Google Scholar] [CrossRef]
- Revankar, S.; Majumdar, P. Fuel Cells e Principles, Design and Analysis; CRC Press: New York, NY, USA, 2014; p. 750. Available online: https://www.routledge.com/Fuel-Cells-Principles-Design-and-Analysis/Revankar-Majumdar/p/book/9781420089684 (accessed on 18 September 2022).
- Singhal, S.C.; Kendall, K. High Temperature Solid Oxide Fuel Cells-Fundamentals, Design and Applications; Elsevier: Oxford, UK, 2004; p. 406. ISBN 9780080508085. Available online: https://cds.cern.ch/record/640123/files/1856173879_TOC.pdf (accessed on 18 September 2022).
- Hacker, V.; Mitsushima, S. Fuel Cells and Hydrogen; Elsevier: Amsterdam, The Netherlands, 2018; p. 296. ISBN 9780128115374. [Google Scholar]
- Brandon, N. Solid Oxide Fuel Cells e Lifetime and Reliability; Elsevier: 2017. Available online: https://www.elsevier.com/books/fuel-cells-and-hydrogen/hacker/978-0-12-811459-9 (accessed on 18 September 2022).
- Albarbar, A.; Alrweq, M. Proton Exchange Membrane Fuel Cell-Design, Modelling and Performance Assessment Techniques; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-319-70727-3. [Google Scholar] [CrossRef]
- Habibollahzade, A.; Rosen, M.A. Syngas-fueled solid oxide fuel cell functionality improvement through appropriate feedstock selection and multi-criteria optimization using Air/O2-enriched-air gasification agents. Appl. Energy 2021, 286, 116497. [Google Scholar] [CrossRef]
- Jia, J.; Li, Q.; Luo, M.; Wei, L.; Abudula, A. Effects of gas recycle on performance of solid oxide fuel cell power systems. Energy 2011, 36, 1068–1075. [Google Scholar] [CrossRef]
- Gadsbøll, R.; Thomsen, J.; Bang-Møller, C.; Ahrenfeldt, J.; Henriksen, U.B. Solid oxide fuel cells powered by biomass gasification for high efficiency power generation. Energy 2017, 131, 198–206. [Google Scholar] [CrossRef] [Green Version]
- D’Andrea, G.; Gandiglio, M.; Lanzini, A.; Santarelli, M. Dynamic model with experimental validation of a biogas-fed SOFC plant. Energy Convers. Manag. 2017, 135, 21–34. [Google Scholar] [CrossRef]
- Subotić, V.; Baldinelli, A.; Barelli, L.; Scharler, R.; Pongratz, G.; Hochenauer, C.; Anca-Couce, A. Applicability of the SOFC technology for coupling with biomass-gasifier systems: Short- and long-term experimental study on SOFC performance and degradation behaviour. Appl. Energy 2019, 256, 113904. [Google Scholar] [CrossRef]
- Langnickel, H.; Rautanen, M.; Gandiglio, M.; Santarelli, M.; Hakala, T.; Acri, M.; Kiviaho, J. Efficiency analysis of 50 kWe SOFC systems fueled with biogas from waste water. J. Power Sources Adv. 2020, 2, 100009. [Google Scholar] [CrossRef]
- Bicer, Y.; Khalid, F. Life cycle environmental impact comparison of solid oxide fuel cells fueled by natural gas, hydrogen, ammonia and methanol for combined heat and power generation. Int. J. Hydrog. Energy 2018, 45, 3670–3685. [Google Scholar] [CrossRef]
- Boretti, A. Production of hydrogen for export from wind and solar energy, natural gas, and coal in Australia. Int. J. Hydrog. Energy 2020, 45, 3899–3904. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.; Xiong, X.; Tsang, D.C.; Zhang, S.; Clark, J.H.; Hu, C.; Ng, Y.H.; Shang, J.; Ok, Y.S. Biorenewable hydrogen production through biomass gasification: A review and future prospects. Environ. Res. 2020, 186, 109547. [Google Scholar] [CrossRef]
- Cormos, C.-C. Hydrogen and power co-generation based on coal and biomass/solid wastes co-gasification with carbon capture and storage. Int. J. Hydrog. Energy 2012, 37, 5637–5648. [Google Scholar] [CrossRef]
- Stambouli, A.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Larminie, J.; Dicks, A. Medium and High Temperature Fuel Cells. In Fuel Cell Systems Explained; John Wiley & Sons, Ltd: Chichester, UK, 2003; ISBN 9781118878330. [Google Scholar]
- Sazali, N.; Wan Salleh, W.N.; Jamaludin, A.S.; Mhd Razali, M.N. New Perspectives on Fuel Cell Technology: A Brief Review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrog. Energy 2021, 47, 26238–26264. [Google Scholar] [CrossRef]
- Barbieri, G.; Violante, V.; Di Maio, F.P.; Criscuoli, A.; Drioli, E. Methane Steam Reforming Analysis in a Palladium-Based Catalytic Membrane Reactor. Ind. Eng. Chem. Res. 1997, 36, 3369–3374. [Google Scholar] [CrossRef]
- Tong, J.; Matsumura, Y. Pure hydrogen production by methane steam reforming with hydrogen-permeable membrane reactor. Catal. Today 2006, 111, 147–152. Available online: https://link.springer.com/article/10.1007/s11244-008-9129-5 (accessed on 18 September 2022). [CrossRef]
- Algieri, C.; Coppola, G.; Mukherjee, D.; Shammas, M.; Calabro, V.; Curcio, S.; Chakraborty, S. Catalytic Membrane Reactors: The Industrial Applications Perspective. Catalysts 2021, 11, 691. [Google Scholar] [CrossRef]
- Uvarov, V.I.; Kapustin, R.D.; Fedotov, A.S.; Kirillov, A.O. Synthesis of Porous Ceramic Materials for Catalytically Active Membranes by Technological Combustion and Sintering. Glas. Ceram. 2020, 77, 221–225. [Google Scholar] [CrossRef]
- Fedotov, A.S.; Uvarov, V.I.; Tsodikov, M.V.; Moiseev, I.I.; Paul, S.; Heyte, S.; Simon, P.; Marinova, M.; Dumeignil, F. Synthesis of 1,3-Butadiene from 1-Butanol on a Porous Ceramic [Fe,Cr]/γ-Al2O3(K,Ce)/α-Al2O3 Catalytic Converter. Kinet. Catal. 2020, 61, 390–404. [Google Scholar] [CrossRef]
- Eliseev, O.L. Gas-to-liquid technologies. Russ. J. Gen. Chem. 2009, 79, 2509–2519. [Google Scholar] [CrossRef]
- Zoppi, G.; Pipitone, G.; Gruber, H.; Weber, G.; Reichhold, A.; Pirone, R.; Bensaid, S. Aqueous phase reforming of pilot-scale Fischer-Tropsch water effluent for sustainable hydrogen production. Catal. Today 2020, 367, 239–247. [Google Scholar] [CrossRef]
- Zoppi, G.; Pipitone, G.; Pirone, R.; Bensaid, S. Aqueous phase reforming process for the valorization of wastewater streams: Application to different industrial scenarios. Catal. Today 2021, 387, 224–236. [Google Scholar] [CrossRef]
- D’Angelo, M.F.N.; Ordomsky, V.; Schouten, J.C.; Van Der Schaaf, J.; Nijhuis, T.A. Carbon-Coated Ceramic Membrane Reactor for the Production of Hydrogen by Aqueous-Phase Reforming of Sorbitol. ChemSusChem 2014, 7, 2007–2015. [Google Scholar] [CrossRef]
- Jiaying, Z. Kinetic study of carbon monoxide methanation over mesoporous Ni-Mo catalyst prepared by a hydrothermal method. Prog. React. Kinet. Mech. 2019, 44, 3–17. [Google Scholar] [CrossRef]
- Mutschler, R.; Moioli, E.; Luo, W.; Gallandat, N.; Züttel, A. CO2 hydrogenation reaction over pristine Fe, Co, Ni, Cu and Al2O3 supported Ru: Comparison and determination of the activation energies. J. Catal. 2018, 366, 139–149. [Google Scholar] [CrossRef]
- Stabnikov, V.N. Distillation and Rectification of Ethyl Alcohol, 2nd ed.; Food Industry Publishing House: Moscow, Russia, 1969; p. 224. [Google Scholar]
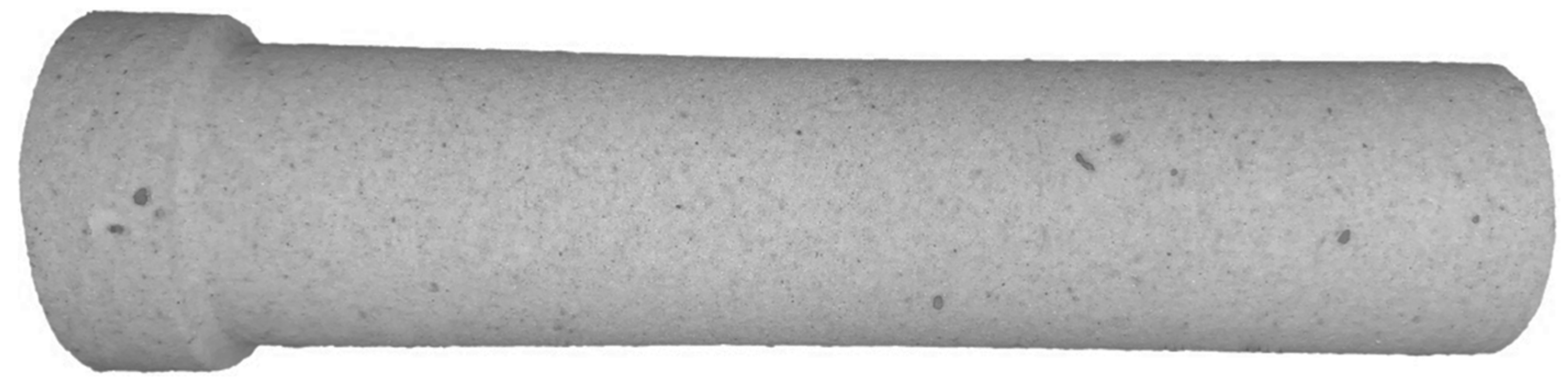
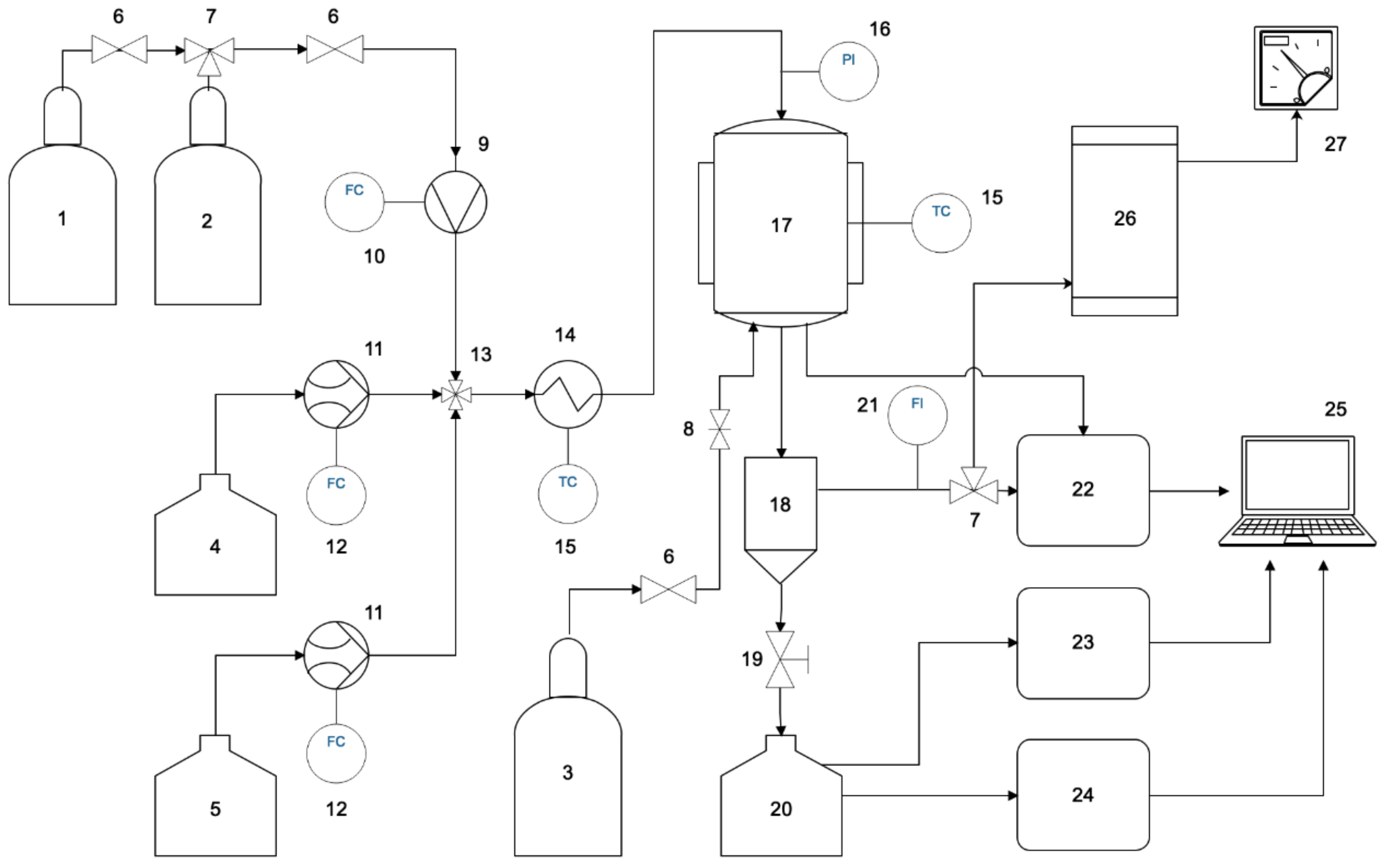

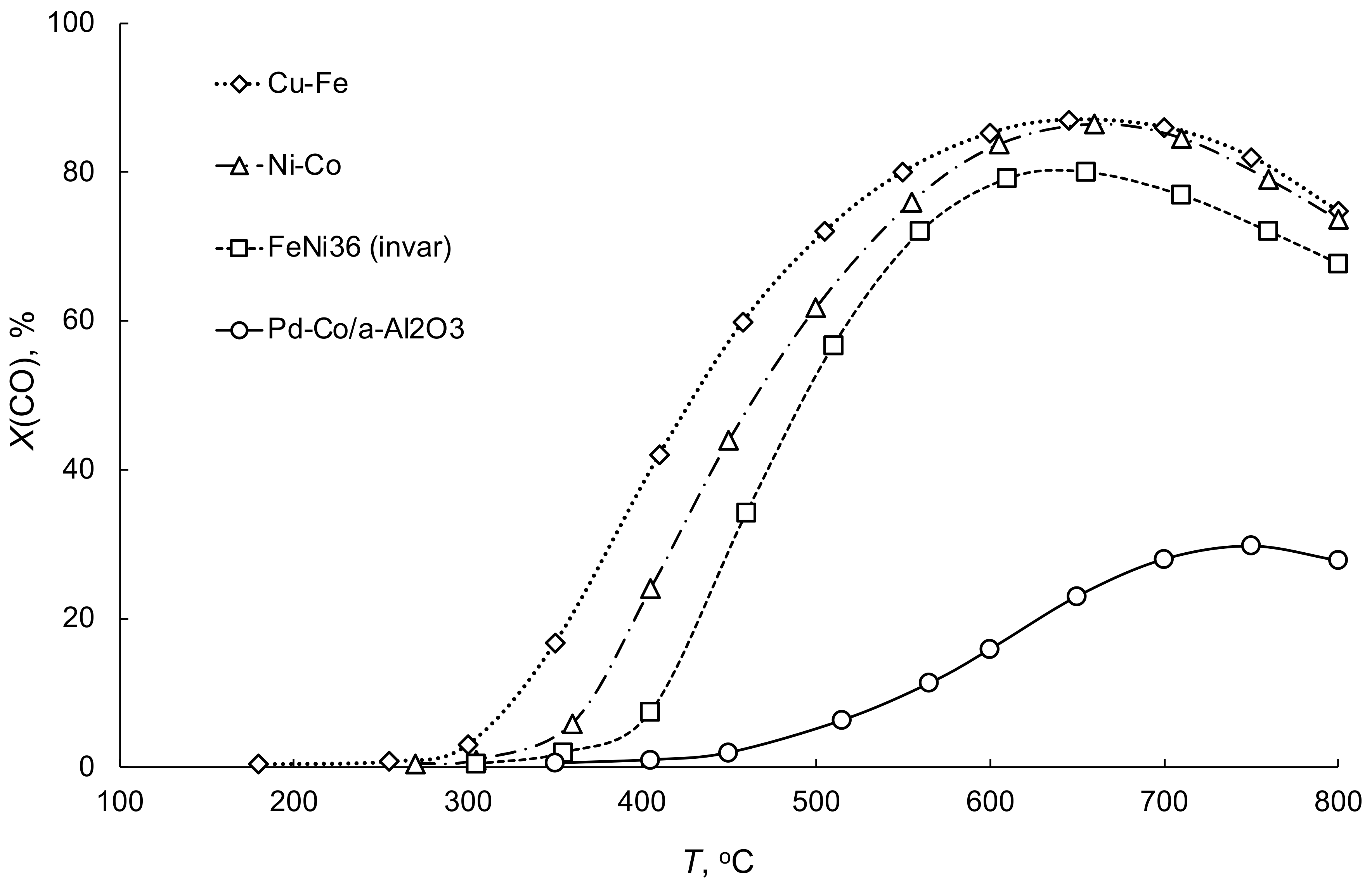
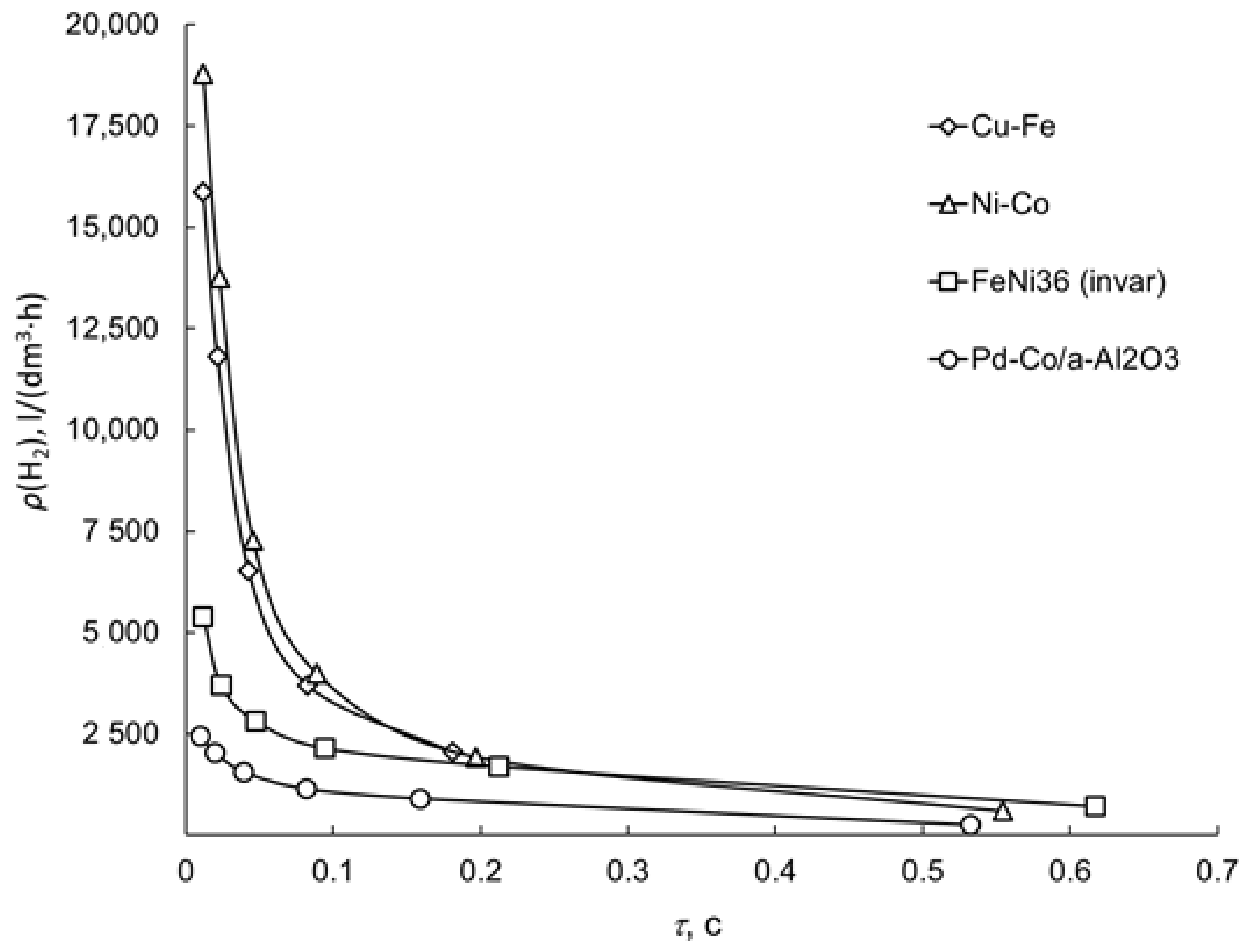

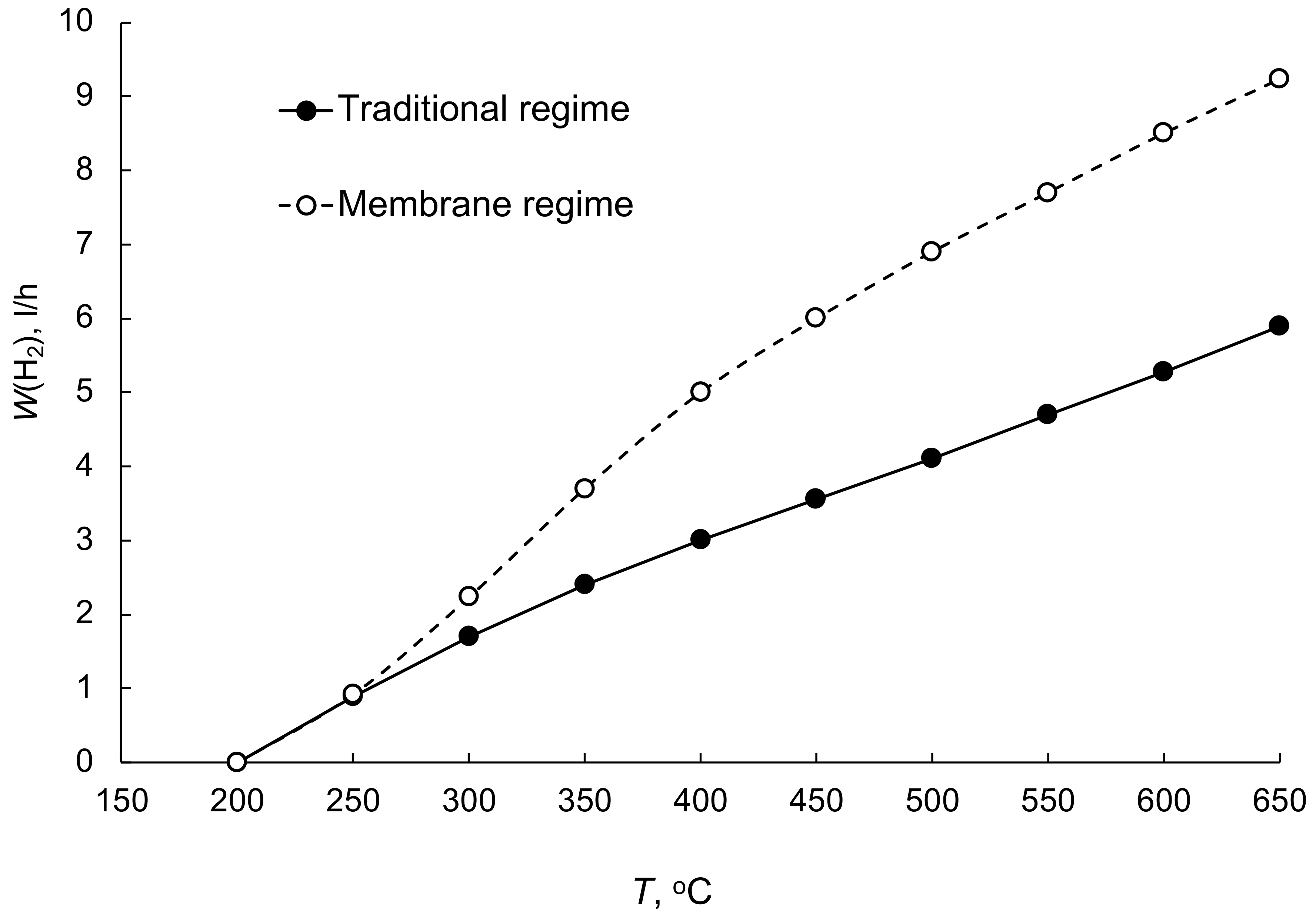
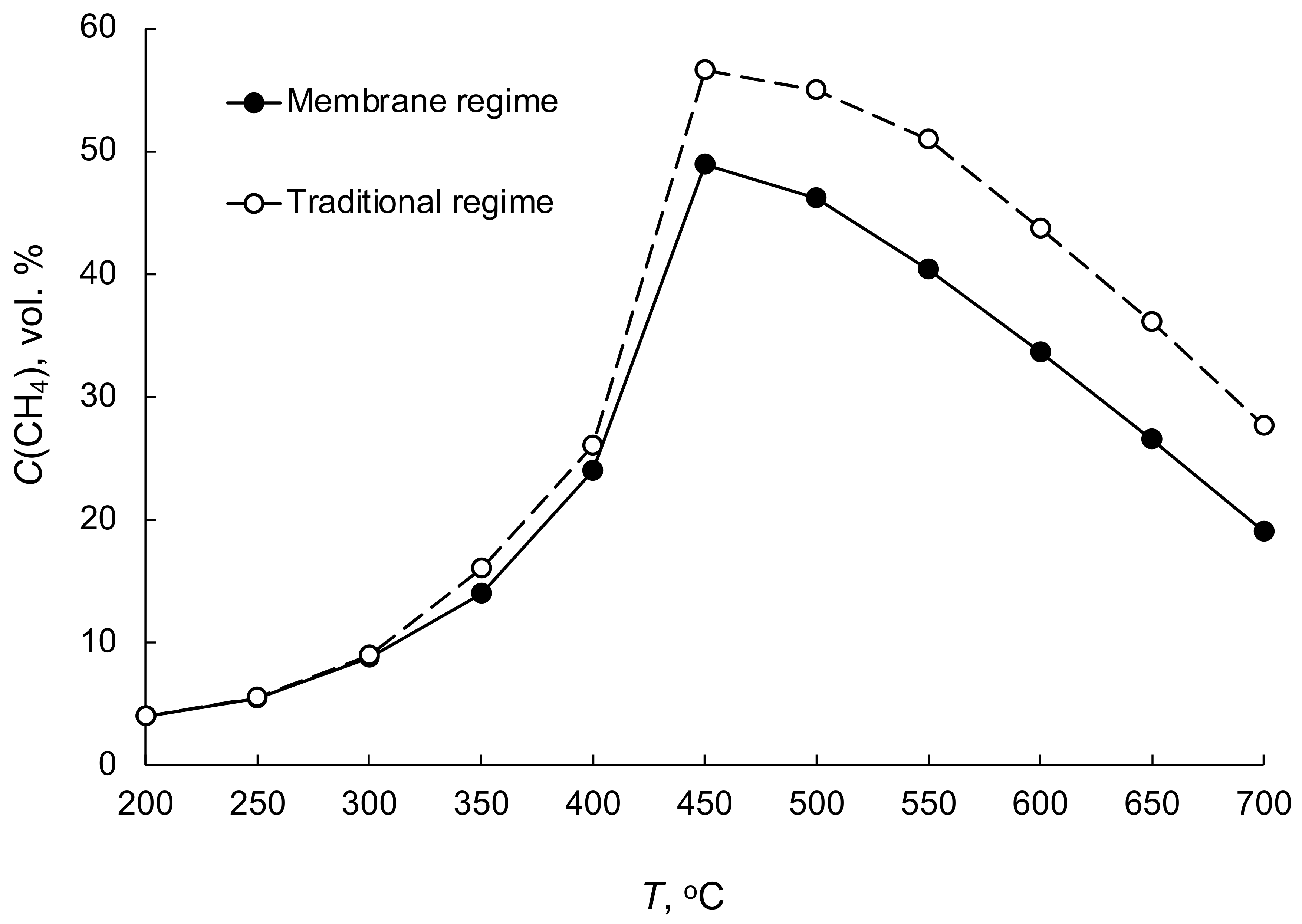
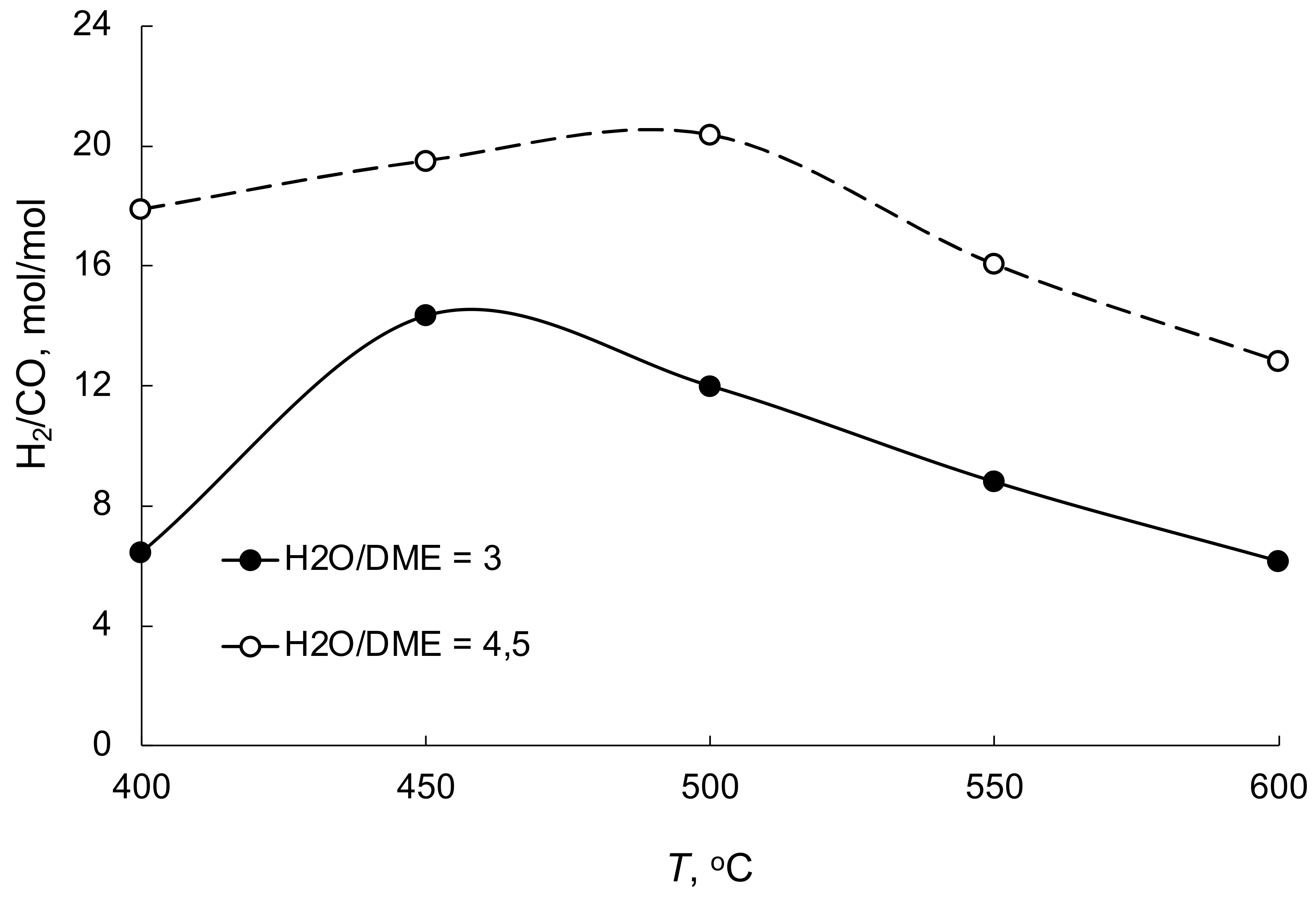

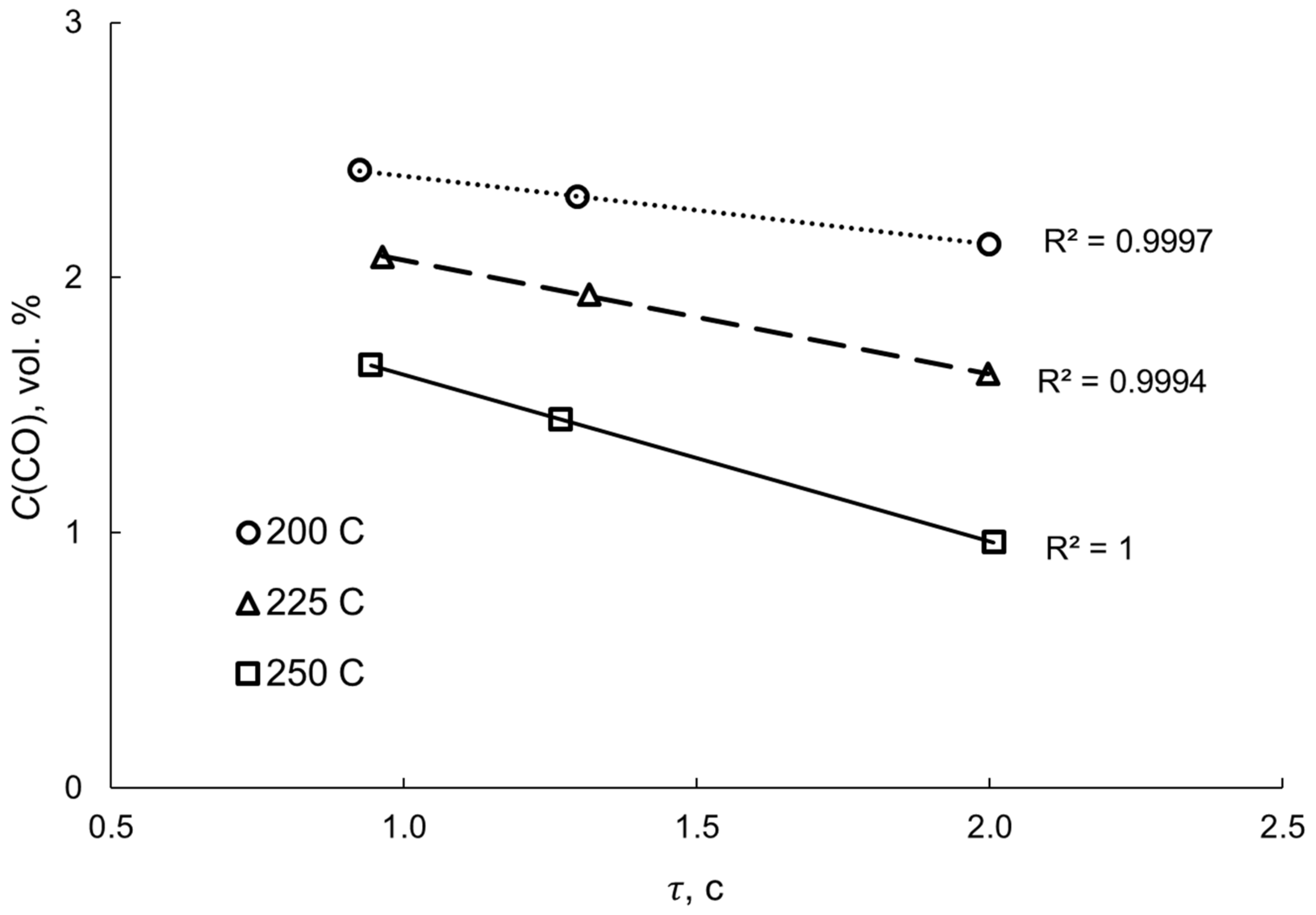

| No | Components | Component Contents, wt. % | Preparation Method |
|---|---|---|---|
| 1 | Ni(Al)-Co | 45(5)–50 | SHS |
| 2 | Ni-Co | 50–50 | SHS |
| 3 | Cu-Fe | 50–50 | SHS |
| 4 | FeNi36 (invar) | 64–36 | SHS |
| 5 | Pd-Co/α-Al2O3 | 0.034–0.017/α-Al2O3 | SHS + sol-gel |
| 6 | Pd/Ni-Al | 0.07/α-Al2O3 | SHS + sol-gel |
| 7 | Pd-Co/Ni-Al | 0.034–0.017/Ni-Al | SHS + sol-gel |
| 8 | Mn/Ni-Al | 0.07/α-Al2O3 | SHS + sol-gel |
| Converter | Component Concentrations in the Reaction Products, vol. % | |||
|---|---|---|---|---|
| H2 | CO | CH4 | CO2 | |
| Cu-Fe | 70.6 | 11.3 | 0.0 | 18.1 |
| Ni-Co | 47.0 | 7.2 | 0.2 | 45.7 |
| FeNi36 (invar) | 45.4 | 11.4 | 1.0 | 42.2 |
| Pd-Co/α-Al2O3 | 22.8 | 55.8 | 0.0 | 21.4 |
| Converter | Process Parameters | Concentration of Components, vol. % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T, °C | V, h−1 | X(CO), % | Initial Mixture | Reaction Products | |||||
| H2 | CO | H2 | CO | CH4 | CO2 | ||||
| Ni-Co | 450 | 7000 | 88.5 | 81.5 | 18.5 | 83.06 | 1.95 | 1.56 | 13.43 |
| Ni-Co | 500 | 7000 | 91.1 | 93.5 | 6.5 | 92.84 | 0.64 | 3.51 | 3.01 |
| 3500 | 96.5 | 91.56 | 0.31 | 5.53 | 2.60 | ||||
| Cu-Fe | 500 | 7000 | 55.0 | 94.72 | 2.40 | 1.37 | 1.53 | ||
| 3500 | 68.8 | 94.70 | 1.65 | 2.27 | 1.38 | ||||
| Ni-Co | 550 | 7000 | 92.4 | 98.2 | 1.8 | 97.51 | 0.19 | 1.48 | 0.82 |
| 3500 | 96.0 | 97.77 | 0.08 | 1.95 | 0.20 | ||||
| Alcohols | Ketones | Acids | ||||
|---|---|---|---|---|---|---|
| Methanol | Ethanol | Butanol | Pentanol | Acetone | Butanone | Acetic Acid |
| 2.8 | 18.2 | 1.3 | 0.4 | 1.3 | 4.0 | 5.0 |
| Q, h−1 | XCH4, % | Ρsyngas, l/(h∙dm3) | H2/CO, mol/mol |
|---|---|---|---|
| 16,000 | 99 | 7000 | 0.8 |
| 32,000 | 96 | 13,000 | 0.9 |
| 64,000 | 85 | 23,000 | 1.1 |
| Q, h−1 | Concentration, vol. % | |||
|---|---|---|---|---|
| H2 | CO | CH4 | CO2 | |
| 16,000 | 17.8 | 22.7 | 0.1 | 59.4 |
| 32,000 | 18.6 | 21.1 | 0.3 | 56.0 |
| 64,000 | 19.0 | 18.0 | 1.0 | 62.0 |
| Q, h−1 | Concentration, % | ||||||
|---|---|---|---|---|---|---|---|
| Alcohols | Ketones | Acids | |||||
| Methanol | Ethanol | Butanol | Pentanol | Acetone | Butanone | Acetic Acid | |
| 16,000 | 0 | 0.001 | 0 | 0 | 0 | 0 | 0 |
| 32,000 | 0.001 | 0.001 | 0 | 0 | 0 | 0 | 0 |
| 64,000 | 0.001 | 0.002 | 0 | 0 | 0 | 0 | 0 |
| Residual Combustible Components of the Mixture | C0, vol. % | Pd/Ni-Al | Pd-Co/Ni-Al | Mn/Ni-Al | |||
|---|---|---|---|---|---|---|---|
| C, vol. % | X, % | C, vol. % | X, % | C, vol. % | X, % | ||
| H2 | 0 | 38.85 | - | 35.85 | - | 36.23 | - |
| CO | 6.63 | 8.19 | 4.99 | 6.37 | 20.42 | 6.46 | 21.26 |
| CH4 | 2.12 | 0.29 | 89.60 | 0.76 | 70.50 | 0.65 | 76.00 |
| C2H4 | 4.16 | 0 | 100 | 0 | 100 | 0 | 100 |
| C3H6 | 0.72 | 0 | 100 | 0 | 100 | 0 | 100 |
| C4H10 | 0.56 | 0 | 100 | 0 | 100 | 0 | 100 |
| Model Mixture No. | Concentration, vol. % | |||
|---|---|---|---|---|
| H2 | CO | CH4 | CO2 | |
| 1 | 59.0 | 1.8 | 17.4 | 21.8 |
| 2 | 78.6 | 2.6 | 18.8 | - |
| Current Density | 300 mA/cm2 |
|---|---|
| Power density | 150 mW/cm2 |
| Specific volume | 3.3 L/kW |
| Specific weight | 1.6 kg/kW |
| Component | Concentration, vol. % |
|---|---|
| ethanol | 80 |
| propanol | 5 |
| n-butanol | 5 |
| n-pentanol | 10 |
| Substrate | H2O/sub., vol./vol. | Q, h−1 | T, °C | Reaction Products, vol. % | |||
|---|---|---|---|---|---|---|---|
| H2 | CO | CH4 | CO2 | ||||
| methane | 2 | 7000 | 800 | 73.0 | 13.0 | 1.0 | 14.0 |
| ethanol | 1,5 | 10,000 | 72.0 | 13.7 | 0.6 | 13.7 | |
| fermentation products | 7 | 15,000 | 73.8 | 3.6 | 0.6 | 22.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedotov, A.S.; Tsodikov, M.V.; Yaroslavtsev, A.B. Hydrogen Production in Catalytic Membrane Reactors Based on Porous Ceramic Converters. Processes 2022, 10, 2060. https://doi.org/10.3390/pr10102060
Fedotov AS, Tsodikov MV, Yaroslavtsev AB. Hydrogen Production in Catalytic Membrane Reactors Based on Porous Ceramic Converters. Processes. 2022; 10(10):2060. https://doi.org/10.3390/pr10102060
Chicago/Turabian StyleFedotov, A. S., M. V. Tsodikov, and A. B. Yaroslavtsev. 2022. "Hydrogen Production in Catalytic Membrane Reactors Based on Porous Ceramic Converters" Processes 10, no. 10: 2060. https://doi.org/10.3390/pr10102060
APA StyleFedotov, A. S., Tsodikov, M. V., & Yaroslavtsev, A. B. (2022). Hydrogen Production in Catalytic Membrane Reactors Based on Porous Ceramic Converters. Processes, 10(10), 2060. https://doi.org/10.3390/pr10102060







